Abstract
Background:
Radiotracer imaging can assess microvascular foot perfusion and identify regional perfusion abnormalities in patients with critical limb ischemia (CLI) and diabetes mellitus (DM), but the relationship between perfusion response to revascularization and subsequent clinical outcomes has not been evaluated.
Objectives:
Evaluate the prognostic value of single photon emission computed tomography (SPECT)/computed tomography (CT) imaging of angiosome foot perfusion for predicting amputation outcomes in patients with CLI and DM.
Methods:
Patients with CLI, DM, and non-healing foot ulcers (n=25) were prospectively enrolled for SPECT/CT perfusion imaging of the feet before and after revascularization. CT images were used to segment angiosomes (i.e. three-dimensional vascular territories) of the foot. Relative changes in radiotracer uptake after revascularization were evaluated within the ulcerated angiosome. Incidence of amputation was assessed at 3 and 12 months after revascularization.
Results:
SPECT/CT detected a significantly lower microvascular perfusion response for patients who underwent amputation compared to those who remained amputation free at 3 (p=0.01) and 12 (p=0.01) months after revascularization. The cutoff percent change in perfusion for predicting amputation at 3 months was 7.55%, and 11.56% at 12 months. The area under the curve based on the amputation outcome was 0.799 at 3 months and 0.833 at 12 months. The probability of amputation-free survival was significantly higher at 3 (p = 0.002) and 12 months (p = 0.03) for high perfusion responders than low perfusion responders to revascularization.
Conclusions:
SPECT/CT imaging detects regional perfusion responses to lower extremity revascularization and provides prognostic value in patients with CLI.
Keywords: critical limb ischemia, perfusion imaging, diabetes, single photon emission computed tomography, revascularization
INTRODUCTION
Peripheral arterial disease (PAD) consists of atherosclerosis of non-coronary arteries and affects approximately 8.5 million adults in the United States and 200 million adults worldwide (1,2). Critical limb ischemia (CLI) is the most severe manifestation of lower extremity PAD and is characterized by lower limb ischemic rest pain and/or the presence of ischemic ulcers or necrotic tissue. Additionally, CLI is associated with a high risk for cardiovascular events, death, lower extremity amputation, and poor quality of life, as well as high treatment costs (3,4). Therefore, relief of ischemic pain, healing of wounds, and preservation of functional limbs are the primary therapeutic goals for CLI patients. Ultimately, improving microvascular perfusion and non-invasively quantifying those improvements within specific regions of the ischemic limb is essential for achieving therapeutic goals. Indeed, the importance and need for standard noninvasive tools that can assess limb microvascular perfusion in the setting of CLI was recently highlighted by the American Heart Association (5). However, the majority of clinically available non-invasive tools only allow for evaluation of the hemodynamic and anatomical improvements associated with therapy, while the available tools that do allow for evaluation of microvascular perfusion are restricted to superficial measurements (e.g. transcutaneous oxygen pressure) or a limited anatomical region of tissue (e.g. toe pressure). Non-invasive assessment of lower extremity microvascular perfusion is particularly important for CLI patients who often have diabetes mellitus (DM), which exacerbates PAD by promoting microvascular disease and ultimately contributes to increased mortality and increased risk for lower extremity amputation (6–9).
Several imaging modalities and approaches are currently being developed and investigated to evaluate lower extremity microvascular perfusion in PAD patients, with each of these modalities possessing relative strengths and limitations. For example, modalities such as hyperspectral, laser Doppler, and transcutaneous oxygen pressure are relatively low cost and enable efficient bedside evaluation, but these techniques only allow for superficial assessment of tissue perfusion (~2–3 mm depth) (10). Contrast-enhanced ultrasound (CEUS) techniques (11,12) and magnetic resonance-based methods such as arterial spin labeling (13,14) also exist and permit the quantitative evaluation of stress-induced abnormalities in calf skeletal muscle perfusion, which is potentially beneficial for assessing PAD patients who suffer from exercise-induced claudication; however, CEUS lacks widespread expertise while magnetic resonance-based methods are time consuming and provide poor signal-to-noise ratio under resting conditions (15). Although radiotracer-based imaging techniques such as single photon emission computed tomography (SPECT)/computed tomography (CT) expose patients to ionizing radiation, these methods provide assessment of volumetric perfusion in the foot and offer evaluation of microvascular perfusion under resting conditions (16,17), which may be particularly advantageous in patients with CLI and DM who may present with resting ischemic pain and have functional exercise limitations due to the presence of foot wounds.
Our research team has recently demonstrated the utility of SPECT/CT imaging for detecting and quantifying abnormalities in resting volumetric microvascular perfusion within specific angiosomes, or three-dimensional vascular territories of the feet, in patients with CLI and DM (17). Additionally, in a recent case report, we have shown proof-of-concept for using SPECT/CT imaging to quantify perfusion responses to revascularization in specific three-dimensional angiosomes of the foot in a patient with CLI (16). While angiosome-guided endovascular revascularization is considered an effective treatment option for CLI patients that leads to favorable clinical outcomes (18–20), no studies to date have evaluated quantitative volumetric changes in microvascular perfusion within ulcerated angiosomes of the foot in a population of CLI patients undergoing revascularization procedures. We hypothesized that SPECT/CT imaging would allow for angiosome-specific evaluation of perfusion responses to revascularization in CLI patients and predict subsequent lower extremity amputation outcomes. Therefore, in the current study, we performed serial SPECT/CT imaging of microvascular perfusion in CLI patients with DM to quantify perfusion responses to endovascular revascularization within ulcerated angiosomes of the foot. Furthermore, we assessed the prognostic value of SPECT/CT perfusion imaging for predicting early- (3 months) and late-term (12 months) limb salvage outcomes.
METHODS
Research Subjects
Patients with CLI, DM, and non-healing foot ulcers who were scheduled for lower extremity endovascular revascularization (n=25) were prospectively enrolled into an ongoing clinical study focused on evaluating the prognostic utility of SPECT and positron emission tomography (PET) perfusion imaging approaches in the setting of PAD (https://clinicaltrials.gov, NCT03622359). The criteria for DM included fasting plasma glucose measures greater than 126 mg/dL on 2 separate occasions, a glycated hemoglobin (HbA1c) greater than 6.5%, or a two-hour plasma glucose ≥200 mg/dL in an oral glucose tolerance test. CLI status was determined based on the presence of a non-healing foot ulcer, tissue necrosis, or ischemic rest pain in the foot or calf, along with evidence of significant obstructive disease for one or multiple lower extremities arteries, as identified by prior abnormal CT angiography, magnetic resonance angiography, duplex ultrasound, toe pressure (<50 mmHg), or ankle-brachial index (<0.9 or ≥1.3). Patients were not eliminated from enrollment consideration due to common diabetic foot conditions, such as cellulitis, osteomyelitis, Charcot foot, and stasis dermatitis.
The study protocol was approved by the Yale University Institutional Review Board for Human Subjects Research and Review Committee as well as the Radiation Safety Committee and was in accordance with the guidelines set forth by the Declaration of Helsinki. All individuals provided written informed consent after receiving an explanation of the experimental procedures and potential risks associated with participating in the study.
