Abstract
Increased carotid intima-media thickness (cIMT) is associated with heart failure (HF) in previous studies, but it is not known whether the association of cIMT differs between HF with reduced (HFrEF) versus preserved ejection fraction (HFpEF). We studied 6699 participants (mean age 62 ± 10 years, 47% male, and 38% white) from the Multi-Ethnic Study of Atherosclerosis (MESA) with baseline cIMT measurements. We classified HF events as HFrEF (EF <50%) or HFpEF (EF ≥ 50%) at the time of diagnosis. Cox proportional hazard regression was used to compute hazard ratios (HR), and 95% confidence intervals (CI) for the association between the IMT Z-score (measured maximum IMT of Internal Carotid (IC) and Common Carotid (CC) sites as the mean of the maximum IMT of the near and far walls of right and left sides), and incident HFrEF or HFpEF. Models were adjusted for covariates and interim coronary artery disease (CAD) events. A total of 191 HFrEF and 167 HFpEF events occurred during follow-up. In multivariable analysis, each 1 standard deviation increase in the measured maximum IMT (Z-score) was associated with both HFrEF and HFpEF in the unadjusted and demographically adjusted models [HR, 95% CI 1.57 (1.43-1.73)] and [HR, 95% CI 1.61 (1.47-1.77)] but not in the fully adjusted models [HR, 95% CI 1.11 (0.96-1.28)] and [HR, 95% CI 1.13 (0.98-1.30)]. In conclusion, cIMT was significantly associated with incident HF, but the association is partially attenuated with adjustment for demographic factors and becomes non-significant after adjustment for other traditional heart failure risk factors and interim CAD events. There was no difference in the association of IMT measures with HFrEF versus HFpEF.
Keywords: Carotid intima-media thickness, heart failure, coronary artery disease
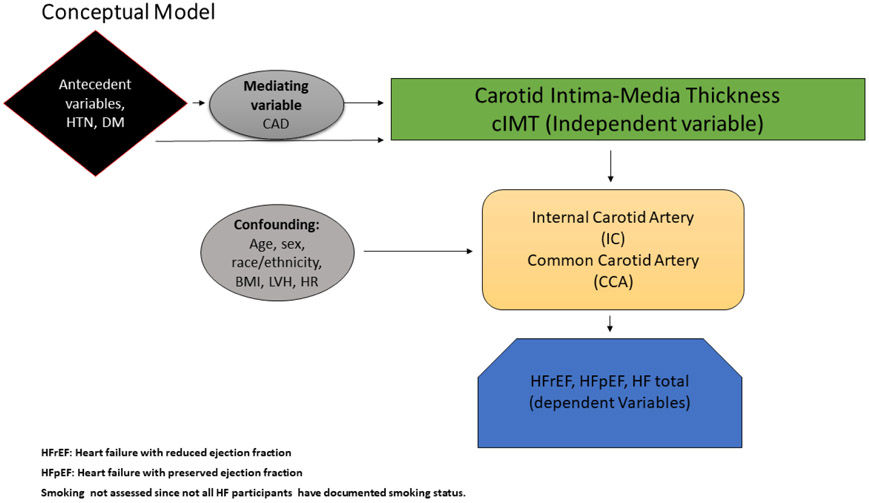
Heart failure (HF) related mortality, morbidity, health care costs, and poor quality of life are major public health problems in the United States as the prevalence and incidence of HF continue to rise.1-4 The prevalence and the rates of adverse clinical outcomes for both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF) are generally similar.5-7 Most studies of cIMT have focused on its relationship with coronary artery disease (CAD).8,9 However, cIMT has been shown to be associated with incident HF10. cIMT may be associated with risk of HFrEF due to shared atherosclerotic pathways.11,12 On the other hand, cIMT may be associated with HFpEF through mechanisms other than myocardial ischemia or infarct13. For instance, an increase in cIMT is associated with a decrease in arterial distensibility, which in turn leads to increased pressure afterload, pressure wave propagation, and diastolic dysfunction.14,15 Therefore, utilizing data from MESA we studied the association between cIMT and HF both overall and stratified by HFrEF versus HFpEF. We also studied the relationship between ICA versus CCA IMT with HFrEF and HFpEF (Figure 1).
Figure 1.
Conceptual Model showing Comparison of Relationship of Carotid Intima-Media thickness with Heart Failure All and Heart Failure Phenotypes.
