Abstract
Neurotrophic keratopathy is a degenerative disease in which damage to the corneal nerves leads to corneal hypoesthesia. Injuries to neurotrophic corneas are notoriously difficult to treat and have traditionally been approached with supportive management. However, recent progress in the field of corneal neurotization has given new direction for addressing nerve loss directly by stimulating new nerve growth onto the cornea from nearby sensory nerves transferred to the perilimbal region. Herein, we review the surgical techniques utilized in corneal neurotization, including direct transfers and the use of nerve grafts. Considerations in surgical approach, as well as factors that influence prognosis and outcomes of the surgical intervention are also discussed.
Keywords: neurotization, neurotrophic keratopathy, corneal nerve, nerve coaptation, anesthetic cornea, neurotrophic cornea, herpetic keratopathy
I. INTRODUCTION
Corneal nerves play an essential role in directing growth and repair of the cornea. Injuries to neurotrophic corneas are notoriously difficult to treat and are often associated with poor visual outcomes. Traditional treatment paradigms call for supportive management, as the root cause of the problem – loss of corneal nerves and important growth factors – could not be addressed. Recently, exciting progress in the field of corneal neurotization has given new direction for addressing nerve loss directly by stimulating new nerve growth onto the cornea from nearby sensory nerves transferred to the perilimbal region [1,2].
Corneal neurotization was first described in 1972 by Samii, where a sural nerve was used as an interpositional graft between the greater occipital nerve and the transected ophthalmic nerve [3,4]. The described technique via frontal craniotomy was complex and required a long procedure time, and thus was not readily incorporated into routine clinical practice. In 2009, Terzis et al. described direct transfer of the supratrochlear and supraorbital nerves to the corneoscleral limbus of the denervated cornea to restore corneal sensation [1]. Over the past decade, refinements in this surgical technique have led to promising results in restoring corneal function [5–8]. A comprehensive review of the advancements in technique, the clinical outcomes, and recent insights into the mechanisms are discussed.
II. METHODS
The authors searched PubMed and Google Scholar with the terms “corneal neurotization”, “corneal neurotisation”, and “neurotrophic keratopathy”. Articles and abstracts published in English between December 2008 and November 2020 were included in the literature search. After selecting for studies reporting on surgical techniques, 45 articles were included for review.
III. PATHOPHYSIOLOGY OF NEUROTROPHIC CORNEA
Neurotrophic keratopathy is a degenerative disease in which damage to the corneal nerves leads to corneal hypoesthesia. Neurotrophic keratopathy has a prevalance of 1.6 to 11/10,000, with the most frequent cause of neurotrophic keratopathy being trigeminal nerve injury from herpetic keratopathy [84]. Other conditions such as diabetes, dry eye syndrome, topical medications like chronic glaucoma treatment, familial dysautonomia, leprosy, tumors, chemical, and surgical trauma, including refractive surgery, can also affect the trigeminal nerve at different levels [9–12, 80].
Corneal nerves are essential in maintaining ocular surface homeostasis, as they play a role in tear production and epithelial regeneration [9]. Corneal nerves secrete substance P and calcitonin gene-related factors which play important roles in epithelial proliferation and wound healing [9,10, 80]. In turn, corneal epithelial cells secrete growth factors such as neurotrophin 3, neurotrophin-like nerve growth factor, and ciliary neurotrophic factor which promote the survival of corneal nerves [9,13]. These factors and others including glial cell-derived neurotrophic factor and epidermal growth factor help stimulate nerve and epithelial cell regeneration after injury [80].
Additionally, corneal sensation is essential for reflex blinking and tearing. Decreased corneal sensation increases the risk for corneal micro trauma and corneal epithelial breakdown and is associated with delayed healing [9,14,15,80]. Abnormal innervation of the cornea reduces tear production and compromises epithelial function, further reducing expression of epitheliotrophic mediators and neurotrophic growth factors [80]. For instance, patients with neurogenic deregulation as a result of long-standing diabetes and chronic inflammation showed an instability of their tear film and reduction of corneal sensitivity [85]. Thus, a neurotrophic cornea – lacking properly functioning nerves or sensation- makes the eye vulnerable to developing severe complications such as non-healing ulcers, thinning, infection, melting, and perforation; this can lead to scarring and vascularization of the cornea which ultimately limits vision [9,13–15, 80].
Until recently, the traditional approach to managing neurotrophic corneas has been supportive rather than addressing the root cause. This includes frequent lubrication with preservative free artificial tears, autologous serum tears, and therapeutic soft or hard contact lenses, including BostonSight PROSE contact lenses [11,13,16, 81]. Surgical options include amniotic membrane transplantation which can reduce vascularization and surface inflammation [13,17,18, 80]. Tarsorrhaphy is another surgical option for non-healing corneal ulcers. Despite these supportive treatments, neurotrophic corneal ulcers have a protracted course with permanent sequelae.
Replacement of nerve growth factors by either external application (such as with topical insulin-derived growth factor, human recombinant nerve growth factor, and others) or via neurotization is different from the aforementioned supportive methods in that it addresses the root cause of the cornea’s poor ability to heal itself and promotes reinnervation [19–25, 80,81,82]. While administration of topical nerve growth factors has shown improvement in nerve regeneration and corneal sensitivity in animal models, randomized clinical trials have shown efficacy in wound healing but failed to show statistically significant improvement in corneal sensation in humans [26–29]. Serum tears and other blood derivates which contain and provide multiple growth factors have also been shown to aid epithelial healing with mild improvement of corneal sensation, but the risk of contamination and accessibility have limited their use [80,83]. Corneal neurotization, on the other hand, confers a durable improvement in corneal sensation, and maybe a more permanent solution.
IV. LESSONS FROM PERIPHERAL NERVE REPAIR
Repair of peripheral nerve injuries has historically been performed by coapting the proximal severed nerve end to the distal end either directly, or through the use of a tubule (i.e., conduit), or an interpositional nerve graft for tension free repair. The success of peripheral nerve coaptation is based on the regeneration of axons across the neurorrhaphy from the proximal injured nerve end into the distal nerve stump or directly into the target tissue. This process is initiated by Wallerian degeneration and followed by Schwann cell dedifferentiation and proliferation aided by tissue macrophages and inflammatory cells [30]. Studies of sensory nerve reconstruction have shown that younger age and a shorter distance between the transected or injured nerve ends (gap length) are associated with improvements in static two-point discrimination [31–34]. Sensory end-organs can survive for an extended period of time; as such, surgical repairs can take place several years after the initial injury and still yield successful reinnervation outcomes, unlike motor nerve repairs where the timing of surgery can alter the success rate by as much as 54% [15,33,35,36]. Use of nerve autografts as interpositional grafts have shown high rates of success in digit reconstruction [34]. Processed nerve allografts are a viable alternative and comparable results have been reported [34].
The cause of peripheral nerve injury is associated with success of reinnervation. Local mechanical insult (i.e., acute stretch/compression or blunt crush) that leads to neuropraxia (i.e., local myelin injury only) or axonotmesis (i.e., axonal transection with sparing of epineurium) has a good prognosis overall and can achieve spontaneous recovery within weeks to years [35,37]. Spontaneous recovery can occur in up to 70% of closed radial nerve injuries [38]. In contrast, injuries that cause neurotmesis (i.e., division of axons, endoneurium, perineurium, and epineurium) have poor prognosis although sharp injuries may still fare better [33]. In the case of blast injuries, successful reinnervation has been reported in less than 50% of cases [37]. When surgery is successful, significant progress is typically seen within the first year [32,33]. A longer duration of time is associated with a more stable level of sensory recovery and is thought to be related to the time needed for functional reorganization of the somatosensory cortex, which is seen usually after a period of 6 months [33]. Sensory improvements have been observed up to 3 years after surgery [32,33]. In an experimental rat study, diabetes appeared to delay functional nerve recovery [39]. However, it is unclear to what extent this translates to human nerve repair.
When the distal nerve stump is not available, direct tissue neurotization is an alternative option where the donor nerve or an interpositional nerve graft is directly implanted into the target tissue [30,40]. With this technique, it is hypothesized that the proximal nerve directly sprouts axons to reinnervate the target tissue [15]. Rat studies support this hypothesis through findings of increased acetylcholinesterase activity, higher combined muscle action potential amplitude, and greater nerve fiber count after peripheral nerve neurotization [40,41]. Although innervation via neurotization is a slower process than direct nerve repair or grafted coaptation, it is a viable method for reinnervation in cases where nerve-to-nerve reconnection is not possible (e.g., chronic neural degeneration, distal nerve injury or distal neural atrophy, neurotrophic cornea, etc.) [42].