Clinical SPECT/CT Imaging Protocol
All patients reported to the hospital following an 8 hour fast and underwent resting SPECT/CT imaging of the feet before and 1–3 days after lower extremity revascularization using a conventional hybrid SPECT/CT imaging system with large field-of-view sodium iodide detectors (Infinia Hawkeye, GE Healthcare). During each imaging visit, patients received an intravenous injection of technetium-99m (99mTc)-tetrofosmin (554.6 ± 35.8 MBq) under resting conditions and underwent SPECT imaging of the feet 15 minutes later. SPECT images were acquired using a 360° step and shoot acquisition with a 140.5 keV±10% window, 3° projections, and 30 seconds per stop. Immediately following the SPECT acquisition, CT images were acquired with a slice thickness of 5 mm, at 140 kVp, and 2.5 mA for the purposes of attenuation correction and future image segmentation of angiosomes. All SPECT images were reconstructed using 2 iterations and 10 subsets of ordered subset expectation and maximization algorithm, applying corrections for attenuation, scatter, and resolution loss. SPECT/CT images were reconstructed using commercially available system software (Xeleris, GE Healthcare, Buckinghamshire, UK).
SPECT/CT Imaging Processing
CT attenuation images were used to segment and define three-dimensional angiosomes of the feet using a recently developed approach that allows for regional evaluation of angiosome perfusion in the feet of patients with CLI (16,17). Reconstructed SPECT images were co-registered with CT images and the average 99mTc-tetrofosmin uptake on SPECT images was assessed for the CT-defined 3D angiosome that contained the non-healing ulcer, with additional normalization of the SPECT value to the injected radiotracer dose (mCi) and patient body weight (kg). To ensure precise serial analysis of perfusion within the angiosome-defined region of the foot, pre-and post- revascularization CT images were co-registered using a previously developed semi-automated approach that allows for serial registration of foot images (16,17,21). The relative percent change in perfusion (i.e. 99mTc-tetrofosmin uptake) from pre- to post-revascularization time point was quantified for the angiosome containing the non-healing wound. Additional evaluation of foot perfusion was similarly performed on the contralateral untreated foot to assess test-retest reliability of SPECT perfusion measures from the pre- to post-revascularization study visit.
Patient Demographics and Clinical Outcomes
Baseline patient characteristics including age, gender, body mass index, comorbidities, cardiovascular and immunosuppressive medications, and standard laboratory values were recorded at the time of enrollment. Short- (3 months) and long-term (12 months) patient outcomes were obtained from clinic visits, chart review, and hospital admissions during the 12 months after each patient’s revascularization procedure. Specific clinical outcomes of interest included the occurrence of lower extremity amputation (including minor and/or major amputation) and death from any cause.
Statistical Analysis
Continuous variables are presented as either means and standard deviations or means and interquartile range (IQR), while categorical variables are presented as counts and percentages. Due to the skewed nature of the perfusion responses across the patient population, differences in the angiosome perfusion change between patients with and without amputation at both outcome time points were tested via a Wilcoxon Rank Sum Test (also known as the Mann-Whitney U Test). Receiver operating characteristic (ROC) curve was used to determine a cutoff for change in perfusion value at both time points based on the occurrence of amputation or death. Several optimization criteria were examined when choosing cutoffs, including misclassification-cost term, efficiency, correct classification rate (CCR), the Youden index, sensitivity, and specificity. The cutoff based on the 3-month amputation-free survival outcome maximized all of these criteria, and the cutoff based on the 12-month amputation-free survival outcome was chosen to maximize CCR while also balancing between sensitivity and specificity. Area under the curve (AUC) was calculated to assess the discriminability of amputation using change in perfusion. Kaplan-Meier curves were estimated and data were analyzed via a log rank test to evaluate the cutoffs chosen for low and high change in perfusion. Additionally, Cox regression was performed to explore associations between variables of interest and amputation-free survival. Serial SPECT perfusion measures acquired before and after revascularization in the contralateral untreated foot were assessed for the reliability and consistency of the repeated 2 measures using Bland-Altman plot and intraclass correlation coefficient.
A p-value of <0.05 was considered statistically significant. Estimates are presented with 95% confidence intervals (CI). All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC) or R, version 3.6.1 (R Core Team, 2019).
RESULTS
Patient Demographics and Revascularization Procedures
All patients (n=25) presented with CLI, previously diagnosed type 2 DM (HbA1c, 8.5±2.0%), and multiple cardiovascular co-morbidities that included hypertension, hyperlipidemia, coronary artery disease, renal disease, or prior stroke (Table 1). Additionally, some patients presented with Charcot foot (n=2) and osteomyelitis (n=2). Ankle-brachial index measurements were only available in 14 patients while toe-brachial index was only obtainable in 15 patients due to the presence of wounds and non-compressible arteries. Among the 25 patients, 13 patients presented with single vessel disease while 12 had multi-vessel disease. With regard to lesion location, 12 of the 25 patients had infrapopliteal (below-the-knee), 1 had a combination of infrapopliteal and femoropopliteal disease, 12 had femoropopliteal disease, and 1 had aortoiliac disease. A total of 39 lesions were treated, with 8 lesions treated by self-expanding stents and all remaining lesions being treated by percutaneous transluminal balloon angioplasty. Table 1 provides a full summary of patient demographics.
TABLE 1.
Patient Characteristics
| Demographics | |
| Age (yrs) | 64.7 ± 13.8 |
| Gender (Male) | 18 (72%) |
| BMI (kg/m2) | 30.1 ± 6.5 |
| HbA1c (%) | 8.5 ± 2.0 |
| Systolic blood pressure (mmHg) | 137 ± 20 |
| Diastolic blood pressure (mmHg) | 75 ± 14 |
| Ankle-brachial index | 0.62 ± 0.19 |
| Toe-brachial index | 0.44 ± 0.18 |
| Risk Factors | |
| Diabetes Mellitus | 25 (100%) |
| Hypertension | 23 (92%) |
| Hyperlipidemia | 16 (64%) |
| Tobacco Use | 15 (60%) |
| Comorbidities | |
| Coronary Artery Disease | 13 (52%) |
| Renal Disease | 10 (40%) |
| Prior Stroke | 4 (16%) |
N=25 patients. Values are mean ± SD or n (%).
BMI = body mass index.
SPECT/CT Imaging of Angiosome Perfusion and Clinical Outcomes
Qualitative assessment of 99mTc-tetrofosmin SPECT/CT images demonstrated the ability to non-invasively detect serial changes in resting foot perfusion in response to endovascular revascularization and differentiated low versus high perfusion responders to revascularization (Central Illustration). Quantitative evaluation of serial SPECT/CT perfusion imaging demonstrated that the average and median percent changes in angiosome foot perfusion following revascularization were 9.9 ± 9.5% and 7.5% (4.9–10.2%), respectively.
Central Illustration. SPECT/CT perfusion imaging of perfusion response to lower extremity revascularization in the setting of DM and CLI.
