Abstract
Cancer is the second deadliest disease worldwide. Although recent advances applying precision treatments with targeted (molecular and immune) agents are promising, the histological and molecular heterogeneity of cancer cells and huge mutational burdens (intrinsic or acquired after therapy) leading to drug resistance and treatment failure are posing continuous challenges. These recent advances do not negate the need for alternative approaches such as chemoprevention, the pharmacological approach to reverse, suppress or prevent the initial phases of carcinogenesis or the progression of premalignant cells to invasive disease by using nontoxic agents. Although data are limited, the success of several clinical trials in preventing cancer in high-risk populations suggests that chemoprevention is a rational, appealing, and viable strategy to prevent carcinogenesis. Particularly among higher risk groups the use of safe, nontoxic agents is the utmost consideration since these individuals have not yet developed invasive disease. Natural dietary compounds present in fruits, vegetables and spices are especially attractive for chemoprevention and treatment due to their easy availability, high margin of safety, relatively low cost and wide-spread human consumption. Hundreds of such compounds have been widely investigated for chemoprevention and treatment in the last few decades. Previously, we reviewed the most widely studied natural compounds and their molecular mechanisms, which were highly exploited by the cancer research community. In the time since our initial review, many promising new compounds have been identified. In this review, we critically review these promising new natural compounds, their molecular targets and mechanisms of anti-cancer activity which may create novel opportunities for further design and conduct of preclinical and clinical studies.
Keywords: Chemoprevention, Natural Compounds, Molecular Targets, Apoptosis
Introduction
Cancer is a major public health concern worldwide and is the second leading cause of deaths in the United States. In 2020, approximately 1,806,590 new cancer cases and 606,520 cancer deaths are projected to occur in the United States, which translates into about 1,663 deaths per day [1]. At the same time, cancer is taking an enormous toll in dollars around the world and is becoming a growing economic threat among low to middle-income countries. While there have been substantial improvements in cancer survival, the economic burden of medical costs for cancer treatments in the US is increasing significantly due to the increasing age of the population and trends in treatment patterns following cancer diagnosis. The estimated and projected economic burden of cancer, including health care expenditures, productivity loss, and morbidity for patients and their families, has become an alarming issue for health care policy makers, healthcare systems, physicians, employers, and the society overall [2]. Further, despite the advances of ongoing cancer treatments including surgery, radiation therapy, conventional chemotherapy, hormone therapy, immune therapy and targeted therapy, the overall disease-free survival rate is still disappointing and complete recovery remains a dream for many cancer patients. Moreover, drug-associated toxicities such as gastrointestinal, musculoskeletal or constitutional symptoms, hair loss, heart or kidney problems, lung tissue damage or nerve damage, infertility etc. are posing additional challenges. Therefore, the search for non-toxic alternative remedies including use of non-toxic natural agents for chemoprevention and treatment in addition to changing dietary habits and lifestyle are drawing increasing attention as a cost effective means to reduce society’s cancer burden. Due to their easy availability, cheaper price and wide-margin of safety, plant-derived natural products have made a tremendous impact in drug discovery endeavors, receiving US Food and Drug Administration approval and are gaining increasing attention for chemoprevention as well as treatment [3–6]. Natural compounds generally exhibit multi-targeted effects affecting diverse molecular targets including transcription factors, cytokines, chemokine’s, adhesion molecules, growth factor receptors, and inflammatory enzymes etc. [7–9]. Moreover, the combination of natural compounds with standard chemotherapeutic drugs have significantly improved patient survival by making cancer cells more sensitive to chemotherapy and radiotherapy [10]. In the current review, we focus on an update of the biology and molecular targets of emerging natural compounds which possess high potential to combat cancer.
Overview of established natural products and prospective new natural compounds
The increase in cancer incidence along with undesirable side effects observed with conventional chemotherapy drugs demands the discovery of new, safe and effective agents. Nature has traditionally served as a rich repository of chemicals. Sesquiterpenes, flavonoids, alkaloids, diterpenoids, and polyphenols represent a diverse group of compounds available in fruits and vegetables. These compounds, derived from medicinal plants, have been a vital source of anticancer therapies [11]. The anti-cancer activity of dietary botanicals, including cruciferous vegetables such as cabbage and broccoli, Allium vegetables such as garlic and onion, green tea, Citrus fruits, soybeans, tomatoes, berries, and ginger, as well as medicinal plants have been well established preclinically [4, 12, 13].
More than 3000 plant species containing hundreds of active compounds have been reported to possess anticancer activities and about thirty plant-derived compounds have been isolated so far that have been tested in clinical trials [14]. The list includes curcumin (from turmeric), resveratrol (from red grapes or red wine), epigallocatechin gallate (EGCG, from green tea), genistein (from soybeans), lycopene (from tomatoes), luteolin (from green vegetables), pomegranate (from pomegranate fruit), n-3-polyunsaturated fatty acid (from corn oil, sunflower oil), brassinin (from cruciferous vegetables), and indole-3-carbinol (from broccoli) etc. Having previously reviewed these compounds and some other extensively investigated compounds in detail, these compounds are excluded from this review [4, 13]. However, these compounds are summarized in Table 1. Most of these compounds selectively inhibit cell proliferation and induce growth arrest and apoptosis by targeting multiple cellular signaling pathways including transcription factors, growth factors, tumor cell survival factors, inflammatory cytokines, protein kinases, and angiogenic factors that are frequently deregulated in cancers (Table 1). Despite the potential activity against tumorigenesis and malignancy, poor pharmacokinetics including low bioavailability, limited tissue distribution, rapid metabolism and excretion from body impede the success of many natural compounds in clinical applications. To date none of them have been approved for clinical application. However, in last decade, many other promising natural compounds have emerged. The current article will discuss these natural compounds with potential anti-cancer activities. The following section of this review is intended to provide a flavor of some of these promising natural compounds. We have chosen those compounds with solid evidence of anti-cancer properties supported by multiple preclinical in vitro and in vivo studies (Fig. 1, Table 2). We anticipate that some promising compounds and references are not included in this review due to word and reference limits. This is completely unintentional and we regret to those investigators whose studies are not included.
Table 1:
Sources, mechanism of action, molecular targets and limitations of well-studied natural compounds that are tested clinically
| Active Compound | Chemical Structure | Natural Source | Active against | Mechanism of Action | Limitations |
|---|---|---|---|---|---|
| Curcumin | 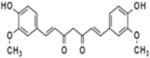 |
Turmeric | Lung, Colon, Lymphoma, Pancreas, Skin, Oral cavity, Small intestine, prostate, Glioblastoma, and Head & Neck cancer | Anti-proliferative (cell cycle arrest and apoptosis), anti-oxidant, anti-inflammatory, anti-angeogenic, and immunomodulatory | Low bioavailability, limited tissue distribution, rapid metabolism, and rapid excretion from the body |
| Resveratrol | 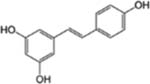 |
Red Grape, Red wine | Colon, Ovary, Prostate, Lung, Gastrointestine, Liver, Leukemia, Breast and Pancreatic cancer | Anti-proliferative (cell cycle arrest and apoptosis), anti-inflammatory and anti-angiogenic effects | Poor bio-availability following oral ingestion, and first metabolically eliminated from the body. |
| Epigallo catechin gallate (EGCG) | 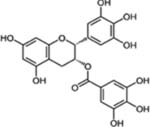 |
Green tea | Ovary, Skin, Colon, Head and Neck, Prostate, Colon Pancreas, Bladder, Liver, and Lung cancer | Anti-invasive, antiproliferative (cell cycle arrest and apoptosis), anti-angiogenic and anti-metastatic effects | Low bioavailability due to its instability under neutral or alkaline conditions |
| Genistein | 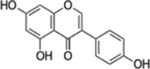 |
Soybean, Red Clover | Prostate, Breast, Colon, Liver, Ovary, Bladder, Gastric, leukemia and Brain, cancer | Anti-invasive, antiproliferative (Cell cycle arrest and apoptosis), anti-metastatic, anti-angiogenic, and anti-inflammatory effects | Poor water solubility after oral administration. Genotoxic, Cytotoxicity to normal cells, Adverse side effects on fertility, fetus and during uterine development |
| Luteolin | 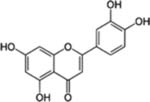 |
Broccoli, Celery, Cabbage, Spinach, Green pepper, and Cauliflower | Upper respiratory tract, Oral, Lung, Colon, Liver, Gastric and Prostate cancer | Anti-proliferative (cell cycle arrest and apoptosis), anti-invasive, anti-angiogenic, and anti-metastatic effects | Associated with risk of lung, prostate, stomach, and breast cancer in humans |
| Lycopene |  |
Tomatoes, Guava, Rosehip, Watermelon, and pink Grapefruit | Prostate, Breast, Lung, and Colon cancer | Anti-proliferative (cell cycle arrest and apoptosis), anti-invasive, and anti-metastatic effects | Lycopene has a special unsaturated structure and poor stability so that it is easy to degrade and isomerize. Its intestinal absorption is far to be complete and highly variable depending on food processing |
| Apigenin | 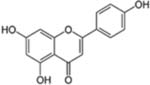 |
Chamomile, Cloves, Peppermint, Red wine, Artichokes and Spinach | Colon, Breast, Cervical, Hematologic, Lung, Ovary, Prostate, Liver, and Brain cancer | Anti-mutagenic, anti-inflammatory, antiproliferative (cell cycle arrest and apoptosis) effects | Apigenin in its pure form is unstable and is not very soluble in water or organic solvents, rapid metabolism, low bioavailability and quick elimination through bile or urine |
| Honokiol | 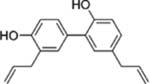 |
Bark or seed cones of the Magnolia Tree | Lung, Breast, ovarian, Prostate, Colon, Brain, Leukemia, Multiple myeloma and Head and neck cancer | Anti-proliferative (cell cycle arrest and apoptosis), anti-angiogenic effects | Poor aqueous solubility otherwise the limitation of Honokiol has not |
| been well studied | |||||
| Ellagic acid | 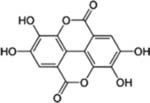 |
Grapes, Pomegranate, Red raspberry, Strawberry, Blueberry and Walnuts | Brest, Osteosarcoma, Pancreatic, Prostate, ovarian cancer, and Lymphoma | Anti-proliferative (cell cycle arrest and apoptosis), anti-metastatic, and antiinvasion effects | Low bioavailability, poor absorption from the gut, rapid metabolism, and lack of transport to the target organs upon oral administration |
| Lupeol | 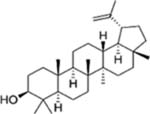 |
Cabbage, Pepper, Cucumber, Tomato, Olive, Fig, Mango, Strawberry, Red grapes. | Colon, Breast, Prostate, Skin, and pancreatic cancer | Anti-proliferative effects (cell cycle arrest and apoptosis) | Has not been well studied |
Figure 1:
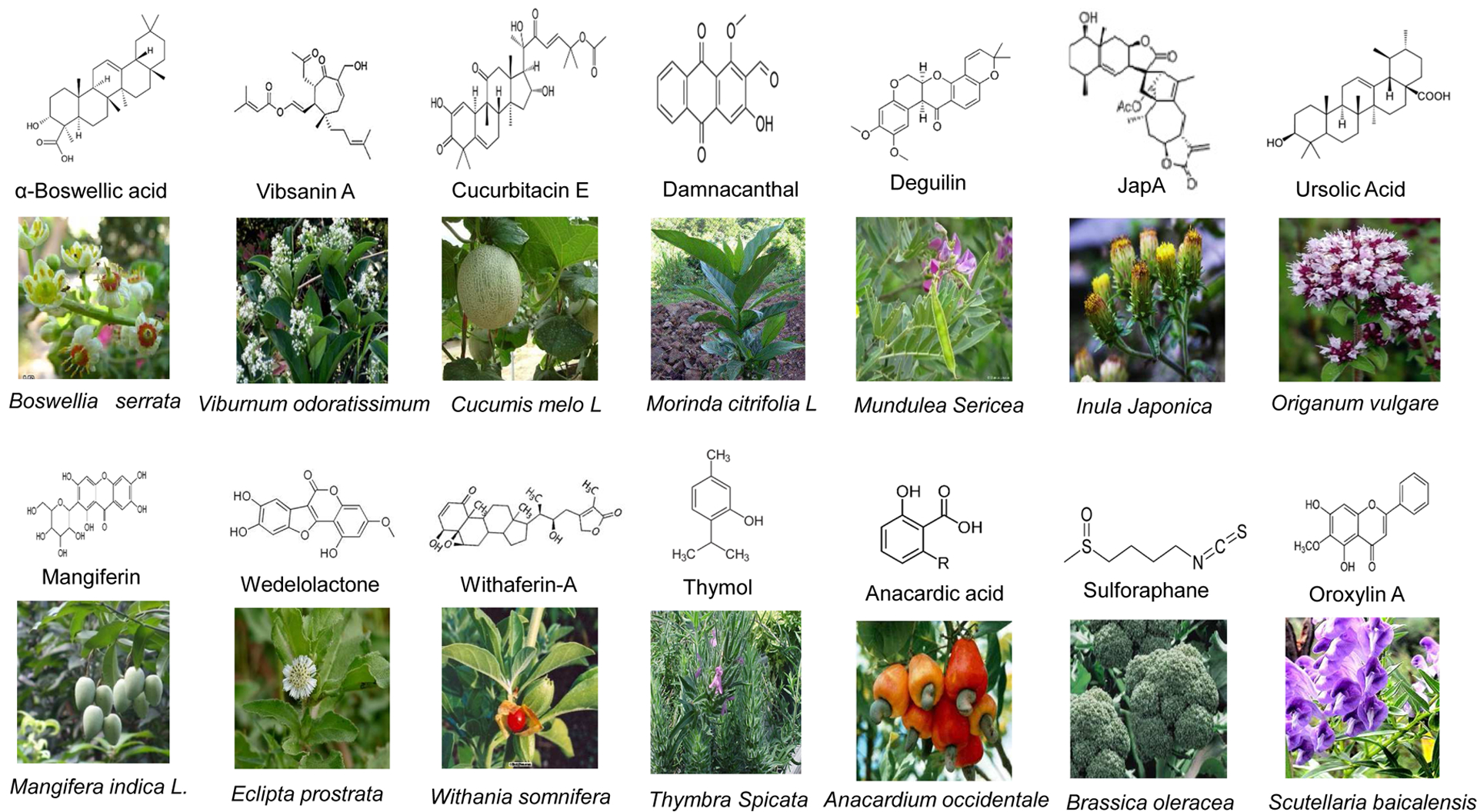
Structures of updated natural compounds and images of their source medicinal plants.
