Abstract
Field release of Wolbachia-infected Aedes aegypti has emerged as a promising solution to manage the transmission of dengue, Zika and chikungunya in endemic areas across the globe. Through an efficient self-dispersing mechanism, and the ability to induce virus-blocking properties, Wolbachia offers an unmatched potential to gradually modify wild Ae. aegypti populations turning them unsuitable disease vectors. Here we describe a proof-of-concept field trial carried out in a small community of Niterói, greater Rio de Janeiro, Brazil. Following the release of Wolbachia-infected eggs, we report here a successful invasion and long-term establishment of the bacterium across the territory, as denoted by stable high-infection indexes (> 80%). We have also demonstrated that refractoriness to dengue and Zika viruses, either thorough oral-feeding or intra-thoracic saliva challenging assays, was maintained over the adaptation to the natural environment of Southeastern Brazil. These findings further support Wolbachia’s ability to invade local Ae. aegypti populations and impair disease transmission, and will pave the way for future epidemiological and economic impact assessments.
Subject terms: Ecology, Microbiology
Introduction
The mosquito Aedes aegypti (= Stegomyia aegypti) holds a core status among tropical disease vectors, being able to host and transmit a broad variety of viruses, such as those causing dengue, Zika and chikungunya1,2. Dengue virus (DENV) is certainly the most prevalent, with a global distribution spectrum including 128 countries3 and approximately 400 million infections annually4. Brazil accounts for a large fraction of these cases, with more than 1.5 million infections only in 20195. In spite of being historically less prevalent, chikungunya (CHIKV) and Zika (ZIKV) viruses, restricted to Africa and Asia until the early 2000’s, were recently emerging in new territories, with significant impact on public health6–9. The introduction of CHIKV in Central and South America, for example, led to about one million suspected disease cases between 2013 and 201410. Similarly, ZIKV first records in America date to the end of 2013, after which it rapidly spread through the continent11. In 2015, the World Health Organization (WHO) declared a Public Health Emergency of International Concern following a serious ZIKV outbreak in Northeast Brazil, associated with high rates of microcephaly in newborns12.
Since effective vaccines or therapeutic drugs are still under development13–15 and not currently available to fight outbreaks of Zika, dengue and chikungunya, public health initiatives rely entirely on vector control. Traditionally, this is achieved by the mechanical elimination of breeding sites and the use of chemical insecticides to reduce Ae. aegypti populations. However, both methods have proven inefficient and unsustainable for the long term, mostly due to the myriad artificial breeding sites used by this species in urban landscapes16,17 and the advent of naturally insecticide resistant allelic variants18–20. In face of these challenges, the development of new solutions is, therefore, a critical need for a more efficient control of Ae. aegypti populations and/or disease transmission.
In recent years, an innovative approach using the endosymbiont Wolbachia pipientis to block arbovirus transmission has been proposed and successfully tested in Ae. aegypti21–24, gaining momentum as a viable and sustainable alternative to traditional control methods. Naturally found in around 40% of all arthropods25, Wolbachia are maternally-inherited intracellular bacteria which exploits host reproductive biology to increase its transmission rates and dispersal in nature. For some bacterial strains, this is achieved by triggering a phenomenon called cytoplasmic incompatibility (CI), which turns the progeny unviable when an infected male copulates with an uninfected female26. Remarkably, Wolbachia-host association can also lead to pathogen interference (PI) phenotypes27,28, particularly relevant to vector control applied studies. In view of this, stable and heritable lines, harboring different Wolbachia strains, have been created following artificial transinfection of Ae. aegypti21,29–32. Through pathways involving the modulation of host immune system33 and metabolite sequestration34,35, Wolbachia-harboring lines exhibit high-levels of refractoriness to DENV, CHIKV, ZIKV, and other medically relevant arboviruses30,36–38. As such, releasing some of these lines in the field to gradually replace virus-susceptible wild populations could potentially reduce transmission and human infection rates, as recently reported39.
Successful field release trials using Ae. aegypti lines infected with Wolbachia wMel strain have been reported in Northern Australia, Indonesia and more recently in Southeastern Brazil22,40,41. In the latter, a small community in Rio de Janeiro (RJ) was subject to a rolling out strategy based on adult releases, which led to the invasion and long-term establishment of the bacterium in the field. Nonetheless, important vector competence data following the invasion is still lacking and needs to be assessed in order to provide evidence of virus-blocking maintenance.
Accumulating evidence suggest that eggs can also be released in the field, thus providing an alternative carrier for Wolbachia introduction into wild Ae. aegypti populations41–43. When compared to adult-driven methods, egg releases thrive on its simpler, more natural approach. Replacing a large, all-at-once, pulse of mosquitoes by a slow and gradual release from Mosquito Release Containers (MRCs) tends to alleviate undesirable social effects and increase community acceptance41.
In this study, we report the results of a trial in which Wolbachia-harboring eggs were released in Jurujuba, a small suburb of Niterói (Rio de Janeiro State). Besides evaluating Wolbachia’s ability to invade mosquito populations, we investigated the bacterium density level and the vector competence for ZIKV and DENV in post-release field samples, contributing to a better characterization of targeted populations in Southeastern Brazil.
Results
Egg releases successfully promote Wolbachia invasion in Jurujuba, Niterói (RJ)
To evaluate the efficiency of egg releases as a method of Wolbachia field deployment in Brazil, we carried out a pilot study in Jurujuba, a suburban neighborhood of Niterói (Rio de Janeiro State). Using MRCs, Wolbachia-infected eggs (wMelRio Ae. aegypti strain40) were distributed over all seven Jurujuba’s sectors (i.e. sub-areas within the neighborhood) (Fig. 1, Supplementary Fig. S1, Supplementary Table S1). BG-sentinels were spatially distributed (Supplementary Fig. S2, Supplementary Table S2) to collect Ae. aegypti field specimens for Wolbachia molecular diagnosis, before calculation of prevalence rates (percent infected individuals).
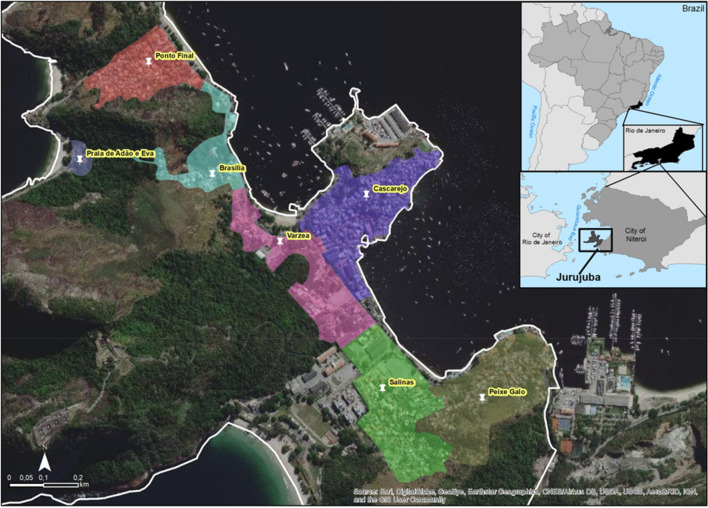
Figure 1.
Map of Jurujuba release areas. Satellite view of Jurujuba, a suburban neighborhood of Niterói (RJ). With an estimated population of 2797 in 2.53 km2, Jurujuba was divided into seven release areas (highlighted) according to local sectors: Ponto Final, Várzea, Brasília, Cascarejo, Praia de Adão e Eva, Peixe-Galo and Salinas. Map was created with ArcGIS Desktop 10.7 (Esri Inc., https://www.esri.com/en-us/arcgis/products/arcgis-desktop/overview) using Google Earth (Google LLC) source code, under the license and in accordance with the fair use described in ‘https://about.google/brand-resource-center/products-and-services/geo-guidelines’.