In peripheral nerve reconstruction, epineurial repair, where the epineurium of the proximal and distal nerve is connected, is the most common method of coaptation. The nerve ends can be connected via an end-to-end (ETE), end-to-side (ETS), or side-to-side (STS) approach (Figure 1) [43–46]. In digital reconstruction, both ETE and ETS techniques showed good to excellent outcomes in the majority of cases, with ETE having a small but significant improvement compared to ETS coaptation [32]. In an experimental rat tibial neurorrhaphy study, all methods resulted in functional recovery. The ETE method had overall the best functional recovery, which was associated with higher nerve fiber counts, area, and density [44]. Ultimate choice of surgical technique depends on the relative sizes of the donor and recipient nerves, as a similar caliber is needed for good alignment in the ETE approach. The ETS approach may be more suitable when there are significant differences in nerve caliber, the donor nerve is critical to function, or the proximal nerve end is not available [47,48]. In an ETS neurorrhaphy, an epineurial window is created on the donor nerve to which the epineurium of the distal nerve stump is connected. This maneuver may increase the risk of axonal injury, a significant concern if a critical nerve is used as a donor, but it appears to be beneficial in stimulating axonal regeneration [44,49–52]. More recently, an STS “bridging” approach has shown promise in rat studies as another method of repairing peripheral nerve injuries.
Figure 1.
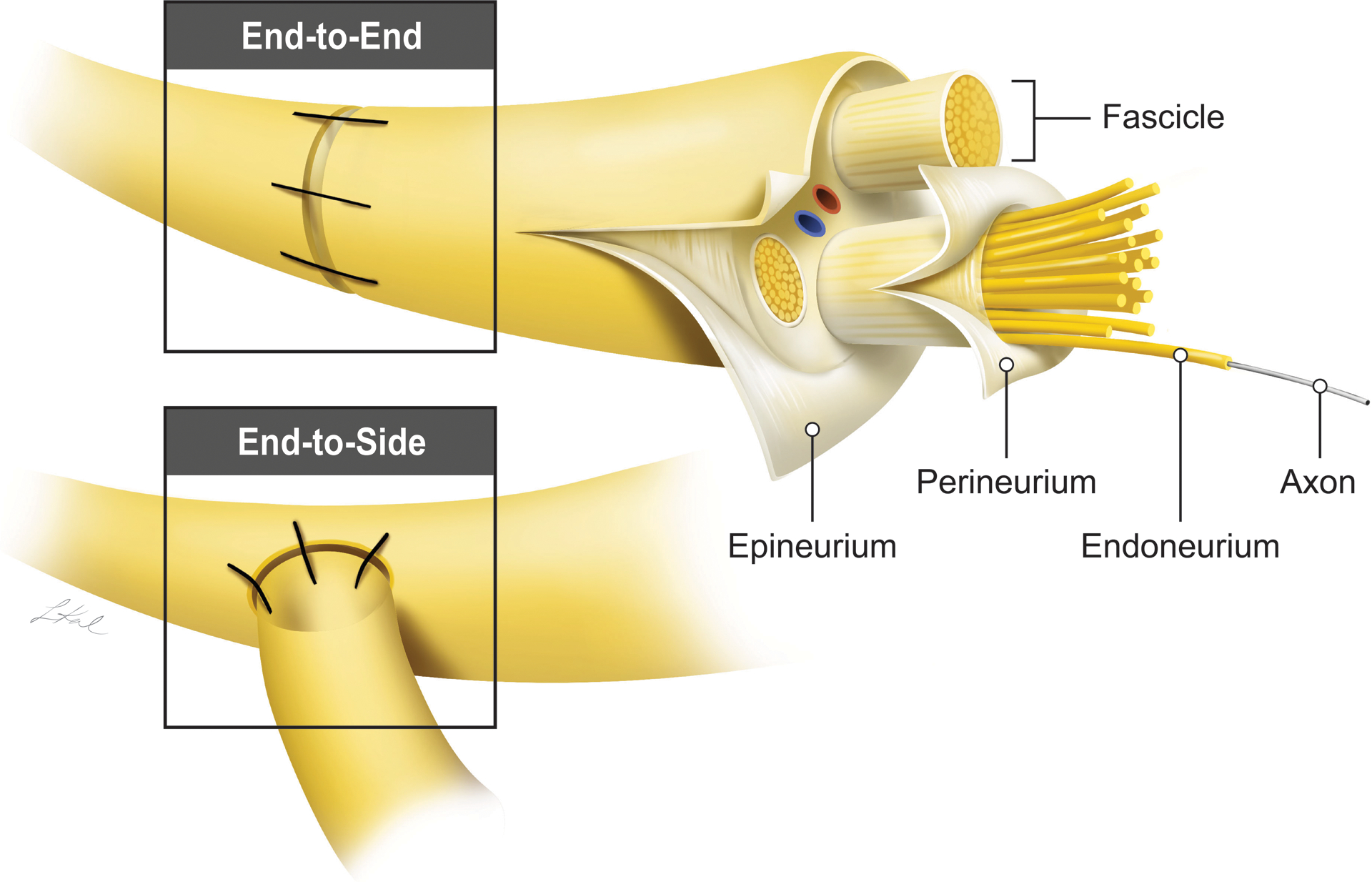
A model of end-to-end (ETE) and end-to-side (ETS) anastomosis commonly used in nerve repair. For the ETS, a window is created in the epineurium prior to the anastomosis.
V. CORNEAL NEUROTIZATION TECHNIQUES
In corneal neurotization, an intact donor sensory nerve can be transferred directly to the affected cornea or indirectly using interpositional nerve graft (Figure 2). An interpositional nerve graft can be harvested as an autograft or an allograft which is decellularized and processed prior to use. Various techniques have been described, and donor nerves ipsilateral and contralateral to the affected cornea have been used. Selection of an approach takes into account several factors – the availability of a sensate donor site, the size of the donor nerve which can individually vary, the surgeońs experience and preference, and the distance between the end (or side) of the donor nerve and the cornea (gap length).
Figure 2.
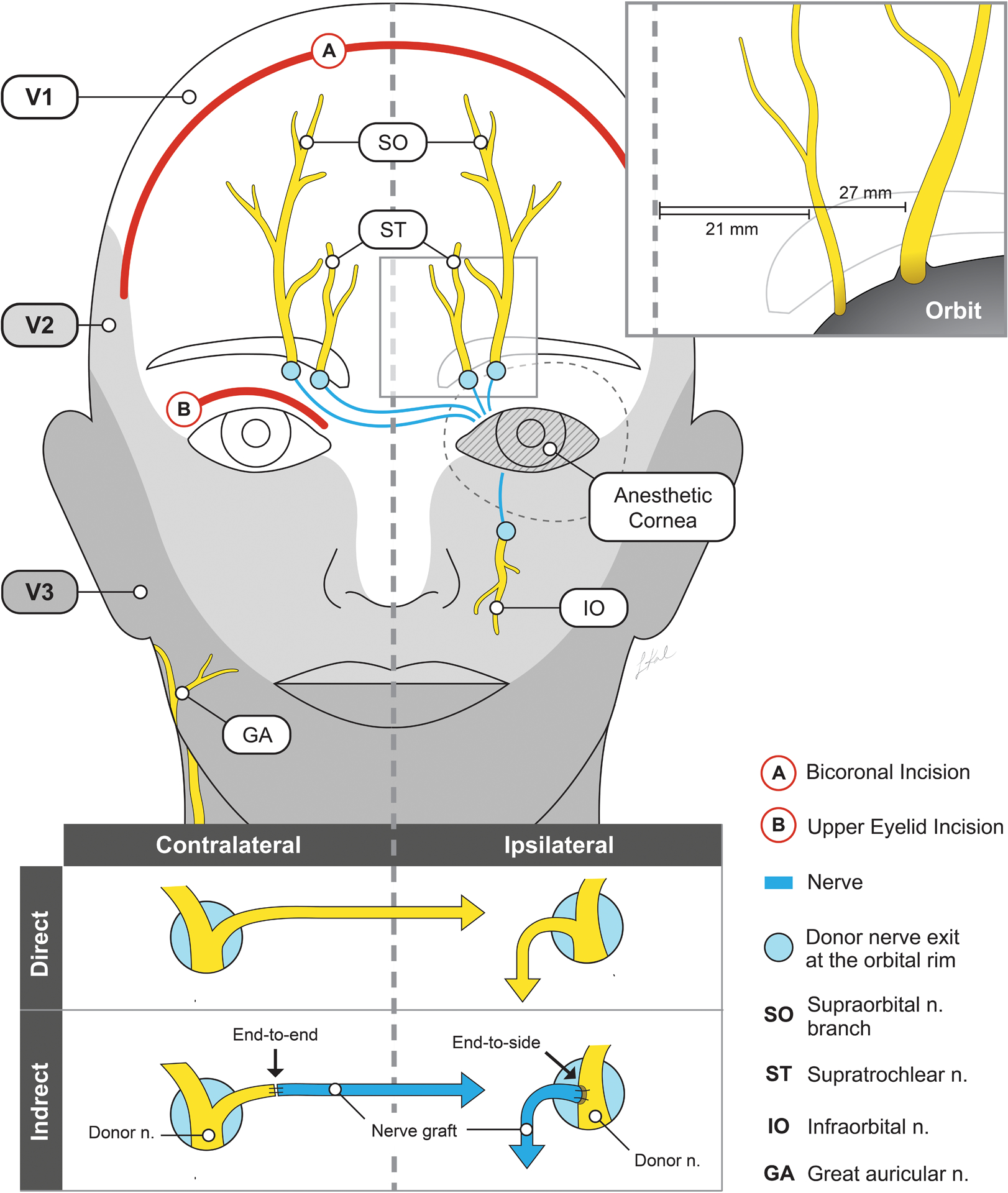
Common sites for direct and indirect corneal neurotization. Donor sites include supraorbital, supratrochlear, infraorbital, and great auricular nerves. The supraorbital nerve exits at the superior orbital rim lateral to the supratrochlear nerve and has a larger caliber (upper inset). The deep branch of the supraorbital nerve has a more consistent course. In the case of direct nerve transfer, coronal incisions (in red, labeled “A”), or minimally invasive endoscopic incisions are made. For indirect nerve transfers, the exit site of the donor nerve is exposed (for example by an eyelid crease incision in red, labeled “B”), and the nerve graft is anastomosed to the donor by an end-to-end or end-to-side approach (lower inset). Selection of contralateral or ipsilateral donor sites depends on the availability and condition of sensate nerves.