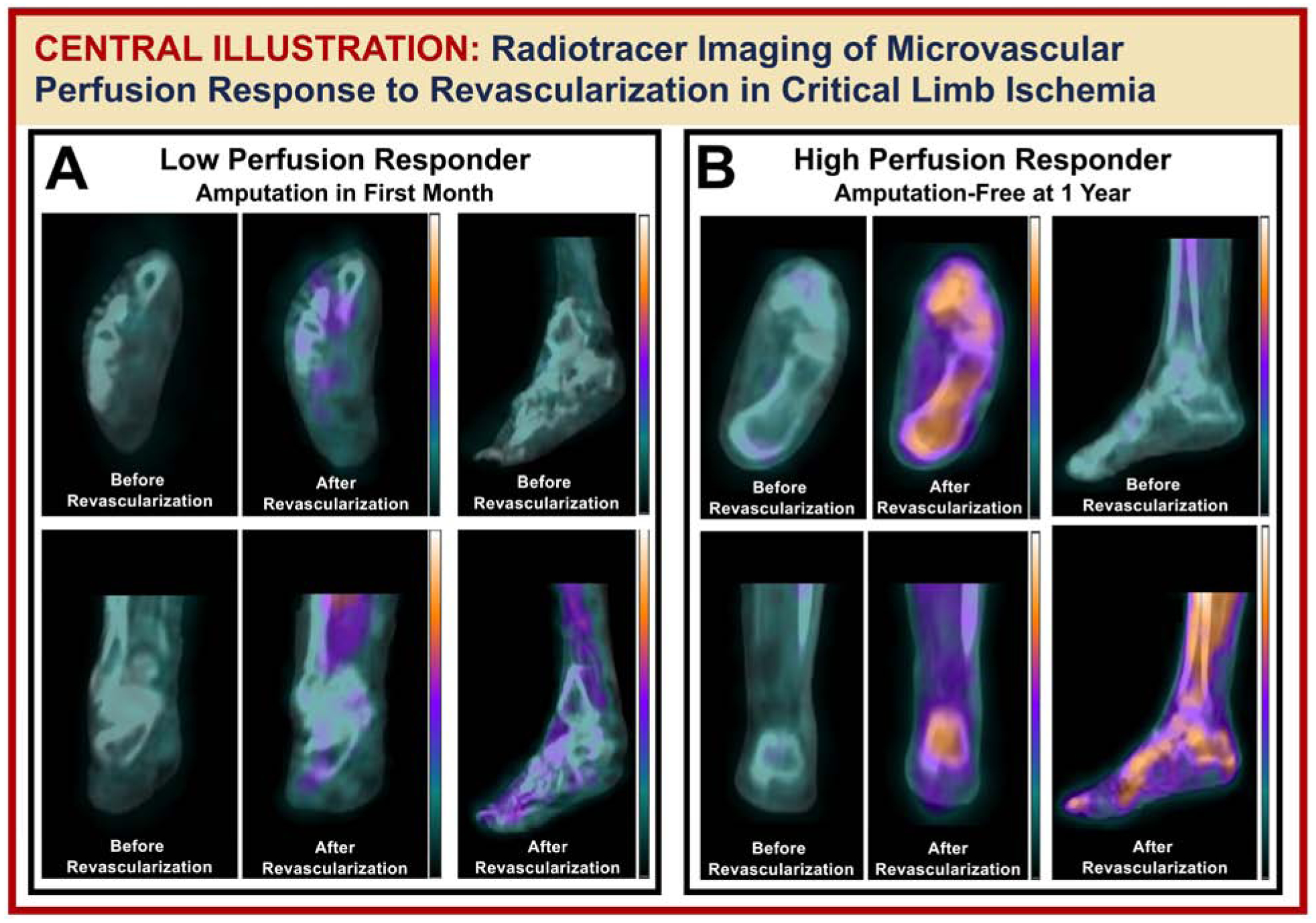
Pictured is (A) a patient who demonstrated a low perfusion response to revascularization (i.e. 5%) and underwent eventual minor amputation in the first month, and (B) a patient who alternatively demonstrated a high perfusion response (i.e. 29%) and did not receive an amputation in the 12 months after revascularization.
At 3 months after revascularization, 14 (56%) patients had undergone at least one amputation while the remaining 11 (44%) patients did not require an amputation. All 14 amputations in the first 3 months were minor amputations, which were defined as toe or transmetatarsal amputations. The median change in angiosome perfusion was significantly lower in patients who had an amputation procedure in the first 3 months after revascularization [5.78%, IQR: (3.06–7.55%)] when compared to patients who did not undergo an amputation [9.99%, IQR: (7.69–22.47%); p=0.011] (Figure 1A). At 12 months after revascularization, 19 (76%) patients had at least one amputation or were deceased, while 6 (24%) patients remained amputation free. Of the 19 patients who had amputations or were deceased at 12 months after revascularization, 5 patients had major amputations (above- or below-the-knee), 12 had minor amputations (toe or transmetatarsal), and 2 were deceased. The median revascularization-induced change in angiosome perfusion was significantly lower in patients who had an amputation or were deceased at 12 months [5.83%, IQR: (3.06–10.01%)] when compared to patients who did not undergo an amputation procedure [9.99%, IQR: (7.69–22.47%); p=0.014] (Figure 1B).
Figure 1. Comparison of SPECT/CT imaging-derived changes in angiosome perfusion in response to lower extremity revascularization between patients who remained amputation free versus patients who received an amputation.
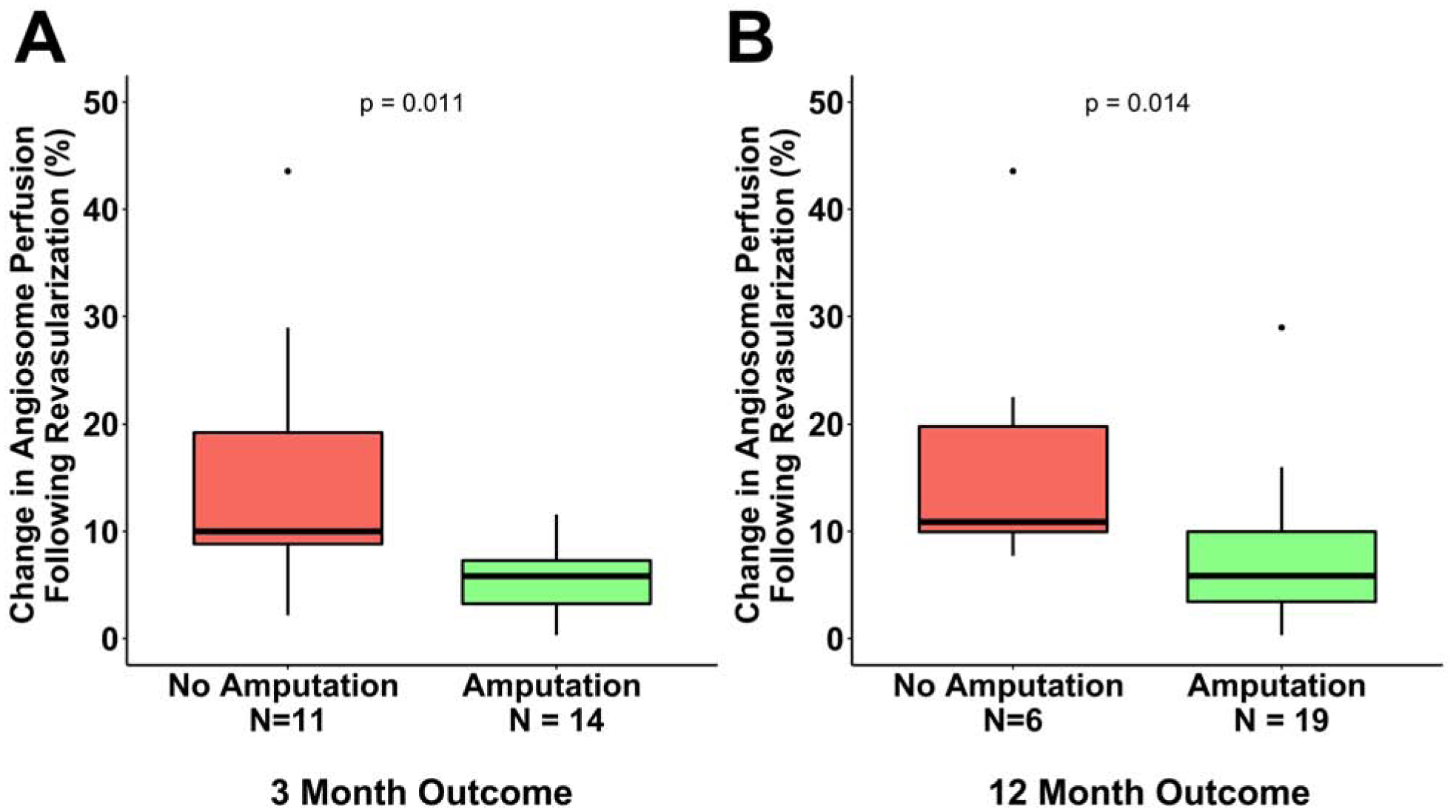
SPECT/CT perfusion imaging detected significantly lower percent change in angiosome perfusion for patients who received an amputation in the initial (A) 3 and (B) 12 months after revascularization.