Situated at the furthermost housing area of Jurujuba’s peninsula, Ponto Final was selected the starting sector for field release, from which adjacent ones would derive following a rollout strategy. As such, all related activity planning, including community engagement, territorial mapping, release and monitoring sites allocation, and mass-rearing production scale was initially tuned to Ponto Final. Here, Wolbachia-infected eggs were released over 25 consecutive weeks, from August 2015 to February 2016, during which prevalence rates rapidly increased from basal to high levels (> 80%) (Fig. 2). In fact, by week 17, infection rates hit 81% and, by the end of the release period, 88%. Post-release prevalence rates were maintained at high levels in subsequent months, suggesting a successful invasion at this particular sector and paving the way to cover others, fulfilling our initial design.
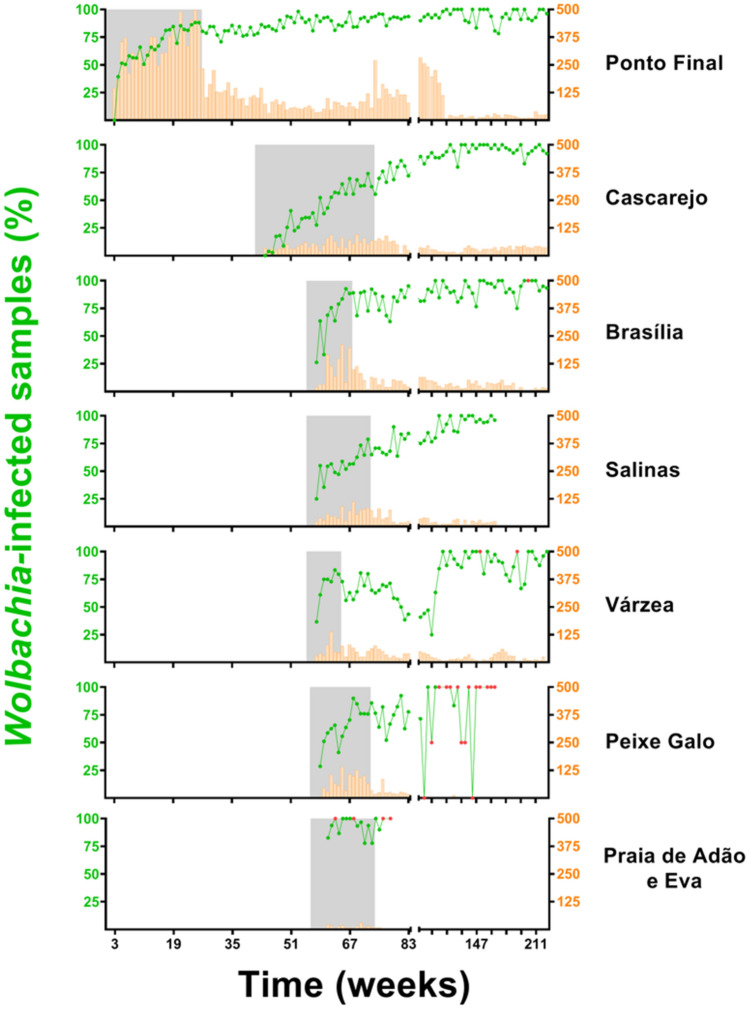
Figure 2.
Wolbachia’s spread across Niteroi’s suburb Jurujuba. Over a period spanning 73 weeks, wMel-infected eggs were gradually released across all sectors of Jurujuba: Ponto Final, Cascarejo, Brasília, Salinas, Várzea, Peixe Galo and Praia de Adão e Eva (release interventions depicted under grey shaded areas). Infection indexes (%) are plotted as line-connected dots (green), following the vertical left axis, whereas the sample sizes are plotted as histograms (orange), following the vertical right axis. Small-sized samples (N < 5) were marked in red. The horizontal axis represents time, in number of weeks, since the beginning of egg-releases in Ponto final (August 2015) until more recent days (December 2019). Note that the horizontal axis is split into two fragments, in which the data account for 1-week (left fragment) or 4-week bins (right fragment). Ticks are scaled accordingly to represent a 16-week bin. Graphs were created with GraphPad Prism 8 (https://www.graphpad.com).
Following new planning and schedules, the release of Wolbachia-infected eggs was extended to Cascarejo, in June 2016, and to Brasília, Salinas, Várzea, Peixe-Galo and Praia de Adão e Eva, in September 2016 (Fig. 2). The release period duration and number of MRCs allocated in each sector varied slightly (Supplementary Table S1), due to their intrinsic properties (i.e. housing area and human population density). By mid-January 2017, egg release ceased in whole Jurujuba, with most sectors recording mid-to-high rates of Wolbachia infection (60–90%). Mimicking Ponto Final, the post-release phase generally followed the positive trends of the release period and featured increasing infection rates towards near fixation levels (90–100%) (Fig. 2). This was clearly the case for Cascarejo, Brasília and Salinas. A notable exception was Várzea, which recorded an erratic invasion profile, with high infection rates by the end of the release period (i.e. ~ 80% in eight weeks) and a gradual decrease over the following months, when rates hit less than 40%. Here, a complete recovery and stability at high rates was achieved only after 10 months into the post-release phase. Peixe Galo also revealed a slightly erratic profile but, unlike Várzea, it did not resemble a negative trend. Instead, mid-to-high level oscillation was sustained over the post-release phase, and part of the noise might be attributed to low sampling. Lastly, Praia de Adão e Eva exhibited consistently high rates over the release period and the following few weeks, suggesting a standard invasion profile. However, given its low Ae. aegypti abundance, as well as a relatively small area and human population, post-release monitoring was suspended, yielding no long-term data and preventing a more assertive analysis for this sector.
By aggregating weekly infection rates from different sectors, an overall post-release profile of Jurujuba was revealed (Fig. 3). Confirming previous data analysis, in which sectors were individually treated, the overall profile revealed that Jurujuba consistently recorded high infection rates (80–100%) along the post-release phase, from mid-January 2017 until December 2019. Data encompassing this period, of almost three years, suggest that Wolbachia successfully invaded Jurujuba, being able to sustain infection rates in the long-term.
Figure 3.
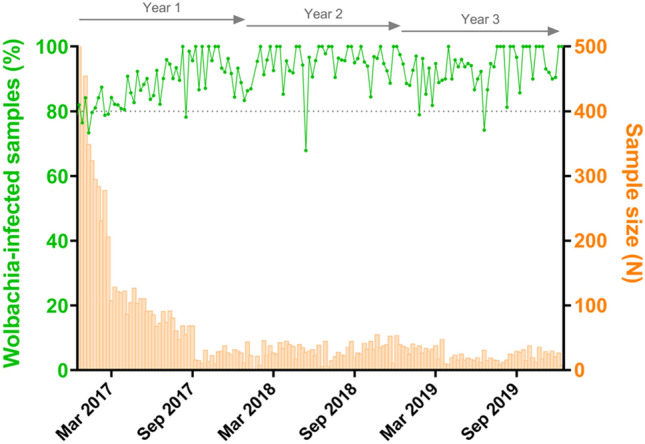
Post-release establishment of Wolbachia in Jurujuba. Following egg-release cessation in Jurujuba, Wolbachia-infection was monitored over the following three years, during which a self-sustained field establishment was observed. Infection indexes (%) are observed as line-connected dots (green), following the vertical left axis, whereas the sample sizes are plotted as histograms (orange), following the vertical right axis. The horizontal axis denotes time (weeks), with ticks scaled for 6-month bins. Top arrows mark yearly post-release periods. The dashed line, positioned at the 80% index mark, denotes a threshold for high-percent infection. This graph was generated in GraphPad Prism 8 (https://www.graphpad.com).