Table 2.
Sources, mechanism of action and molecular targets of updated natural compounds.
| Active | Natural | Effective against | Mechanism of action | Molecular targets |
|---|---|---|---|---|
| compound | source | |||
| α-Boswellic acid | Boswellia serrata | Prostate, colon, breast, lung and pancreatic cancer and leukaemia. | Antiproliferative (cell cycle arrest and apoptosis), anti-inflammatory, antiangiogcnic, anti-invasive and anti-metastatic effects | AKT, STAT3, ERK, Bcl-2, Bcl-xL, DR4, DR5, cyclin D, cyclin E, Rb, NF-κB, COX-2, MMP-9, CXCR4, VEGF and SHP-1 |
| Cucurbitacin E | Cucumis melo L | Colon, breast, thyroid, liver, gastric and pancreatic cancer, leukaemia, glioblastoma, medulloblastoma and meningioma | Anti-inflammatory, antimicrobial, antiproliferative (cell cycle arrest and apoptosis), antipolymerization, antipcrmeability and antiadhesion effects. | AKT, ERK, STAT3, STAT5, JAK2, c-myc, cyclins, survivin, p53, Bcl-xL, GADD45-γ, XIAP, Bcl-2 and Mcl-1 |
| Deguclin | Mundulea sericea | Lung, breast, colon, prostate, thyroid and pancreatic cancer, melanoma and leukaemia. | Antiproliferative (cell cycle arrest and apoptosis), anti-inflammatory, antiangiogcnic, antiinvasion and anti-metastatic effects. | PI3K-AKT, IκBα, NF-κB, AMPK, mTOR, P27, p21, cyclin E, pRb, E2F1, HIF-lα-VEGF, CD44, MMP2, MMP9, cyclin D, pRb |
| Mangiferin | Man gif era indica (L) | Leukaemia, glioma, neuroblastoma and nasopharyngeal, prostate, liver, breast, skin, lung, colon and ovarian cancer | Antiproliferative (cell cycle arrest and apoptosis), anti-inflammatory, antioxidant, immunomodulatory, antiangiogcnic and anti-metastatic effects | ATR, Chkl, Weel, AKT, Erkl/2, Cdc2, cyclinBl, protein kinase C, NF-κB, MMP-7, MMP-9, Bcl-2, VEGF, COX2, FGF and β-catenin |
| Withaferin A | Withania somnifera | Glioblastoma, melanoma, leukaemia and prostate, breast, colon, thyroid, cervical, pancreatic and ovarian cancer | Antiproliferative (cell cycle arrest and apoptosis), anti-oxidative, anti-inflammatory and antiangiogcnic effects | AKT, Bim, Bid, DR5, caspase-8, Bax, Bak, p53, p21, Bcl-2, XIAP, PARP |
| Oroxylin A | Scutellaria baicalensis | Colon, lung, breast, liver and cervical cancer, leukaemia and glioblastoma | Antiproliferative (cell cycle arrest and apoptosis), anti-metastatic, antioxidant, anti-viral, anti-invasion and anti-inflammatory effects | MMP-9, c-Src, AKT, HK II, GSK-3β, vimentin, AKT, ERK, p53, mTOR. STAT3, notch-1, Mcl-1, Beclin 1, SIRT3 |
| Ursolic acid | Origanum vulgare | Colon, prostate, lung, breast, bladder, liver, pancreatic, cervical and ovarian cancer, myeloma and leukaemia | Anti-inflammatory, antiproliferative (cell cycle arrest and apoptosis), antiangiogcnic, anti-metastatic effects | JNK, Gfi-1/Stat5, Bcl-xL, Bcl-2, cFLIP, survivin, cyclin Dl, MMP-9, VEGF, ICAM-1, NF-kB, STAT3, β-catenin, DR4, DR5, TNF-alpha, STAT3, Beclin-1, AKT/mTOR |
| Sulforaphanc | Brassica oleracea | Bladder, brain, breast, colon, prostate, liver and pancreatic cancer | Antiproliferative (cell cycle arrest and apoptosis), antiangiogcnic, anti-metastatic effects | HD AC, p21, caspase-9 and 7, DR5, Bax, Bcl-2, Bcl-xl and Bad |
STAT, signal transducer and activator of transcription; ERK, extracellular signal-regulated kinase; DR, death receptor; NF-κB, nuclear factor-κB; COX-2, cyclo-oxygenase 2; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor; SHP-1, Src homology region 2 domain—containing phosphatase-1; Rb, retinoblastoma protein; Bel, B-ccll lymphoma; CXCR4, C-X-C chemokine receptor type 4; GADD45, growth arrest and DNA damage—inducible 45; Mcl-1, myeloid cell leukaemia 1; AMPK, 5’ adenosine monophosphate—activated protein kinase; mTOR, mammalian target of rapamycin; HIF-lα, hypoxic inducing factor-lα; FGF, fibroblast growth factor; ATR, ataxia telangiectasia and Rad3-rclatcd protein; XIAP, X-linked inhibitor of apoptosis protein; PARP, poly (ADP-ribose) polymerase; HK II, hexokinase-II; GSK-3β, glycogen synthase kinase-3β; SIRT3, sirtuin-3; JNK, c-Jun-N-terminal kinase; ICAM-1, intercellular adhesion molecule 1; TNF-α, tumour necrosis factor-α; HDAC, histone deacetylase.
Boswellic acids
Boswellic acids (BA) such as acetyl-β BA, 11-keto-β-BA, acetyl-11-keto-β-BA (AKBA) are a series of pentacyclic triterpene molecules and are the major components of the resins produced by plants in the genus Boswellia specifically from Boswellia carterri and Boswellia serrata. BAs make up about 30% of the resin of Boswellia serrate (Fig. 1). Commonly known as Indian frankincense or olibanum, salai guggal, loban, or kundur. Boswellia have long-standing application as traditional remedies for various ailments, especially inflammatory diseases (asthma, arthritis, chronic bowel diseases), cerebral edema, chronic pain syndrome and have been shown to exhibit immense potential in combating cancer [15–17]. At the molecular level, BAs modulate multiple molecular targets functionally characterized as kinases, transcription factors, enzymes, receptors, growth factors, and others which are directly or indirectly associated with carcinogenesis [17].
The anti-cancer potential of BAs have been demonstrated against multiple cancer types and have been shown to induce cell cycle arrest and apoptosis, inhibit cell proliferation, survival, angiogenesis, tissue invasion, and metastasis. Molecularly, BAs exert their anti-cancer actions by inhibition of growth factor mediated activation of AKT and extracellular signal regulated kinase (ERK), inactivation of the transcription factors nuclear factor- κB (NF-κB) and signal transducer and activator of transcription (STAT)-3, inhibition of cell cycle regulatory proteins cyclin D and cyclin E, and downregulation of anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 (Table 2 and Fig. 2) [17, 18]. AKBA induced apoptosis of prostate cancer cells in a death receptor 5 (DR5)- and caspase-8- dependent, but DR4- or Fas- independent mechanisms [19]. AKBA also modulated specific cancer-related miRNAs, particularly miR-34a and miR-27a in colorectal cancer cells, which had been tested in-vivo using a xenograft mouse model [20]. BAs decreased cyclin D, cyclin E, and Cyclin dependent kinase CDK2 and CDK4, along with significant reductions in phosphorylated Rb (pRb) in HCT116 colon cancer cells [21]. In pancreatic cancer cell lines, AKBA inhibited constitutively active NF-κB and NF-κB regulated genes such as COX-2, MMP-9, CXCR4, and VEGF [22]. 3-α-propionyloxy-β-BA-induced cell cycle arrest and apoptosis of cancer cells of varied tissue origin and tumor regression in murine models [23]. Chemically modified BA (cyano enone of methyl boswellates) showed cytotoxic activity on a number of cancer cell lines with IC50 ranging from 0.2 to 0.6 μM, and inhibits DNA synthesis and induces apoptosis in A549 cell lines [24]. AKBA inhibited constitutively active STAT3 and STAT3-targeted genes involved in cell proliferation, survival, and angiogenesis by activating Src homology region 2 domain-containing phosphatase 1 (SHP-1), which is responsible for dephosphorylation of STAT-3 [25].
Figure 2:
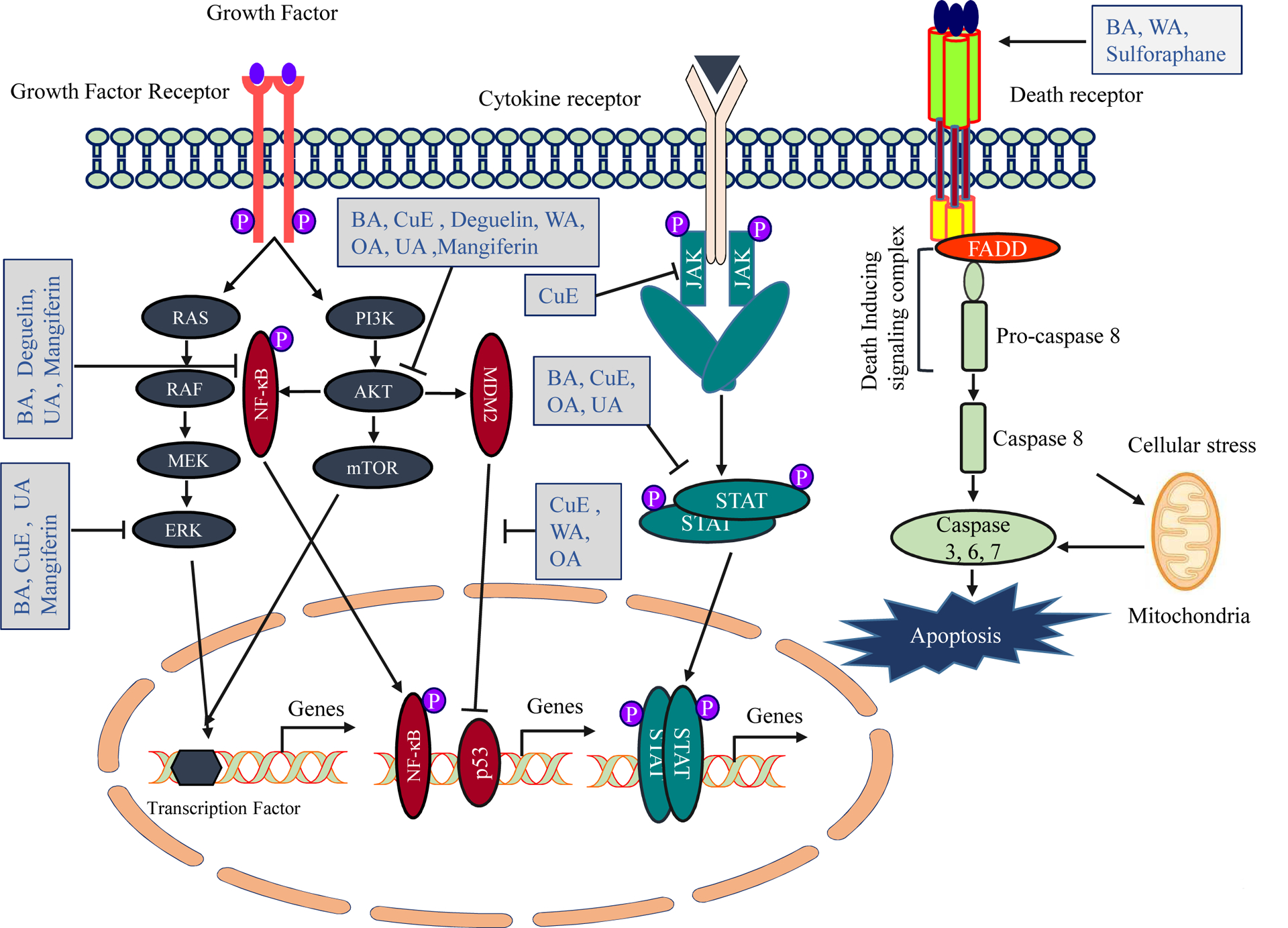
Molecular targets of updated natural compounds. Each natural compound affects (activation or inhibition) multiple pathways. Cell surface receptors and their downstream signaling pathways associated with carcinogenesis are illustrated along with natural compounds that effect these pathways.
The anti-cancer efficacy of BAs has also been demonstrated in vivo in colorectal, pancreatic, leukemia and prostate cancers [15, 22, 26–28]. In a HT-29 colon cancer xenograft model, AKBA activated apoptotic proteins, suppressed inflammatory cytokines, and modulated epidermal growth factor receptor (EGFR) and ATM/p53 signaling pathways resulting in inhibition of adenocarcinoma growth, G1-phase cell cycle arrest, and induction of apoptosis [21, 26, 29]. Additionally, AKBA prevented spontaneous intestinal polyposis and induced apoptosis in intestinal adenocarcinomas through inhibition of the Wnt/β-catenin and NF-κB/cyclooxygenase-2 signaling pathways in APC min/+ mice models [26, 30, 31]. Acetyl-β-BA and AKBA promoted apoptosis, inhibited cell proliferation and prostate tumor burden in PC-3 transplanted NMRI/nu-nu mice, PC-3 xenograft and PC-3 xenotransplanted mouse models [28, 32, 33]. Further in vivo studies revealed that 3-α-butyryloxy-β-BA and AKBA induced cancer cell specific apoptosis, inhibited metastasis and suppressed tumor growth of leukemia and pancreatic tumors [22, 27].