Methods
MESA is a multi-ethnic, multicenter, prospective observational cohort 16 of 6,814 men and women aged 45 to 84 years without clinical CVD at baseline (participation rate was 60% among those eligible), who were recruited between July 2000 and August 2002 from 6 US communities (Forsyth county, NC, Baltimore, MD, Chicago, IL, Los Angeles County, CA, northern Manhattan, NY; and St. Paul, MN). All participants provided written informed consent and the study was approved by the institutional review boards at all field centers. For this analysis, participants (N=88) were excluded if they were missing baseline cIMT data.
Participant’s characteristics were collected during the initial MESA visit. Age, sex, race/ethnicity, and education were self-reported. Education was categorized as high school or less or some college or more. Smoking was defined as ever (current or former) versus never smoker. Blood samples were obtained after a 12-hour fast, and measurements of total cholesterol, high-density lipoprotein cholesterol, and plasma glucose were used. Diabetes mellitus was defined as fasting glucose values ≥126 mg/dl or a history of diabetes medication use. Blood pressure was measured for each participant after 5 minutes in the seated position, and systolic measurements were recorded 3 separate times, and the mean of the last 2 values was used. The use of aspirin, statins, and antihypertensive medications was collected by medication inventory. Body mass index was computed as the weight in kilograms divided by the square of height in meters. Resting heart rate was obtained from baseline ECGs.
The participants were imaged supine with their head rotated 45 degrees away from the side being imaged, and the images were recorded on superVHS videotape. The CCA was imaged at 45 degrees from the vertical with the beginning of the bulb shown to the left of the image. The ICA was imaged in three projections centered on the ICA flow divider: anterior, lateral (at 45 degrees), and posterior.17 A matrix array probe (M12L, General Electric, Waukesha, WI) was used.18 cIMT was measured on near and far walls of the common carotid (1 projection) and the ICA (3 projections) using hand-drawn continuous tracings of the intima-lumen and media-adventitia interfaces that were then processed using a previously described algorithm.19 The average of the mean far wall CC IMT and the maximum of the near and far wall IC IMT values seen on either side or projection were used for these analyses and it was consistent with prior studies.17,20
In addition, we created a composite Z score for overall maximal IMT by summing the maximum IMT from the two carotid IMT sites (right and left if both were measured) after standardization (subtraction of the mean and division by standard deviation of each measure), and then dividing by the standard deviation of the sum. If only one of the two measures were available, it was used. The resulting variable is hereafter referred to as Z score maximum IMT.21
The ascertainment of incident HF events in MESA has been described previously.22 Participants were contacted by telephone every 9 to 12 months or at MESA follow up examinations and data obtained for interim hospitalizations, outpatient diagnoses and deaths from baseline through December 31, 2013. Two physicians reviewed each record for independent endpoint classification and assignment of event dates. Incident HF was defined as including symptoms of HF, a physician diagnosis of HF, and another objective feature of HF (dilated or poor LV function, pulmonary edema by chest radiograph, heart failure treatment, or evidence of diastolic dysfunction). HF events were identified per the MESA events committee and they provide information on EF. HFpEF events were defined as cases with ejection fraction ≥50% per ACC/AHA guidelines, which classify patients with a LVEF of ≥50% as having a preserved EF.23 Comprehensive statistics were performed to characterize the data, and baseline characteristics were compared by HF status. Categorical variables were reported as frequency and percentage, whereas continuous variables were recorded as mean ±SD. Statistical significance for categorical variables was tested using χ2 method and the ANOVA procedure for continuous variables.