Wolbachia density is higher in post-release field samples
To investigate whether Wolbachia whole-body density (i.e. titer) was affected over time in Jurujuba’s environment, field samples were tested a few months after releases were finalized (March–May 2017) and one year later (March–May 2018). Counterpart wMelRio colony samples (i.e. collected at equivalent time periods) were also tested to assess data from individuals reared under laboratory environmental conditions and control generation-dependent variation in density, which could possibly mask evolutionary changes in this trait. Density data were plotted to reveal possible differences between field and colony samples, as well as details of their distribution (Fig. 4). Indeed, data suggest that Wolbachia density vary among groups, which was further corroborated by Kruskal–Wallis statistical test (H = 340.2, P < 0.0001). Subsequent multiple comparisons revealed that ‘field’ densities are higher than in ‘colony’ originating mosquitoes, in samples both from the beginning (P < 0.0001) or from ~ one year into the post-release phase (P < 0.0001). Moreover, when field samples are compared, a significant increase in density over time is detected (P < 0.0001), suggesting an evolving Wolbachia-host relationship in Jurujuba’s environment.
Figure 4.
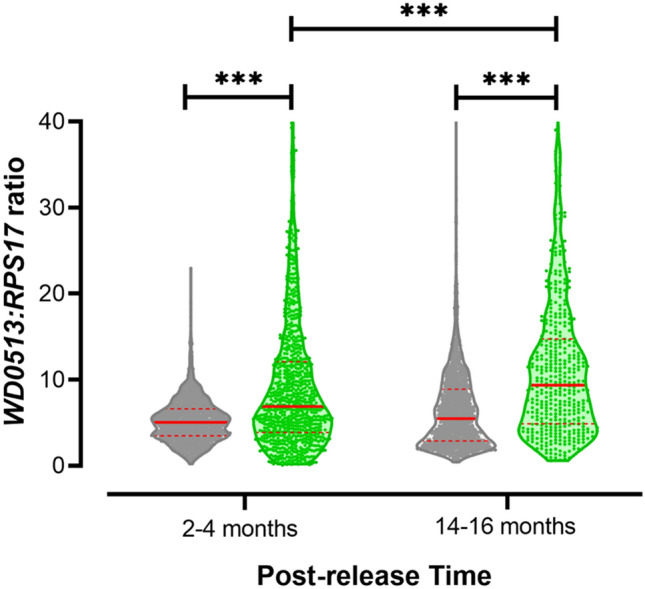
Wolbachia whole-body density increases subsequent to field establishment. Wolbachia whole-body density was molecularly assayed in whole-body extracts from colony (grey) and Jurujuba samples (green), collected over the post-release phase. Density or titration levels (vertical axis) are relative quantification indexes, reflecting the copy number ratio between wMel WD0513 and the endogenous ribosomal maker RPS17. Data were aggregated in 12-weeks pools and represented by violin plots, with medians and quartiles in solid and dashed red lines, respectively. Statistical inferences were performed by the non-parametric Kruskal–Wallis test followed by Dunn’s post-hoc multiple comparisons. Asterisks highlight differences between groups, considering a significance level (α) equal to 0.05, and its numbers reflects probability ranges: *P < 0.05, **P < 0.01, ***P < 0.001. Graph and statistics were made with GraphPad Prism 8 (https://www.graphpad.com).
Wolbachia inhibits DENV e ZIKV replication in the head and thorax of field samples
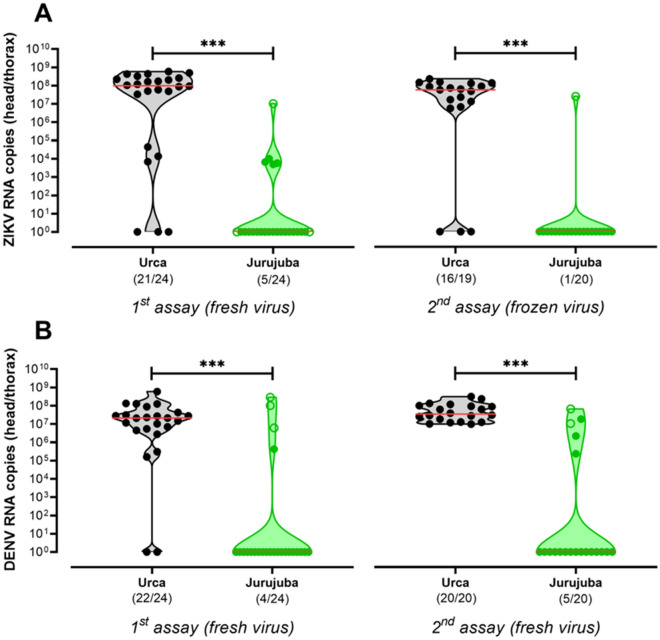
Following the invasion and long-term stability of Wolbachia in Jurujuba, Ae. aegypti field samples were submitted to vector competence assays. Jurujuba specimen eggs were collected in Ponto Final over three months, from April to June 2017, which correspond to 14–16 months into the post-release phase of this particular sector. Specimens eggs from Urca, a Wolbachia-free area in the neighboring city, Rio de Janeiro, were collected at the same time period and served as experimental controls. F1 adult females, from Jurujuba and Urca, were orally challenged with ZIKV or DENV, and viral titers were assessed 14 days post infection (dpi) in head/thorax individual extracts. Two independent assays were performed for each virus.
Our results revealed that oral challenging with ZIKV could promote infection and high viral titers in most samples from Urca, but could not elicit a similar outcome in samples from Jurujuba, which were mostly negative (Fig. 5a). Mann–Whitney U tests corroborate the significant reduction of ZIKV titers in Jurujuba samples, in both first (U = 47.5, P < 0.0001) and second assays (U = 36.5, P < 0.0001). The oral challenging with DENV led to an almost identical picture, with most samples from Urca being infected with high viral titers, whilst in samples from Jurujuba only a few were infected (Fig. 5b). Once again, significant differences between Urca and Jurujuba were found in the first (U = 74, P < 0.0001) and second assays (U = 20.5, P < 0.0001). In addition, we double-checked the Wolbachia status of the same F1 samples, further confirming its large presence in Jurujuba (~ 88% rate; 55 out of 62 individuals tested positive for Wolbachia) and complete absence in Urca (Supplementary Fig. S3). Altogether, our data suggest that the oral exposure with ZIKV or DENV is less prone to trigger and disseminate infection in Wolbachia-harboring Jurujuba specimens than in Wolbachia-negative Urca correlates, and that this effect is bacterium-driven.
Figure 5.
Wolbachia inhibits ZIKV and DENV tissue replication following oral-infection of field samples. Following the long-term establishment of Wolbachia in Jurujuba, field samples were brough to the laboratory and orally challenged with ZIKV and DENV, either fresh or frozen. Wolbachia-mediated inhibition of ZIKV and DENV replication was evaluated by comparing samples from Jurujuba (green) and Urca (grey), a Wolbachia-free non-targeted area in the suburbs of Rio de Janeiro. Violin plots represent absolute quantifications of (A) DENV and (B) ZIKV titers in head/thorax extracts, at 14 dpi. Median and quartiles are depicted in solid and dashed red lines, respectively. The proportion of infected individuals are shown as fractions underneath. Wolbachia– Jurujuba individuals were flagged by empty dots. Non-parametric Mann–Whitney U test highlighted differences between Urca and Jurujuba. Asterisks denote significant effects, with α equal to 0.05, and levels varying according to probability ranges: *P < 0.05, **P < 0.01, ***P < 0.001. Graph and statistics were made with GraphPad Prism 8 (https://www.graphpad.com).