ROC analysis based on the occurrence of amputations or death was used to examine potential cutoff points for the change in angiosome perfusion that could predict lower extremity amputation at 3 and 12 months after revascularization. The cutoff percent change in angiosome perfusion for predicting amputation at 3 months was 7.55%, and the area under the curve (AUC) based on the amputation outcome at 3 months was 0.799 (95% CI: 0.613–0.985) (Figure 2). The CCR based on this cutoff was 80% (95% CI: 64.3–95.7%), while sensitivity and specificity were 78.6% (95% CI: 57.1–100%) and 81.8% (95% CI: 59.0–100%), respectively. The cutoff for percent change in angiosome perfusion for predicting amputation-free survival at 12 months was 11.56%, and the AUC based on amputation outcome at 12 months was 0.833 (95% CI: 0.670–0.996) (Figure 2). The CCR based on this cutoff was also 80% (95% CI: 64.3–95.7%), while sensitivity and specificity were 89.5% (95% CI: 75.7–100%) and 50% (95% CI: 10.0–90.0%), respectively. Patients with percent change in angiosome perfusion below the selected cutoffs were classified as low responders and those with values greater than the cutoffs were classified as high responders. 12 (48%) patients were classified as high responders using the 3-month cutoff and 9 patients (36%) were classified as high responders using the 12-month cutoff. The probability of amputation-free survival was significantly higher at 3 (p = 0.002) (Figure 3A) and 12 months (p = 0.03) (Figure 3B) for patients who were high perfusion responders than in patients who were low perfusion responders to revascularization, confirming that the cutoffs were good indicators of both short- and long-term clinical outcomes.
Figure 2. Receiver-operating characteristic curve analysis for determining the cutoff for percent change in angiosome perfusion value based on the occurrence of lower extremity amputation or death at 3 and 12 months after revascularization.
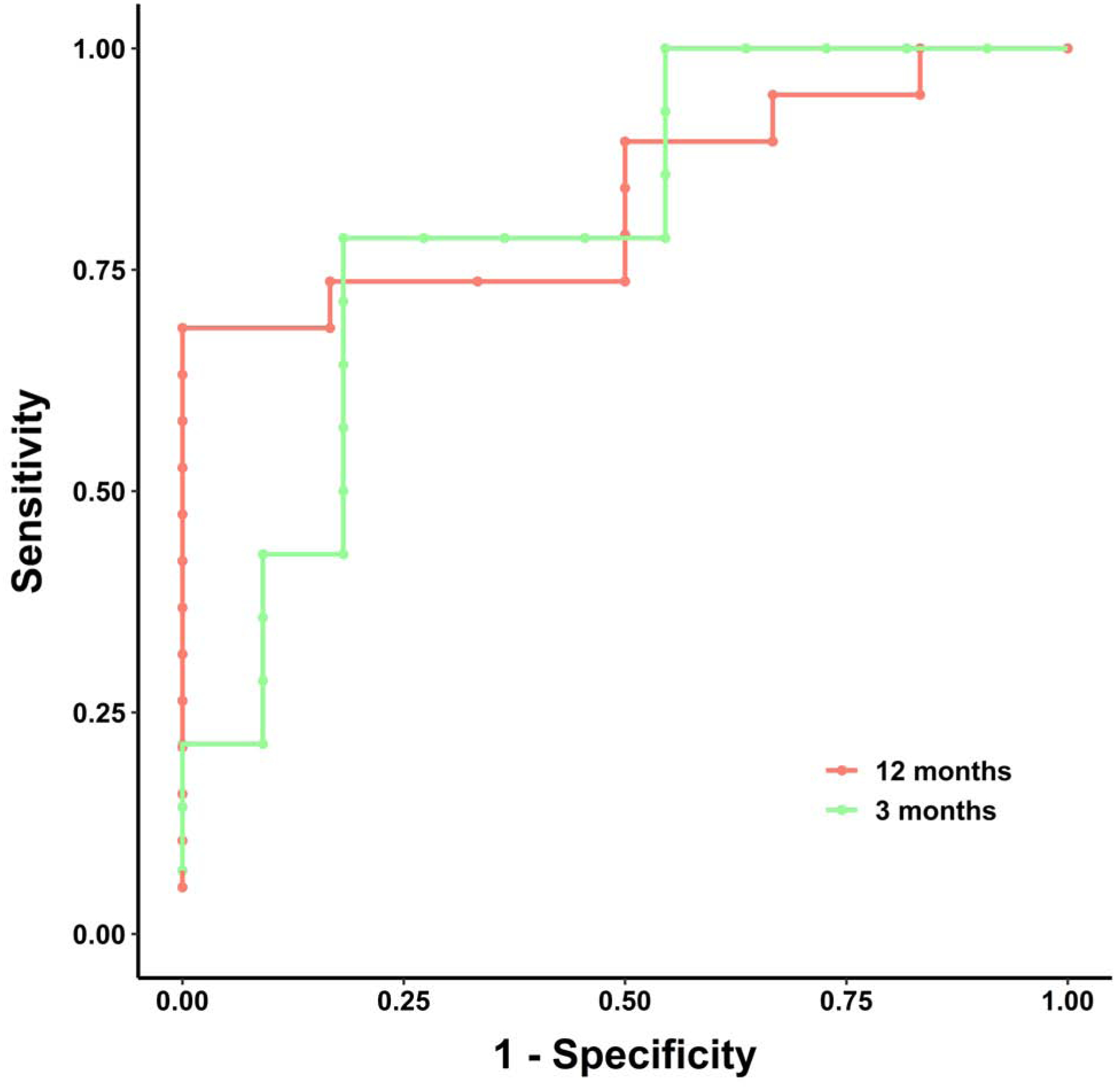
(A) At 3 months after revascularization, the cutoff value for percent change in perfusion that predicted amputation-free survival was 7.5% and the area under the ROC was 0.799. (B) At 12 months after revascularization, the cutoff value for percent change in perfusion was 9.94%, and the area under the ROC curve was 0.833.