Cucurbitacins
Cucurbitacins (Cu) are a group of tetracyclic triterpenes derived from the climbing-stems of members of the pumpkin and gourd families (Fig. 1) [34]. They have been extensively used as traditional folk medicines throughout Asia and have antipyretic, analgesic, anti-inflammatory, antimicrobial and anticancer activities with selective activities against carcinogenesis [35–37]. However, low therapeutic indices, nonspecific cytotoxicity and poisoning in humans have dampened the initial interest of using crude extracts [37–40]. Cus contain 17 principal compounds differing in their side chains (A to T), and hundreds of derivatives. Among them, Cu B, D, E, I, and derivatives have been studied extensively for their anticancer potential [37]. Several in vitro and in vivo studies have demonstrated the anti-cancer efficacy of Cu E and Cu B against colon, gastric, breast, thyroid, pancreatic and hepatocellular cancer, glioblastoma, medulobastom, leptomenigial and meningioma, and B cell leukemia (Table 2) [41–45].
Cus induce cell cycle arrest, apoptosis and inhibit growth of many cancer cells in a cell-type specific manner. Multiple studies revealed that Cu I or JSI-124 is a potent JAK-STAT inhibitor and blocks the tyrosine phosphorylation of STAT-3 and JAK2 and affect other oncogenic signaling pathways, such as the Akt/PKB or MAPK/ERK pathways (Fig. 2) [46–48]. Cu B inhibited the tyrosine phosphorylation of STAT-3, STAT-5, and JAK-2 in pancreatic cancer cell lines in vitro and in xenografts in vivo [49]. The inhibition of JAK-STAT pathway resulted in the alteration of many downstream targets such as growth stimulating signals (e.g., c-myc, cyclins, survivin) and apoptosis (e.g., p53, Bcl-xL, Bcl-2) [48, 50]. Cu E has inhibitory effects on cancer cell proliferation, actin polymerization, and permeability [35, 51–53]. It induced G2/M cell cycle arrest in human malignant glioma cells, colorectal cancer cell lines as well as pharyngeal and nasopharyngeal carcinoma cells by inducing GADD45-γ [54–56]. Furthermore, Cu E inhibited growth of triple negative breast cancer cells and induced cell cycle arrest and apoptosis by inhibiting AKT and ERK activation and cyclin D1, survivin, XIAP, Bcl-2, Mcl-1 expression in MDA-MB-468 and SW527 cells [57]. Taken together, Cus are promising multi-targeted natural compounds that require further in vitro and in vivo studies against a variety of cancers to pave the way for clinical trials.
Deguelin
Deguelin is a flavonoid isolated from leguminous plants such as Derris trifoliata Lour. and Mundulea sericea (Fig. 1). It has been used as a commercial insecticide and pesticide [58], but also possesses antitumor potential as supported by multiple in vivo and in vitro studies (Table 2). Deguelin modulates various signaling pathways and affects tumor cell proliferation with little or no toxicity. Deguelin induced apoptosis of cancer cells by blocking anti-apoptotic pathways, such as PI3K-Akt, IKK-IκBα-NF-κB and AMPK-mTOR-survivin, while inhibiting tumor cell propagation and malignant transformation through p27-cyclinE-pRb-E2F1 cell cycle control and HIF-1α-VEGF anti-angiogenic pathways (Fig. 2) [59–63].
In vitro studies using lung, breast and leukemia cancer cell lines suggest that degueilin induced apoptosis by disrupting the PI3K-Akt cell survival pathway - inhibition of PI3K activity and phosphorylation of Akt, and upregulation of pro-apoptotic Bad and Bax [64–67]. Furthermore, deguelin induces apoptosis of lung squamous cell carcinoma cells by decreasing the expression of Galectin-1 in vitro and in vivo [68]. Deguelin also inhibits cell proliferation, and cell invasion and metastasis by reorganization of the actin cytoskeleton, and decreased filopodia and lamellipodia formation as a result of decreased expression of tumor metastasis related genes such as CD44, MMP2 and MMP9 at protein and mRNA levels [69]. In colon cancer and pre-malignant human bronchial epithelial cells, deguelin induces G1/S cell cycle arrest through increased expression of cell cycle regulatory proteins p21 and p27 [70, 71]. Similarly, deguelin induced G1/S cell cycle arrest in breast cancer cell line MDA-MB-231 and lymphoma Daudi cells by reducing cyclinD/pRb expression [72].
In the two-stage 7,12-dimethylbenz(a)anthracene/TPA skin carcinogenesis model with CD-1 mice, at a dose of 33 μg, deguelin decreased tumor incidence from 60% to 10% and tumor multiplicity from 4.2 to 0.1 as compared to untreated controls [60]. At 330 μg, no tumors were observed in the treatment group. In N-methylnitrosourea mammary carcinogenesis model with Sprague Dawley rats, intragastric administration of 2 or 4 mg of deguelin/kg, 5 days/week, reduced tumor multiplicity from 6.8 tumors/rat in the control group to 5.1 or 3.2 tumors/animal, respectively [60]. In A/J mice, deguelin significantly reduced tumor multiplicity, tumor volume and overall tumor burden with exposure to the tobacco-specific carcinogen benzo(a)pyrene (Bap) and other carcinogens, with no detectable toxicity [62, 63]. Deguelin also inhibits vasculogenic function of endothelial progenitor cells (EPCs) in tumor progression and metastasis via suppression of focal adhesion kinase (FAK) [73]. In a mouse xenograft model, tumor growth, lung metastasis and tumor-induced circulating EPCs were suppressed by deguelin treatment with 2 mg/kg dose [74]. A study evaluating the anti-metastatic potential of deguelin in vivo and in-vitro using TGFβ1-stimulated PanC-1 cells demonstrated that tumor growth, peritoneal-dissemination and liver/lung metastasis of orthotopically implanted PanC-1-luc cells were significantly reduced in deguelin-treated mice along with the induction of apoptosis [75]. Furthermore, deguelin-treated tumors showed an increased epithelial signature such as increased expression of E-Cadherin and cytokeratin-18 and decreased expression of Snail [75].
Mangiferin
Mangiferin, a bioactive polyphenolic compound primarily derived from the Cashew (Anacardiaceae) and Gentian (Gentianaceae) families, though also found in mangoes and honeybush tea, has been extensively studied for its therapeutic properties. Salicia chinesis (saptarangi) and Mangifera indica (mango), widely used in Indian Ayurvedic medicine, also contain high levels of mangiferin (Fig. 1) [76]. Salicia chinesis has been extensively investigated for its hypo-lipidaemic, anti-diabetic, hepatoprotective and antioxidant properties [77]. Mangifera indica is used not only in Indian ayurvedic medicine but also used in Cuba, China and throughout East Asia for its anti-inflammatory, anti-viral, anti-diabetic properties. Mangiferin is gaining attention for its chemopreventive as well as chemotherapeutic properties against various types of cancer [78–80]. Mangiferin has been shown to inhibit the initiation, promotion, and metastasis of cancers by targeting multiple proinflammatory transcription factors, cell-cycle regulatory proteins, growth factors, kinases, cytokines, chemokines, adhesion molecules, and inflammatory enzymes (Table 2 and Fig. 2) [81].
In vitro studies demonstrated that mangiferin induces G2/M cell cycle arrest through modulation of multiple proteins and signaling pathways including ATR, Chk1, Wee1, Akt, Erk1/2, cdc2 and cyclinB1 in breast, glioma, leukemia, hepatocellular, and nasopharyngeal carcinoma [82–84]. Recent in vitro and in vivo studies have shown that mangiferin triggered G2/M cell cycle arrest and apoptosis of lung cancer cells via down regulating the cdk1-cyclin B1 and PKC-NF-κB pathways and significantly reduced tumor burden of A549 xenografts in mice [85]. Mangiferin reduced cell proliferation and epithelial to mesenchymal transition through the modulation of β-catenin signaling, MMP-7 and MMP-9 [82, 86]. In human prostate cancer cells, mangiferin inhibits cell proliferation and induces apoptosis by downregulation of Bcl-2 and upregulation of miRNA-182 [87]. In vitro and in vivo studies suggest that mangiferin upregulates the expression of various detoxifying enzymes, resulting in enhanced clearance of reactive oxygen species (ROS). In N2A neuroblastoma cells, mangiferin reduced oxidative stress and protected cells from 1-methyl-4-phenylpyridine (MPP+) induced cytotoxicity by restoring glutathione action and reducing the expression of superoxide dismutase (SOD) and catalase [76]. Furthermore, mangiferin time- and dose-dependently inhibits telomerase activity and induces apoptosis by upregulating the expression of Fas mRNA and protein in K562 cells [88]. Mangiferin aglycon, a metabolite of mangiferin, reduced UBV-induced skin cancer in mice primarily through interaction with the ERK pathway [89]. Mangiferin also reduced tumor volume in a breast cancer xenograft model by disruption of MMP expression and β-catenin signaling pathway [86]. In summary, mangiferin is primarily implicated in down-regulating inflammation, causing cell cycle arrest, reducing proliferation/metastasis, and promoting apoptosis in malignant cells and protecting against oxidative stress and DNA damage.
Withaferin-A
Withaferin-A (WA) is isolated from the plant Withania somnifera, commonly known as Indian Winter cherry or Ashwagandha and has long been used in a wide variety of ayurvedic formulations and herbal remedies for the treatment of cancers, inflammation, and neurological disorders (Fig. 1) [90–92]. In vitro and in vivo studies have revealed that WA suppresses experimentally induced carcinogenesis, largely by virtue of its potent anti-oxidative, anti-inflammatory, anti-proliferative and proapoptotic properties (Table 2, Fig. 2). Moreover, WA sensitizes resistant cancer cells to existing chemotherapeutic agents [93]. WA-induced G2/M cell cycle arrest in colorectal cancers through degradation of Mad2 and cdc2 thus interfering with spindle assembly causing delayed mitosis [94]. WA inhibited AKT activity in AKT overexpressing colorectal cancer cells leading to inhibition of cell proliferation, migration and invasion and downregulation of EMT markers in vitro and significantly suppressed AKT-induced aggressive tumor growth in a xenograft model [95]. In head and neck squamous cell carcinoma (HNSCC) cells, WA induced apoptosis by increasing the expression of pro-apoptotic Bim, truncation of Bid, and activation of the DR5- caspase-8 pathway [96]. In breast cancer cells, WA induces apoptosis and down regulates cell proliferation through upregulation of proapoptotic Bim, Bax and Bak, cell cycle regulatory proteins p53 and p21, and downregulation of anti-apoptotic Bcl-2 and apoptosis inhibitory proteins XIAP, c1IAP and survivin [97–99]. Furthermore, in lung cancer cells, WA decreased the expression of Bcl-2, phospho Akt and active caspase-2, and increased cleavage of PARP resulting in Go/G1 cell cycle arrest and apoptosis [100]. Potential anti-tumor activity of WA has been also tested in other cancers such as glioblastoma, cervical, melanoma, leukemia and pancreatic cancer cells [101–105]. WA also exhibits synergistic effects with other drugs. WA sensitizes ovarian cancer cells to cisplatin-induced cytotoxicity [106] and synergizes the activity of doxorubicin against ovarian cancer cell lines, thus reducing doxorubicin dose as well as doxorubicin-induced adverse effects [107]. Further, WA in combination with doxorubicin reduced tumor growth of ovarian tumor xenografts 70%−80% in nude mice [107].
Administration of WA reduced the growth of Ehrlich ascites tumor in Swiss albino mice and prolonged the survival of mouse sarcoma and ascites tumor-bearing mice [108, 109]. WA also reduced the growth of human prostate cancer (PC3) cell tumor xenografts in nude mice by blocking angiogenesis and inducing apoptosis [110]. WA inhibits the growth of human breast cancer cells injected subcutaneously into nude mice [97] and 4T mouse mammary tumor cells implanted orthotopically into Balb/c mice [111]. This activity is linked to FOXO3a-Bim-dependant apoptosis and modulation of vimentin disassembly and phosphorylation. WA inhibits growth and metastasis of orthotopic ovarian tumors [106], pancreatic cancer xenograft tumors [105] and HCT116 colon cancer xenograft tumors [112] in nude mice. In vivo anti-tumor efficiency was also tested in other cancers such as melanoma, thyroid, mouse mesothelioma, and glioblastoma cancer [113–115]. Taken together, both in vitro and in vivo studies establish that WA is a promising candidate for further preclinical and clinical development as an anticancer agent.
Oroxylin A
Oroxylin A (OA) is a flavonoid isolated from the root of Chinese Skullcap (Scutellaria baicalensis, Fig. 1). OA exhibits multiple pharmacological activities, including antioxidant, anti-inflammatory, anti-viral and anti-tumor properties (Table 2) [116]. Multiple studies have demonstrated the potential of OA as a promising anticancer drug. OA induces apoptosis, cell cycle arrest, and inhibits metastasis (Fig. 2) [117–119]. OA inhibits glycolysis and glycolysis-dependent proliferation of human breast cancer cells by sirtuin-3 (SIRT3) dependent binding of hexokinase II (HK II) in mitochondria [116] and promotes SIRT3-mediated superoxide dismutase (SOD2) transcription and HIF1α destabilization [120]. OA also modulates mitochondrial function, inducing apoptosis via mitochondrial translocation of wild-type p53 in vitro, and significantly inhibits tumor volume in vivo in HCT-116 tumors transplanted in BALB/C nude mice [121].
OA decreased the ATP level, inhibited glycolysis and sensitized A549 lung cancer cells to anoikis by deactivating the c-Src/AKT/HK II pathway in addition to inducing dissociation of HK II from mitochondria and inhibited lung metastasis in vivo in nude mice [122]. Another study demonstrated that OA inhibits invasion and migration through suppressing ERK/GSK-3β signaling in snail-expressing non-small-cell lung cancer cells, and inhibits the growth and lung metastasis in A549 orthotopic models [123]. OA also induces autophagy in human malignant glioma cells. Mechanistically, OA inhibits phosphorylation of AKT, ERK, mTOR and STAT-3, and expression of Notch-1 and Mcl-1 but upregulates Beclin 1, the key autophagy-related protein [124]. OA has also been found to be beneficial in other cancers such as acute myeloid leukemia cells, hepatocellular carcinoma, and cervical cancer cells [117, 125, 126].