Wolbachia largely attenuates saliva transmission of DENV and ZIKV by field samples
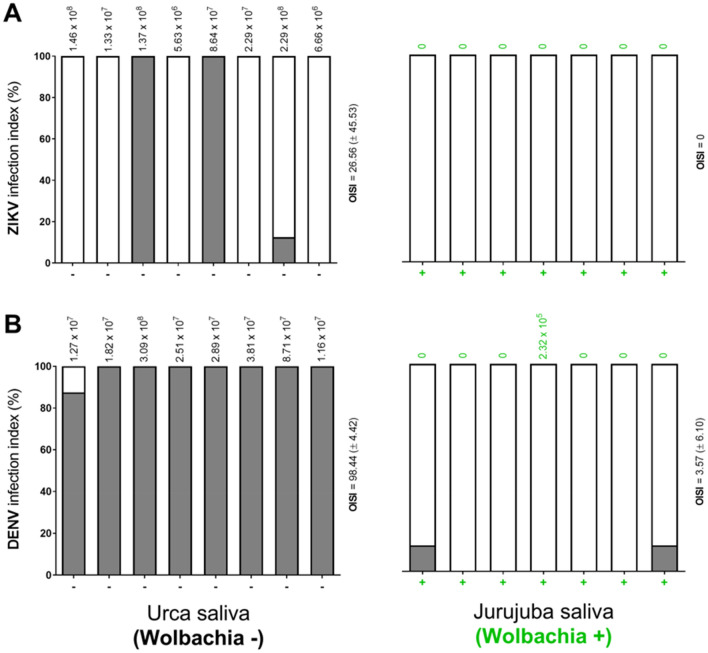
To further understand the extent of Wolbachia-mediated refractoriness to ZIKV and DENV, we investigated the infective potential of saliva from orally-challenged Urca and Jurujuba samples. At 14 dpi, saliva samples were harvested and intrathoracically injected into groups of naïve Urca specimens (n = 8) to evaluate the degree of which infective particles could be transmitted (Fig. 6). Infected individual counts were assessed at 5 dpi, for ZIKV, or at 7 dpi, for DENV, and their percent representation in each group was the metric used for comparisons, along with an overall intrathoracic saliva infection index (OISI; see “Methods” for more details). We also measured ZIKV and DENV titers in the head/thorax of which saliva were harvested, and plotted the values at the top of each infected group. As previously observed in extracts following oral-infection, ZIKV and DENV titers were high in head/thorax samples from Urca, but virtually undetectable in those from Jurujuba (except for one sample with low titer). However, it is important to note that these titers reflect the background infection status, not necessarily translating to the saliva.
Figure 6.
Wolbachia-harboring field samples impair saliva transmission of ZIKV and DENV. ZIKV and DENV transmission by the saliva is affected by the presence of Wolbachia in Jurujuba. Saliva harvested from Wolbachia−Urca (grey) or Wolbachia+ Jurujuba samples (green), orally-challenged with ZIKV (A) or DENV (B), were intrathoracically injected into groups of naïve Urca individuals (n = 8) and consequent infection was determined at 5 dpi, for ZIKV, or at 7 dpi, for DENV. Groups are represented by stacked columns of infected (filled in grey) or non-infected individuals (clear), percent transformed. Viral titers of head/thorax extracts, of corresponding saliva samples, are shown at the top of each column. OISI, the overall saliva intrathoracic infection indexes (± SD) are represented at the right of each graph. Graphs were created and customized with GraphPad Prism 8 (https://www.graphpad.com).
Our results revealed that saliva samples from orally-infected Urca individuals were carrying ZIKV and DENV infectious particles. The infection rates for ZIKV and DENV, however, seemed to differ. While three out of the eight groups challenged with ZIKV-infected saliva had at least one individual positive for the virus (OISI = 26.56 ± 45.53) (Fig. 6a), all eight groups challenged with DENV-infected saliva elicited this response (OISI = 98.44 ± 4.42) (Fig. 6b). This evidence suggests that despite being susceptible to both viruses, Urca population might be less competent to transmit ZIKV than DENV.
In contrast, saliva samples from orally-infected Jurujuba individuals (Wolbachia+) were usually not carrying ZIKV and DENV infectious particles. In fact, all seven groups challenged with saliva from ZIKV-infected individuals did not elicit a single infection (OISI = 0) (Fig. 6a), whilst only two out of seven groups challenged with saliva from DENV-infected individuals were positive for the virus (a single infection in each group) (OISI = 3.57 ± 6.10) (Fig. 6b). It is curious, though, that these specific saliva samples had no detectable titers in their corresponding head/thorax extracts, once again indicating that saliva and background infection status do not always correlate. Here, one could speculate that such Wolbachia+ head/thorax extracts harbor very low titers, often below qPCR sensitivity threshold, and which could have been depleted to an even lower level by preceding saliva harvesting. Overall, our findings support the view that the Wolbachia-harboring Jurujuba population likely has low susceptibility to ZIKV and DENV infection, with reduced viral multiplication and dissemination within key tissues, not being able to efficiently transmit the virus through saliva inoculation.
Discussion
The global burden of dengue, Zika and chikungunya places Ae. aegypti at the top of the list encompassing medically relevant mosquito vectors1,4,44. Since human immunization is not an option to date, public health authorities focus their efforts on vector suppression campaigns using long-standing protocols with major constraints. Mechanical removal of breeding sites, for instance, are labor intensive and usually leave some hotspots untouched, in which dry quiescent eggs remain viable until more favorable conditions resume45–47. Deployment of chemical pesticides have also proven inefficient, given the lack of precision and the surge of resistant variants19,48. To tackle some of these constraints and fulfil the urgent need for more efficient strategies, possible solutions have emerged in recent years22,40–42,49–51.
One promising solution lies on the field-release of lab-reared Wolbachia-infected individuals to gradually replace wild uninfected populations. The concept is fundamentally based on both the CI and PI bacterium-driven effects, arising from complex interactions with the mosquito host52. While the first favors bacterium inheritance towards fixation, the second confers refractoriness to several arboviruses. Should these effects translate to wild populations, then Wolbachia offers an unprecedented strategy, both natural and sustainable, to control mosquito-borne disease transmission.
Over the last decade, the World Mosquito Program (formerly ‘Eliminate Dengue: Our Challenge’), field-release trials have been performed to assess whether Wolbachia can live up to expectations in diverse real-world scenarios22,40–42,53. Interestingly, trials have revealed several challenges for a successful Wolbachia invasion and long-term stability in natural populations. As highlighted by pilot studies in Northern Australia and Vietnam, choosing a bacterium strain associated with high fitness costs to the host, like the virulent wMelPop, may impair a population replacement strategy54. Even though it could still be used in alternative strategies to transiently suppress Ae. aegypti populations and local transmission of arboviruses55,56, its field application is not sustainable and presumes continuous release of large quantities of individuals57. In contrast, the utilization of strains associated with low-to-mild fitness costs, albeit with generally lower PI, allow fewer individuals to be released in the field in order to promote an efficient invasion. Fulfilling these criteria, the strain wMel has been advocated as a choice for field release, collecting successful trials in Australia, Brazil and Indonesia22,23,39–43. Nonetheless, due to its beneficial attributes in warmer climates, keeping density levels high and stable, wAlbB has proven a second option and suitable alternative to wMel, as shown by a recent trial held in Malaysia53. Interestingly, a superinfected line hosting both wMel and wAlbB could also be an alternative for future interventions, as judged by preliminary analysis pointing to a higher PI while not increasing fitness costs58. Thus, the investigation and field application of new strains or combinations of existing ones has been encouraged to broaden the available options, and help building a Wolbachia toolset that suits diverse needs32,58.