VI.A.1. Open direct transfer
Terzis et al. was the first to describe an open technique for the isolation of the contralateral supratrochlear and supraorbital nerves [1]. The dissection was carried to the superior orbital rim through a coronal incision, where branches of the exiting supratrochlear and supraorbital nerves were isolated, transected distally, tunneled across the nasal bridge, and externalized through an eyelid crease incision superior to the affected cornea (Figure 2). The branches were then tunneled through the superior fornix onto the ocular surface. Incisions in the bulbar conjunctiva allow nerve branches to be placed around the limbus between the Tenon’s and sclera. The branches were then sutured into place with 10–0 nylon before the conjunctiva was closed over them. This procedure was staged with motor reanimation of the orbicularis muscle in an adult with a CN V and VII paralysis after neuroma resection [53]. A hemicoronal modification has also been described with isolation of the deep (periosteal) branch of the ipsilateral supraorbital nerve [8].
VI.A.2. Endoscopic and minimally invasive direct transfer
Endoscopic and minimally invasive direct transfers allow for smaller incisions that may offer faster healing time and less scarring at the incision site. Using this technique, two 1 cm sagittal incisions can be made in the scalp instead of a coronal incision, and dissection carried in the subperiosteal or subgaleal plane with the aid of an endoscope until the superior orbital rim is reached [6,7] (Video 1). An upper eyelid crease incision is then made, with continued dissection superiorly to allow direct visualization of the supraorbital nerve at its exit point at the rim. The deep branch, which has a more reliable course and has more densely populated nerve axons, is dissected with the aid of an endoscope for a total of about 7–9 cm [54]. For ipsilateral transfers, dissection of the entire length of the donor nerve segment can be achieved through the upper lid incision only. It is then transected and tunneled to the affected cornea. Either an ipsilateral or contralateral supraorbital nerve can be used depending on the preoperative sensibility testing. The ipsilateral infraorbital nerve can also be used as long as pre-op testing shows intact sensation, in which case, about 4–5 cm of nerve length is needed to transfer directly to the corneal limbus [5,55].
Video 1.
Minimally invasive direct nerve transfer of the contralateral supraorbital nerve.
This video was published in Ophthalmic Plastic and Reconstructive Surgery, volume 56, Wisely CE et al., Clinical and morphologic outcomes of minimally invasive direct corneal neurotization, copyright Wolters Kluwer Health, Inc., 2020.
VI.A.3. Direct transfer considerations
While initial cases reported objective improvement of sensibility at an average of 2.8 years, others have observed evidence of objective improvement by 3–8 months post direct transfer [1,5,8,53,55–57]. The small number of cases reported in the literature thus far limits the conclusions that can be drawn. However, denervation time does not appear to influence the success rate or recovery time. Majority of reported cases have shown success in improving corneal sensation, reducing corneal neovascularization and opacity, and/or reducing Mackie score (see section below).
Despite transection of the supratrochlear and supraorbital nerve branches, numbness of the forehead resolved over a median of 3–9 months in all cases, likely because of collaterals from intact branches. Rare but possible adverse effects include subgaleal hematoma and development of a neuroma, scarring at the incision site, and alopecia from coronal incisions. Bony overgrowth at the supraorbital notch was reported in 1 case [1,5].
VI.B.1. Indirect transfer with interpositional nerve autograft
Interpositional nerve autografts have been successfully used to connect donor nerve to the affected cornea. Second site locations for harvest include the purely sensory sural nerve, great auricular nerve, and lateral antebrachial cutaneous nerve [15,56,58–65]. Most commonly, the contralateral supratrochlear nerve has been accessed by a sub-brow or eyelid crease incision and dissection to the superior orbital rim (Figure 2). The supraorbital nerve or infraorbital nerve can also be used [63] (Video 2). If the nerve is small, an ETE coaptation can be made between the transected donor nerve and the harvested autograft. Alternatively, an epineurial window could be created for ETS coaptation. Typically, a harvested autograft must be 10–15 cm or longer to enable tension-free anastomosis and promote nerve sprouting [58,60,66]. If the contralateral side is used, the autograft is tunneled to the affected side and passed through an eyelid crease incision, with or without a protective sheath [67]. This is then tunneled to the superior conjunctival fornix onto the bulbar conjunctiva. The epineurium of the autograft is then opened, and the fascicles dissected (typically 4–8) [56,58,59,62] (Figure 1). A superior bulbar conjunctival incision is created for perilimbal positioning of the dissected fascicles. Both sutures and corneoscleral tunneling have been described as methods to secure the fascicles, after which point the conjunctiva is closed over the fascicles to minimize desiccation, scarring, and inflammation [15,58–60].
Video 2.
Indirect nerve transfer using the ipsilateral infraorbital donor nerve and a sural nerve autograft.
VI.B.2. Indirect transfer with interpositional processed nerve allograft
Another technique that has been described is the use of a processed nerve allograft as a substitute for an autograft [57,75]. The caliber of the allograft as well as the donor nerve can vary, but both ETE and ETS neurorrhaphies have been described from the ipsilateral or contralateral supraorbital, supratrochlear, or infraorbital nerves [57,75]. Access to the infraorbital nerve can be achieved by a transconjunctival fornix approach and unroofing of the infraorbital canal to expose the infraorbital nerve for ETS anastomosis. For anastomoses, a nerve connector can be used, and is theorized to protect the neurorrhaphy [57]. The epineurium of the allograft is similarly opened, the fascicles divided, and secured to the sclera in the perilimbal region.
VI.B.3. Indirect transfer considerations
Following the indirect nerve transfer method, subjective improvement in sensation has been reported as early as 1-month post-op, with the majority of cases showing objective improvement in corneal function by 6–9 months. Reports note the importance of continuing topical treatment during the recovery period even after the return of corneal sensation [15]. In the early stages of recovery, worsening discomfort in the affected eye may occur as the damaged epithelium of the cornea begins to be reinnervated [58,59]. Referred sensation to the site of the donor nerve upon stimulation of the cornea may also occur early on in the post-operative period [57,58]. This typically resolves within 3–6 months, although a 10-year period of ocular discomfort has been reported in one case [58,59]. Numbness in the donor nerve territory improved in all reported cases described over the course of 3–6 months. Similarly, numbness at the secondary harvest site either improved with time or was tolerable [56,58,64,65]. Other uncommon complications include hyperesthesia, neuropathic pain, and significant scarring.