Ursolic Acid
Ursolic acid (UA) is a pentacyclic triterpene acid present in many foods and medicinal herbs, usually in the bark, leaves or fruit peels. UA exhibits a wide array of pharmacological activities such as protective effects on lungs, kidneys, liver and brain, anti-inflammatory properties, anabolic effects on skeletal muscles and the ability to suppress bone density loss leading to osteoporosis. Fruit peel of apple, leaves of marjoram, oregano (Fig. 1), rosemary, sage, thyme, coffee, hawthorn leaves and flowers of lavender, leaves and bark of eucalyptus and black elder, and the wax layer of many edible fruits contain high amounts of UA [127, 128]. Multiple studies have demonstrated the potential anticancer effects of UA against various types of cancers [129].
UA displays chemopreventive and anticancer properties through the inhibition of multiple signaling pathways resulting in the suppression of proliferation of a number of tumor cells, induction of apoptosis, and inhibition of angiogenesis and metastasis (Table 2, Fig. 2) [130–133]. UA induced apoptosis of human leukemia HL-60 cells through the up-regulation of intracellular Ca2+ release [134]. Further studies demonstrated that UA induces apoptosis of human leukemia cells and exhibits anti-leukemic activity in nude mice through the PKB pathway [134, 135]. Another study demonstrates that UA induced apoptosis of K562 cells via modulation of Gfi-1/Stat5/Akt pathway and downregulation of Mcl-1 and Bcl-xL and synergizes with imatinib in both K562 and imatinib-resistant K562/G01 cells [136]. UA induced apoptosis of breast cancer cells by activating Fas receptor, caspase 3 and PARP in the mitochondrial death pathway, and suppressed migration and invasion by modulating JNK, Akt and mTOR signaling [137, 138]. UA inhibited tumor size by inhibiting cell proliferation, inducing apoptosis and modulating AKT/mTOR signaling pathway in an in vivo study in which MMTV-Wnt-1 mammary tumors were injected into the mammary fat pad of ovariectomized female C57BL/6 mice [139].
UA induced cell death and modulated autophagy through the JNK pathway in apoptosis-resistant colorectal cancer cells [140]. Another study demonstrates that UA induced apoptosis of colorectal cancer cells by inhibiting constitutively active NF-κB and down-regulation of cell survival (Bcl-xL, Bcl-2, cFLIP, and survivin), proliferation (cyclin D1), and metastatic (MMP-9, VEGF, and ICAM-1) proteins [141]. UA inhibited colonic adenocarcinomas in an orthotopic mouse model by reducing the proliferation marker Ki-67 and microvessel density marker CD-31. UA triggered the concomitant suppression of NF-κB, STAT-3, β-catenin, cell survival proteins and induction of DR4 and DR5 [141, 142]. In prostate cancer, UA suppressed TNF-α induced NF-κB activation and IL-6-induced STAT-3 activation in LnCaP cells [143], induced autophagy in PC3 cells [144], and induced apoptosis via Beclin-1 and Akt/mTOR pathways [145]. Further, UA suppressed subcutaneously implanted DU145 prostate cancer cells in male nude mice, reduced the expression of VEGF and increased the expression of caspase-3 in tumor tissues [143]. The in vivo and in vitro efficacy of UA has also been tested against other cancers such as lung, bladder, cervical, pancreatic, ovarian, multiple myeloma, and hepatocellular carcinoma [146–150]. All these studies suggest that UA modulates multiple molecular targets that play vital roles in carcinogenesis.
Sulforaphane
Sulforaphane (SFN) is an organosulfur isothiocyanate found abundantly in cruciferous vegetables such as broccoli, brussel sprouts, and cabbage (Fig. 1) and is produced through enzymatic hydrolysis by myrosinase enzyme during chewing or harvesting [151–153]. SFN has been extensively studied due to its apparent health-promoting properties in disease and limited toxicity in normal tissues. SFN interferes with the multistage process of carcinogenesis through the modulation and/or regulation of critical cellular mechanisms (Table 2, Fig. 2). SFN inhibits phase I enzymes (such as multiple cytochrome P450s) responsible for the activation of pro-carcinogens and induces phase II enzymes (such as UDP glucuronosyl transferase 1A1, glutathione S transferase A1, NADPH:quinone oxidoreductase 1) critical for mutagen elimination [154–156]. Furthermore, SFN modulates a number of anticancer processes, including activation of apoptosis, inhibition of cell proliferation, and induction of cell cycle arrest [157, 158].
Numerous cell culture and animal model studies have shown that SFN induces apoptosis of bladder, brain, breast, colon, pancreatic and prostate cancers through modulation of critical signaling molecules essential for tumor survival and progression. SFN induces mitochondria-dependent apoptosis of glioblastoma cells through release of cytochrome C, and activation of casepage-3 resulting in a favorable Bax:Bcl-2 ratio [159]. It induces apoptosis of colon cancer cells by inhibition HDAC, expression of cell cycle regulatory protein p21, activation of caspase-9 and 7, and expression of Bcl-xL and Bax [160]. In human hepatoma HepG2 cells, SFN induced apoptosis via both intrinsic and extrinsic apoptotic pathways as indicated by expression of DR5, fragmentation of DNA, activation of caspase-3 and modulation of Bax, Bcl-2 and Bcl-xL protein expression [161, 162]. SFN decreased tumor mass, increased TUNEL positive cells, cleaved PARP and increased expression of pro-apoptotic BID and Bax in BALB/C mice implanted with mammary carcinoma cells, and in immune compromised nude mice xenografted with PC-3 prostate cancer cells [163, 164].
SFN induced the G1 cell cycle arrest of androgen-dependent LnCaP and androgen-independent DU-145 cells [165, 166]. Furthermore, it induced G2/M arrest in HT-29 colon cancer cell, PC-3 prostate cancer cells, sarcomatid mammary F311 cells [163], MCF-7 mammary cancer cells, human T-cell leukemia [167], and human pancreatic cancer cells [159]. In vivo studies clearly demonstrate the ability of SFN to inhibit cell proliferation [168]. In addition, SFN interferes with essential steps in the progression of the carcinogenesis process, such as the progression of benign tumors to malignant tumors, angiogenesis, and metastasis. Taken together, the anti-cancer properties of SFN makes these compounds attractive multipotent agents, and indicate an important new avenue for future researches on clinical applicability of SFN to cancer patients. Two clinical trials using SFN have been listed in www.clinicaltrials.gov (NCT03232138; NCT03665922)
Other potential natural compounds for cancer prevention and therapy
Beside the aforementioned compounds, there are many other dietary natural compounds that are currently under investigation for their potential anti-cancer effects. For example, damnacanthal, JapA, vibsanin-A, wedolactone and thymol, and others have shown promising anticancer properties (Fig. 1). Damnacanthal, an anthraquinone present in the roots of Noni plants (Morinda citrifolia L.), exhibits numerous pharmacological activities, including anti-cancer activity. It targets several tyrosine kinases and signal transduction pathways that play a role in cell growth and apoptosis of cancer cells [169, 170]. Damnacanthal inhibits activation of c-Met, decreases phosphorylation of Akt and inhibits MMP-2 secretion in HepG2 cells, and inhibits the growth and clonogenic potential and induces apoptosis [170]. A further study demonstrated that damnacanthal induced inhibition of cell growth through the degradation of cyclin D1 in colon, breast and prostate cancer cells [169]. Ex vivo and in vivo experiments revealed that damnacanthal possesses anti-angiogenic properties mediated via three different kinases: vascular endothelial growth factor (VEGF) receptor-2, c-Met and FAK [171].
JapA is a structurally unique dimeric sesquiterpenoid compound isolated from the above ground part of Inula japonica Thunb, a plant that has been widely used in traditional Chinese medicine for the treatment of inflammation, diabetes, digestive disorders, and bronchitis [172–174]. JapA bears similar chemical structural features with artemisinin and parthenolide, which are currently under preclinical and clinical studies for cancer therapy [175]. However, due to dimerization and unique mechanisms of action, JapA is considered to be more effective than these analogs as an anticancer drug. JapA decreased cell proliferation and induced G2/M cell cycle arrest and apoptosis through down regulation of MDM2, regardless of p53 status both in vitro and in vivo without causing any host toxicity in breast cancer models [176]. Further molecular studies revealed that JapA inhibits transcription factor NFAT1 which regulates transcription of MDM2 and contributes to the anti-cancer activity of JapA [177]. JapA is safe and effective in treating other cancers expressing high levels of MDM2, e.g., Burkitt lymphoma and lung cancer [178, 179].
Vibsanin A (VA), a vibsane-type diterpenoid isolated from the leaves of Viburnum odoratissimum, has been observed to induce differentiation of myeloid leukemia cells through direct interaction with and activation of PKC [180]. In mouse xenograft models of acute myeloid leukemia, VA administration prolonged host survival and inhibited PKC-mediated inflammatory responses correlated with promotion of skin tumors in mice; recommending VA as a myeloid differentiation-inducing compound, with potential application as an antileukemic agent [180]. Wedelolactone is a known inhibitor of the IκB kinase II (IKK II) [181], an upstream kinase and activator of NF-κB by mediating phosphorylation and degradation of IκBα and has many different bioactivities including anti-hepatotoxic, anti-hypertensive and anti-tumor properties [182, 183]. Wedelolactone has been shown to reduce growth of various cancer cells such as prostate, breast, ovarian cancers and myeloma. Wedelolactone suppressed growth and induced apoptosis of androgen receptor-negative MDA-MB-231 breast cancer cells at concentrations that did not inhibit NF-κB activity [184]. Wedelolactone also inhibited anchorage-independent growth and suppressed cell motility and invasion of MDA-MB-231 cells [185]. Another study demonstrated that wedelolactone downregulates the expression of c-Myc mRNA in prostate cancer cells. Further in-vitro studies demonstrated that wedelolactone dramatically decreases the protein level, nuclear accumulation, DNA-binding, and transcriptional activities of c-Myc which is consistent with downregulation of c-Myc in prostate cancer xenograft model [186].
Mechanisms and molecular targets of natural compounds
Human cancers evolve through a multistage process driven by the progressive accumulation of genetic and epigenetic abnormalities leading to aberrant expression of genes and proteins that support cancer promotion, progression and acquiring resistance to cancer therapy. Molecular-based targeted cancer therapies with high impact on patient’s outcome is one of the major medical advances in the last few decades and has been foundational in the success of precision medicine. Moreover, the fact that cancer’s multistage progression requires that numerous signaling pathways be compromised, targeting a single gene is unlikely to produce meaningful outcomes. A significant advantage of natural compounds is that most of them are multi-targeted, often in a context dependent process that inhibits and/or activates many different signaling pathways. The key signaling pathways modulated by these natural compounds are illustrated in (Table 2, Fig. 2), and described in the following part of this article.
p53 tumor suppressor
p53, also known as the “guardian of the genome”, is a transcription factor and a tumor suppressor and is among the most inactivated genes in cancers [187]. Inactivation occurs through deletion, mutations or disruption of regulatory pathways that regulate p53 gene or protein expression. Perturbations in p53 signaling pathways are believed to be required for the development of most cancers, and there is evidence to suggest that restoration or reactivation of p53 function will have significant therapeutic benefits [188]. As the “guardian of the genome,” p53 conserves genomic stability by preventing genome mutations from propagating. Activated p53 regulates genes including p21, 14-3-3, Noxa, Puma, Fas, Bax, and many others to direct cellular processes such as cell cycle arrest, apoptosis, genetic stability, and inhibition of angiogenesis [4, 189]. p53 controls cell cycle arrest in G1 phase in response to DNA damage and thus protects against genomic instability, abnormal DNA replication and chromosome segregation [190, 191]. Loss of or reductions in p53 expression is highly associated with increased genetic instability during tumorigenesis in HNSCC [190], breast [192], ovarian [193] and renal cancers [194].
Many natural compounds mentioned in this article induce cell cycle arrest and apoptosis by activating p53 and its target genes. For example, AKBA modulated ATM/p53 signaling pathways in HT-29 colon cancer xenograft model resulting in inhibition of adenocarcinoma growth, G1-phase cell cycle arrest, and induction of apoptosis [21, 26, 29]. Deguelin increased the expression of p53 and downstream p21 and p27 to induce G1/S cell cycle arrest [63, 70]. WA upregulates the expression of p53 and p21 to inhibit breast cancer cell growth and induction of apoptosis [99]. OA induced apoptosis in human colon cancer cells by stimulating mitochondrial translocation of wild-type p53 [121]. JapA inhibited cell growth, decreased cell proliferation and induced G2/M cell cycle arrest and apoptosis through down regulation of the p53 negative regulator MDM2, thus increasing the expression of p53 [176].
Nuclear Factor-κB
NF-κB is an oncogene and a master transcription factor that functions as a regulator of cell proliferation, differentiation, apoptosis, inflammation, stress response, cell signaling, and other physiological processes. Accumulated evidence suggests that dysregulation of these physiological processes has been linked to the onset of many cancers and has been described as one of the major culprits in carcinogenesis. Experimental in vitro and in vivo studies have demonstrated that downregulation of NF-κB activity by natural or synthetic compounds suppresses the development of carcinogen-induced tumors, inhibits the growth of cancer cells, and induces apoptosis by altering gene expression crucial for the control of carcinogenesis and cancer cell survival [195]. In pancreatic cancer cell lines, AKBA inhibited the constitutively active NF-κB and the expression of NF-κB target genes such as COX-2, MMP-9, CXCR4, and VEGF [22]. Deguelin induces apoptosis of cancer cells by blocking the IKK-IκBα-NF-κB pathway [59]. The PKC-NF-κB pathway is a key pathway inhibited by Mangiferin in lung cancer cells. Further, UA induced apoptosis of colorectal cancer cells by inhibiting constitutively active NF-κB [141].
Signal Transducers and Activators of Transcription
Members of the STAT protein family are transcription factors aberrantly activated downstream of Janus kinases (JAKs) during carcinogenesis. The JAK/STAT signaling pathway mediates cellular responses to many cytokines and growth factors. The STAT family modulates transcription of genes controlling cellular processes including proliferation, differentiation and apoptosis [196]. Dysregulated activation of STAT3 and STAT5 contributes to the initiation and progression of many solid tumors including HNSCC and hematologic malignancies such as multiple myeloma, lymphoma and leukemia [197]. Interruption of STATs activation by natural and dietary compounds leads to decreased protein expression critically involved in the regulation of the cell cycle and apoptosis. AKBA inhibited constitutively active STAT3 in human multiple myeloma cells by the induction of SHP-1, a phosphatase responsible for STAT3 dephosphorylation [25]. The inhibition of STAT3 activation leads to the suppression of genes involved in cell proliferation, survival, and angiogenesis. Multiple studies confirmed that Cu I is a JAK-STAT3 pathway inhibitor and blocks tyrosine phosphorylation of STAT3 and JAK2 in various human cancers [46–48]. Further studies revealed that Cu B inhibited tyrosine phosphorylation of STAT3, STAT5 and JAK2 in pancreatic cancer cell lines and in xenograft models [49]. In prostate cancer, UA suppressed IL-6-induced STAT3 activation in LnCaP cells [143].