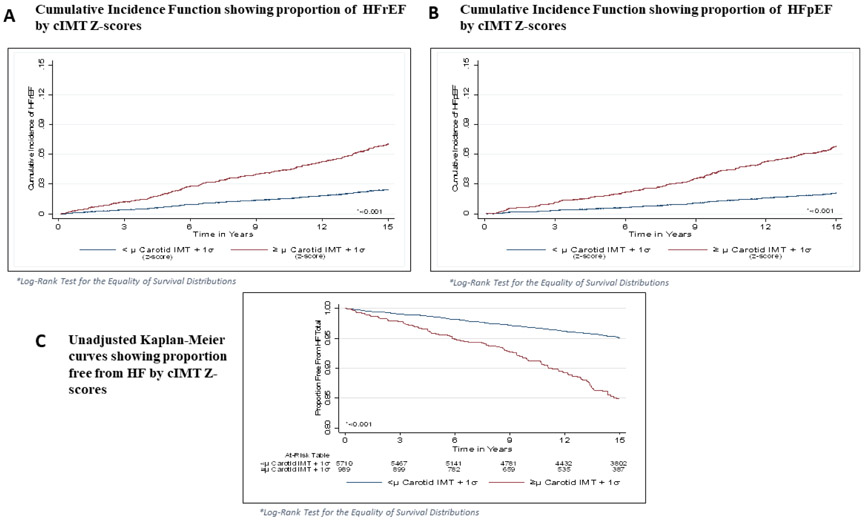
Follow-up time was defined as the time between the baseline cIMT measurement until a diagnosis of HF, death, loss to follow-up, or end of study follow-up (December 31, 2013). Cox regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CI) for the association of each CCA IMT and ICA IMT measurement with HF. P values for the HRs were computed using the likelihood ratio method. Separate analyses were conducted for HFrEF and HFpEF outcomes. In another set of analyses, Cox regression was used to compute HRs and 95% CI for the association between Z-score for maximal IMT with HF total, HFrEF and HFpEF (Figure 2), in which Z-score for maximal IMT (measured maximum IMT of the ICA and CCA sites) of the near and far walls of the right and left sides. A sequence of nested multivariable models were constructed as follows: model 1 adjusted for age, sex and race/ethnicity; model 2 adjusted for model 1 covariates plus body mass index (BMI), diabetes mellitus (DM), systolic blood pressure, left ventricular hypertrophy and heart rate; model 3 adjusted for model 1 and model 2 covariates in addition to interim CAD events. The Fine-Gray model was used to account for competing risk of developing HFrEF and HFpEF. This method allowed us to model time to first HF with either HFrEF or HFpEF as the main event of interest and the alternative as the competing risk. This method allowed mutual exclusivity of the classification of HF event types.24 Statistical significance was defined as p<0.05 SAS version 9.4 (Cary, NC, United States) was used for all analyses.
Figure 2.
Cumulative Incidence Function of proportion of HFrEF by cIMT Z-scores (A) Proportion of HFpEF by cIMT Z-scores (B) and of unadjusted Kaplan-Meier curves showing proportion free from HF by cIMT Z-scores (C).
Results
A total of 6699 participants (mean age 62 ± 10 years, 47% male, 38% whites, 20% blacks, 24% Hispanics, 14% Chinese American) were included in the final analysis. Over a median follow-up of 12.1 years, a total of 385 HF cases (incidence rate per 1000 person-years: 4.16) were identified. Of these, 191 (50%) were HFrEF and 167 (43%) were HFpEF. Baseline characteristics stratified by the development of HF are shown in Table 1. As shown, participants who did not develop HF were more likely to be younger, to be female, to have higher educational attainment, and to have fewer cardiovascular risk factors than those who developed HFrEF or HFpEF. Participants with incident HFpEF were more likely to be older, to be female, to report smoking, and to have higher systolic blood pressure and resting heart rate than participants with incident HFrEF.
Table 1.
Baseline characteristics by heart failure phenotypes
| No Heart Failure | ||||
|---|---|---|---|---|
| (N=6341) | HFrEF (N=191) | HFpEF (N=167) | P Value | |
| Age (years) | 61.8 +/− 10.2 | 67.2 +/− 9.0 | 61.8 +/− 10.2 | <0.001 |
| Men | 2952 (46.6%) | 128 (67.0%) | 78 (46.7%) | <0.001 |
| < 0.001 | ||||
| White | 2436 (38.4%) | 73 (38.2%) | 74 (44.3%) | 0.001 |
| Chinese | 773 (12.2%) | 5 (2.6%) | 17 (10.2%) | |
| Black | 1732 (27.3%) | 72 (37.7%) | 43 (25.8%) | |
| Hispanic | 1400 (22.1%) | 41 (21.5%) | 33 (19.8%) | |