There is increasing evidence that processed nerve allografts are viable alternatives to nerve autografts in corneal neurotization. A multicenter retrospective study using processed nerve allografts reported similar time course and level of sensation return compared to the use of autografts, with a mean time to first gain of sensation of 3.7 months and maximal gain of 6.6 months [75]. Comparable outcomes were achieved with ETE and ETS coaptations, as well as location and laterality of donor nerve [75].
VI.C. Surgical considerations:
Choice of approach needs to be individualized when considering surgical repair, and takes into account donor nerve sensory function, nerve caliber and axon count, anatomical proximity to the recipient cornea, surgeon’s preference, and the surgical accessibility.
Pre-operative sensation testing should include the supraorbital nerve (supplies the skin of the lateral forehead, anterior hair bearing scalp, and upper eyelid, as well as the conjunctiva of the upper eyelid), the supratrochlear nerve (supplies the medial portion of the forehead and upper eyelid and the bridge of the nose), and the infraorbital nerve (supplies the skin of the cheek, upper lip, lateral aspect of the nose, and upper gingiva and teeth). If the function of these nerves is decreased or absent, additional testing should be performed to check the skin innervation by the ipsilateral greater auricular nerve which supplies the skin inferior to the external auditory meatus including the mastoid process and parotid gland regions. Neurotrophic keratopathy due to a localized, ocular cause, may still exhibit intact sensation in the V1 distribution on the same side of the anesthetic cornea so that ipsilateral supraorbital or supratrochlear nerves may still be considered, while a more central cause may not, in which case a contralateral donor nerve may be required [6].
Other considerations for donor nerve selection include the distance from donor to the affected cornea and the nerve caliber and axon count (Figure 2). Matching donor and graft caliber aids coaptation [57]. The supraorbital nerve, with approximately 6000 myelinated axons exiting at the supraorbital rim, and the infraorbital nerve are closest to an ipsilaterally affected cornea, and are also of larger caliber and have a more consistent anatomical path than the supratrochlear nerve (approximately 2500 myelinated axons at the orbital rim) [54].
The surgical approach to the supraorbital and supratrochlear nerves are through an upper lid crease or sub brow incision, while isolation of the infraorbital nerve requires an inferior orbitotomy and unroofing of the infraorbital canal to expose the nerve. An ETS coaptation is typically done if the infraorbital nerve is selected, given the significant morbidity upon neurotmesis (Video 2) [57].
A couple theories of the mechanism of reinnervation has been proposed – a paracrine-like release of nerve growth factors provided by the donor nerve and direct sprouting of axons [1,68]. As nerve growth occurs at a rate of up to 1 mm/day, a paracrine-like effect was proposed to explain the relatively fast subjective recovery in many patients [68]. Several lines of evidence however support the second theory of direct axonal sprouting. Clinically, stimulation of the affected cornea triggered a referred tactile sensation in the contralateral donor nerve territory [58]. On magnetoencephalography, stimulation of the operated cornea evoked a response in the area of the brain corresponding to the location of the contralateral donor nerve, and not the ipsilateral trigeminal nerve [15]. Lastly, labeled corneal nerves post neurotization traced back to the contralateral donor nerve in a rat model, suggesting a continuous axonal connection to the donor neurons [14].
Given the supporting evidence for direct axonal sprouting, limiting the distance between the donor nerve and the affected cornea ideally minimizes the distance that the newly sprouted nerves have to travel. Indeed, gap length is correlated with recovery time in peripheral nerve repair [31,69]. Fibrin tissue glue with synthetic nerve conduits, and/or amniotic membrane wraps, have been used in the literature to help optimize nerve regeneration at the site of the neurorrhaphy [13,30,57,60,67,76,77]. Based on animal studies suggesting improved outcomes, amniotic membrane wraps have been used to reduce inflammation and scarring at coaptation sites, as well as protecting these sites to prevent potential aberrant axonal escape, which can cause neuromas [13,18,57,67,78,79].
VII. OUTCOMES OF CORNEAL RE-INNERVATION
Direct and indirect corneal neurotization methods have both shown high rates of recovery of corneal defects [70,76]. On the Mackie grading scale for neurotrophic keratopathy, a statistically significant mean improvement from grade 2.46 +/− 0.77 to 0.86 +/−0.79 pre and post neurotization is noted in a recent meta-analysis, with improvements seen typically within the first 6 months and may continue to progress [11,15,70,74]. There appears to be no significant long-term difference in these outcome measures, and selection of the surgical technique should be made based on each patient’s individual needs and surgeon’s experience [70,76].
VII.A. Return of Sensation
Whereas corneal sensation does not return with topical recombinant human nerve growth factor, a significant degree of sensation does return with corneal neurotization. In a prospective comparative case series, successful reinnervation was observed in 80% for direct and 83% for indirect neurotization [70]. Subjective perception of sensation occurs earlier than objective improvement by esthesiometry measurement [1]. Patients report pain and discomfort starting several weeks after the operation, whereas objective improvement is documented after around 5–6 months [1,8,59,65,71]. Improvements continue for about a year after the procedure, though a longer time course has been reported in earlier studies [1,8]. Return of sensation is significant, though not complete compared to the contralateral cornea [65,71]. More long-term studies are needed, but the duration of effect appears to be strong [1].
VII.B. Corneal Morphologic Changes
In the cornea, nerve bundles divide multiple times before entering the corneal stroma radially and then move to the most superficial layers ending in free nerve endings (Figure 3). These provide afferent input for blinking and lacrimal reflex as well as the neurotrophic factors for corneal growth and homeostasis [11,65]. The number and density of subbasal nerves are correlated with corneal sensitivity [12,72]. Additionally, the distance of the injured nerve to the healthy nerve graft (or distal nerve) has been shown to affect the speed of corneal recovery. An explanation for this may be due to the migration of trophic factors which aid healing process, as studies have previously noted that nerves regenerate predominantly in the corneal periphery [15,65,68,71,73].
Figure 3.
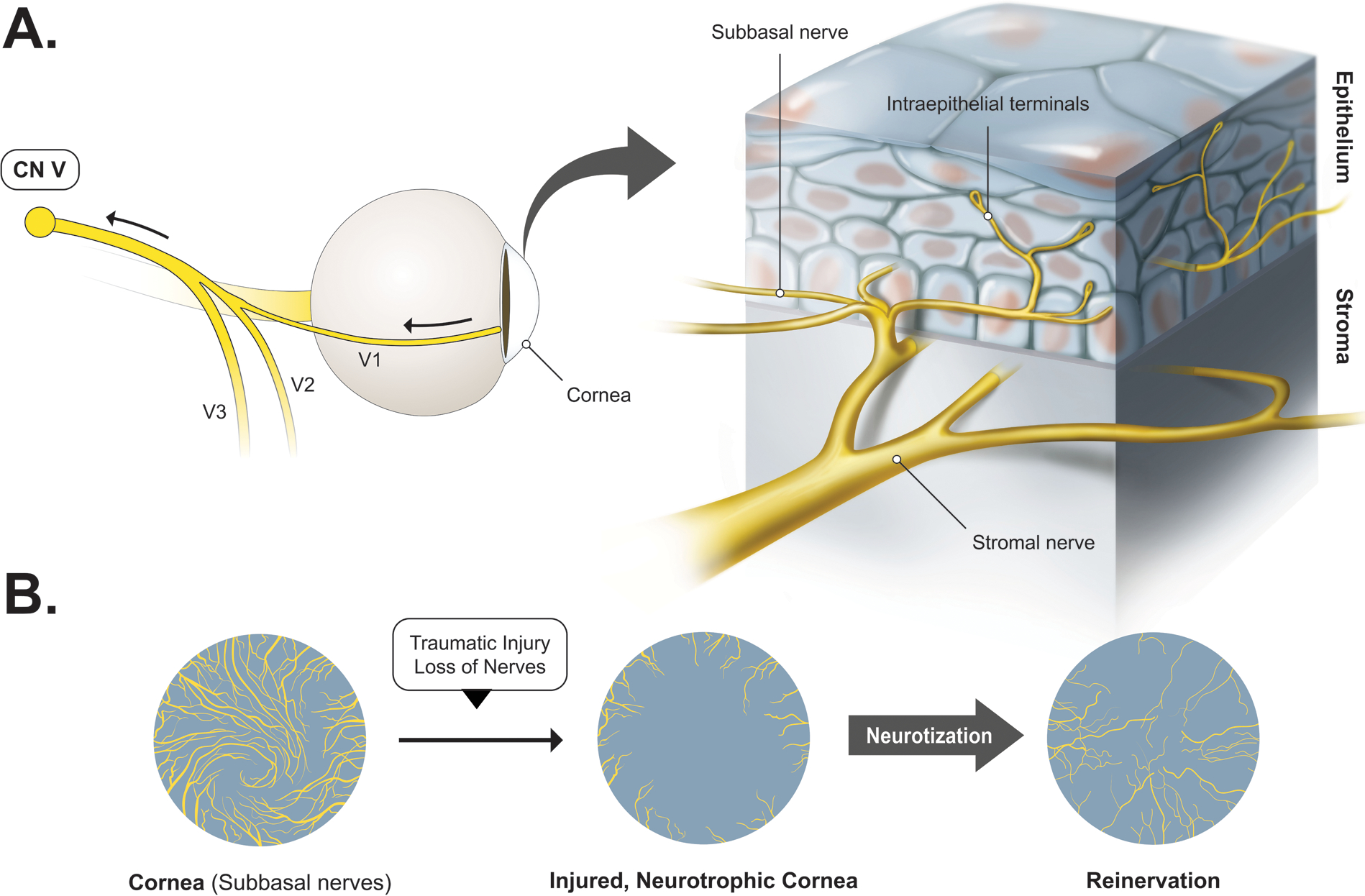
Model of reinnervation post corneal neurotization based on current research. A. The cornea is innervated by ciliary nerves. Nerve bundles divide before entering the corneal stroma radially in a whorl-like pattern, and progressively move superficially as the subbasal nerve plexus, and ending in free nerve endings in the epithelium, where neurotrophic factors are secreted. B. Model of corneal structural changes after corneal neurotization. There is regrowth of subepithelial and stromal nerves, but nerve density throughout the cornea is not as uniform, and there is loss of the characteristic whorl pattern [15,65]. Axons are thinner, and a larger number of myelinated fibers are present, as opposed to the unmyelinated fibers typically found in the uninjured cornea.