Receptor Activated Signaling Pathways
Cell surface receptors are the key players for molecular communication between cells or intracellular organelles essential for various aspects of life. This process involves receiving, promoting and sending signals by means of elaborate signal transduction networks between extracellular and intracellular regions. In cancers, members of cell surface receptors including receptor tyrosine kinases (RTK), G-protein coupled receptors (GPCR), cytokines receptors, integrin receptors as well as death receptors are associated with regulation of cell fates. RTKs such as EGFR, platelet derived growth factor receptor, fibroblast growth factor receptor, hepatocyte growth factor receptor, insulin like growth factor receptor-1 receptor, nerve growth factor receptor and others have been associated with carcinogenesis and play a central role by modulating a wide array of biological functions such as cell migration, proliferation, survival or differentiation [198]. As a consequence of growth factor receptor activation, several downstream signaling pathways, the most important of which are PI3K-AKT and Ras-MAPK, are activated and have significant impacts on tumorigenesis. Tumor associated cell surface receptors and downstream molecules have become targets of many natural agents. For example, BA induces apoptosis through inhibition of the growth factor mediated activation of AKT and ERK resulting inactivation of NF-κB and STAT-3, inhibition of cyclin D and E, Bcl-2, Bcl-xL, and Mcl-1 proteins [17, 18]. Others include, AKBA induced apoptosis through activation of DR5- caspase-8 pathway [19] and degueilin induced apoptosis by disrupting PI3K/Akt pathway [63–66]. WA inhibits constitutively active AKT leading to the inhibition of cell proliferation, migration and invasion. Oral administration of WA significantly suppressed AKT-induced aggressive tumor growth in a xenograft model [95]. WA also induces apoptosis through DR5 - caspase-8 pathway [96]. Damnacanthal inhibited cell growth and induced apoptosis by inactivation of c-Met and AKT [170]. Finally, the anti-angiogenic effects of Damnacanthal are mediated via inhibition of VEGFR2, c-Met and FAK [171].
Host Immunity/ Immuneprevention
The host immune system serves as a unique natural defense to detect and destroy abnormal cells, thus preventing the development of cancers. However, cancer cells acquire the ability to evade detection and destruction by the host immune system. Reactivating the host immune system’s power (known as immuneprevention or immunetherapy) to eliminate damaged cells before tumor onset or to destroy cancer cells is the most recent development to in efforts in the “war on cancer”. Effective cytotoxic T cells represent a crucial component of the adaptive immune system that have the ability to kill specifically chosen target cells. It is now established that the absence of functional T cells or T cell–derived cytokines, such as interferon (IFN)-gamma, enhances the onset of spontaneous and carcinogen-induced tumors [199]. Thus, activation of functional T cells or production of several cytokines resulting in the improvement of anti-tumor microenvironment might contribute to cancer prevention and therapy. The transcription factor NF-κB, a critical signaling molecule in host innate immunity, is activated in response to the formation of an inflammatory tumor microenvironment during malignant progression and upregulates the expression of tumor promoting cytokines, such as IL-6 or TNF-α, and survival genes, such as Bcl-XL [200]. BA, mangiferin, WA, and UA have anti-inflammatory activity, inhibiting NF-κB activation and its downstream targets such as COX-2, MMP-9, CXCR4, and VEGF expression [22]. In prostate cancer, UA suppressed TNF-α induced NF-κB activation and IL-6-induced STAT3 activation in LnCaP cells [132]. Although, some of the aforementioned natural compounds have been found to modulate host factors which are important for their chemopreventive or anti-tumor potential, very few studies have been performed to allow for a sufficient to understanding of the role of these compounds in host immune systems. More direct studies are required for complete characterization of natural compounds in modulating host immunity, thus immnoprevention or immunetherapy of cancers.
Conclusion and Future directions
After FDA approval of tamoxifen and raloxifene for breast cancer risk reduction, increasing attention has been paid on chemoprevention research with the possibility of applying chemopreventive agents for individuals who are at high risk of neoplastic development. Various epidemiological and preclinical findings, as well as results from several early clinical studies convincingly demonstrate that natural dietary compounds have practical advantages in the treatment and possible prevention of cancers with regard to availability, suitability for oral application, regulatory approval and mechanisms of action. Targeting multiple intracellular signaling pathways with the use of combinatorial approach has been a promising strategy for anti-cancer drug development. Many of the above natural agents interrupt multiple signal transduction pathways depending on the cancer origin. However, researchers are struggling with key challenges to take advantage of these mechanisms for effective cancer treatment and prevention in populations with different cancer risks. Moreover, poor pharmacokinetic activity like low potency, short bioavailability and limited tissue distribution of dietary agents pose further challenges. Formulation of the parent compounds or development of synthetic analogs with favorable pharmacokinetics might be ways to overcome the limitations of current natural compounds. Moreover, prospective natural compounds with potential anti-cancer activity should be evaluated for further drug design and preclinical and clinical trials. The mentioned natural compounds in this review have promising anti-cancer activity evidenced by both in-vitro and in-vivo studies and some of them are in very preliminary clinical studies. For example, Boswellic acid (BA) exerts its potent anti-cancer effect through modulation of multiple molecular targets such as kinases, transcription factors, enzymes, receptors, growth factors, and others involved in cell survival and proliferation. Several in vitro and in vivo studies documented the anti-cancer efficiency of Cu E and Cu B against many cancer cells such as bladder cancer, hepatocellular carcinoma, pancreatic cancer, breast cancer, HNSCC, osteosarcoma, and myeloid leukemia and leukemia [53, 201–204]. WA sensitizes resistant cancer cells to existing chemotherapeutic agents [93]. As single agent the majority of these natural compounds target multiple signal transduction pathways and thus testing combinations either with other compounds or with already validated agents could be an effective approach to enhance efficacy, sensitivity, and bioavailability while reducing unexpected toxicities. Administration of drugs locally rather than systemically (affecting the whole body) is a proven way to decrease side effects and drug toxicity while maximizing a treatment’s impact. So, introduction of modern technologies to improve local drug delivery might be more effective way to obtain the best response and overcome limitations associated with natural compounds. Since many of the limitations of these compounds have not been well studied more preclinical studies are certainly needed to validate the usefulness of these agents either alone or in combination with existing therapies.
Highlights.
Table of natural compounds studied through preclinical and early clinical studies
Comprehensive review of new promising natural compounds & their anti-cancer effects
Summary of cellular processes affected by new promising compounds
Comprehensive review of the molecular targets of promising new compounds
Assessment of the future perspectives about these compounds
Acknowledgement:
This work was supported by the start-up funding from Faculty Research Support grant from Marshall University School of Pharmacy and the WV-INBRE grant (P20GM103434) to Ruhul Amin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no potential conflict of Interest.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA Cancer J Clin, 69 (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Yabroff KR, Lund J, Kepka D, Mariotto A, Economic burden of cancer in the United States: estimates, projections, and future research, Cancer Epidemiol Biomarkers Prev, 20 (2011) 2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ramos S, Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways, Mol Nutr Food Res, 52 (2008) 507–526. [DOI] [PubMed] [Google Scholar]
- [4].Amin AR, Kucuk O, Khuri FR, Shin DM, Perspectives for cancer prevention with natural compounds, J Clin Oncol, 27 (2009) 2712–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Newman DJ, Natural products as leads to potential drugs: an old process or the new hope for drug discovery?, J Med Chem, 51 (2008) 2589–2599. [DOI] [PubMed] [Google Scholar]
- [6].Dias DA, Urban S, Roessner U, A historical overview of natural products in drug discovery, Metabolites, 2 (2012) 303–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shanmugam MK, Kannaiyan R, Sethi G, Targeting cell signaling and apoptotic pathways by dietary agents: role in the prevention and treatment of cancer, Nutr Cancer, 63 (2011) 161–173. [DOI] [PubMed] [Google Scholar]
- [8].Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S, Targeting cell signaling pathways for drug discovery: an old lock needs a new key, J Cell Biochem, 102 (2007) 580–592. [DOI] [PubMed] [Google Scholar]
- [9].Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB, From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer, J Soc Integr Oncol, 5 (2007) 25–37. [DOI] [PubMed] [Google Scholar]
- [10].Shanmugam MK, Lee JH, Chai EZ, Kanchi MM, Kar S, Arfuso F, Dharmarajan A, Kumar AP, Ramar PS, Looi CY, Mustafa MR, Tergaonkar V, Bishayee A, Ahn KS, Sethi G, Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds, Semin Cancer Biol, 40–41 (2016) 35–47. [DOI] [PubMed] [Google Scholar]
- [11].Millimouno FM, Dong J, Yang L, Li J, Li X, Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature, Cancer Prev Res (Phila), 7 (2014) 1081–1107. [DOI] [PubMed] [Google Scholar]
- [12].Park W, Amin AR, Chen ZG, Shin DM, New perspectives of curcumin in cancer prevention, Cancer Prev Res (Phila), 6 (2013) 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O, Cancer prevention with natural compounds, Semin Oncol, 37 (2010) 258–281. [DOI] [PubMed] [Google Scholar]
- [14].Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N, Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis, Apoptosis, 20 (2015) 1531–1562. [DOI] [PubMed] [Google Scholar]
- [15].Takahashi M, Sung B, Shen Y, Hur K, Link A, Boland CR, Aggarwal BB, Goel A, Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family, Carcinogenesis, 33 (2012) 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M, Frankincense (ru xiang; boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases, J Tradit Complement Med, 3 (2013) 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roy NK, Deka A, Bordoloi D, Mishra S, Kumar AP, Sethi G, Kunnumakkara AB, The potential role of boswellic acids in cancer prevention and treatment, Cancer Lett, 377 (2016) 74–86. [DOI] [PubMed] [Google Scholar]
- [18].Park YS, Lee JH, Bondar J, Harwalkar JA, Safayhi H, Golubic M, Cytotoxic action of acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells, Planta Med, 68 (2002) 397–401. [DOI] [PubMed] [Google Scholar]
- [19].Lu M, Xia L, Hua H, Jing Y, Acetyl-keto-beta-boswellic acid induces apoptosis through a death receptor 5-mediated pathway in prostate cancer cells, Cancer Res, 68 (2008) 1180–1186. [DOI] [PubMed] [Google Scholar]
- [20].Toden S, Okugawa Y, Buhrmann C, Nattamai D, Anguiano E, Baldwin N, Shakibaei M, Boland CR, Goel A, Novel Evidence for Curcumin and Boswellic Acid-Induced Chemoprevention through Regulation of miR-34a and miR-27a in Colorectal Cancer, Cancer Prev Res (Phila), 8 (2015) 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu JJ, Huang B, Hooi SC, Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells, Br J Pharmacol, 148 (2006) 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Park B, Prasad S, Yadav V, Sung B, Aggarwal BB, Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets, PLoS One, 6 (2011) e26943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [23].Qurishi Y, Hamid A, Sharma PR, Wani ZA, Mondhe DM, Singh SK, Zargar MA, Andotra SS, Shah BA, Taneja SC, Saxena AK, PARP cleavage and perturbance in mitochondrial membrane potential by 3-alpha-propionyloxy-beta-boswellic acid results in cancer cell death and tumor regression in murine models, Future Oncol, 8 (2012) 867–881. [DOI] [PubMed] [Google Scholar]
- [24].Ravanan P, Singh SK, Rao GS, Kondaiah P, Growth inhibitory, apoptotic and anti-inflammatory activities displayed by a novel modified triterpenoid, cyano enone of methyl boswellates, J Biosci, 36 (2011) 297–307. [DOI] [PubMed] [Google Scholar]