In addition to the Wolbachia strain, other determining factors impacting the invasion dynamics, hence the success of a field trial, include the genetic background of the host, the rear and release method per se (space–time release schedule, quantity and quality/fitness of released individuals) and the density of local Ae. aegypti populations40,42. Of particular importance, the genetic background of the host not only establishes unique interactions with Wolbachia strains, but also harbors bacterium-independent fitness traits that may dictate the adaptation to wild environments. Therefore, background homogenization was key to revert a failed attempt to deploy wMel in Tubiacanca, a small community of Rio de Janeiro (RJ), Southeastern Brazil40. Here, mimicking prior successful trials held in Australia22,23 was not enough to drive a sustainable invasion, and soon after adult release ceased Wolbachia prevalence dropped. Neither increasing the quantity nor the quality of released individuals (i.e. larger, longer-living individuals) could revert this outcome, suggesting that fitness-related nuances could be limiting Wolbachia’s spread. After a thorough investigation of the infected line, ruling out putative variations in traits affecting mating and reproduction success40,59,60, or Wolbachia’s maternal transmission rate, it was revealed that its genetic footprint of insecticide resistance had been largely attenuated over laboratory adaptation, becoming particularly less fit to survive in areas with high insecticide usage like those found in Southeastern Brazil40. With the re-introduction of resistant alleles, matching the frequency found in local populations, the reformed line was then able to switch the negative trend to a successful invasion in a second trial40. Thus, by narrowing disparities between lab-reared infected lines and wild populations, genetic background homogenization has been perceived as a good practice prior to current field release efforts.
In this study, we report the successful introduction and long-term establishment of Wolbachia into Ae. aegypti populations from Jurujuba, a suburban community of Niterói (RJ). Sitting by the shores of Guanabara bay, Jurujuba represents an additional site for Wolbachia release trials in Rio de Janeiro (RJ) and surrounding areas, launched some years before in Tubiacanga40. Jurujuba is enclosed in a greener landscape with softly connected housing clusters (Fig. 1), and Tubiacanga in a more organized and uniform housing display. Contrary to the latter, in which adult-releases were undertaken, Jurujuba held an alternative egg-release method, providing a chance to evaluate challenges and outcomes of each strategy, and trace future perspectives of trials in the country. The relatively small distance between both sites (19 km on a straight line over water) also proved valuable by allowing the field release of the same Wolbachia-infected line (i.e. wMelRio)40, disregarding the need for a whole genetic background swap. Instead, we carried out only minor quality control (e.g. genetic monitoring of insecticide resistance alleles) and re-introduction of local genetic variability in every few generations (e.g. addition of wild-caught males to the colony) to secure background homogeneity.
Following a rollout strategy, Wolbachia-containing eggs were deployed across all seven sectors of Jurujuba, revealing an overall invasion trend characterized by sustained high indexes at the end of the release period. However, a thorough observation of each sector uncovers distinct invasion profiles (Fig. 2), with some featuring accentuated trends and reaching early high infection indexes, and others showing less pronounced trends along the release period. The phenomena underlying distinct Wolbachia invasion dynamics could be linked to the density and spatial distribution of local Ae. aegypti populations and, ultimately, to human occupation51,61. Thus, it is reasonable to speculate that sectors featuring accentuated invasion trends have smaller populations of Ae. aegypti, as a result of better management of breeding sites or even fewer inhabitants. An opposing scenario might explain less pronounced, more resilient, invasion trends. Speculating on Várzea’s erratic profile, however, is challenging since none of the above causes seem reasonably suited to explain it assertively, leaving room to non-controlled events (e.g. indoors insecticide-spraying). Interestingly though, Várzea constitutes a stretch of houses surrounded by forest, and connecting three other sectors: Brasília, Cascarejo and Salinas (Fig. 1). Thus, it is possible that migration from these adjacent sectors could have contributed to Várzea’s profile, first with non-infected individuals and then with infected ones, respectively dampening and recovering rates over the post-release phase.
Our data corroborate to a stable, self-sustaining, and long-term persistence of Wolbachia infection in Ae. aegypti from Jurujuba. We have shown that over the post-release phase, spanning mid-January 2017 to December 2019, Wolbachia infection of field specimens were sustained at near-fixation indexes with only minor fluctuation (80–100%) (Fig. 3). Curiously, while experiencing and adapting to the natural habitat, Wolbachia’s association with infected hosts seems to have evolved to higher whole-body densities (Fig. 4), corroborating previous findings of a trial held in Australia62. When a comparison is drawn to colony-reared individuals, a significant increase in this parameter can be observed after only a few months (i.e. 2–4) in the field, becoming even more pronounced after one year. Considering that density levels has been positively correlated to maternal transmission rates32,63, as well as to the strength of CI and PI21,64–67, our findings suggest Wolbachia-host association affects have not been alleviated due to co-evolution in the field, and shall endure in the years to come.
Indeed, further supporting this view, our vector competence analysis suggests that PI is maintained in Wolbachia-positive Jurujuba samples collected slightly over one year into the post-release phase (Figs. 5, 6). In both oral and saliva challenging assays, Jurujuba samples were highly refractory to ZIKV or DENV, impairing not only the replication of viral particles but also its dissemination to tissues playing key roles in transmission to humans (e.g. salivary glands). Since most samples from Jurujuba were Wolbachia-positive (~ 88%) (Supplementary Fig. S3) and those from Urca, a Wolbachia-free area, were highly susceptible to both viruses, we could endorse that the refractory effect was bacterium driven. Despite numerous studies of pathogen interference in wMel-harboring lines, including some on a Brazilian background context36,68, the data presented here account for the first evidence of ZIKV- and DENV-blocking in samples from Rio de Janeiro and surrounding areas, notably Jurujuba and Tubiacanga, which have been subjected to release trials in recent years. Most important, it adds an important validation to the undergoing control strategy, leaving to epidemiological analysis the last verdict.
Corroborating previous trials in Australia and Indonesia41–43, the release of Wolbachia-infected eggs in Jurujuba proved an efficient method to introduce and disseminate the bacterium into Brazilian populations of Ae. aegypti. Volunteers and community members could actively participate, with low-demanding basic training, in field deployment schedules, naturally enhancing engagement and the strategy’s successful outcome43. Altogether, these beneficial features highly encourage the release of Wolbachia-infected eggs as part of control strategies in Brazil and other countries, particularly in those sites lacking proper infra-structure or financial support.
Ultimately, this work adds a new chapter on a successful story of Wolbachia field releases in Southeastern Brazil. When associated with a local genetic background, and continually monitored for homogeneity, wMel’s costs on fitness could be overridden by its efficient drive mechanism and spread into wild populations of Rio de Janeiro. Its long-term stability in the field, as shown by persistent high-infection indexes and pathogen interference, further reinforces the method’s sustainability and constitutes solid grounds to future epidemiological studies. Should we observe a significant impact on humans, then Wolbachia’s deployment shall gain momentum in public health initiatives and pave the way to cover larger areas in the country.
Methods
Mosquito lines and maintenance
To introduce Wolbachia into Brazilian Ae. aegypti, an Australian line infected with the wMel strain21 was backcrossed for 8 generations to a natural mosquito population of Rio de Janeiro, Brazil24. Following the genetic background introgression, additional crosses and knockdown resistance (kdr) screening were undertaken to replicate natural insecticide resistance profiling and generate the line wMelRio. To assure a minimal variation in this profiling overtime, and sustain a homogeneous genetic background, wMelRio colony was refreshed with 10% wild males once in every five generations40.
To maintain wMelRio, immatures (i.e. larval stages L1 to L4) were reared in dechlorinated water, at 28 °C, and fed Tetramin Flakes (Tetra GmbH, Herrenteich, Germany) until pupal formation. Following adult emersion, groups of 1000 females and 800 males were sorted and kept in BugDorm cages (MegaView Science Co Ltd., Taiwan) at 25 °C, with 10% sucrose solution ad libitum. Every three days, females were fed human blood (from blood donation centers; see details under ‘ethical considerations’), through Hemotek artificial feeders (Hemotek Ltd, UK). Note that, to avoid arboviral contamination of our colony, all blood samples were formerly tested negative for DENV, ZIKV, CHIKV, MAYV and YFV by multiplex qPCR assays36,68. Egg-laying was induced by placing dampened strips of filter paper (i.e. partially immersed in water-containing cups) inside the cages for 2–3 days, after which they were gradually dried at room temperature. Strips loaded with eggs (i.e. ovistrips) were kept at room temperature until further use, either for colony maintenance or field release. Eggs older than 40 days were discarded due to a decay in overall quality60.