A rat model of corneal neurotization showed a significant increase in central corneal nerve density in the subbasal and stromal nerve plexus from regeneration post-procedure [15]. Similar changes in the subbasal nerve plexus have also been seen in a human study using confocal microscopy, starting as early as 3 months post neurotization with progressive improvement seen over the course of 3–6 months before stabilizing [65]. In this study, there was a lag in the detection of corneal sensation at 6–8 months after first nerve fibers become visible on confocal microscopy, suggesting that time is needed to establish neural connections with the somatosensory cortex [65]. However, blinking reflex was not restored after neurotization.
Some structural differences between normal and post-neurotization corneas were noted from the rat study. Post-procedure, density was not as uniformly seen throughout the cornea, axons were thinner, and there was no restoration of the characteristic whorl pattern of the subbasal plexus [15]. After neurotization, a larger number of myelinated fibers were shown to be present, in contrast to the unmyelinated C fibers that are typical for an uninjured cornea [15].
VII.C. Visual Prognosis
Improvement in visual acuity after corneal neurotization is mostly limited by corneal scarring, amblyopia, and other underlying ocular conditions [15,16,57,62]. Therefore, some authors advocate performing corneal neurotization at earlier stages of neurotrophic keratopathy before irreversible corneal scarring and amblyopia (in children) sets in [2,16,31]. Age appears to be correlated with final visual acuity and reestablishment of corneal sensation, with those younger than 18 years old demonstrating faster and more complete recovery [16] (Figure 4). Successful corneal transplants after neurotization have been performed simultaneously with corneal neurotization, though most surgeons wait at least 2 years [5,15,63,71]. In the few cases that have been reported, complete re-epithelization was achieved in 4–12 weeks after surgery, and return of central corneal sensation to pre-transplant levels was achieved in 6–12 months [5,15,63,71]. Reported complications include transient epithelial defects and bacterial keratopathy.
Figure 4.
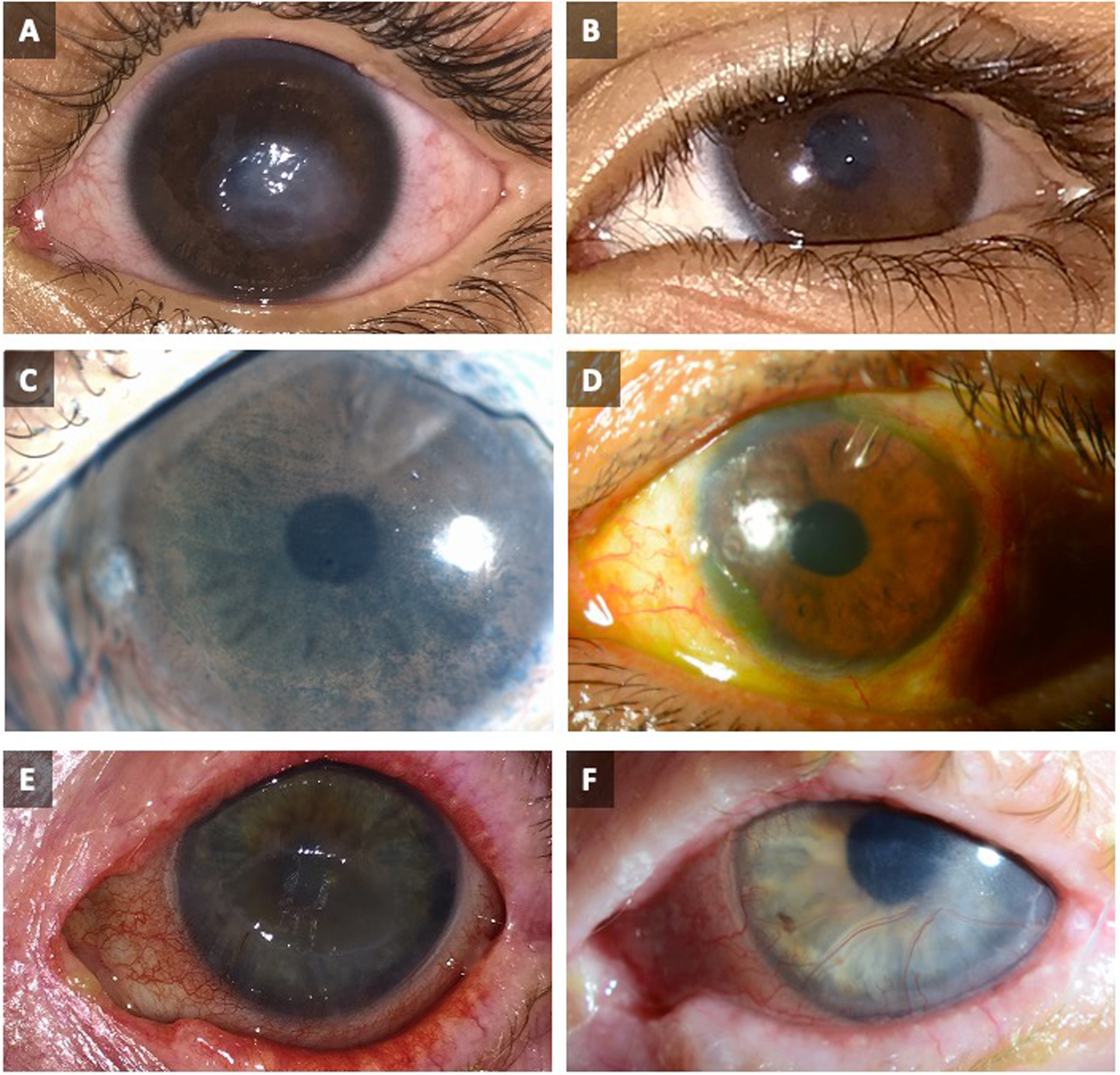
Pre- and post-operative images of patients who have undergone corneal neurotization surgery.
(A) 5-year-old boy with a 2-year history of corneal denervation, seen prior to minimally invasive direct corneal neurotization and (B) 11 months post-op.
(C) A 63-year-old male with ocular history that includes glaucoma with multiple drainage devices, type 2 diabetes mellitus with proliferative diabetic retinopathy status post panretinal photocoagulation, and severe dry eye syndrome, seen prior to corneal neurotization with end-to-side coaptation with diffuse lysamine green staining and (D) 6 months after indirect infraorbital nerve transfer, showing clear cornea without epithelial defects.
(E) An 86-year-old woman with herpes zoster keratitis and supraorbital postherpetic neuralgia, seen prior to minimally invasive direct corneal neurotization, and (F) 15 months post-op.
This figure was adapted from a previous publication in Ophthalmic Plastic and Reconstructive Surgery, volume 56, Wisely CE et al., Clinical and morphologic outcomes of minimally invasive direct corneal neurotization, copyright Wolters Kluwer Health, Inc., 2020.
