Abstract
OBJECTIVES
This study aimed to characterize trends in technetium Tc 99m pyrophosphate (99mTc-PYP) scanning for amyloid transthyretin cardiac amyloidosis (ATTR-CA) diagnosis, to determine whether patients underwent appropriate assessment with monoclonal protein and genetic testing, to evaluate use of single-photon emission computed tomography (SPECT) in addition to planar imaging, and to identify predictive factors for ATTR-CA.
BACKGROUND
99mTc-PYP scintigraphy has been repurposed for noninvasive diagnosis of ATTR-CA. Increasing use of 99mTc-PYP can facilitate identification of ATTR-CA, but appropriate use is critical for accurate diagnosis in an era of high-cost targeted therapeutics.
METHODS
Patients undergoing 99mTc-PYP scanning 1 h after injection at a quaternary care center from 2010 to 2019 were analyzed; clinical information was abstracted; and SPECT results were analyzed.
RESULTS
Over the decade, endomyocardial biopsy rates remained stable with scanning rates peaking at 132 in 2019 (p < 0.001). Among 753 patients (516 men, mean age 77 years), 307 (41%) had a visual score of 0, 177 (23%) of 1, and 269 (36%) of 2 or 3. Of 751 patients with analyzable heart to contralateral chest ratios, 249 (33%) had a ratio ≥1.5. Monoclonal protein testing status was assessed in 550 patients, of these, 174 (32%) did not undergo both serum immunofixation and serum free light chain analysis tests, and 331 (60%) did not undergo all 3 tests—serum immunofixation, serum free light chain analysis, and urine protein electrophoresis. Of 196 patients with confirmed ATTR-CA, 143 (73%) had genetic testing for transthyretin mutations. In 103 patients undergoing cardiac biopsy, grades 2 and 3 99mTc-PYP had sensitivity of 94% and specificity of 89% for ATTR-CA with 100% specificity for grade 3 scans. With respect to SPECT as a reference standard, planar imaging had false positive results in 16 of 25 (64%) grade 2 scans.
CONCLUSIONS
Use of noninvasive testing with 99mTc-PYP scanning for evaluation of ATTR-CA is increasing, and the inclusion of monoclonal protein testing and SPECT imaging is crucial to rule out amyloid light chain amyloidosis and distinguish myocardial retention from blood pooling.
Keywords: cardiac amyloidosis, cardiac scintigraphy, heart failure with preserved ejection fraction, transthyretin amyloidosis
Systemic amyloidosis is a disorder that results from deposits of amyloid fibrils in various organs, including the heart, vasculature, nervous system, gastrointestinal tract, kidneys, skin, and lungs. The type of amyloid fibrils determines the pathophysiology, clinical presentation, genetics, prognosis, and treatment of the disorder and includes amyloid transthyretin (ATTR), amyloid light chain (AL), and inflammatory amyloidosis. Both initial symptoms and cardiac imaging findings tend to be nonspecific, and the disorder is not commonly recognized until it has reached an advanced stage. Historically, the treatment of amyloid transthyretin cardiac amyloidosis (ATTR-CA) was limited to supportive care and heart and liver transplantation in highly selected patients. In 2019, tafamidis became the first ATTR-CA stabilizer to be approved by the U.S. Food and Drug Administration after the drug was shown to reduce mortality in patients with the disease (1). Further investigations are underway studying alternative TTR stabilizers, TTR silencers, and amyloid fibril degraders (2). This revolution in treatment has spurred efforts to recognize the diagnosis prior to the onset of significant organ dysfunction particularly because the TTR therapies approved to date prevent or slow amyloidogenesis but do not address the existing disease burden.