- [25].Kunnumakkara AB, Nair AS, Sung B, Pandey MK, Aggarwal BB, Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1, Mol Cancer Res, 7 (2009) 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [26].Yuan Y, Cui SX, Wang Y, Ke HN, Wang RQ, Lou HX, Gao ZH, Qu XJ, Acetyl-11-keto-beta-boswellic acid (AKBA) prevents human colonic adenocarcinoma growth through modulation of multiple signaling pathways, Biochim Biophys Acta, 1830 (2013) 4907–4916. [DOI] [PubMed] [Google Scholar]
- [27].Qurishi Y, Hamid A, Sharma PR, Wani ZA, Mondhe DM, Singh SK, Zargar MA, Andotra SS, Shah BA, Taneja SC, Saxena AK, NF-kappaB down-regulation and PARP cleavage by novel 3-alpha-butyryloxy-beta-boswellic acid results in cancer cell specific apoptosis and in vivo tumor regression, Anticancer Agents Med Chem, 13 (2013) 777–790. [DOI] [PubMed] [Google Scholar]
- [28].Syrovets T, Gschwend JE, Buchele B, Laumonnier Y, Zugmaier W, Genze F, Simmet T, Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo, J Biol Chem, 280 (2005) 6170–6180. [DOI] [PubMed] [Google Scholar]
- [29].Liu JJ, Nilsson A, Oredsson S, Badmaev V, Zhao WZ, Duan RD, Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells, Carcinogenesis, 23 (2002) 2087–2093. [DOI] [PubMed] [Google Scholar]
- [30].Liu HP, Gao ZH, Cui SX, Wang Y, Li BY, Lou HX, Qu XJ, Chemoprevention of intestinal adenomatous polyposis by acetyl-11-keto-beta-boswellic acid in APC(Min/+) mice, Int J Cancer, 132 (2013) 2667–2681. [DOI] [PubMed] [Google Scholar]
- [31].Wang R, Wang Y, Gao Z, Qu X, The comparative study of acetyl-11-keto-beta-boswellic acid (AKBA) and aspirin in the prevention of intestinal adenomatous polyposis in APC(Min/+) mice, Drug Discov Ther, 8 (2014) 25–32. [DOI] [PubMed] [Google Scholar]
- [32].Pang X, Yi Z, Zhang X, Sung B, Qu W, Lian X, Aggarwal BB, Liu M, Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis, Cancer Res, 69 (2009) 5893–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Buchele B, Zugmaier W, Estrada A, Genze F, Syrovets T, Paetz C, Schneider B, Simmet T, Characterization of 3alpha-acetyl-11-keto-alpha-boswellic acid, a pentacyclic triterpenoid inducing apoptosis in vitro and in vivo, Planta Med, 72 (2006) 1285–1289. [DOI] [PubMed] [Google Scholar]
- [34].Rios JL, Andujar I, Escandell JM, Giner RM, Recio MC, Cucurbitacins as inducers of cell death and a rich source of potential anticancer compounds, Curr Pharm Des, 18 (2012) 1663–1676. [DOI] [PubMed] [Google Scholar]
- [35].Huang WW, Yang JS, Lin MW, Chen PY, Chiou SM, Chueh FS, Lan YH, Pai SJ, Tsuzuki M, Ho WJ, Chung JG, Cucurbitacin E Induces G(2)/M Phase Arrest through STAT3/p53/p21 Signaling and Provokes Apoptosis via Fas/CD95 and Mitochondria-Dependent Pathways in Human Bladder Cancer T24 Cells, Evid Based Complement Alternat Med, 2012 (2012) 952762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Baumgarth M, [New pharmacologically interesting natural substances (author’s transl)], Planta Med, 39 (1980) 297–335. [DOI] [PubMed] [Google Scholar]
- [37].Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX, Cucurbitacins and cucurbitane glycosides: structures and biological activities, Nat Prod Rep, 22 (2005) 386–399. [DOI] [PubMed] [Google Scholar]
- [38].Newman DJ, Cragg GM, Natural products as sources of new drugs over the last 25 years, J Nat Prod, 70 (2007) 461–477. [DOI] [PubMed] [Google Scholar]
- [39].Metcalf RL, Coevolutionary adaptations of rootworm beetles (Coleoptera: Chrysomelidae) to cucurbitacins, J Chem Ecol, 12 (1986) 1109–1124. [DOI] [PubMed] [Google Scholar]
- [40].Pezzuto JM, Plant-derived anticancer agents, Biochem Pharmacol, 53 (1997) 121–133. [DOI] [PubMed] [Google Scholar]
- [41].Song J, Liu H, Li Z, Yang C, Wang C, Cucurbitacin I inhibits cell migration and invasion and enhances chemosensitivity in colon cancer, Oncol Rep, 33 (2015) 1867–1871. [DOI] [PubMed] [Google Scholar]
- [42].Deng C, Zhang B, Zhang S, Duan C, Cao Y, Kang W, Yan H, Ding X, Zhou F, Wu L, Duan G, Shen S, Xu G, Zhang W, Chen M, Huang S, Zhang X, Lv Y, Ling T, Wang L, Zou X, Low nanomolar concentrations of Cucurbitacin-I induces G2/M phase arrest and apoptosis by perturbing redox homeostasis in gastric cancer cells in vitro and in vivo, Cell Death Dis, 7 (2016) e2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yuan G, Yan SF, Xue H, Zhang P, Sun JT, Li G, Cucurbitacin I induces protective autophagy in glioblastoma in vitro and in vivo, J Biol Chem, 289 (2014) 10607–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ishdorj G, Johnston JB, Gibson SB, Cucurbitacin-I (JSI-124) activates the JNK/c-Jun signaling pathway independent of apoptosis and cell cycle arrest in B leukemic cells, BMC Cancer, 11 (2011) 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Qi J, Xia G, Huang CR, Wang JX, Zhang J, JSI-124 (Cucurbitacin I) inhibits tumor angiogenesis of human breast cancer through reduction of STAT3 phosphorylation, Am J Chin Med, 43 (2015) 337–347. [DOI] [PubMed] [Google Scholar]
- [46].Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM, Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice, Cancer Res, 63 (2003) 1270–1279. [PubMed] [Google Scholar]
- [47].Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M, Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells, Biochem Pharmacol, 73 (2007) 56–67. [DOI] [PubMed] [Google Scholar]
- [48].Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM, Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity, Oncogene, 24 (2005) 3236–3245. [DOI] [PubMed] [Google Scholar]
- [49].Thoennissen NH, Iwanski GB, Doan NB, Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, Said JW, Koeffler HP, Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells, Cancer Res, 69 (2009) 5876–5884. [DOI] [PubMed] [Google Scholar]
- [50].Yu H, Jove R, The STATs of cancer--new molecular targets come of age, Nat Rev Cancer, 4 (2004) 97–105. [DOI] [PubMed] [Google Scholar]
- [51].Abbas S, Vincourt JB, Habib L, Netter P, Greige-Gerges H, Magdalou J, The cucurbitacins E, D and I: investigation of their cytotoxicity toward human chondrosarcoma SW 1353 cell line and their biotransformation in man liver, Toxicol Lett, 216 (2013) 189–199. [DOI] [PubMed] [Google Scholar]
- [52].Zhang T, Li J, Dong Y, Zhai D, Lai L, Dai F, Deng H, Chen Y, Liu M, Yi Z, Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion, Breast Cancer Res Treat, 135 (2012) 445–458. [DOI] [PubMed] [Google Scholar]
- [53].Sorensen PM, Iacob RE, Fritzsche M, Engen JR, Brieher WM, Charras G, Eggert US, The natural product cucurbitacin E inhibits depolymerization of actin filaments, ACS Chem Biol, 7 (2012) 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hsu YC, Chen MJ, Huang TY, Inducement of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene from climbing stem of Cucumis melo L., through GADD45gamma in human brain malignant glioma (GBM) 8401 cells, Cell Death Dis, 5 (2014) e1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hsu YC, Huang TY, Chen MJ, Therapeutic ROS targeting of GADD45gamma in the induction of G2/M arrest in primary human colorectal cancer cell lines by cucurbitacin E, Cell Death Dis, 5 (2014) e1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hung CM, Chang CC, Lin CW, Chen CC, Hsu YC, GADD45gamma induces G2/M arrest in human pharynx and nasopharyngeal carcinoma cells by cucurbitacin E, Sci Rep, 4 (2014) 6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kong Y, Chen J, Zhou Z, Xia H, Qiu MH, Chen C, Cucurbitacin E induces cell cycle G2/M phase arrest and apoptosis in triple negative breast cancer, PLoS One, 9 (2014) e103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fang N, Casida JE, Cube resin insecticide: identification and biological activity of 29 rotenoid constituents, J Agric Food Chem, 47 (1999) 2130–2136. [DOI] [PubMed] [Google Scholar]
- [59].Wang Y, Ma W, Zheng W, Deguelin, a novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest and anti-angiogenesis for cancer chemoprevention, Mol Clin Oncol, 1 (2013) 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Udeani GO, Gerhauser C, Thomas CF, Moon RC, Kosmeder JW, Kinghorn AD, Moriarty RM, Pezzuto JM, Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid, Cancer Res, 57 (1997) 3424–3428. [PubMed] [Google Scholar]
- [61].Gills JJ, Kosmeder J 2nd, Moon RC, Lantvit DD, Pezzuto JM, Effect of deguelin on UVB-induced skin carcinogenesis, J Chemother, 17 (2005) 297–301. [DOI] [PubMed] [Google Scholar]
- [62].Yan Y, Wang Y, Tan Q, Lubet RA, You M, Efficacy of deguelin and silibinin on benzo(a)pyrene-induced lung tumorigenesis in A/J mice, Neoplasia, 7 (2005) 1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lee HY, Oh SH, Woo JK, Kim WY, Van Pelt CS, Price RE, Cody D, Tran H, Pezzuto JM, Moriarty RM, Hong WK, Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis, J Natl Cancer Inst, 97 (2005) 1695–1699. [DOI] [PubMed] [Google Scholar]
- [64].Chun KH, Kosmeder JW 2nd, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee HY, Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells, J Natl Cancer Inst, 95 (2003) 291–302. [DOI] [PubMed] [Google Scholar]
- [65].Chen Y, Wu Q, Cui GH, Chen YQ, Li R, Deguelin blocks cells survival signal pathways and induces apoptosis of HL-60 cells in vitro, Int J Hematol, 89 (2009) 618–623. [DOI] [PubMed] [Google Scholar]
- [66].Bortul R, Tazzari PL, Billi AM, Tabellini G, Mantovani I, Cappellini A, Grafone T, Martinelli G, Conte R, Martelli AM, Deguelin A PI3K/AKT inhibitor, enhances chemosensitivity of leukaemia cells with an active PI3K/AKT pathway, Br J Haematol, 129 (2005) 677–686. [DOI] [PubMed] [Google Scholar]
- [67].Lee HY, Molecular mechanisms of deguelin-induced apoptosis in transformed human bronchial epithelial cells, Biochem Pharmacol, 68 (2004) 1119–1124. [DOI] [PubMed] [Google Scholar]
- [68].Yan B, Zhao D, Yao Y, Bao Z, Lu G, Zhou J, Deguelin Induces the Apoptosis of Lung Squamous Cell Carcinoma Cells through Regulating the Expression of Galectin-1, Int J Biol Sci, 12 (2016) 850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhao H, Jiao Y, Zhang Z, Deguelin inhibits the migration and invasion of lung cancer A549 and H460 cells via regulating actin cytoskeleton rearrangement, Int J Clin Exp Pathol, 8 (2015) 15582–15590. [PMC free article] [PubMed] [Google Scholar]
- [70].Murillo G, Salti GI, Kosmeder JW 2nd, Pezzuto JM, Mehta RG, Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest, Eur J Cancer, 38 (2002) 2446–2454. [DOI] [PubMed] [Google Scholar]
- [71].Lee HY, Suh YA, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM, Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells, Clin Cancer Res, 10 (2004) 1074–1079. [DOI] [PubMed] [Google Scholar]
- [72].Murillo G, Peng X, Torres KE, Mehta RG, Deguelin inhibits growth of breast cancer cells by modulating the expression of key members of the Wnt signaling pathway, Cancer Prev Res (Phila), 2 (2009) 942–950. [DOI] [PubMed] [Google Scholar]
- [73].Nguyen MP, Lee D, Lee SH, Lee HE, Lee HY, Lee YM, Deguelin inhibits vasculogenic function of endothelial progenitor cells in tumor progression and metastasis via suppression of focal adhesion, Oncotarget, 6 (2015) 16588–16600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim WY, Chang DJ, Hennessy B, Kang HJ, Yoo J, Han SH, Kim YS, Park HJ, Seo SY, Mills G, Kim KW, Hong WK, Suh YG, Lee HY, A novel derivative of the natural agent deguelin for cancer chemoprevention and therapy, Cancer Prev Res (Phila), 1 (2008) 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Boreddy SR, Srivastava SK, Deguelin suppresses pancreatic tumor growth and metastasis by inhibiting epithelial-to-mesenchymal transition in an orthotopic model, Oncogene, 32 (2013) 3980–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kavitha M, Nataraj J, Essa MM, Memon MA, Manivasagam T, Corrigendum to “Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice” [Chem. Biol. Interact. 206 (2013) 239–247], Chem Biol Interact, 331 (2020) 109214. [DOI] [PubMed] [Google Scholar]
- [77].Ghadage DM, Kshirsagar PR, Pai SR, Chavan JJ, Extraction efficiency, phytochemical profiles and antioxidative properties of different parts of Saptarangi (Salacia chinensis L.) - An important underutilized plant, Biochem Biophys Rep, 12 (2017) 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Matkowski A, Kus P, Goralska E, Wozniak D, Mangiferin - a bioactive xanthonoid, not only from mango and not just antioxidant, Mini Rev Med Chem, 13 (2013) 439–455. [PubMed] [Google Scholar]
- [79].Tolosa L, Rodeiro I, Donato MT, Herrera JA, Delgado R, Castell JV, Gomez-Lechon MJ, Multiparametric evaluation of the cytoprotective effect of the Mangifera indica L. stem bark extract and mangiferin in HepG2 cells, J Pharm Pharmacol, 65 (2013) 1073–1082. [DOI] [PubMed] [Google Scholar]
- [80].Zou T, Wu H, Li H, Jia Q, Song G, Comparison of microwave-assisted and conventional extraction of mangiferin from mango (Mangifera indica L.) leaves, J Sep Sci, 36 (2013) 3457–3462. [DOI] [PubMed] [Google Scholar]
- [81].Rajendran P, Rengarajan T, Nandakumar N, Divya H, Nishigaki I, Mangiferin in cancer chemoprevention and treatment: pharmacokinetics and molecular targets, J Recept Signal Transduct Res, 35 (2015) 76–84. [DOI] [PubMed] [Google Scholar]
- [82].Xiao J, Liu L, Zhong Z, Xiao C, Zhang J, Mangiferin regulates proliferation and apoptosis in glioma cells by induction of microRNA-15b and inhibition of MMP-9 expression, Oncol Rep, 33 (2015) 2815–2820. [DOI] [PubMed] [Google Scholar]
- [83].Peng ZG, Yao YB, Yang J, Tang YL, Huang X, Mangiferin induces cell cycle arrest at G2/M phase through ATR-Chk1 pathway in HL-60 leukemia cells, Genet Mol Res, 14 (2015) 4989–5002. [DOI] [PubMed] [Google Scholar]