Egg releases
Mass-reared wMel-infected Brazilian Ae. aegypti, wMelRio, were released as eggs in Jurujuba (22°56′ 00″ S, 43°07′ 00″ W), a lower-middle-class community in the city of Niterói (state of Rio de Janeiro, Brazil). Located by the shores of Guanabara bay, this community has grown from a typical fisherman settlement, with informal occupancy, to a total population of 2797 residents in 890 houses. Jurujuba encompasses a total area of 2.53 km2, divided into seven smaller sectors (i.e. sub-areas or localities within the neighborhood): Ponto Final, Várzea, Brasília, Cascarejo, Praia de Adão e Eva, Peixe-Galo and Salinas.
wMelRio eggs were released in the field through special deployment devices, referred to as mosquito release containers (MRCs), which consisted of small white plastic buckets (19 cm height × 18 cm top diameter × 15.5 cm base diameter) with four small holes on the side wall, only a few centimeters away from the top lid. Each MRC was loaded with 1 L of water, 0.45 g of Tetramin Tropical Tablets (i.e. one and a half tablet) (Tetra GmbH, Herrenteich, Germany) and an ovistrip containing approximately 150–300 eggs. After six to seven days, about 80% of the immatures were pupae, and after 11 to 12 days, most of the adults had already emerged and left the device from the wall holes. Every 15 days, MRCs were checked and reloaded so that another rearing and release cycle could take place. Release sites were spatially distributed as evenly as possible (Supplementary Fig. S1), so as to maximize the spread of Wolbachia-harboring individuals and promote mating with their wild peers. The release strategy was optimized by splitting the sites into two groups, A and B, with alternate MRC loading schedules. Thus, while MRCs from group A were releasing adults, those from group B were being loaded with new ovistrips, water and food. In the following week, an opposite situation occurred, with MRCs from group B releasing adults. The release schedules, as well as the number of allocated MRCs, varied according to each Jurujuba’s sector (Supplementary Table S1).
Ethical considerations
All methods were carried out in accordance with relevant guidelines and regulations. Study protocol for Wolbachia field release was approved by the National Research Ethics Committee (CONEP, CAAE 02524513.0.1001.0008) and three government agencies: IBAMA (Ministry of Environment), Anvisa (Ministry of Health) and MAPA (Ministry of Agriculture, Livestock and Supply) to obtain the RET (Special Temporary Registry, 25351.392108/2013-96). Prior to mosquito releases, an informed consent was obtained from 70% of Jujuruba households. Also, a written informed consent was obtained from households that hosted BG-sentinel mosquito traps.
For the maintenance and mass-rearing of Wolbachia-infected Ae. aegypti, adult females were fed human blood from a donation center (Hospital Antonio Pedro, Rio de Janeiro State University), with supporting regulatory approval (CONEP, CAAE 59175616.2.0000.0008) We only used blood bags which would have been discarded by the donation center, mainly due to insufficient volume to meet their quality assurance policy. Samples had no information on donor’s identity, sex, age and any clinical condition, but were tested negative for several diseases, including Hepatitis B, Hepatitis C, Chagas disease, syphilis, HIV and HTLV, as part of the Brazilian Government routine screening.
For vector competence assays, human blood was obtained from Fundação Hemominas as part of a research agreement with Instituto René Rachou (Fiocruz Minas) (OF.GPO/CCO-Nr224/16).
Wolbachia field monitoring and density level assessment
Ae. aegypti field population was monitored with BG-Sentinel traps (Biogents AG, Regensburg, Germany), spread across Jurujuba in a semi-homogeneous fashion (Supplementary Fig. S2, Supplementary Table S2, Supplementary Datasheet S1). These monitoring sites were chosen among suitable households who formally agreed with hosting of a trap, and had to be reallocated according to necessity (i.e. household quits hosting the trap). Working traps were checked weekly by removing the catch bags (e.g. small meshed envelopes placed inside the BG-Sentinels to collect trapped insects) and bringing them to the laboratory for species identification and Wolbachia screening. Catch bags were barcoded according to the trap ID and site, so as to create a pipeline for field samples.
Screening for Wolbachia in Ae. aegypti samples was undertaken by qPCR. Briefly, individual DNA was extracted by homogenizing head/thorax body parts in Squash Buffer (10 mM Tris–Cl, 1 mM EDTA, 25 mM NaCl, pH 8.2) supplemented with Proteinase K (200 ug/ml) and incubating at 56 °C for 5 min. Extraction ended by enzyme inactivation at 98 °C for 15 min. DNA amplifications were carried out with FastStart Essential DNA Probes Master (Roche), using specific primers and probes to Wolbachia pipientis WD0513 and Ae. aegypti rps17 markers (Supplementary Table S3). Thermocycling conditions were set on a LightCycler 96 Instrument (Roche), as follows: 95 °C for 10 min (initial denaturation), and 40 cycles of 95 °C for 15 s and 60 °C for 30 s. Samples were analyzed using absolute quantification, by comparison to serial dilutions of either gene product, cloned and amplified in the pGEMT-Easy plasmid (Promega). Negative control samples were normalized between plates, and were used as reference to determine a minimum threshold for positive samples.
DENV and ZIKV isolation and replication in mosquito cells
ZIKV was kindly provided by Instituto Aggeu Magalhães (IAM, Fiocruz) through viral isolation of a symptomatic patient sample from Recife (PE, Brazil) in 2015 (ZikV/H.sapiens/Brazil/BRPE243/2015). DENV was sourced following a viral isolate from a patient sample diagnosed with Dengue type 1 in Contagem (MG, Brazil), also in 2015 (Den1/H.sapiens/Brazil/BRMV09/2015). Both ZIKV and DENV samples were accompanied by patients’ written consent (CONEP, reference number 862.912), being further catalogued into the national database of genetic patrimony and associated knowledge (SISGEN, access number AA1D462).
In vitro culture of viral particles were done as previously described36. Briefly, ZIKV and DENV were replicated in Aedes albopictus C6/36 cells, grown at 28 °C in Leibovitz L-15 medium (ThermoFisher) supplemented with 10% fetal bovine serum (FBS) (ThermoFisher). After seven days, supernatants were harvested and virus titers were assessed, first by Reverse Transcription (RT)-qPCR, and later by plaque assay with VERO cells grown under 37 °C, 5% carbon dioxide, in Dulbecco’s Modified Eagle Medium (DMEM) (ThermoFisher) supplemented with 3% Carboxymethylcellulose (Synth) and 2% FBS.
Vector competence assays
To perform vector competence assays with field samples of Ae. aegypti, ovitraps were mounted in both Ponto Final (Jurujuba) and Urca, a Wolbachia-free area in Rio de Janeiro. Ovitraps were collected from the field over 13 weeks, from April to June 2017, which corresponds to the time-frame between 14 and 16 months along the post-release phase in Ponto Final. Once in the insectary, eggs samples were reared to the adult stage in a controlled insectary environment (refer to ‘mosquito lines and maintenance’ for details).