| Education, high school or less | 5205 (82.3%) | 151 (79.5%) | 127 (76.1%) | 0.07 |
| Body mass index (kg/m2) | 28.2 +/− 5.4 | 29.3 +/− 5.3 | 30.2 +/− 6.2 | <0.001 |
| Diabetes mellitus | 742 (11.7%) | 53 (27.8%) | 44 (26.4%) | <0.001 |
| Total cholesterol (mg/dL) | 194.4 +/− 35.9 | 189.4 +/− 35.0 | 189.5 +/− 33.5 | 0.04 |
| Low-density lipoprotein cholesterol (mg/dL) | 117 +/− 31 | 114 +/− 32 | 112 +/− 29 | 0.079 |
| High-density lipoprotein cholesterol (mg/dL) | 51.1 +/− 14.9 | 47.3 +/− 13.0 | 49.9 +/− 13.7 | 0.002 |
| Lipid-lowering medication use | 1010 (15.9%) | 41 (21.5%) | 32 (19.2%) | 0.07 |
| Healthy diet* | 2858 (47.0%) | 85 (47.8%) | 84 (52.2%) | 0.42 |
| Systolic blood pressure (mmHg) | 126.9 +/− 21.2 | 137.0 +/− 22.7 | 139.3 +/− 22.9 | <0.001 |
| Heart rate (beat-per-minute) | 63.0 +/− 9.6 | 63.3 +/− 10.7 | 65.4 +/− 9.8 | 0.001 |
| Glomerular Filtration Rate (mL/min/1.73m2) | 74.6 +/− 16.3 | 70.9 +/− 19.2 | 71.3 +/− 19.3 | <0.001 |
| Left Ventricular Hypertrophy | 54 (0.86%) | 7 (3.7%) | 54 (0.86%) | <0.001 |
| Anti-hypertensive medication use | 2261 (36.0%) | 112 (58.6%) | 98 (58.7%) | <0.001 |
| Family history of coronary heart disease | 2516 (42.3%) | 91 (51.7%) | 69 (44.0%) | 0.04 |
| Cigarette smoking | ||||
| Never | 3217 (50.9%) | 80 (42.1%) | 72 (43.1%) | |
| Former | 2281 (36.1%) | 81 (42.6%) | 78 (46.7%) | |
| Current | 824 (13.0%) | 29 (15.3%) | 17 (10.2%) | |
| Alcohol use | <0.001 | |||
| Never | 1302 (20.7%) | 27 (14.2%) | 33 (19.8%) | |
| Former | 1469 (23.3%) | 64 (33.7%) | 58 (34.7%) | |
| Current | 3525 (56.0%) | 99 (52.1%) | 76 (45.5%) | |
| Internal Carotid IMT (mm) | 1.1 +/− 0.59 | 1.3 +/− 0.71 | 1.4 +/− 0.73 | <0.001 |
| Common Carotid IMT (mm) | 0.87 +/− 0.19 | 0.95 +/− 0.21 | 0.95 +/− 0.18 | <0.001 |
| Z- Score Maximum IMT | −0.025 +/− 0.989 | 0.487 +/− 1.099 | 0.512 +/− 1.025 | <0.001 |
Continuous variables presented as mean (standard deviation) and categorical variables as count (percentage).
Healthy diet consisted of adequate quantities of 5 items identified by American heart Association (fruits and vegetables, fish, wholegrains, sodium <1500 mg/day, and sugar-sweetened beverages ≤450 kcal (36 oz) per week).
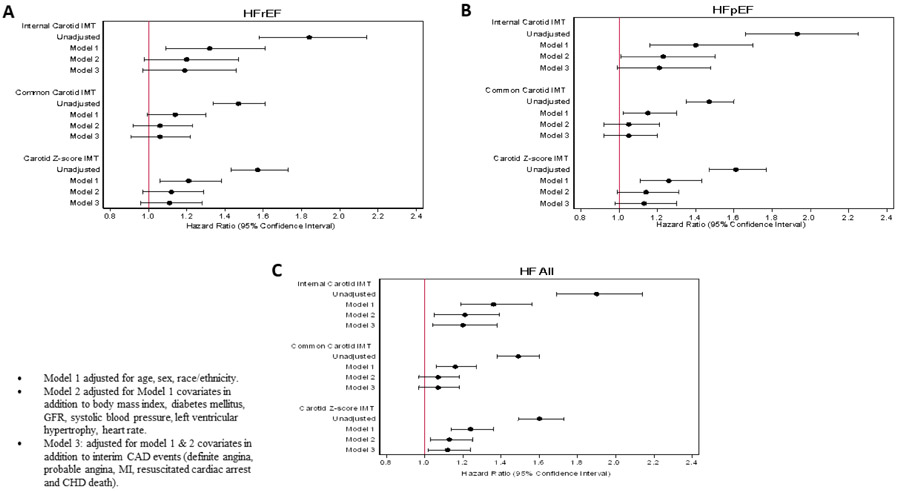
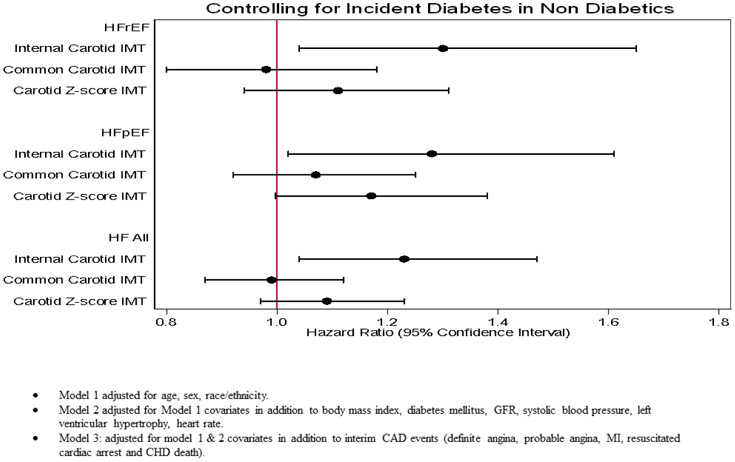
In multivariable analysis, we computed hazard ratios (HR), and 95% confidence intervals (CI) for the association between the ICA IMT and incident HF, HFrEF and HFpEF. Models were adjusted for covariates and interim CAD. In the unadjusted model, ICA IMT was significantly associated with total HF (HR= 1.90, 95% CI: 1.69 to 2.14), HFrEF (HR= 1.84, 95% CI: 1.58 to 2.14), and HFpEF (HR= 1.93, 95% CI: 1.66 to 2.25) p<0.001. The strength of this association was partially attenuated with adjustment for demographic factors, total HF, HFrEF and HFpEF (Table 2, figure 3). Furthermore, after adjustment for other traditional risk factors and interim CAD events, there were no significant associations between ICA IMT and HFrEF or HFpEF (Table 2, Figure 3 a,b). Moreover, there was a nominal positive association between ICA IMT and total HF (Figure 3c). Finally, after controlling for diabetes in non-diabetic there was a statistically significant association between ICA IMT and total HF, HFrEF and HFpEF (Figure 4).