Topical recombinant human nerve growth factor is an alternative treatment option for neurotrophic corneas. The REPARO trial included patients with Mackie stage 2–3 neurotrophic corneas [27]. 76% of the patients showed complete resolution of their epithelial defects as opposed to 54% in the placebo group. The rate of recurrence after complete healing was 3.4–3.6% at 48-week follow up after treatment, compared to 4.8% recurrence in the placebo-treated group. Corneal sensation testing did not show statistical significance compared to the control group. Thus, corneal neurotization may offer a more permanent solution, though long-term studies are needed to understand the changes that occur in sensation over time [27].
VIII. CORNEAL NEUROTIZATION IN POST-HERPETIC NEUROTROPHIC KERATOPATHY
A growing body of literature has sought to address the role of corneal neurotization in the treatment of neurotrophic keratopathy caused by herpetic infection. To limit the risks of reactivation and reinfection, immediate pre-operative and post-operative administration of treatment dose antivirals is recommended, followed by extended lifelong prophylactic dosing [2]. There are no reports of reactivation to date. Both intact ipsilateral and contralateral donor nerves have been used with similar success. Direct or indirect methods are both similarly effective [2,56,60,64]. In a series with long denervation time (average 15.2 years), 78% showed resolution of presenting corneal pathology, with a shift from 70% of patients presenting initially with Mackie stage III to 53% with Mackie stage I post-operatively [56]. In a series with shorter denervation times (83% of cases less than 2 years), significant improvements in corneal sensation were seen at 3 months post op with maximal potential achieved in most by 6 months post op [2]. Recurrence of persistent epithelial defect occurred occasionally, but resolved with traditional management [2,56]. Majority of the persistent epithelial defects occurred within the first 6 months of corneal neurotization before re-establishment of protective corneal sensation. As in many cases described, visual acuity was often limited by other ocular comorbidities. Optimal timing of surgery is still a matter of debate. When the eye is free from reactivation and is stable, earlier intervention may halt progressive disease and can potentially improve visual outcomes [2].
IX. FUTURE DIRECTIONS
In the short time that corneal neurotization has become an option in the treatment of neurotrophic corneas, significant advances have been made to the surgical technique and understanding of the mechanism of action. There are still many unanswered questions regarding the long-term durability of corneal sensation and repair mechanisms. Additionally, optimal timing, risk of reactivation, and antiviral regimen of corneal neurotization in herpetic cases still need to be addressed. The effect of human recombinant nerve growth factor in promoting nerve growth post neurotization is also unclear. Further research into these areas will help provide better treatment options for this difficult to treat condition.
Grant Support:
Andrea C Arteaga, M. Soledad Cortina, Vinay K. Aakalu, Catherine Y. Liu:
Unrestricted Grant. Research to Prevent Blindness, NY, NY.
P30 EY001792; National Eye Institute, National Institutes of Health.
Financial Disclosures:
Vinay Aakalu: Consultant, Horizon Pharma; Patents- University of Illinois.
Other grant support- Department of Defense, National Eye Institute, Veterans Administration Office of Research and Development.
Catherine Y. Liu: Patent pending – Bristol Drug Delivery.
Ilya Leyngold: Consultant, AxoGen Inc., Consultant, Osteomed, Ad Hoc Advisory Board, Horizon Therapeutics
Footnotes
Declaration of interest:
Conflicts of interest: No conflicting relationship exists for any author except for Dr. Leyngold who serves as a medical consultant for AxoGen Inc.
REFERENCES
- [1].Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg 2009;123:112–20. doi: 10.1097/PRS.0b013e3181904d3a. [DOI] [PubMed] [Google Scholar]
- [2].Kim JS, Rafailov L, Leyngold IM. Corneal neurotization for postherpetic neurotrophic keratopathy: Initial experience and clinical outcomes. Ophthalmic Plastic and Reconstructive Surgery 2020:1. doi: 10.1097/IOP.0000000000001676. [DOI] [PubMed] [Google Scholar]
- [3].Samii M [Operative reconstruction of injured nerves]. Langenbecks Arch Chir 1972;332:355–62. doi: 10.1007/BF01282653. [DOI] [PubMed] [Google Scholar]
- [4].Samii M, Jannetta PJ, editors. The cranial nerves. Berlin, Heidelberg: Springer Berlin Heidelberg; 1981. doi: 10.1007/978-3-642-67980-3. [DOI] [Google Scholar]
- [5].Wisely CE, Rafailov L, Cypen S, Proia AD, Boehlke CS, Leyngold IM. Clinical and morphologic outcomes of minimally invasive direct corneal neurotization. Ophthalmic Plast Reconstr Surg 2020. doi: 10.1097/IOP.0000000000001586. [DOI] [PubMed] [Google Scholar]
- [6].Leyngold I, Weller C, Leyngold M, Espana E, Black KD, Hall KL, et al. Endoscopic corneal neurotization: Cadaver feasibility study. Ophthalmic Plastic and Reconstructive Surgery 2018;34:213–6. doi: 10.1097/IOP.0000000000000913. [DOI] [PubMed] [Google Scholar]
- [7].Leyngold I, Weller C, Leyngold M, Tabor M. Endoscopic corneal neurotization: Technique and initial experience. Ophthalmic Plast Reconstr Surg 2018;34:82–5. doi: 10.1097/IOP.0000000000001023. [DOI] [PubMed] [Google Scholar]
- [8].Jacinto F, Espana E, Padilla M, Ahmad A, Leyngold I. Ipsilateral supraorbital nerve transfer in a case of recalcitrant neurotrophic keratopathy with an intact ipsilateral frontal nerve: A novel surgical technique. Am J Ophthalmol Case Rep 2016;4:14–7. doi: 10.1016/j.ajoc.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC. Neurotrophic keratitis: current challenges and future prospects. Eye Brain 2018;10:37–45. doi: 10.2147/EB.S117261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoşal BM, Ornek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 2005;19:1276–9. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- [11].Koaik M, Baig K. Corneal neurotization. Curr Opin Ophthalmol 2019;30:292–8. doi: 10.1097/ICU.0000000000000578. [DOI] [PubMed] [Google Scholar]
- [12].Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci 2012;53:4926–31. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- [13].Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol 2014;8:571–9. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Catapano J, Antonyshyn K, Zhang JJ, Gordon T, Borschel GH. Corneal neurotization improves ocular surface health in a novel rat model of neurotrophic keratopathy and corneal neurotization. Invest Ophthalmol Vis Sci 2018;59:4345. doi: 10.1167/iovs.18-24843. [DOI] [PubMed] [Google Scholar]
- [15].Catapano J, Fung SSM, Halliday W, Jobst C, Cheyne D, Ho ES, et al. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: Long-term clinical outcomes and evidence of corneal reinnervation. Br J Ophthalmol 2019;103:1724–31. doi: 10.1136/bjophthalmol-2018-313042. [DOI] [PubMed] [Google Scholar]
- [16].Park JK, Charlson ES, Leyngold I, Kossler AL. Corneal neurotization: A review of pathophysiology and outcomes. Ophthalmic Plast Reconstr Surg 2020. doi: 10.1097/IOP.0000000000001583. [DOI] [PubMed] [Google Scholar]
- [17].Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea 2005;24:654–60. doi: 10.1097/01.ico.0000153102.19776.80. [DOI] [PubMed] [Google Scholar]
- [18].Kruse FE, Rohrschneider K, Völcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology 1999;106:1504–10; discussion 1511. doi: 10.1016/S0161-6420(99)90444-X. [DOI] [PubMed] [Google Scholar]
- [19].Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol 2010;94:584–91. doi: 10.1136/bjo.2009.164780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yoon KC. Use of umbilical cord serum in ophthalmology. Chonnam Med J 2014;50:82–5. doi: 10.4068/cmj.2014.50.3.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yoon K-C, Heo H, Im S-K, You I-C, Kim Y-H, Park Y-G. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol 2007;144:86–92. doi: 10.1016/j.ajo.2007.03.016. [DOI] [PubMed] [Google Scholar]