For virus challenging assays through oral-feeding, young females (4–6 days old) were starved for 20 to 24 h, and subsequently offered culture supernatant containing ZIKV or DENV mixed with human red blood cells (2:1 ratio), using an artificial membrane feeding system36. It is important to mention that, as for the colony maintenance protocol, blood samples used here were also submitted to quality control prior to its use in the assays, mainly due to putative arbovirus contaminations which could affect the experimental outputs. Likewise, all samples were tested negative for DENV, ZIKV, CHIKV, MAYV and YFV by multiplex qPCR assays36,68. Oral-infections were performed twice for each virus. ZIKV was offered first from fresh (initial virus titer of 4.8 × 108 PFU/mL) and second from frozen culture supernatant (initial virus titer of 7.6 × 106 PFU/mL). In contrast, DENV was offered from fresh supernatants only (virus titers of 2 × 106 PFU/mL and 6.5 × 107 PFU/mL), since frozen versions failed to infect. Specimens were allowed to feed for one hour, after which engorged females were selected and maintained with 10% sucrose solution ad libitum, during the whole extrinsic incubation period. At 14 days post-infection (dpi), viral loads were assessed in heads/thorax extracts by RT-qPCR (refer to ‘Viral diagnosis’ for more details).
For saliva-mediated virus challenging assays, ZIKV and DENV pre-exposed females (14 dpi) from Jurujuba (Wolbachia +) and Urca (Wolbachia −) were starved for about 16 h (overnight) before being knocked down and kept at 4 °C for wings and legs removal. Salivation was induced by introducing a 10 µL sterile filter tip, pre-loaded with 5 µl of a solution [30% sucrose (w/v) diluted in 50% fetal bovine serum (FBS) and 50% DMEM medium], into the mosquito proboscis for 30 min. Saliva samples were individually collected, and 276 nL was intrathoracically injected into young naive females (Urca) using a Nanoject II hand held injector (Drummond), as previously described36,68. Each saliva sample was used to inoculate 8–14 naïve Wolbachia-free Ae. aegypti specimens, of which 8 were screened for infective particles. ZIKV and DENV were quantified by RT-qPCR at 5 dpi and 7 dpi, respectively (refer to ‘Viral diagnosis’ for more details). Overall Intrathoracic Saliva Infection index (OISI) was obtained by averaging the percentages (± SD) of infected individuals in each group.
Viral diagnosis
To identify ZIKV and DENV particles in individual samples, whole specimens were processed into head/thorax homogenates for RNA/DNA extraction with the High Pure Viral Nucleic Acid Kit (Roche), according to manufacturer’s instructions30. Extracted samples were diluted in nuclease-free water to a concentration of 50 ng/μL. ZIKV, DENV and Wolbachia levels, in vector competence assays, were quantified by RT-qPCR using TaqMan Fast Virus 1-Step Master Mix (ThermoFisher) and specific primers and probes (Supplementary Table S3). Reactions were run on a LightCycler 96 Instrument (Roche), using the following thermocycling conditions: 50 °C for 5 min (initial RT step), 95 °C for 20 s (RT inactivation/DNA initial denaturation), and then 40 cycles of 95 °C for 3 s and 60 °C for 30 s. Each RNA/DNA sample was used in two reactions, one with ZIKV, DENV or Wolbachia primers, and another with Ae. aegypti rps17 endogenous control30. Absolute quantification was achieved by comparing amplification profiles with standard curves generated by serial dilutions of their respective gene products, amplified from a cloned sequence in pGEM-T Easy vector (Promega). Negative control samples (no virus RNA) served as reference to fix a minimum threshold for positive ones. ZIKV and DENV loads were defined as their copy number per sample (head/thorax or saliva), while Wolbachia loads were relative quantifications to the rps17 reference gene. Here, it is worth noting that, while Wolbachia titer is naturally variable and dependent on its whole-body density, the overall expression of rps17 is stable and particularly suitable for internal controls in assays with adult females69, as demonstrated previously by us and others30,62,68.
Map creation and source codes
The satellite image map of Jurujuba was created with ArcGIS Desktop 10.7 (Esri Inc., https://www.esri.com/en-us/arcgis/products/arcgis-desktop/overview) using Google Earth (Google LLC) source code, under the license and in accordance with the fair use described in ‘https://about.google/brand-resource-center/products-and-services/geo-guidelines/’. Maps with geotagged MRCs and BG-Sentinel traps were created with ArcGIS Desktop 10.7 and OpenStreetMap source code (OpenStreetMap contributors), under the license CC-BY-SA 2.0.
Statistical analyses
Graphs and statistical analyzes were performed in GraphPad Prism 8 (GraphPad Software Inc., https://www.graphpad.com). Kruskal–Wallis test followed by Dunn’s post-hoc multiple comparisons were used to analyze Wolbachia density data from field-collected and colony samples. ZIKV and DENV loads in head/thorax extracts, from both oral and saliva-challenging samples, were compared using the Mann–Whitney U test. For all statistical inferences, ⍺ was set to 0.05.
Supplementary Information
Acknowledgements
We would like to thank all members of the Mosquitos Vetores research group, for critical and helpful reviews, as well as past and present team members of the World Mosquito Program, whose dedication was key to the positive outcome of this study. Special thanks to Flavia Teixeira, for ethical and regulatory compliance, Roberto Peres and Catia Cabral, for field deployment and mass-rearing supervision and Simon Kutcher (WMP Global) for overall technical inputs, during early days of our implementation. We are also grateful for the field assistance provided by public agents of the Health Municipality of Niterói, and for the incredible support by Jurujuba community members.
Author contributions
G.S.R., M.N.R., F.B.S.D. and L.A.M. conceived the study. J.S.M.G., G.S.R., M.N.R., F.B.S.D., J.P., F.D.C. and T.N.P. performed the investigation, data curation and analysis. L.A.M. managed the project supervision, validation and funding. J.S.M.G. and L.A.M. drafted the manuscript, and all authors reviewed and approved its final version.
Funding
LAM is a fellow of CNPq. This work was funded by the Brazilian Ministry of Health (SVS and SCTIE), and a grant to Monash University from the Bill and Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All relevant data generated or analyzed during this study are included in this manuscript (and its Supplementary Information file).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: João Silveira Moledo Gesto, Gabriel Sylvestre Ribeiro and Marcele Neves Rocha.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-89409-8.
References
- 1.Kraemer MUG, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Global Vector Control Response 2017–2030. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.Brady OJ, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SVS. Boletim Epidemiológico 38. Secretaria de Vigilância em Saúde - Ministério da Saúde50, 9–39 (2019).