Table 2.
Hazard ratios (95% confidence interval) for the association between internal carotid (IC), common carotid (CC) intimal-media thickness (IMT) and incident heart failure with reduced and preserved ejection fractions
| Internal carotid IMT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Events (n) |
Unadjusted | Model 1 | Model 2 | Model 3 | ||||
| HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
||
| HFrEF | 191 |
1.84 (1.58-2.14) |
<0.001 |
1.32 (1.09-1.61) |
0.005 | 1.20 (0.98-1.47) |
0.08 | 1.19 (0.97-1.46) |
0.11 |
| HFpEF | 167 |
1.93 (1.66-2.25) |
<0.001 |
1.40 (1.16-1.70) |
<0.001 | 1.23 (1.01-1.50) |
0.04 | 1.21 (0.99-1.48) |
0.069 |
| HF, total | 385 |
1.90 (1.69-2.14) |
<0.001 |
1.36 (1.19-1.56) |
<0.001 |
1.21 (1.05-1.39) |
0.008 |
1.20 (1.04-1.38) |
0.02 |
| Common carotid IMT | |||||||||
| Outcome | Events (n) |
Unadjusted | Model 1 | Model 2 | Model 3 | ||||
| HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
||
| HFrEF | 191 |
1.47 (1.34, 1.61) |
<0.001 | 1.14 (0.995, 1.30) |
0.059 | 1.06 (0.92, 1.23) |
0.43 | 1.06 (0.91, 1.22) |
0.91 |
| HFpEF | 167 |
1.47 (1.35, 1.60) |
<0.001 |
1.15 (1.02, 1.30) |
0.02 | 1.05 (0.92, 1.21) |
0.454 | 1.05 (0.92, 1.20) |
0.476 |
| HF, total | 385 |
1.49 (1.38, 1.60) |
<0.001 |
1.16 (1.06, 1.27) |
0.002 | 1.07 (0.97, 1.18) |
0.17 | 1.07 (0.97, 1.18) |
0.20 |
• Model 1 adjusted for age, sex, race/ethnicity.
• Model 2 adjusted for Model 1 covariates in addition to body mass index, diabetes mellitus, systolic blood pressure, left ventricular hypertrophy, heart rate.
• Model 3: adjusted for model 1 & 2 covariates in addition to interim CAD events (definite angina, probable angina, MI, resuscitated cardiac arrest and CHD death).
• Bolded items are significant.
Figure 3.
Showing Comparison of Relationship HR (95% CI) of Carotid IMT Subsegment and Z-score IMT with HF All (C) and HF Phenotypes (A, B).
Figure 4.
Showing Comparison of Relationship HR (95% CI) of Carotid IMT Subsegment and Z-score IMT with HF All and HF Phenotypes after Controlling for Incident Diabetes in Non-Diabetics.
In a similar manner we computed HR, and 95% CI for the association between the CCA IMT and incident HF, HFrEF and HFpEF. In the unadjusted model, (1 SD, 0.19 mm increase in IMT) was significantly associated with total HF (HR= 1.49, 95% CI: 1.38 to 1.60), HFrEF (HR= 1.47, 95% CI: 1.34 to 1.61), and HFpEF (HR= 1.47, 95% CI: 1.35 to 1.60) p<0.001. The strength of association between CCA IMT with incident HF and HFrEF was partially attenuated with adjustment for demographic factors, total HF and HFrEF (Table 2, figure 3). Furthermore, after adjustment for other traditional risk factors and interim CAD events, there were no significant associations between CCA IMT and total HF, HFrEF or HFpEF (Table 2, figure 3).