Figure 3. Kaplan-Meier plots for amputation-free survival based on revascularization-induced change in angiosome perfusion.
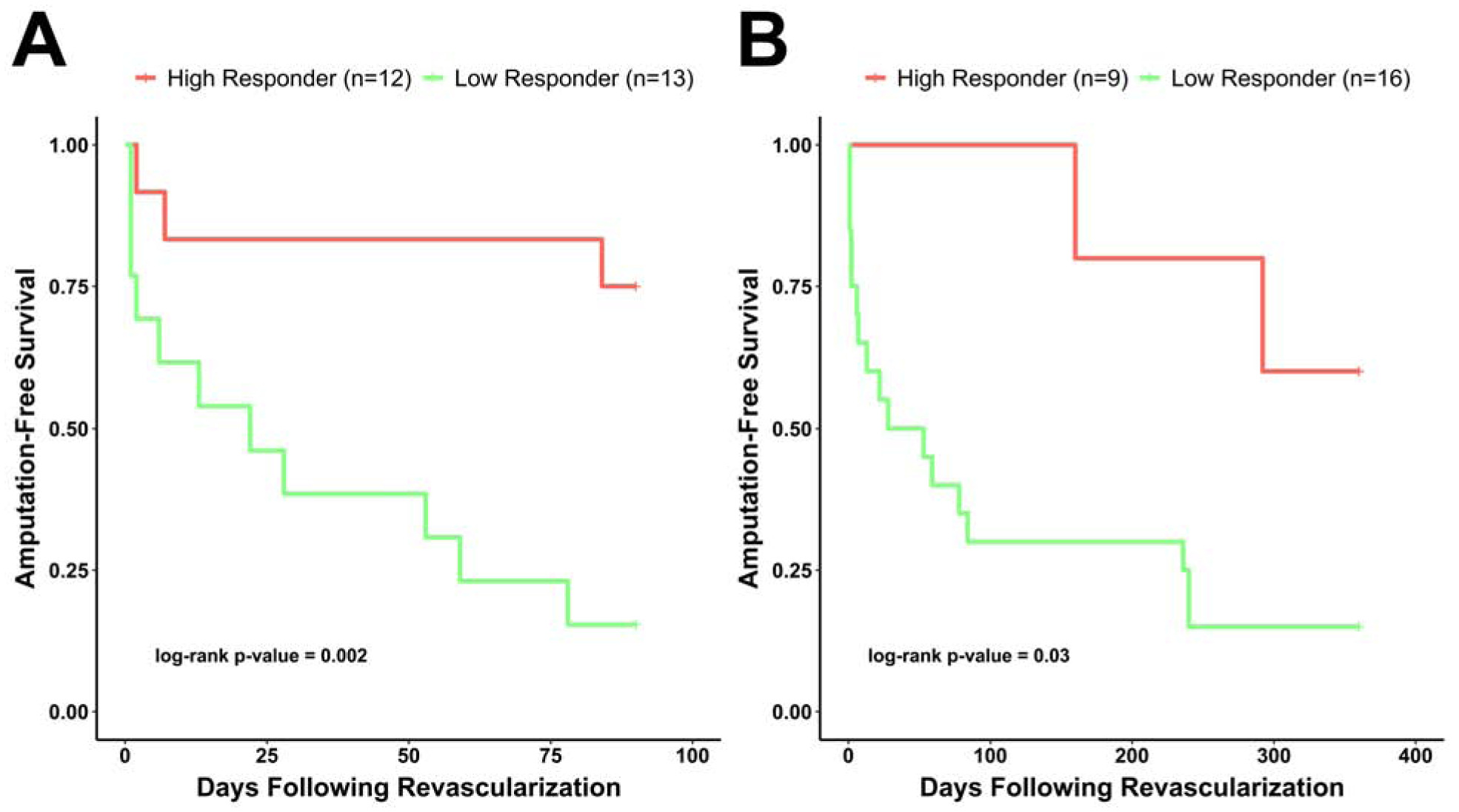
Amputation-free survival in the high perfusion responder patient group was significantly higher than in the low perfusion responder group at (A) 3 months (log-rank test, p = 0.002) and (B) 12 months (p = 0.03) after lower extremity revascularization.
Univariate Predictors of Lower Extremity Amputation
Univariate Cox proportional hazards regression analyses revealed that the change in angiosome perfusion was associated with amputation-free survival at 12 months after revascularization (p=0.03; Figure 4). Specifically, the risk of an amputation event was 62% lower for a 1 standard deviation increase in perfusion of 9.5% for the 12-month outcome time point. In addition to percent change in perfusion being associated with amputation-free survival, hemoglobin (p = 0.02) and hematocrit (p = 0.047) were significant predictors of amputation-free survival at 12 months after revascularization (Figure 4).
Figure 4. Forest plot of hazard ratios (HR) for lower extremity amputation in patients with CLI during the first 12 months after endovascular revascularization.
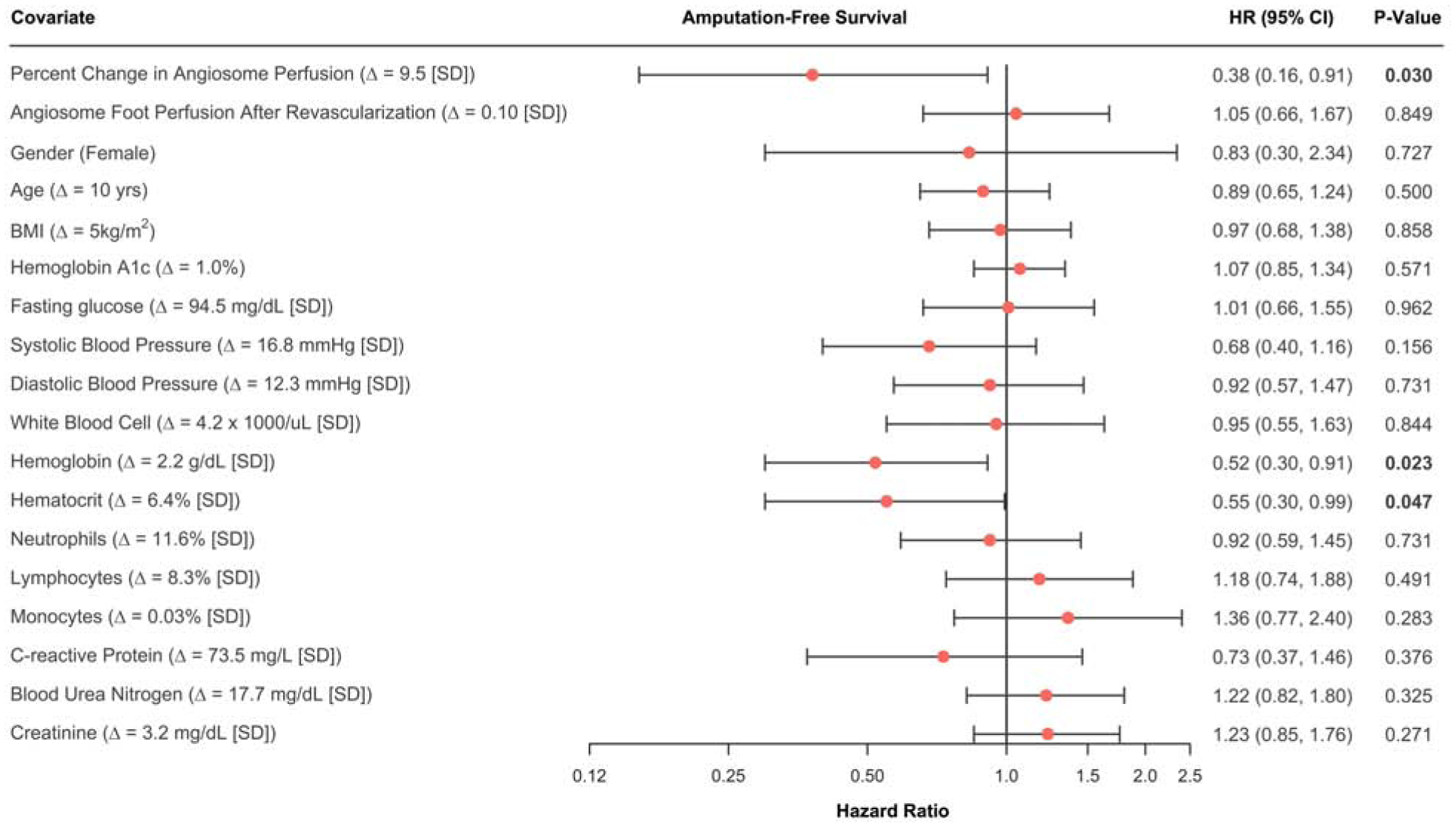
HR calculations are based on univariate Cox proportional hazards regression models.