- [84].Pan LL, Wang AY, Huang YQ, Luo Y, Ling M, Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line, Asian Pac J Cancer Prev, 15 (2014) 7065–7068. [DOI] [PubMed] [Google Scholar]
- [85].Shi W, Deng J, Tong R, Yang Y, He X, Lv J, Wang H, Deng S, Qi P, Zhang D, Wang Y, Molecular mechanisms underlying mangiferin-induced apoptosis and cell cycle arrest in A549 human lung carcinoma cells, Mol Med Rep, 13 (2016) 3423–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li H, Huang J, Yang B, Xiang T, Yin X, Peng W, Cheng W, Wan J, Luo F, Li H, Ren G, Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and beta-catenin signaling pathway, Toxicol Appl Pharmacol, 272 (2013) 180–190. [DOI] [PubMed] [Google Scholar]
- [87].Li M, Ma H, Yang L, Li P, Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182, Oncol Lett, 11 (2016) 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hu XY, Deng JG, Wang L, Yuan YF, Synthesis and anti-tumor activity evaluation of gallic acid-mangiferin hybrid molecule, Med Chem, 9 (2013) 1058–1062. [DOI] [PubMed] [Google Scholar]
- [89].Li J, Malakhova M, Mottamal M, Reddy K, Kurinov I, Carper A, Langfald A, Oi N, Kim MO, Zhu F, Sosa CP, Zhou K, Bode AM, Dong Z, Norathyriol suppresses skin cancers induced by solar ultraviolet radiation by targeting ERK kinases, Cancer Res, 72 (2012) 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Vyas AR, Singh SV, Molecular targets and mechanisms of cancer prevention and treatment by withaferin a, a naturally occurring steroidal lactone, AAPS J, 16 (2014) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bhattacharya SK, Satyan KS, Ghosal S, Antioxidant activity of glycowithanolides from Withania somnifera, Indian J Exp Biol, 35 (1997) 236–239. [PubMed] [Google Scholar]
- [92].Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS, Withaferin A is a potent inhibitor of angiogenesis, Angiogenesis, 7 (2004) 115–122. [DOI] [PubMed] [Google Scholar]
- [93].Lee IC, Choi BY, Withaferin-A--A Natural Anticancer Agent with Pleitropic Mechanisms of Action, Int J Mol Sci, 17 (2016) 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Das T, Roy KS, Chakrabarti T, Mukhopadhyay S, Roychoudhury S, Withaferin A modulates the Spindle assembly checkpoint by degradation of Mad2-Cdc20 complex in colorectal cancer cell lines, Biochem Pharmacol, 91 (2014) 31–39. [DOI] [PubMed] [Google Scholar]
- [95].Suman S, Das TP, Sirimulla S, Alatassi H, Ankem MK, Damodaran C, Withaferin-A suppress AKT induced tumor growth in colorectal cancer cells, Oncotarget, 7 (2016) 13854–13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lee HE, Shin JA, Jeong JH, Jeon JG, Lee MH, Cho SD, Anticancer activity of Ashwagandha against human head and neck cancer cell lines, J Oral Pathol Med, 45 (2016) 193–201. [DOI] [PubMed] [Google Scholar]
- [97].Stan SD, Hahm ER, Warin R, Singh SV, Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo, Cancer Res, 68 (2008) 7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hahm ER, Singh SV, Withaferin A-induced apoptosis in human breast cancer cells is associated with suppression of inhibitor of apoptosis family protein expression, Cancer Lett, 334 (2013) 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang X, Mukerji R, Samadi AK, Cohen MS, Down-regulation of estrogen receptor-alpha and rearranged during transfection tyrosine kinase is associated with withaferin a-induced apoptosis in MCF-7 breast cancer cells, BMC Complement Altern Med, 11 (2011) 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cai Y, Sheng ZY, Chen Y, Bai C, Effect of Withaferin A on A549 cellular proliferation and apoptosis in non-small cell lung cancer, Asian Pac J Cancer Prev, 15 (2014) 1711–1714. [DOI] [PubMed] [Google Scholar]
- [101].Grogan PT, Sarkaria JN, Timmermann BN, Cohen MS, Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mTOR pathway inhibitory modulation, Invest New Drugs, 32 (2014) 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C, Lemaire C, Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2, Apoptosis, 16 (2011) 1014–1027. [DOI] [PubMed] [Google Scholar]
- [103].Munagala R, Kausar H, Munjal C, Gupta RC, Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells, Carcinogenesis, 32 (2011) 1697–1705. [DOI] [PubMed] [Google Scholar]
- [104].Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, Suri KA, Qazi GN, Singh J, Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine, Apoptosis, 12 (2007) 2115–2133. [DOI] [PubMed] [Google Scholar]
- [105].Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, Li Y, Gunatilaka AA, Zhan CG, Sun D, Withaferin A targets heat shock protein 90 in pancreatic cancer cells, Biochem Pharmacol, 79 (2010) 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kakar SS, Jala VR, Fong MY, Synergistic cytotoxic action of cisplatin and withaferin A on ovarian cancer cell lines, Biochem Biophys Res Commun, 423 (2012) 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS, Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer, PLoS One, 7 (2012) e42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Shohat B, Gitter S, Abraham A, Lavie D, Antitumor activity of withaferin A (NSC-101088), Cancer Chemother Rep, 51 (1967) 271–276. [PubMed] [Google Scholar]
- [109].Shohat B, Shaltiel A, Ben-Bassat M, Joshua H, The effect of withaferin A, a natural steroidal lactone, on the fine structure of S-180 tumor cells, Cancer Lett, 2 (1976) 71–77. [DOI] [PubMed] [Google Scholar]
- [110].Lahat G, Zhu QS, Huang KL, Wang S, Bolshakov S, Liu J, Torres K, Langley RR, Lazar AJ, Hung MC, Lev D, Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies, PLoS One, 5 (2010) e10105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [111].Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, Tighiouart M, Vertino PM, Harvey RD, Garcia A, Marcus AI, Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation, Int J Cancer, 129 (2011) 2744–2755. [DOI] [PubMed] [Google Scholar]
- [112].Choi BY, Kim BW, Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity, J Cancer Prev, 20 (2015) 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Devi PU, Kamath R, Rao BS, Radiosensitization of a mouse melanoma by withaferin A: in vivo studies, Indian J Exp Biol, 38 (2000) 432–437. [PubMed] [Google Scholar]
- [114].Yang H, Wang Y, Cheryan VT, Wu W, Cui CQ, Polin LA, Pass HI, Dou QP, Rishi AK, Wali A, Withaferin A inhibits the proteasome activity in mesothelioma in vitro and in vivo, PLoS One, 7 (2012) e41214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chang E, Pohling C, Natarajan A, Witney TH, Kaur J, Xu L, Gowrishankar G, D’Souza AL, Murty S, Schick S, Chen L, Wu N, Khaw P, Mischel P, Abbasi T, Usmani S, Mallick P, Gambhir SS, AshwaMAX and Withaferin A inhibits gliomas in cellular and murine orthotopic models, J Neurooncol, 126 (2016) 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wei L, Zhou Y, Dai Q, Qiao C, Zhao L, Hui H, Lu N, Guo QL, Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma, Cell Death Dis, 4 (2013) e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liu W, Mu R, Nie FF, Yang Y, Wang J, Dai QS, Lu N, Qi Q, Rong JJ, Hu R, Wang XT, You QD, Guo QL, MAC-related mitochondrial pathway in oroxylin-A-induced apoptosis in human hepatocellular carcinoma HepG2 cells, Cancer Lett, 284 (2009) 198–207. [DOI] [PubMed] [Google Scholar]
- [118].Yang Y, Hu Y, Gu HY, Lu N, Liu W, Qi Q, Zhao L, Wang XT, You QD, Guo QL, Oroxylin A induces G2/M phase cell-cycle arrest via inhibiting Cdk7-mediated expression of Cdc2/p34 in human gastric carcinoma BGC-823 cells, J Pharm Pharmacol, 60 (2008) 1459–1463. [DOI] [PubMed] [Google Scholar]
- [119].Lu Z, Lu N, Li C, Li F, Zhao K, Lin B, Guo Q, Oroxylin A inhibits matrix metalloproteinase-2/9 expression and activation by up-regulating tissue inhibitor of metalloproteinase-2 and suppressing the ERK1/2 signaling pathway, Toxicol Lett, 209 (2012) 211–220. [DOI] [PubMed] [Google Scholar]
- [120].Wei L, Zhou Y, Qiao C, Ni T, Li Z, You Q, Guo Q, Lu N, Oroxylin A inhibits glycolysis-dependent proliferation of human breast cancer via promoting SIRT3-mediated SOD2 transcription and HIF1alpha destabilization, Cell Death Dis, 6 (2015) e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Qiao C, Lu N, Zhou Y, Ni T, Dai Y, Li Z, Guo Q, Wei L, Oroxylin A modulates mitochondrial function and apoptosis in human colon cancer cells by inducing mitochondrial translocation of wild-type p53, Oncotarget, 7 (2016) 17009–17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wei L, Dai Q, Zhou Y, Zou M, Li Z, Lu N, Guo Q, Oroxylin A sensitizes non-small cell lung cancer cells to anoikis via glucose-deprivation-like mechanisms: c-Src and hexokinase II, Biochim Biophys Acta, 1830 (2013) 3835–3845. [DOI] [PubMed] [Google Scholar]
- [123].Wei L, Yao Y, Zhao K, Huang Y, Zhou Y, Zhao L, Guo Q, Lu N, Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3beta signaling in snail-expressing non-small-cell lung cancer cells, Mol Carcinog, 55 (2016) 2121–2134. [DOI] [PubMed] [Google Scholar]
- [124].Zou M, Hu C, You Q, Zhang A, Wang X, Guo Q, Oroxylin A induces autophagy in human malignant glioma cells via the mTOR-STAT3-Notch signaling pathway, Mol Carcinog, 54 (2015) 1363–1375. [DOI] [PubMed] [Google Scholar]
- [125].Hui H, Zhang X, Li H, Liu X, Shen L, Zhu Y, Xu J, Guo Q, Lu N, Oroxylin A, a natural anticancer flavonoid compound, induces differentiation of t(8;21)-positive Kasumi-1 and primary acute myeloid leukemia cells, J Cancer Res Clin Oncol, 142 (2016) 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Li HN, Nie FF, Liu W, Dai QS, Lu N, Qi Q, Li ZY, You QD, Guo QL, Apoptosis induction of oroxylin A in human cervical cancer HeLa cell line in vitro and in vivo, Toxicology, 257 (2009) 80–85. [DOI] [PubMed] [Google Scholar]
- [127].Jager S, Trojan H, Kopp T, Laszczyk MN, Scheffler A, Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts, Molecules, 14 (2009) 2016–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Szakiel A, Paczkowski C, Pensec F, Bertsch C, Fruit cuticular waxes as a source of biologically active triterpenoids, Phytochem Rev, 11 (2012) 263–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhu Z, Qian Z, Yan Z, Zhao C, Wang H, Ying G, A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors, Int J Nanomedicine, 8 (2013) 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A, Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies, Biochem Pharmacol, 85 (2013) 1579–1587. [DOI] [PubMed] [Google Scholar]
- [131].Wang J, Liu L, Qiu H, Zhang X, Guo W, Chen W, Tian Y, Fu L, Shi D, Cheng J, Huang W, Deng W, Ursolic acid simultaneously targets multiple signaling pathways to suppress proliferation and induce apoptosis in colon cancer cells, PLoS One, 8 (2013) e63872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, Lim LH, Kumar AP, Hui KM, Sethi G, Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model, Int J Cancer, 129 (2011) 1552–1563. [DOI] [PubMed] [Google Scholar]
- [133].Kiran MS, Viji RI, Sameer Kumar VB, Sudhakaran PR, Modulation of angiogenic factors by ursolic acid, Biochem Biophys Res Commun, 371 (2008) 556–560. [DOI] [PubMed] [Google Scholar]
- [134].Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS, Son HC, Kim KW, Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells, Int J Cancer, 73 (1997) 725–728. [DOI] [PubMed] [Google Scholar]
- [135].Gao N, Cheng S, Budhraja A, Gao Z, Chen J, Liu EH, Huang C, Chen D, Yang Z, Liu Q, Li P, Shi X, Zhang Z, Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway, Br J Pharmacol, 165 (2012) 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Lin Z, Jiang J, Liu XS, Ursolic acid-mediated apoptosis of K562 cells involves Stat5/Akt pathway inhibition through the induction of Gfi-1, Sci Rep, 6 (2016) 33358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kim KH, Seo HS, Choi HS, Choi I, Shin YC, Ko SG, Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells, Arch Pharm Res, 34 (2011) 1363–1372. [DOI] [PubMed] [Google Scholar]
- [138].Yeh CT, Wu CH, Yen GC, Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling, Mol Nutr Food Res, 54 (2010) 1285–1295. [DOI] [PubMed] [Google Scholar]
- [139].De Angel RE, Smith SM, Glickman RD, Perkins SN, Hursting SD, Antitumor effects of ursolic acid in a mouse model of postmenopausal breast cancer, Nutr Cancer, 62 (2010) 1074–1086. [DOI] [PubMed] [Google Scholar]
- [140].Xavier CP, Lima CF, Pedro DF, Wilson JM, Kristiansen K, Pereira-Wilson C, Ursolic acid induces cell death and modulates autophagy through JNK pathway in apoptosis-resistant colorectal cancer cells, J Nutr Biochem, 24 (2013) 706–712. [DOI] [PubMed] [Google Scholar]
- [141].Prasad S, Yadav VR, Sung B, Reuter S, Kannappan R, Deorukhkar A, Diagaradjane P, Wei C, Baladandayuthapani V, Krishnan S, Guha S, Aggarwal BB, Ursolic acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: chemosensitization with capecitabine, Clin Cancer Res, 18 (2012) 4942–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Prasad S, Yadav VR, Kannappan R, Aggarwal BB, Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors. EVIDENCE FOR THE ROLE OF REACTIVE OXYGEN SPECIES AND JNK, J Biol Chem, 291 (2016) 16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Shanmugam MK, Rajendran P, Li F, Nema T, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Ho PC, Hui KM, Sethi G, Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice, J Mol Med (Berl), 89 (2011) 713–727. [DOI] [PubMed] [Google Scholar]