Cox proportional hazard regression was used to compute HR, and 95% CI for the association between the Z-score IMT (measured maximum IMT of ICA and CCA sites as the mean of the maximum IMT of the near and far walls of right and left sides), and incident HF, HFrEF and HFpEF, models were adjusted for covariates and interim CAD events (Table 3). In the unadjusted model (Figure 2), Z-score IMT (1 SD, 0.18 mm increase in IMT) was significantly associated with total HF (HR= 1.60, 95% CI: 1.49 to 1.73), HFrEF (HR= 1.57, 95% CI: 1.43 to 1.73), and HFpEF (HR= 1.61, 95% CI: 1.47 to 1.77) p<0.001 (Table 3). The strength of association between Z-score IMT with incident HF and its phenotypes is partially attenuated with adjustment for demographic factors, total HF, HFrEF and HFpEF (Table 3). Furthermore, after adjustment for other traditional risk factors and interim CAD events, there were no significant associations between Z-score IMT and HFrEF or HFpEF (Table 3, Figure 3 a,b). Moreover, there was a nominal positive association between Z-score IMT and total HF (Figure 3c). Finally, after controlling for diabetes in non-diabetics there was no significant association between Z-score IMT and total HF including HFrEF and HFpEF (Figure 4).
Table 3.
Unadjusted and Adjusted HRs (95% CI) for Incident HFrEF, HFpEF and HF total per 1 SD (0.18 mm) increase in Carotid intima-media thickness (IMT)*
| Outcome | Unadjusted | Model 1 | Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) |
p-value | HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
|
| HFrEF | 1.57 (1.43-1.73) | <0.001 | 1.21 (1.06-1.38) | 0.004 | 1.12 (0.97-1.29) | 0.122 | 1.11 (0.96-1.28) | 0.15 |
| HFpEF | 1.61 (1.47-1.77) | <0.001 | 1.26 (1.11-1.43) | <0.001 | 1.14 (0.99-1.31) | 0.068 | 1.13 (0.98-1.30) | 0.100 |
| HF, total | 1.60 (1.49-1.73) | <0.001 | 1.24 (1.14-1.36) | <0.001 | 1.13 (1.03-1.25) | 0.01 | 1.12 (1.02, 1.24) | 0.018 |
Z-score for maximal IMT (measured maximum IMT of the IC and CC sites as the mean of the maximum IMT of the near and far walls of the right and left sides).
Model 1 adjusted for age, sex, race/ethnicity.
Model 2 adjusted for Model 1 covariates in addition to body mass index, diabetes mellitus, systolic blood pressure, left ventricular hypertrophy, heart rate.
Model 3: adjusted for model 1 & 2 covariates in addition to interim CAD events.
Bolded items are significant.
Discussion
The objective of this study was to investigate the association of carotid IMT with incident HF and HF phenotypes. There were significant unadjusted associations between carotid IMT combined measurement and incident HFrEF, HFpEF and HF total, but these were partially attenuated with adjustment for demographic factors and became non-significant after adjustment for other traditional HF risk factors and interim CAD events. Similarly, neither ICA nor CCA IMT was significantly associated with HFrEF and HFpEF after adjustment for traditional HF risk factors.
Carotid IMT is a well validated measure of pre-clinical atherosclerosis.25,26 In this community based study, we found mean carotid IMT to be 0.87±0.19 mm. Relatively similar mean far wall estimates have been reported in other populations of similar age groups; in the Carotid Atherosclerosis Progression Study27 and Malmo Diet and Cancer Study,8 mean far wall IMT was 0.73±0.16 mm and 0.77±0.15 mm, respectively. The present study has shown that carotid IMT is not independently associated with incident HF, with IMT modeled as combined measured (Z-score) variable and by carotid location subtype (CCA IMT, ICA IMT) variables, after taking into account potential confounding by age, gender, race, BMI, diabetes mellitus, systolic blood pressure, left ventricular hypertrophy, heart rate and interim CAD events. Our results differ somewhat from those described by Engstrom et al,28 who reported a significant association of increased IMT and HF hospitalizations in a sample of 4691 subjects with 75 cases of HF. We show no association after adjustment for prevalent and incident cases of CAD, which is a major cause of HFrEF.10 Findings from a recent study from the Atherosclerosis Risk in Communities Study (ARIC)29 that evaluated the association between cIMT and incident HF showed that cIMT was a weaker predictor of incident HF among individuals with normal fasting glucose than those with impaired fasting glucose or type 2 DM. (HR per SD increase in cIMT for DM 1.12, 95% CI: 1.05 to 1.21; compared with HR for normal fasting glucose 1.27, 95% CI: 1.20 to 1.34 and for impaired fasting glucose 1.18 (1.11 to 1.25)) p=0.015. However, in our study the fully adjusted models show no associations between carotid IMT combined measurement and incident HFrEF, HFpEF and HF total.