- [22].Yamada N, Matsuda R, Morishige N, Yanai R, Chikama T -i, Nishida T, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol 2008;92:896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- [23].Erdem E, Yagmur M, Harbiyeli I, Taylan-Sekeroglu H, Ersoz R. Umbilical cord blood serum therapy for the management of persistent corneal epithelial defects. Int J Ophthalmol 2014;7:807–10. doi: 10.3980/j.issn.2222-3959.2014.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guerra M, Marques S, Gil JQ, Campos J, Ramos P, Rosa AM, et al. Neurotrophic keratopathy: Therapeutic approach using a novel matrix regenerating agent. J Ocul Pharmacol Ther 2017;33:662–9. doi: 10.1089/jop.2017.0010. [DOI] [PubMed] [Google Scholar]
- [25].Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol 2016;100:22–7. doi: 10.1136/bjophthalmol-2015-306842. [DOI] [PubMed] [Google Scholar]
- [26].Bonini S, Lambiase A, Rama P, Filatori I, Allegretti M, Chao W, et al. Phase I trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology 2018;125:1468–71. doi: 10.1016/j.ophtha.2018.03.004. [DOI] [PubMed] [Google Scholar]
- [27].Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology 2018;125:1332–43. doi: 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- [28].Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol 2012;23:296–302. doi: 10.1097/ICU.0b013e3283543b61. [DOI] [PubMed] [Google Scholar]
- [29].Lambiase A, Mantelli F, Sacchetti M, Rossi S, Aloe L, Bonini S. Clinical applications of NGF in ocular diseases. Arch Ital Biol 2011;149:283–92. doi: 10.4449/aib.v149i2.1363. [DOI] [PubMed] [Google Scholar]
- [30].Kadakia S, Helman S, Saman M, Cooch N, Wood-Smith D. Concepts in neural coaptation: Using the facial nerve as a paradigm in understanding principles surrounding nerve injury and repair. J Craniofac Surg 2015;26:1304–9. doi: 10.1097/SCS.0000000000001566. [DOI] [PubMed] [Google Scholar]
- [31].Kim JS, Bonsu N-Y, Leland HA, Carey JN, Patel KM, Seruya M. A systematic review of prognostic factors for sensory recovery after digital nerve reconstruction. Ann Plast Surg 2018;80:S311–6. doi: 10.1097/SAP.0000000000001440. [DOI] [PubMed] [Google Scholar]
- [32].Paprottka FJ, Wolf P, Harder Y, Kern Y, Paprottka PM, Machens H-G, et al. Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: Meta-analysis and systematic review. Plastic Surgery International 2013;2013:1–17. doi: 10.1155/2013/704589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mermans JF, Franssen BBGM, Serroyen J, Van der Hulst RRWJ. Digital nerve injuries: a review of predictors of sensory recovery after microsurgical digital nerve repair. HAND 2012;7:233–41. doi: 10.1007/s11552-012-9433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herman ZJ, Ilyas AM. Sensory outcomes in digital nerve repair techniques: An updated meta-analysis and systematic review. Hand (N Y) 2020;15:157–64. doi: 10.1177/1558944719844346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Menorca RMG, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin 2013;29:317–30. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Martin E, Senders JT, DiRisio AC, Smith TR, Broekman MLD. Timing of surgery in traumatic brachial plexus injury: a systematic review. J Neurosurg 2018:1–13. doi: 10.3171/2018.1.JNS172068. [DOI] [PubMed] [Google Scholar]
- [37].Isaacs J Major peripheral nerve injuries. Hand Clinics 2013;29:371–82. doi: 10.1016/j.hcl.2013.04.006. [DOI] [PubMed] [Google Scholar]
- [38].Shao YC, Harwood P, Grotz MRW, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: A SYSTEMATIC REVIEW. The Journal of Bone and Joint Surgery British Volume 2005;87-B:1647–52. doi: 10.1302/0301-620X.87B12.16132. [DOI] [PubMed] [Google Scholar]
- [39].Pham VM, Tu NH, Katano T, Matsumura S, Saito A, Yamada A, et al. Impaired peripheral nerve regeneration in type-2 diabetic mouse model. Eur J Neurosci 2018;47:126–39. doi: 10.1111/ejn.13771. [DOI] [PubMed] [Google Scholar]
- [40].Swanson AN, Wolfe SW, Khazzam M, Feinberg J, Ehteshami J, Doty S. Comparison of neurotization versus nerve repair in an animal model of chronically denervated muscle. The Journal of Hand Surgery 2008;33:1093–9. doi: 10.1016/j.jhsa.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jia X, Chen C, Yang J, Yu C. End-to-side neurotization with the phrenic nerve in restoring the function of toe extension: an experimental study in a rat model. J Plast Surg Hand Surg 2018;52:185–8. doi: 10.1080/2000656X.2017.1408017. [DOI] [PubMed] [Google Scholar]
- [42].McNamara MJ, Garrett WE, Seaber AV, Goldner JL. Neurorrhaphy, nerve grafting, and neurotization: A functional comparison of nerve reconstruction techniques. The Journal of Hand Surgery 1987;12:354–60. doi: 10.1016/S0363-5023(87)80003-5. [DOI] [PubMed] [Google Scholar]
- [43].Hendry JM, Alvarez-Veronesi MC, Snyder-Warwick A, Gordon T, Borschel GH. Side-to-side nerve bridges support donor axon regeneration into chronically denervated nerves and are associated with characteristic changes in Schwann cell phenotype. Neurosurgery 2015;77:803–13. doi: 10.1227/NEU.0000000000000898. [DOI] [PubMed] [Google Scholar]
- [44].Rönkkö H, Göransson H, Taskinen H-S, Paavilainen P, Vahlberg T, Röyttä M. Comparison of peripheral nerve regeneration with side-to-side, end-to-side, and end-to-end repairs: An experimental study. Plast Reconstr Surg Glob Open 2016;4:e1179. doi: 10.1097/GOX.0000000000001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rönkkö H, Göransson H, Taskinen H-S, Paavilainen P, Vahlberg T, Röyttä M. Protective distal side-to-side neurorrhaphy in proximal nerve injury-an experimental study with rats. Acta Neurochir (Wien) 2019;161:645–56. doi: 10.1007/s00701-019-03835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wan H, Zhang L, Li D, Hao S, Feng J, Oudinet JP, et al. Hypoglossal-facial nerve “side”-to-side neurorrhaphy for persistent incomplete facial palsy. J Neurosurg 2014;120:263–72. doi: 10.3171/2013.9.JNS13664. [DOI] [PubMed] [Google Scholar]
- [47].Frey M, Giovanoli P. End-to-side neurorrhaphy of sensory nerves. Eur J Plast Surg 2003;26:85–8. doi: 10.1007/s00238-003-0475-z. [DOI] [Google Scholar]
- [48].Tos P, Colzani G, Ciclamini D, Titolo P, Pugliese P, Artiaco S. Clinical applications of end-to-side neurorrhaphy: An update. Biomed Res Int 2014;2014. doi: 10.1155/2014/646128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Geuna S, Papalia I, Ronchi G, d’Alcontres FS, Natsis K, Papadopulos NA, et al. The reasons for end-to-side coaptation: how does lateral axon sprouting work? Neural Regen Res 2017;12:529–33. doi: 10.4103/1673-5374.205081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Viterbo F, Brock RS, Maciel F, Ayestaray B, Garbino JA, Rodrigues CP. End-to-side versus end-to-end neurorrhaphy at the peroneal nerve in rats. Acta Cir Bras 2017;32:697–705. doi: 10.1590/s0102-865020170090000002. [DOI] [PubMed] [Google Scholar]
- [51].Gao W, Liu Q, Li S, Zhang J, Li Y. End-to-side neurorrhaphy for nerve repair and function rehabilitation. J Surg Res 2015;197:427–35. doi: 10.1016/j.jss.2015.03.100. [DOI] [PubMed] [Google Scholar]