- [144].Shin SW, Kim SY, Park JW, Autophagy inhibition enhances ursolic acid-induced apoptosis in PC3 cells, Biochim Biophys Acta, 1823 (2012) 451–457. [DOI] [PubMed] [Google Scholar]
- [145].Zhang Y, Kong C, Zeng Y, Wang L, Li Z, Wang H, Xu C, Sun Y, Ursolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro, Mol Carcinog, 49 (2010) 374–385. [DOI] [PubMed] [Google Scholar]
- [146].Gao YS, Yuan Y, Song G, Lin SQ, Inhibitory effect of ursolic acid and oleanolic acid from Eriobotrya fragrans on A549 cell viability in vivo, Genet Mol Res, 15 (2016). [DOI] [PubMed] [Google Scholar]
- [147].Zheng QY, Li PP, Jin FS, Yao C, Zhang GH, Zang T, Ai X, Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells, Cell Signal, 25 (2013) 206–213. [DOI] [PubMed] [Google Scholar]
- [148].Li J, Liang X, Yang X, Ursolic acid inhibits growth and induces apoptosis in gemcitabine-resistant human pancreatic cancer via the JNK and PI3K/Akt/NF-kappaB pathways, Oncol Rep, 28 (2012) 501–510. [DOI] [PubMed] [Google Scholar]
- [149].Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, Guha S, Sethi G, Aggarwal BB, Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells, Mol Cancer Res, 5 (2007) 943–955. [DOI] [PubMed] [Google Scholar]
- [150].Tian Z, Lin G, Zheng RX, Huang F, Yang MS, Xiao PG, Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana, World J Gastroenterol, 12 (2006) 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Fahey JW, Zhang Y, Talalay P, Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens, Proc Natl Acad Sci U S A, 94 (1997) 10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Huang TY, Chang WC, Wang MY, Yang YR, Hsu YC, Effect of sulforaphane on growth inhibition in human brain malignant glioma GBM 8401 cells by means of mitochondrial- and MEK/ERK-mediated apoptosis pathway, Cell Biochem Biophys, 63 (2012) 247–259. [DOI] [PubMed] [Google Scholar]
- [153].Zhang Y, Talalay P, Cho CG, Posner GH, A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure, Proc Natl Acad Sci U S A, 89 (1992) 2399–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Maheo K, Morel F, Langouet S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A, Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes, Cancer Res, 57 (1997) 3649–3652. [PubMed] [Google Scholar]
- [155].Bacon JR, Williamson G, Garner RC, Lappin G, Langouet S, Bao Y, Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes, Carcinogenesis, 24 (2003) 1903–1911. [DOI] [PubMed] [Google Scholar]
- [156].Jiang ZQ, Chen C, Yang B, Hebbar V, Kong AN, Differential responses from seven mammalian cell lines to the treatments of detoxifying enzyme inducers, Life Sci, 72 (2003) 2243–2253. [DOI] [PubMed] [Google Scholar]
- [157].Lenzi M, Fimognari C, Hrelia P, Sulforaphane as a promising molecule for fighting cancer, Cancer Treat Res, 159 (2014) 207–223. [DOI] [PubMed] [Google Scholar]
- [158].Tortorella SM, Royce SG, Licciardi PV, Karagiannis TC, Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition, Antioxid Redox Signal, 22 (2015) 1382–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Karmakar S, Weinberg MS, Banik NL, Patel SJ, Ray SK, Activation of multiple molecular mechanisms for apoptosis in human malignant glioblastoma T98G and U87MG cells treated with sulforaphane, Neuroscience, 141 (2006) 1265–1280. [DOI] [PubMed] [Google Scholar]
- [160].Myzak MC, Karplus PA, Chung FL, Dashwood RH, A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase, Cancer Res, 64 (2004) 5767–5774. [DOI] [PubMed] [Google Scholar]
- [161].Kim H, Kim EH, Eom YW, Kim WH, Kwon TK, Lee SJ, Choi KS, Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of DR5, Cancer Res, 66 (2006) 1740–1750. [DOI] [PubMed] [Google Scholar]
- [162].Yeh CT, Yen GC, Effect of sulforaphane on metallothionein expression and induction of apoptosis in human hepatoma HepG2 cells, Carcinogenesis, 26 (2005) 2138–2148. [DOI] [PubMed] [Google Scholar]
- [163].Jackson SJ, Singletary KW, Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization, Carcinogenesis, 25 (2004) 219–227. [DOI] [PubMed] [Google Scholar]
- [164].Singh AV, Xiao D, Lew KL, Dhir R, Singh SV, Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo, Carcinogenesis, 25 (2004) 83–90. [DOI] [PubMed] [Google Scholar]
- [165].Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC, Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells, Int J Oncol, 20 (2002) 631–636. [DOI] [PubMed] [Google Scholar]
- [166].Wang L, Liu D, Ahmed T, Chung FL, Conaway C, Chiao JW, Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention, Int J Oncol, 24 (2004) 187–192. [PubMed] [Google Scholar]
- [167].Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P, Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane, Carcinogenesis, 23 (2002) 581–586. [DOI] [PubMed] [Google Scholar]
- [168].Gills JJ, Jeffery EH, Matusheski NV, Moon RC, Lantvit DD, Pezzuto JM, Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion, Cancer Lett, 236 (2006) 72–79. [DOI] [PubMed] [Google Scholar]
- [169].Sukamporn P, Rojanapanthu P, Silva G, Zhang X, Gritsanapan W, Baek SJ, Damnacanthal and its nanoformulation exhibit anti-cancer activity via cyclin D1 down-regulation, Life Sci, 152 (2016) 60–66. [DOI] [PubMed] [Google Scholar]
- [170].Garcia-Vilas JA, Quesada AR, Medina MA, Damnacanthal, a noni anthraquinone, inhibits c-Met and is a potent antitumor compound against Hep G2 human hepatocellular carcinoma cells, Sci Rep, 5 (2015) 8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Garcia-Vilas JA, Pino-Angeles A, Martinez-Poveda B, Quesada AR, Medina MA, The noni anthraquinone damnacanthal is a multi-kinase inhibitor with potent anti-angiogenic effects, Cancer Lett, 385 (2017) 1–11. [DOI] [PubMed] [Google Scholar]
- [172].Qin JJ, Jin HZ, Fu JJ, Hu XJ, Wang Y, Yan SK, Zhang WD, Japonicones A-D, bioactive dimeric sesquiterpenes from Inula japonica Thunb, Bioorg Med Chem Lett, 19 (2009) 710–713. [DOI] [PubMed] [Google Scholar]
- [173].Qin JJ, Jin HZ, Huang Y, Zhang SD, Shan L, Voruganti S, Nag S, Wang W, Zhang WD, Zhang R, Selective cytotoxicity, inhibition of cell cycle progression, and induction of apoptosis in human breast cancer cells by sesquiterpenoids from Inula lineariifolia Turcz, Eur J Med Chem, 68 (2013) 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Hu Z, Qin J, Zhang H, Wang D, Hua Y, Ding J, Shan L, Jin H, Zhang J, Zhang W, Japonicone A antagonizes the activity of TNF-alpha by directly targeting this cytokine and selectively disrupting its interaction with TNF receptor-1, Biochem Pharmacol, 84 (2012) 1482–1491.22981364 [Google Scholar]
- [175].Wang GW, Qin JJ, Cheng XR, Shen YH, Shan L, Jin HZ, Zhang WD, Inula sesquiterpenoids: structural diversity, cytotoxicity and anti-tumor activity, Expert Opin Investig Drugs, 23 (2014) 317–345. [DOI] [PubMed] [Google Scholar]
- [176].Qin JJ, Wang W, Voruganti S, Wang H, Zhang WD, Zhang R, Identification of a new class of natural product MDM2 inhibitor: In vitro and in vivo anti-breast cancer activities and target validation, Oncotarget, 6 (2015) 2623–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Qin JJ, Wang W, Voruganti S, Wang H, Zhang WD, Zhang R, Inhibiting NFAT1 for breast cancer therapy: New insights into the mechanism of action of MDM2 inhibitor JapA, Oncotarget, 6 (2015) 33106–33119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Li X, Yang X, Liu Y, Gong N, Yao W, Chen P, Qin J, Jin H, Li J, Chu R, Shan L, Zhang R, Zhang W, Wang H, Japonicone A suppresses growth of Burkitt lymphoma cells through its effect on NF-kappaB, Clin Cancer Res, 19 (2013) 2917–2928. [DOI] [PubMed] [Google Scholar]
- [179].Du Y, Gong J, Tian X, Yan X, Guo T, Huang M, Zhang B, Hu X, Liu H, Wang Y, Li J, Li M, Japonicone A inhibits the growth of non-small cell lung cancer cells via mitochondria-mediated pathways, Tumour Biol, 36 (2015) 7473–7482. [DOI] [PubMed] [Google Scholar]
- [180].Yu ZY, Xiao H, Wang LM, Shen X, Jing Y, Wang L, Sun WF, Zhang YF, Cui Y, Shan YJ, Zhou WB, Xing S, Xiong GL, Liu XL, Dong B, Feng JN, Wang LS, Luo QL, Zhao QS, Cong YW, Natural Product Vibsanin A Induces Differentiation of Myeloid Leukemia Cells through PKC Activation, Cancer Res, 76 (2016) 2698–2709. [DOI] [PubMed] [Google Scholar]
- [181].Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, Yuan J, Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex, Cell Death Differ, 11 (2004) 123–130. [DOI] [PubMed] [Google Scholar]
- [182].Saxena AK, Singh B, Anand KK, Hepatoprotective effects of Eclipta alba on subcellular levels in rats, J Ethnopharmacol, 40 (1993) 155–161. [DOI] [PubMed] [Google Scholar]
- [183].Diogo LC, Fernandes RS, Marcussi S, Menaldo DL, Roberto PG, Matrangulo PV, Pereira PS, Franca SC, Giuliatti S, Soares AM, Lourenco MV, Inhibition of snake venoms and phospholipases A(2) by extracts from native and genetically modified Eclipta alba: isolation of active coumestans, Basic Clin Pharmacol Toxicol, 104 (2009) 293–299. [DOI] [PubMed] [Google Scholar]
- [184].Benes P, Knopfova L, Trcka F, Nemajerova A, Pinheiro D, Soucek K, Fojta M, Smarda J, Inhibition of topoisomerase IIalpha: novel function of wedelolactone, Cancer Lett, 303 (2011) 29–38. [DOI] [PubMed] [Google Scholar]
- [185].Lee YJ, Lin WL, Chen NF, Chuang SK, Tseng TH, Demethylwedelolactone derivatives inhibit invasive growth in vitro and lung metastasis of MDA-MB-231 breast cancer cells in nude mice, Eur J Med Chem, 56 (2012) 361–367. [DOI] [PubMed] [Google Scholar]
- [186].Sarveswaran S, Ghosh R, Parikh R, Ghosh J, Wedelolactone, an Anti-inflammatory Botanical, Interrupts c-Myc Oncogenic Signaling and Synergizes with Enzalutamide to Induce Apoptosis in Prostate Cancer Cells, Mol Cancer Ther, 15 (2016) 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Levine AJ, p53: 800 million years of evolution and 40 years of discovery, Nat Rev Cancer, 20 (2020) 471–480. [DOI] [PubMed] [Google Scholar]
- [188].Muller PA, Vousden KH, Mutant p53 in cancer: new functions and therapeutic opportunities, Cancer Cell, 25 (2014) 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Bode AM, Dong Z, Post-translational modification of p53 in tumorigenesis, Nat Rev Cancer, 4 (2004) 793–805. [DOI] [PubMed] [Google Scholar]
- [190].Shin DM, Charuruks N, Lippman SM, Lee JJ, Ro JY, Hong WK, Hittelman WN, p53 protein accumulation and genomic instability in head and neck multistep tumorigenesis, Cancer Epidemiol Biomarkers Prev, 10 (2001) 603–609. [PubMed] [Google Scholar]
- [191].Yin Y, Tainsky MA, Bischoff FZ, Strong LC, Wahl GM, Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles, Cell, 70 (1992) 937–948. [DOI] [PubMed] [Google Scholar]
- [192].Eyfjord JE, Thorlacius S, Steinarsdottir M, Valgardsdottir R, Ogmundsdottir HM, Anamthawat-Jonsson K, p53 abnormalities and genomic instability in primary human breast carcinomas, Cancer Res, 55 (1995) 646–651. [PubMed] [Google Scholar]
- [193].Sood AK, Skilling JS, Buller RE, Ovarian cancer genomic instability correlates with p53 frameshift mutations, Cancer Res, 57 (1997) 1047–1049. [PubMed] [Google Scholar]
- [194].Uchida T, Wada C, Wang C, Egawa S, Ohtani H, Koshiba K, Genomic instability of microsatellite repeats and mutations of H-, K-, and N-ras, and p53 genes in renal cell carcinoma, Cancer Res, 54 (1994) 3682–3685. [PubMed] [Google Scholar]
- [195].Sarkar FH, Li Y, NF-kappaB: a potential target for cancer chemoprevention and therapy, Front Biosci, 13 (2008) 2950–2959. [DOI] [PubMed] [Google Scholar]
- [196].Thomas SJ, Snowden JA, Zeidler MP, Danson SJ, The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours, Br J Cancer, 113 (2015) 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ, Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro, J Clin Invest, 102 (1998) 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Fischer OM, Gschwind A, Ullrich AA, Cell surface growth factor receptor molecules as targets for cancer therapy, Discov Med, 4 (2004) 166–171. [PubMed] [Google Scholar]
- [199].Dunn GP, Old LJ, Schreiber RD, The immunobiology of cancer immunosurveillance and immunoediting, Immunity, 21 (2004) 137–148. [DOI] [PubMed] [Google Scholar]
- [200].Karin M, NF-kappaB as a critical link between inflammation and cancer, Cold Spring Harb Perspect Biol, 1 (2009) a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li D, Wu Y, Song Y, Luo J, Pang X, Yi Z, Liu M, Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway, Carcinogenesis, 31 (2010) 2097–2104. [DOI] [PubMed] [Google Scholar]
- [202].Duangmano S, Dakeng S, Jiratchariyakul W, Suksamrarn A, Smith DR, Patmasiriwat P, Antiproliferative effects of cucurbitacin B in breast cancer cells: down-regulation of the c-Myc/hTERT/telomerase pathway and obstruction of the cell cycle, Int J Mol Sci, 11 (2010) 5323–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Kausar H, Munagala R, Bansal SS, Aqil F, Vadhanam MV, Gupta RC, Cucurbitacin B potently suppresses non-small-cell lung cancer growth: identification of intracellular thiols as critical targets, Cancer Lett, 332 (2013) 35–45. [DOI] [PubMed] [Google Scholar]
- [204].Guo J, Wu G, Bao J, Hao W, Lu J, Chen X, Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell cycle arrest in a ROS-dependent manner, PLoS One, 9 (2014) e88140. [DOI] [PMC free article] [PubMed] [Google Scholar]