- 6.Weaver SC. Arrival of chikungunya virus in the new world: Prospects for spread and impact on public health. PLoS Negl. Trop. Dis. 2014;8:e2921. doi: 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahid B, Ali A, Rafique S, Idrees M. Global expansion of chikungunya virus: Mapping the 64-year history. Int. J. Infect. Dis. 2017;58:69–76. doi: 10.1016/j.ijid.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Nugent EK, Nugent AK, Nugent R, Nugent K. Zika Virus: Epidemiology, pathogenesis and human disease. Am. J. Med. Sci. 2017;353:466–473. doi: 10.1016/j.amjms.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Mayer SV, Tesh RB, Vasilakis N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017;166:155–163. doi: 10.1016/j.actatropica.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McSweegan E, et al. The Global Virus Network: Challenging chikungunya. Antiviral Res. 2015;120:147–152. doi: 10.1016/j.antiviral.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faria NR, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barreto ML, et al. Zika virus and microcephaly in Brazil: A scientific agenda. Lancet. 2016;387:919–921. doi: 10.1016/S0140-6736(16)00545-6. [DOI] [PubMed] [Google Scholar]
- 13.Thisyakorn U, Thisyakorn C. Latest developments and future directions in dengue vaccines. Ther. Adv. Vaccines. 2014;2:3–9. doi: 10.1177/2051013613507862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelnabi R, Neyts J, Delang L. Towards antivirals against chikungunya virus. Antiviral Res. 2015;121:59–68. doi: 10.1016/j.antiviral.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin H-H, Yip B-S, Huang L-M, Wu S-C. Zika virus structural biology and progress in vaccine development. Biotechnol. Adv. 2018;36:47–53. doi: 10.1016/j.biotechadv.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Valença MA, Marteis LS, Steffler LM, Silva AM, Santos RLC. Dynamics and characterization of Aedes aegypti (L.) (Diptera: Culicidae) key breeding sites. Neotrop. Entomol. 2013;42:311–316. doi: 10.1007/s13744-013-0118-4. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho FD, Moreira LA. Why is Aedes aegypti Linnaeus so Successful as a Species? Neotrop. Entomol. 2017;46:243–255. doi: 10.1007/s13744-017-0520-4. [DOI] [PubMed] [Google Scholar]
- 18.Linss JGB, et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasites Vectors. 2014;7:25. doi: 10.1186/1756-3305-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maciel-de-Freitas R, et al. Undesirable consequences of insecticide resistance following Aedes aegypti control activities due to a dengue outbreak. PLoS ONE. 2014;9:e92424. doi: 10.1371/journal.pone.0092424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyes CL, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017;11:e0005625. doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker T, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann AA, et al. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl. Trop. Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutra HLC, et al. From lab to field: The influence of urban landscapes on the invasive potential of Wolbachia in Brazilian Aedes aegypti mosquitoes. PLoS Negl. Trop. Dis. 2015;9:e0003689. doi: 10.1371/journal.pntd.0003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 27.Teixeira L, Ferreira Á, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:e1000002. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsey ARI, Bhattacharya T, Newton ILG, Hardy RW. Conflict in the intracellular lives of endosymbionts and viruses: A mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses. 2018;10:141. doi: 10.3390/v10040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 30.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 31.McMeniman CJ, O’Neill SL. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl. Trop. Dis. 2010;4:e748. doi: 10.1371/journal.pntd.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14:e1006815. doi: 10.1371/journal.ppat.1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rancès E, Ye YH, Woolfit M, McGraw EA, O’Neill SL. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caragata EP, Rancès E, O’Neill SL, McGraw EA. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb. Ecol. 2014;67:205–218. doi: 10.1007/s00248-013-0339-4. [DOI] [PubMed] [Google Scholar]
- 35.Geoghegan V, et al. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 2017;8:526. doi: 10.1038/s41467-017-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutra HLC, et al. Wolbachia blocks currently circulating zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia reduces transmission of zika virus by Aedes aegypti. Sci. Rep. 2016;6:28792. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aliota MT, et al. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016;10:e0004677. doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Indriani C, et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: A quasi-experimental trial using controlled interrupted time series analysis [version 1; peer review: 1 approved] Gates Open Res. 2020;4:50. doi: 10.12688/gatesopenres.13122.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia GA, et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl. Trop. Dis. 2019;13:e0007023. doi: 10.1371/journal.pntd.0007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tantowijoyo W, et al. Stable establishment of wMel Wolbachia in Aedes aegypti populations in Yogyakarta, Indonesia. PLoS Negl. Trop. Dis. 2020;14:e0008157–e0008157. doi: 10.1371/journal.pntd.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan P, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2019;3:1547. doi: 10.12688/gatesopenres.13061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Neill SL, et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2019;2:36–36. doi: 10.12688/gatesopenres.12844.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotsakiozi P, et al. Tracking the return of Aedes aegypti to Brazil, the major vector of the dengue, chikungunya and Zika viruses. PLoS Negl. Trop. Dis. 2017;11:e0005653–e0005653. doi: 10.1371/journal.pntd.0005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farnesi LC, Menna-Barreto RFS, Martins AJ, Valle D, Rezende GL. Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: Connection with distinct levels of resistance to desiccation. J. Insect Physiol. 2015;83:43–52. doi: 10.1016/j.jinsphys.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Diniz DFA, de Albuquerque CMR, Oliva LO, de Melo-Santos MAV, Ayres CFJ. Diapause and quiescence: Dormancy mechanisms that contribute to the geographical expansion of mosquitoes and their evolutionary success. Parasites Vectors. 2017;10:310. doi: 10.1186/s13071-017-2235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliva LO, La Corte R, Santana MO, de Albuquerque CMR. Quiescence in Aedes aegypti: Interpopulation differences contribute to population dynamics and vectorial capacity. Insects. 2018;9:111. doi: 10.3390/insects9030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macoris MDL, Martins AJ, Andrighetti MTM, Lima JBP, Valle D. Pyrethroid resistance persists after ten years without usage against Aedes aegypti in governmental campaigns: Lessons from São Paulo State, Brazil. PLoS Negl. Trop. Dis. 2018;12:e0006390–e0006390. doi: 10.1371/journal.pntd.0006390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris AF, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 2012;30:828–830. doi: 10.1038/nbt.2350. [DOI] [PubMed] [Google Scholar]
- 50.Burt A. Heritable strategies for controlling insect vectors of disease. Philos. Trans. R. Soc. Lond. B. 2014;369:20130432. doi: 10.1098/rstb.2013.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt TL, et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017;15:e2001894. doi: 10.1371/journal.pbio.2001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caragata EP, Dutra HLC, Moreira LA. Exploiting intimate relationships: Controlling mosquito-transmitted disease with wolbachia. Trends Parasitol. 2016;32:207–218. doi: 10.1016/j.pt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Nazni WA, et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 2019;29:4241–4248.e5. doi: 10.1016/j.cub.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen TH, et al. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferguson NM, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci. Transl. Med. 2015;7:279ra37. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA. Application of wMelPop Wolbachia Strain to Crash Local Populations of Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e0003930. doi: 10.1371/journal.pntd.0003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasić G, Endersby NM, Williams C, Hoffmann AA. Using Wolbachia-based release for suppression of Aedes mosquitoes: Insights from genetic data and population simulations. Ecol. Appl. 2014;24:1226–1234. doi: 10.1890/13-1305.1. [DOI] [PubMed] [Google Scholar]
- 58.Joubert DA, et al. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gesto JSM, et al. In tune with nature: Wolbachia does not prevent pre-copula acoustic communication in Aedes aegypti. Parasites Vectors. 2018;11:109. doi: 10.1186/s13071-018-2695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farnesi LC, et al. Embryonic development and egg viability of wMel-infected Aedes aegypti. Parasites Vectors. 2019;12:211. doi: 10.1186/s13071-019-3474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hancock PA, et al. Density-dependent population dynamics in Aedes aegypti slow the spread of wMel Wolbachia. J. Appl. Ecol. 2016;53:785–793. doi: 10.1111/1365-2664.12620. [DOI] [Google Scholar]
- 62.Frentiu FD, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl. Trop. Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross PA, et al. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 2017;13:e1006006. doi: 10.1371/journal.ppat.1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu P, Bian G, Pan X, Xi Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 2012;6:e1754. doi: 10.1371/journal.pntd.0001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O’Neill SL, Johnson KN. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl. Environ. Microbiol. 2012;78:6922–6929. doi: 10.1128/AEM.01727-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez J, et al. Symbionts commonly provide broad spectrum resistance to viruses in insects: A comparative analysis of Wolbachia strains. PLoS Pathog. 2014;10:e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terradas G, McGraw EA. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr. Opin. Insect Sci. 2017;22:37–44. doi: 10.1016/j.cois.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 68.Pereira TN, Rocha MN, Sucupira PHF, Carvalho FD, Moreira LA. Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci. Rep. 2018;8:6889. doi: 10.1038/s41598-018-25236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dzaki N, Ramli KN, Azlan A, Ishak IH, Azzam G. Evaluation of reference genes at different developmental stages for quantitative real-time PCR in Aedes aegypti. Sci. Rep. 2017;7:43618. doi: 10.1038/srep43618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data generated or analyzed during this study are included in this manuscript (and its Supplementary Information file).