Although previously only diagnosed by biopsy, there has been a shift within the last decade toward noninvasive diagnosis using bone-seeking radiopharmaceuticals such as technetium Tc 99m pyrophosphate (99mTc-PYP), 99mTc-labeled 3,3-diphos-phono-1,2-propanodicarboxylic acid, or 99mTchydroxymethylene diphosphonate, which are specific for ATTR-CA (3,4). These agents allow for an accurate imaging diagnosis of ATTR-CA so long as AL amyloidosis has been ruled out by testing serum free light chains, serum protein electrophoresis (SPEP) with immunofixation, and urine protein electrophoresis with immunofixation. Based on consensus guidelines, a clinical diagnosis of ATTR-CA can be made in the presence of typical echocardiographic or cardiac magnetic resonance features by positive cardiac scintigraphy with negative monoclonal protein testing (5,6). However, cardiac scintigraphy by planar scanning alone can be subject to false positive results due to blood pooling or overlying bone artifact, and recent multisocietal guidelines have recommended tomographic imaging with single-photon emission computed tomography (SPECT) to confirm myocardial uptake (5,6). However, there is currently a paucity of primary data to support this recommendation.
In this study, we aimed to accomplish the following: 1) study the evolution of how noninvasive testing pathways are being used for the diagnosis of ATTR-CA; 2) review rates of monoclonal protein and genetic testing; and 3) evaluate the role of SPECT imaging to enhance diagnostic accuracy over planar image analysis alone.
METHODS
PATIENTS.
All unique patients who underwent 99mTc-PYP nuclear imaging for possible ATTR-CA at Columbia University Irving Medical Center from December 22, 2010, to October 3, 2019, were included in this study. Although most patients were scanned for clinical purposes, a subset of the patients (n = 175) was scanned from 2014 to 2016 as part of the UNVEIL (Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement) study, which systematically tested patients with severe aortic stenosis who were undergoing transcatheter valve replacement for ATTR-CA (7). Clinical information was abstracted from the medical record.
This study was conducted with approval from the Columbia University Irving Medical Center Institutional Review Board.
99mTc-PYP NUCLEAR IMAGING.
Patients underwent a standardized imaging protocol with 99mTc-PYP imaged at 1 h after injection (8). Scans were graded by experienced, board-certified nuclear cardiologists using semiquantitative and quantitative methods. Planar scans were analyzed using the semiquantitative visual score. The visual score was graded 0 to 3 using both anterior and lateral planar images, with 0 as no myocardial uptake, 1 as myocardial uptake less than ribs, 2 as myocardial uptake equal to ribs, and 3 as myocardial update greater than ribs with mild or absent rib uptake. The quantitative heart to contralateral chest ratio (H:CL) was calculated for each scan by calculating counts in equal-sized regions of interest over the heart and contralateral chest, with either a visual score of 2 or 3 or an H:CL of >1.5 being suggestive of ATTR-CA. The region of interest over the contralateral chest was drawn to avoid the right ventricle or diaphragm. During the decade studied in this protocol, 1-h scanning protocols using only planar imaging were routinely used for diagnosis. In an estimated <10% of the scans, additional scans were conducted at 2 or 3 h to assess for blood pool washout. The final visual score described in this paper is a synthesis of all available information by the reader, including 1-h scans, any additional 2- or 3-h scans, and SPECT imaging. With the increasing recognition that planar scans can be subject to false positive results particularly with 1-h imaging, SPECT scanning was systematically added to the scanning protocol for positive planar scans starting in 2017. SPECT scanning was performed and reconstructed by experienced technologists to create short-axis, vertical long-axis, and horizontal short-axis images using commercially available software (QPS; Cedars-Sinai, Los Angeles, California). In patients undergoing SPECT, computed tomography for attenuation correction was used as needed to ensure image quality such as in obese patients or in those who were unable to hold their arms up. In addition to the primary clinical read, which is included in the main analysis, the SPECT images were reread by 1 of the authors (A.D.) in a subset of 257 patients in the cohort. During this reanalysis, the 1-h planar images were reread and a visual score was rendered. After reviewing the 1-h planar images, the SPECT images were interpreted and the presence or absence of myocardial uptake and blood pooling was documented. This 257 patient cohort included 226 SPECTs performed from 2017 to 2019 and 31 SPECTs performed prior to 2017.
PATIENT DIAGNOSIS DEFINITIONS.