Test-Retest Reliability of SPECT/CT Perfusion Measures
Evaluation of the test-retest reliability of SPECT/CT-derived foot perfusion measures in the contralateral untreated foot revealed the mean difference between pre- and post-revascularization perfusion measures was 0.0007 with 95% agreement limits of −0.020 to 0.021 and an intraclass correlation coefficient of 0.98 (95% CI: 0.6, 0.99) (Figure 5). Thus, assessment of the contralateral untreated limb suggested that serial measures of foot perfusion derived from SPECT/CT images possessed a strong level of agreement. Six patients were excluded from analysis due to pre-existing amputations of the contralateral limb, or procedures being performed on the contralateral limb between the pre- and post-revascularization study visits.
Figure 5. Assessment of the test-retest reliability of SPECT/CT perfusion measurements.
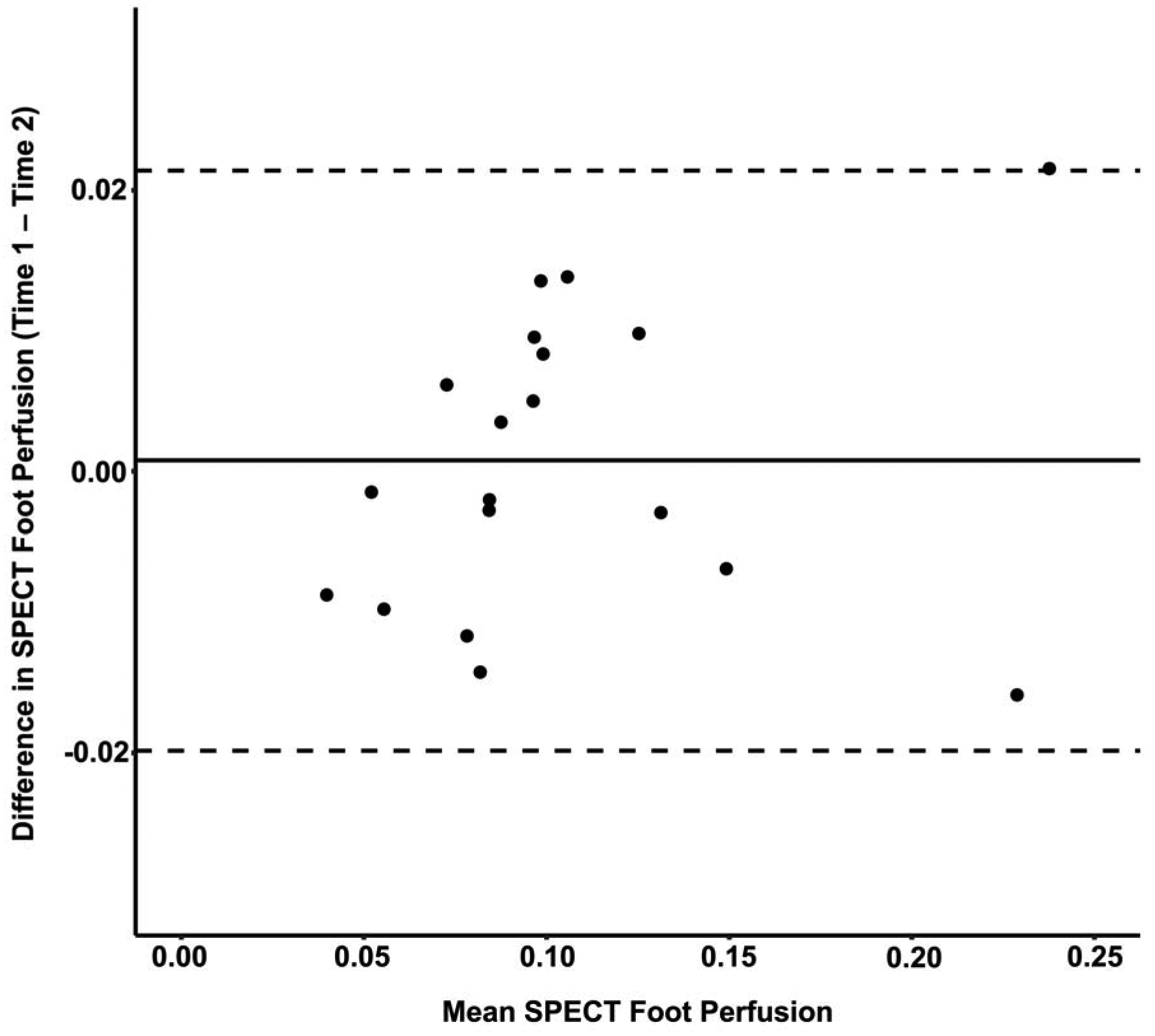
Bland-Altman plot revealed a mean difference of 0.0007 with 95% agreement limits of −0.020 to 0.021 for perfusion measures acquired in the contralateral untreated foot before and after revascularization, which demonstrated excellent agreement level between serial SPECT/CT imaging perfusion measurements. n=19 patients.