Finally, in a Sweden cohort consisting of 4692 subjects the authors examined the association between cIMT and systemic inflammation marker level such as high sensitivity C-reactive protein with incidence of heart failure hospitalization.28 The outcome of their study was hospitalization with a primary diagnosis of acute decompensated total HF with already altered inflammatory biomarkers. Thus, the results of their study support our study findings and could not prove any independent relationship between cIMT and HF.
One potential mechanism linking cIMT and incident heart failure is suggested by studies showing an association between increasing CC IMT with reduced myocardial flow reserve in adults with and without30-32 CAD. Some prospective and cross-sectional studies report an association between increasing carotid IMT and regional LV myocardial systolic and diastolic dysfunction,33,34 as a predictor of HF,35 but none of these studies were able to establish a relationship between carotid IMT and HF. Furthermore, aging and hypertension play a major role in carotid artery thickness, dilatation and remodeling.36 Enlargement and thickening of carotid arteries with aging are generally attributed to fracture of the load-bearing elastin fibers in response to the fatiguing effect of tensile stress.33,37,38 Indeed no study addressed a relationship through aforementioned pathophysiology. At the same time most prior studies they utilized different imaging protocols10,29,39 may be difficult to interpret in a comparison to our approach with IMT modeled as combined measured (Z-score).
By 2030, the prevalence of HF is projected to increase by 23%, with medical costs increasing to nearly $53 billion.40 Accordingly, the identification of at-risk individuals by a low-cost, noninvasive, reproducible and safe measure is important. Although cIMT is associated with incident HF, our study shows this is not independent of other established cardiovascular risk factors. To the best of our knowledge, our study is the first to investigate the association of carotid IMT (including IC and CC IMT) with both incident HFrEF and HFpEF in a large diverse cohort of people who were followed for more than a decade. There are nonetheless a few limitations to this study that should be mentioned. First, although rigorous methods were used to account for all HF cases, some events may have been missed. Second, we did not adjust our analyses for novel biomarkers that could potentially influence the relationship between carotid IMT and HF, such as N-terminal pro-brain natriuretic peptide (NT-proBNP) and high-sensitivity C-reactive protein. However, in a prior published study, the association of carotid IMT with HF remained unaffected by the presence of these markers.24,28 This study demonstrated that increasing IMT is not associated with incident HF (whether HFrEF or HFpEF) independent of traditional cardiovascular risk factors and CAD. Further research may be needed to determine whether other imaging or other tests are able to identify individuals in whom targeted preventive therapies are warranted to reduce the current and future burden of HF.
Acknowledgments
The authors thank the investigators, staff, and participants of MESA (Multi-Ethnic Study of Atherosclerosis) for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Source of Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources. Measurement of ECC was supported by R01 HL071739. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Disclosures:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J 1997;133:703–712. [DOI] [PubMed] [Google Scholar]
- 3.Majani G, Pierobon A, Giardini A, Callegari S, Opasich C, Cobelli F, Tavazzi L. Relationship between psychological profile and cardiological variables in chronic heart failure. The role of patient subjectivity. Eur Heart J 1999;20:1579–1586. [DOI] [PubMed] [Google Scholar]
- 4.Tavazzi L [Practical advice on the treatment of cardiac insufficiency with beta-blockers. Announcement by the Italian Association of Hospital-based Cardiologists]. Rev Port Cardiol 1999;18:556–557. [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 7.Ho JE, Gona P, Pencina MJ, Tu JV, Austin PC, Vasan RS, Kannel WB, D'Agostino RB, Lee DS, Levy D. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J 2012;33:1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med 2005;257:430–437. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 10.Effoe VS, Rodriguez CJ, Wagenknecht LE, Evans GW, Chang PP, Mirabelli MC, Bertoni AG. Carotid intima-media thickness is associated with incident heart failure among middle-aged whites and blacks: the Atherosclerosis Risk in Communities study. J Am Heart Assoc 2014;3:e000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geroulakos G, O'Gorman DJ, Kalodiki E, Sheridan DJ, Nicolaides AN. The carotid intima-media thickness as a marker of the presence of severe symptomatic coronary artery disease. Eur Heart J 1994;15:781–785. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, Tracy R, Gardin JM, Price TR, Furberg CD. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke 1996;27:224–231. [DOI] [PubMed] [Google Scholar]