- [52].Papalia I, Magaudda L, Righi M, Ronchi G, Viano N, Geuna S, et al. Epineurial window is more efficient in attracting axons than simple coaptation in a sutureless (cyanoacrylate-bound) model of end-to-side nerve repair in the rat upper limb: Functional and morphometric evidences and review of the literature. PLoS ONE 2016;11:e0148443. doi: 10.1371/journal.pone.0148443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Allevi F, Fogagnolo P, Rossetti L, Biglioli F. Eyelid reanimation, neurotisation, and transplantation of the cornea in a patient with facial palsy. BMJ Case Rep 2014;2014. doi: 10.1136/bcr-2014-205372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Domeshek LF, Hunter DA, Santosa K, Couch SM, Ali A, Borschel GH, et al. Anatomic characteristics of supraorbital and supratrochlear nerves relevant to their use in corneal neurotization. Eye (Lond) 2019;33:398–403. doi: 10.1038/s41433-018-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gennaro P, Gabriele G, Aboh IV, Cascino F, Menicacci C, Mazzotta C, et al. The second division of the trigeminal nerve for corneal neurotization: A novel one-stage technique in combination with facial reanimation. J Craniofac Surg 2019;30:1252–4. doi: 10.1097/SCS.0000000000005483. [DOI] [PubMed] [Google Scholar]
- [56].Lin C-H, Lai L-J. Herpetic corneal keratopathy management using ipsilateral supratrochlear nerve transfer for corneal neurotization. Ann Plast Surg 2019;83:553–7. doi: 10.1097/SAP.0000000000002120. [DOI] [PubMed] [Google Scholar]
- [57].Leyngold IM, Yen MT, Tian J, Leyngold MM, Vora GK, Weller C. Minimally invasive corneal neurotization with acellular nerve allograft: Surgical technique and clinical outcomes. Ophthalmic Plast Reconstr Surg 2019;35:133–40. doi: 10.1097/IOP.0000000000001181. [DOI] [PubMed] [Google Scholar]
- [58].Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: a new approach to a difficult problem. JAMA Ophthalmol 2014;132:1289–95. doi: 10.1001/jamaophthalmol.2014.2316. [DOI] [PubMed] [Google Scholar]
- [59].Bains RD, Elbaz U, Zuker RM, Ali A, Borschel GH. Corneal neurotization from the supratrochlear nerve with sural nerve grafts: A minimally invasive approach. Plastic and Reconstructive Surgery 2015;135:397e–400e. doi: 10.1097/PRS.0000000000000994. [DOI] [PubMed] [Google Scholar]
- [60].Weis E, Rubinov A, Al-Ghoul AR, Yau FM-K. Sural nerve graft for neurotrophic keratitis: early results. Can J Ophthalmol 2018;53:24–9. doi: 10.1016/j.jcjo.2017.10.044. [DOI] [PubMed] [Google Scholar]
- [61].Sepehripour S, Lloyd MS, Nishikawa H, Richard B, Parulekar M. Surrogate outcome measures for corneal neurotization in infants and children. J Craniofac Surg 2017;28:1167–70. doi: 10.1097/SCS.0000000000003677. [DOI] [PubMed] [Google Scholar]
- [62].Jowett N, Pineda Ii R. Corneal neurotisation by great auricular nerve transfer and scleral-corneal tunnel incisions for neurotrophic keratopathy. Br J Ophthalmol 2019;103:1235–8. doi: 10.1136/bjophthalmol-2018-312563. [DOI] [PubMed] [Google Scholar]
- [63].Fung SSM, Catapano J, Elbaz U, Zuker RM, Borschel GH, Ali A. In vivo confocal microscopy reveals corneal reinnervation after treatment of neurotrophic keratopathy with corneal neurotization. Cornea 2018;37:109–12. doi: 10.1097/ICO.0000000000001315. [DOI] [PubMed] [Google Scholar]
- [64].Bourcier T, Henrat C, Heitz A, Kremer SF, Labetoulle M, Liverneaux P. Lateral antebrachial cutaneous nerve as autologous graft for mini-invasive corneal neurotization (MICORNE). Cornea 2019;38:1029–32. doi: 10.1097/ICO.0000000000002004. [DOI] [PubMed] [Google Scholar]
- [65].Benkhatar H, Levy O, Goemaere I, Borderie V, Laroche L, Bouheraoua N. Corneal neurotization with a great auricular nerve graft: Effective reinnervation demonstrated by in vivo confocal microscopy. Cornea 2018;37:647–50. doi: 10.1097/ICO.0000000000001549. [DOI] [PubMed] [Google Scholar]
- [66].Schmidhammer R, Zandieh S, Hopf R, Mizner I, Pelinka LE, Kroepfl A, et al. Alleviated tension at the repair site enhances functional regeneration: the effect of full range of motion mobilization on the regeneration of peripheral nerves--histologic, electrophysiologic, and functional results in a rat model. J Trauma 2004;56:571–84. doi: 10.1097/01.ta.0000114082.19295.e6. [DOI] [PubMed] [Google Scholar]
- [67].Malhotra R, Elalfy MS, Kannan R, Nduka C, Hamada S. Update on corneal neurotisation. British Journal of Ophthalmology 2019;103:26–35. doi: 10.1136/bjophthalmol-2018-312104. [DOI] [PubMed] [Google Scholar]
- [68].Ting DSJ, Figueiredo GS, Henein C, Barnes E, Ahmed O, Mudhar HS, et al. Corneal neurotization for neurotrophic keratopathy: Clinical outcomes and in vivo confocal microscopic and histopathological findings. Cornea 2018;37:641–6. doi: 10.1097/ICO.0000000000001522. [DOI] [PubMed] [Google Scholar]
- [69].Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol 2008;119:1951–65. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- [70].Fogagnolo P, Giannaccare G, Bolognesi F, Digiuni M, Tranchina L, Rossetti L, et al. Direct versus indirect corneal n eurotization for the treatment of neurotrophic keratopahty: A multicenter prospective comparative study. Am J Ophthalmol 2020. doi: 10.1016/j.ajo.2020.07.003. [DOI] [PubMed] [Google Scholar]
- [71].Giannaccare G, Bolognesi F, Biglioli F, Marchetti C, Mariani S, Weiss JS, et al. In vivo and ex vivo comprehensive evaluation of corneal reinnervation in eyes neurotized with contralateral supratrochlear and supraorbital nerves. Cornea 2020;39:210–4. doi: 10.1097/ICO.0000000000002083. [DOI] [PubMed] [Google Scholar]
- [72].Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci 2007;48:173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- [73].Stevens GA, White RA, Flaxman SR, Price H, Jonas JB, Keeffe J, et al. Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990–2010. Ophthalmology 2013;120:2377–84. doi: 10.1016/j.ophtha.2013.05.025. [DOI] [PubMed] [Google Scholar]
- [74].Swanson M, Swanson R, Clark R, et al. Corneal Neurotization: A Meta-analysis of Outcomes and Patient Selection Factors. Plast Reconstr Surg Glob Open. 2020;8(9 Suppl):32–32. doi: 10.1097/01.GOX.0000720444.85571.69. [DOI] [PubMed] [Google Scholar]
- [75].Sweeney AR, Wang M, Weller CL, Burkat C, Kossler AL, Lee BW, et al. Outcomes of corneal neurotisation using processed nerve allografts: A multicentre case series. Br J Ophthalmol 2020;0:1–5. doi: 10.1136/bjophthalmol-2020-317361. [DOI] [PubMed] [Google Scholar]
- [76].Wolkow N, Habib LA, Yoon MK, Freitag SK. Corneal Neurotization: Review of a New Surgical Approach and Its Developments. Semin Ophthalmol. 2019;34(7–8):473–487. doi: 10.1080/08820538.2019.1648692. Epub 2019 Aug 1. [DOI] [PubMed] [Google Scholar]
- [77].Isaacs J, Browne T. Overcoming short gaps in peripheral nerve repair: Conduits and human acellular nerve allograft. Hand 2014;9(2):131–137. doi: 10.1007/s11552-014-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kim SS, Sohn SK, Lee KY, et al. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur 2010;35:214–9. [DOI] [PubMed] [Google Scholar]
- [79].Meng H, Li M, You F, et al. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci Lett 2011;496:48–53. [DOI] [PubMed] [Google Scholar]
- [80].Ruiz-Lozano RE, Hernandez-Camarena JC, Loya-Garcia D, Merayo-Lloves J, Rodriguez-Garcia A. The molecular basis of neurotrophic keratopathy: Diagnostic and therapeutic implications. The Ocular Surface 2021;19:224–240. [DOI] [PubMed] [Google Scholar]
- [81].Trinh T, Santaella G, Mimouni M, Mednick Z, Cohen E, Sorkin N, Rootman DS, Slomovic AR, Chan CC. Assessment of response to multimodal management of neurotrophic corneal disease. The Ocular Surface 2020. November 12. [DOI] [PubMed] [Google Scholar]
- [82].Di Zazzo A, Coassin M, Varacalli G, Galvagno E, De Vincentis A, Bonini S. Neurotrophic keratopathy: Pros and cons of current treatments. The Ocular Surface 2019. October 1;17(4):619–23. [DOI] [PubMed] [Google Scholar]
- [83].Tsubota Kazuo, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology 106, no. 10 (1999): 1984–1989. [DOI] [PubMed] [Google Scholar]
- [84].Saad S, Abdelmassih Y, Saad R, Guindolet D, el Khoury S, Gabison EE, et al. Neurotrophic keratitis: Frequency, etiologies, clinical management and outcomes. The Ocular Surface 2020;18:231–236. [DOI] [PubMed] [Google Scholar]
- [85].Di Zazzo A, Coassin M, Micerca A, Mori T, De Piano M, Bonini S, et al. Ocular surface diabetic disease: A neurogenic condition? The Ocular Surface 2021;19:218–223. [DOI] [PubMed] [Google Scholar]