DISCUSSION
Previous studies have utilized a variety of radiotracer-based techniques to assess limb perfusion, which have included measuring the first-pass clearance of inert gases (e.g. xenon-133) (22,23), assessing retention of macroaggregated albumin (or microspheres) after intra-arterial delivery (24), and gamma imaging the retention of intravenously administered perfusion radiotracers (i.e. thallium-201 and 99mTc-labeled) (25–32). Perfusion imaging studies have specifically used 2D scintigraphy to detect calf muscle perfusion abnormalities in PAD patients (25–28) and asymptomatic DM patients (29–32), and to evaluate cell-based therapies in CLI patients with non-healing foot ulcers (33,34). More recent research has utilized hybrid 3D SPECT/CT imaging to evaluate resting microvascular perfusion within specific lower extremity muscle groups in a porcine model of PAD (35) as well as angiosomes of the feet in CLI patients (16,17). The present study expanded on prior investigations by demonstrating for the first time the use of serial SPECT/CT imaging for quantifying revascularization-induced changes in angiosome foot perfusion in a population of patients with DM and CLI. We revealed that the variability in patient-specific perfusion responses to revascularization could be non-invasively detected and quantitatively assessed by SPECT/CT imaging. Additionally, we demonstrated with test-retest analysis of the contralateral untreated foot that SPECT/CT perfusion measures were repeatable. Most importantly, however, we showed that microvascular perfusion responses in the foot were significantly higher in CLI patients who did not undergo amputation when compared to patients who did undergo amputation in the initial 3 and 12 months after endovascular revascularization.
In addition to the ability to non-invasively assess angiosome perfusion responses to revascularization, the present study demonstrated that radiotracer-based perfusion imaging possessed sensitivity of 78.6% and specificity of 81.8% for predicting lower extremity amputations in the first 3 months after endovascular revascularization. In the evaluation of 12-month lower extremity amputation outcomes, radiotracer imaging possessed sensitivity of 89.5% and specificity of 50%. Cutoff values for percent change in angiosome perfusion that predicted amputation were determined from ROC analysis. The revascularization-induced percent change in angiosome microvascular perfusion needed to be higher for a patient to achieve limb salvage in the first 12 months (i.e. 11.56%) versus the first 3 months (i.e. 7.55%), thus suggesting a longer-term protective effect associated with more robust treatment-induced perfusion responses. The same cutoff values were selected to classify patients into high and low perfusion responder categories, which revealed that the probability of amputation-free survival was significantly higher at 3 and 12 months for the CLI patients who were high perfusion responders, thereby indicating our selected cutoffs were good indicators of short- and long-term amputation outcomes.
Cox proportional hazards regression analyses demonstrated that SPECT/CT image-derived changes in angiosome perfusion were associated with amputation-free survival in the 12 months after endovascular revascularization, with amputation risk dropping by 62% for every 1 standard deviation (i.e. 9.5%) increase in perfusion. While relative changes in angiosome perfusion were associated with amputation-free survival, the perfusion value obtained after revascularization was not significantly associated with future amputations, thus suggesting that the degree of perfusion change in response to revascularization served as a better predictor of amputation-free survival than the final post-treatment perfusion value. This finding may be due to the patients with DM and CLI in the present study possessing underlying microvascular disease and low levels of baseline microvascular perfusion that inherently limited the threshold for perfusion recovery. Therefore, the robustness of the perfusion response to revascularization likely served as an indicator of lower extremity microcirculation health and hence represented the best metric for predicting short- and long-term incidence of amputation. Along with revascularization-induced changes in angiosome perfusion, hemoglobin and hematocrit were also found to be significant predictors of amputation-free survival in the first 12 months. Thus, higher levels of hemoglobin and hematocrit, which are both related to oxygen transport/delivery and likely connected with perfusion, showed protective effects that were associated with improved amputation-free survival.
Study Limitations
Although the current study demonstrated the utility of serial SPECT/CT perfusion imaging for quantifying relative changes in angiosome microvascular perfusion and predicting short- and long-term amputation outcomes, the number of enrolled patients was relatively small. Thus, the cutoff values for perfusion were both generated and tested within our dataset. We caution that the most rigorous comparison is to assess the cutoff values in independent derivation and validation cohorts. Therefore, studies with larger sample sizes and a wider spectrum of PAD patients are warranted to validate the prognostic value of SPECT/CT perfusion imaging in the setting of limb ischemia. Additionally, while changes in angiosome foot perfusion were associated with lower extremity amputation outcomes in the present study, the decision to amputate is often multifactorial in nature and can be due to reasons aside from perfusion status alone. Therefore, future large-scale trials should consider the role that various pre-existing conditions may play in the decision to amputate. Hemodynamic data (ankle-brachial and toe-brachial indices) and wound healing outcomes also were not always obtainable or documented in the patients enrolled into this trial. Future comprehensive evaluation of wounds is warranted to evaluate the prognostic value of SPECT/CT for predicting wound progression or healing capacity in patients with CLI and DM. Lastly, while SPECT/CT perfusion imaging is more widely available and cost effective, the conventional SPECT/CT system and imaging approach utilized for the present study was limited to assessment of relative changes in perfusion and quantification of absolute perfusion values was not possible. Future investigations utilizing more quantitative PET/CT imaging techniques to evaluate absolute measures of lower extremity perfusion should allow for improved quantitative assessment of lower extremity physiology under baseline conditions as well as following therapeutic interventions.
Conclusions
In summary, the present study demonstrates that 99mTc-tetrofosmin SPECT/CT imaging can evaluate regional changes in microvascular foot perfusion in response to endovascular revascularization procedures in patients with CLI and DM. Additionally, our findings indicate that early changes in angiosome perfusion detected by SPECT/CT imaging may assist in predicting short- and long-term amputation-free survival in the setting of CLI.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
CLI is the severe manifestation of PAD that is associated with high mortality and risk for lower extremity amputation. Quantification of microvascular perfusion in the lower extremities using radiotracer-based imaging techniques show potential for assessing regional perfusion responses to endovascular revascularization in the feet of patients with CLI and DM. The present study suggests our perfusion imaging approach may predict short- and long-term risk for lower extremity amputation in CLI patients.
TRANSLATIONAL OUTLOOK:
Future research should focus on larger-scale application of radiotracer-based perfusion imaging across the disease spectrum to elucidate the prognostic value of radiotracer imaging techniques. Additionally, improved methods for absolute quantitation of muscle perfusion in the setting of limb ischemia could lead to improved risk stratification in the PAD, DM, and CLI patient populations.
Funding:
National Institutes of Health R01 HL135103 (M.R. Stacy)
ABBREVIATIONS LIST:
- AUC
area under the curve
- CCR
correct classification rate
- CEUS
contrast-enhanced ultrasound
- CLI
critical limb ischemia
- CT
computed tomography
- DM
diabetes mellitus
- HbA1c
glycated hemoglobin
- IQR
interquartile range
- PAD
peripheral arterial disease
- ROC
receiver operator curve
- SPECT
single photon emission computed tomography
- 99mTc
technetium-99m
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to declare.