- 13.Sharma K, Al Rifai M, Ahmed HM, Dardari Z, Silverman MG, Yeboah J, Nasir K, Sklo M, Yancy C, Russell SD, Blumenthal RS, Blaha MJ. Usefulness of Coronary Artery Calcium to Predict Heart Failure With Preserved Ejection Fraction in Men Versus Women (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2017;120:1847–1853. [DOI] [PubMed] [Google Scholar]
- 14.Benetos A, Laurent S, Asmar RG, Lacolley P. Large artery stiffness in hypertension. J Hypertens Suppl 1997;15:S89–97. [DOI] [PubMed] [Google Scholar]
- 15.Cuspidi C, Lonati L, Macca G, Sampieri L, Fusi V, Michev I, Severgnini B, Salerno M, Magrini F, Zanchetti A. Prevalence of left ventricular hypertrophy and carotid thickening in a large selected hypertensive population: impact of different echocardiographic and ultrasonographic diagnostic criteria. Blood Press 2001;10:142–149. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 17.Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke 2011;42:3017–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Leary DH, Polak JF, Wolfson SK Jr., Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke 1991;22:1155–1163. [DOI] [PubMed] [Google Scholar]
- 19.Polak JF, Pencina MJ, Herrington D, O'Leary DH. Associations of edge-detected and manual-traced common carotid intima-media thickness measurements with Framingham risk factors: the multi-ethnic study of atherosclerosis. Stroke 2011;42:1912–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB Sr, Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med 2011;365:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 24.Silverman MG, Patel B, Blankstein R, Lima JA, Blumenthal RS, Nasir K, Blaha MJ. Impact of Race, Ethnicity, and Multimodality Biomarkers on the Incidence of New-Onset Heart Failure With Preserved Ejection Fraction (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2016;117:1474–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams MR, Celermajer DS. Detection of presymptomatic atherosclerosis: a current perspective. Clin Sci (Lond) 1999;97:615–624. [PubMed] [Google Scholar]
- 26.Poli A, Tremoli E, Colombo A, Sirtori M, Pignoli P, Paoletti R. Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients. A new model for the quantitation and follow-up of preclinical atherosclerosis in living human subjects. Atherosclerosis 1988;70:253–261. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006;37:87–92. [DOI] [PubMed] [Google Scholar]
- 28.Engstrom G, Melander O, Hedblad B. Carotid intima-media thickness, systemic inflammation, and incidence of heart failure hospitalizations. Arterioscler Thromb Vasc Biol 2009;29:1691–1695. [DOI] [PubMed] [Google Scholar]
- 29.Effoe VS, McClendon EE, Rodriguez CJ, Wagenknecht LE, Evans GW, Chang PP, Bertoni AG. Diabetes status modifies the association between carotid intima-media thickness and incident heart failure: The Atherosclerosis Risk in Communities study. Diabetes Res Clin Pract 2017;128:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dayanikli F, Grambow D, Muzik O, Mosca L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation 1994;90:808–817. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama I, Ohtake T, Momomura S, Nishikawa J, Sasaki Y, Omata M. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation 1996;94:3232–3238. [DOI] [PubMed] [Google Scholar]
- 32.Raitakari OT, Toikka JO, Laine H, Ahotupa M, Iida H, Viikari JS, Hartiala J, Knuuti J. Reduced myocardial flow reserve relates to increased carotid intima-media thickness in healthy young men. Atherosclerosis 2001;156:469–475. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, Bluemke DA, Nasir K, O'Leary DH, Lima JA. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2006;47:2420–2428. [DOI] [PubMed] [Google Scholar]
- 34.Parrinello G, Colomba D, Bologna P, Licata A, Pinto A, Paterna S, Scaglione R, Licata G. Early carotid atherosclerosis and cardiac diastolic abnormalities in hypertensive subjects. J Hum Hypertens 2004;18:201–205. [DOI] [PubMed] [Google Scholar]
- 35.Yan RT, Bluemke D, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, McClelland R, Lima JA. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2011;57:1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation 1999;100:1387–1393. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O'Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2008;28:194–201. [DOI] [PubMed] [Google Scholar]
- 38.Pavlopoulos H, Nihoyannopoulos P. Pulse pressure/stroke volume: a surrogate index of arterial stiffness and the relation to segmental relaxation and longitudinal systolic deformation in hypertensive disease. Eur J Echocardiogr 2009;10:519–526. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG, Group P-IS. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012;379:2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention, Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]