Clinical Trial: clinicaltrials.gov NCT03622359
REFERENCES
- 1.Fowkes FG, Rudan D, Rudan I et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group M, Mozaffarian D, Benjamin EJ et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 3.Teraa M, Conte MS, Moll FL, Verhaar MC. Critical Limb Ischemia: Current Trends and Future Directions. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farber A, Eberhardt RT. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA Surg 2016;151:1070–1077. [DOI] [PubMed] [Google Scholar]
- 5.Misra S, Shishehbor MH, Takahashi EA et al. Perfusion Assessment in Critical Limb Ischemia: Principles for Understanding and the Development of Evidence and Evaluation of Devices: A Scientific Statement From the American Heart Association. Circulation 2019;140:e657–e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowling FL, Rashid ST, Boulton AJ. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol 2015;11:606–16. [DOI] [PubMed] [Google Scholar]
- 7.AD G, W J, RJ M. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J 2007;4:286–287. [DOI] [PubMed] [Google Scholar]
- 8.Howard DP, Banerjee A, Fairhead JF et al. Population-Based Study of Incidence, Risk Factors, Outcome, and Prognosis of Ischemic Peripheral Arterial Events: Implications for Prevention. Circulation 2015;132:1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckman JA, Duncan MS, Damrauer SM et al. Microvascular Disease, Peripheral Artery Disease, and Amputation. Circulation 2019;140:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao P, Eckstein HH, De Rango P et al. Chapter II: Diagnostic methods. Eur J Vasc Endovasc Surg 2011;42 Suppl 2:S13–32. [DOI] [PubMed] [Google Scholar]
- 11.Amarteifio E, Krix M, Wormsbecher S et al. Dynamic contrast-enhanced ultrasound for assessment of therapy effects on skeletal muscle microcirculation in peripheral arterial disease: pilot study. Eur J Radiol 2013;82:640–6. [DOI] [PubMed] [Google Scholar]
- 12.Lindner JR, Womack L, Barrett EJ et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging 2008;1:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollak AW, Meyer CH, Epstein FH et al. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging 2012;5:1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grozinger G, Pohmann R, Schick F et al. Perfusion measurements of the calf in patients with peripheral arterial occlusive disease before and after percutaneous transluminal angioplasty using MR arterial spin labeling. J Magn Reson Imaging 2014;40:980–7. [DOI] [PubMed] [Google Scholar]
- 15.Wang DJ, Chen Y, Fernandez-Seara MA, Detre JA. Potentials and challenges for arterial spin labeling in pharmacological magnetic resonance imaging. J Pharmacol Exp Ther 2011;337:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou TH, Atway SA, Bobbey AJ, Sarac TP, Go MR, Stacy MR. SPECT/CT Imaging: A Noninvasive Approach for Evaluating Serial Changes in Angiosome Foot Perfusion in Critical Limb Ischemia. Adv Wound Care 2020;9:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvelo JL, Papademetris X, Mena-Hurtado C et al. Radiotracer Imaging Allows for Noninvasive Detection and Quantification of Abnormalities in Angiosome Foot Perfusion in Diabetic Patients With Critical Limb Ischemia and Nonhealing Wounds. Circ Cardiovasc Imaging 2018;11:e006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lejay A, Georg Y, Tartaglia E et al. Long-term outcomes of direct and indirect below-the-knee open revascularization based on the angiosome concept in diabetic patients with critical limb ischemia. Ann Vasc Surg 2014;28:983–9. [DOI] [PubMed] [Google Scholar]
- 19.Huang TY, Huang TS, Wang YC, Huang PF, Yu HC, Yeh CH. Direct Revascularization With the Angiosome Concept for Lower Limb Ischemia: A Systematic Review and Meta-Analysis. Medicine 2015;94:e1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iida O, Soga Y, Hirano K et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg 2012;55:363–370.e5. [DOI] [PubMed] [Google Scholar]
- 21.Stacy MR, Qiu M, Papademetris X, Caracciolo CM, Constable RT, Sinusas AJ. Application of BOLD Magnetic Resonance Imaging for Evaluating Regional Volumetric Foot Tissue Oxygenation: A Feasibility Study in Healthy Volunteers. Eur J Vasc Endovasc Surg 2016;51:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassen NA. Muscle Blood Flow in Normal Man and in Patients with Intermittent Claudication Evaluated by Simultaneous Xe-133 and Na24 Clearances. J Clin Invest 1964;43:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassen NA, Lindbjerg J, Munck O. Measurement of Blood-Flow through Skeletal Muscle by Intramuscular Injection of Xenon-133. Lancet 1964;1:686–9. [DOI] [PubMed] [Google Scholar]
- 24.Jones EL, Wagner HN Jr., Zuidema GD. New method for studying peripheral circulation in man. Arch Surg 1965;91:725–34. [DOI] [PubMed] [Google Scholar]
- 25.Seder JS, Botvinick EH, Rahimtoola SH, Goldstone J, Price DC. Detecting and localizing peripheral arterial disease: assessment of 201Tl scintigraphy. AJR Am J Roentgenol 1981;137:373–80. [DOI] [PubMed] [Google Scholar]
- 26.Siegel ME, Stewart CA. Thallium-201 peripheral perfusion scans: feasibility of single-dose, single-day, rest and stress study. Am J Roentgenol 1981;136:1179–83. [DOI] [PubMed] [Google Scholar]
- 27.Sayman HB, Urgancioglu I. Muscle perfusion with technetium-MIBI in lower extremity peripheral arterial diseases. J Nucl Med 1991;32:1700–3. [PubMed] [Google Scholar]
- 28.Miles KA, Barber RW, Wraight EP, Cooper M, Appleton DS. Leg muscle scintigraphy with 99Tcm-MIBI in the assessment of peripheral vascular (arterial) disease. Nucl Med Commun 1992;13:593–603. [PubMed] [Google Scholar]
- 29.Duet M, Virally M, Bailliart O et al. Whole-body (201)Tl scintigraphy can detect exercise lower limb perfusion abnormalities in asymptomatic diabetic patients with normal Doppler pressure indices. Nucl Med Commun 2001;22:949–54. [DOI] [PubMed] [Google Scholar]
- 30.Cosson E, Paycha F, Tellier P et al. Lower-limb vascularization in diabetic patients. Assessment by thallium-201 scanning coupled with exercise myocardial scintigraphy. Diabetes Care 2001;24:870–4. [DOI] [PubMed] [Google Scholar]
- 31.Celen YZ, Zincirkeser S, Akdemir I, Yilmaz M. Investigation of perfusion reserve using 99Tc(m)-MIBI in the lower limbs of diabetic patients. Nucl Med Commun 2000;21:817–22. [DOI] [PubMed] [Google Scholar]
- 32.Kusmierek J, Dabrowski J, Bienkiewicz M, Szuminski R, Plachcinska A. Radionuclide assessment of lower limb perfusion using 99mTc-MIBI in early stages of atherosclerosis. Nucl Med Rev Cent East Eur 2006;9:18–23. [PubMed] [Google Scholar]
- 33.Miyamoto M, Yasutake M, Takano H et al. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transplant 2004;13:429–37. [DOI] [PubMed] [Google Scholar]
- 34.Takagi G, Miyamoto M, Fukushima Y et al. Imaging Angiogenesis Using 99mTc-Macroaggregated Albumin Scintigraphy in Patients with Peripheral Artery Disease. J Nucl Med 2016;57:192–7. [DOI] [PubMed] [Google Scholar]
- 35.Stacy MR, Yu DY, Maxfield MW et al. Multimodality imaging approach for serial assessment of regional changes in lower extremity arteriogenesis and tissue perfusion in a porcine model of peripheral arterial disease. Circ Cardiovasc Imaging 2014;7:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