After chart and 99mTc-PYP scan review, patients were classified into 4 mutually exclusive diagnosis categories: ATTR-CA, light chain cardiac amyloidosis (AL-CA), no CA, or indeterminate, using consensus guidelines (5,6). According to these guidelines, patients were diagnosed with ATTR-CA by either histology with positive cardiac biopsy for ATTR-CA or by imaging criteria that included all of the following criteria: 1) echocardiography or cardiac magnetic resonance findings with increased left ventricular wall thickness consistent with ATTR-CA; 2) grade 2 or 3 cardiac scintigraphy with 99mTc-PYP; and 3) negative monoclonal protein testing with negative serum protein electrophoresis and serum free light chains. Due to concerns that some positive scans may be due to blood pooling and in concordance with new guidelines recommending SPECT confirmation, we required that patients with grade 2 planar scans have myocardial uptake confirmed on SPECT to be classified as ATTR-CA. Patients were considered to have AL-CA if they had an endomyocardial biopsy confirming light chain amyloidosis or an extracardiac biopsy showing systemic light chain amyloidosis with typical cardiac imaging findings. Patients were considered to have no CA if they met the following criteria: negative cardiac biopsy for amyloidosis; grade 0 or 1 99mTc-PYP planar scan; or a grade 2 planar scan with SPECT showing neither blood pooling nor myocardial uptake. All other patients, such as those that did not undergo clinically indicated monoclonal protein testing or patients with grade 2 planar scans where SPECT was not done or showed blood pooling, were categorized as having indeterminate results.
STATISTICAL ANALYSIS.
Descriptive statistics including mean, median, and frequencies were determined for select variables. The Shapiro-Wilk test was used to assess normality of key variables, and where applicable, nonparametric testing was conducted. Categorical variables were assessed using chi-square analysis. No tests had 2 × 2 table cells with expected counts of <5 requiring use of Fisher exact test. Continuous variable means were compared using Student’s t-tests. Trends in testing rate over time were determined using Poisson regression in patients undergoing scans for clinical purposes with exclusion of the 175 patients that were included in the UNVEIL study (7). Other statistical testing was performed in the entire cohort of 753 patients. 99mTc-PYP scan test characteristics including sensitivity and specificity were calculated using 2 × 2 tables against the reference standard of cardiac biopsy. Where assumptions of normality were met, mean, standard deviation, and 95% confidence intervals are described. Nonparametric results are described with median and interquartile ranges. All statistical tests were performed in Python 3.4 (Python Software Foundation, Wilmington, Delaware) and SPSS version 26 (IBM Corp., Armonk, New York).
RESULTS
PATIENT CHARACTERISTICS AND NONINVASIVE TESTING RESULTS.
A total of 753 unique patients underwent 99mTc-PYP scanning over the decade from 2010 to 2019 and were included for analysis. Clinical and demographic characteristics are shown in Table 1, highlighting that the majority were men (n = 513, 68%) with a mean age of 76.7 years. The racial and ethnic background of the patients was non-Hispanic White (n = 537, 71%), Black (n = 106, 14%), Hispanic (n = 49, 6%), and other or unknown (n = 61, 8%). There was no significant difference between patients of White, Black, and Hispanic ethnicity in the rate of ATTR-CA diagnosis.
TABLE 1.
Patient Characteristics
| Total Patients (N = 753) |
Patients Without Cardiac Biopsy (n = 649) |
Patients With Cardiac Biopsy (n = 104) |
|||
|---|---|---|---|---|---|
| Total | ATTR-CA* (n = 127) | No Amyloidosis (n = 434) | ATTR-CA (n = 69) | No Amyloidosis (n = 22) | |
| Age, yrs | 76.7 ± 10.9 | 79.4 ± 8.4 | 76.8 ± 11.4 | 72.9 ± 8.5 | 69.9 ± 8.3 |
| Male | 513 (68) | 96 (76) | 264 (61) | 59 (86) | 17 (77) |
| Racial/ethnic background | |||||
| White | 537 (71) | 86 (68) | 321 (74) | 48 (70) | 15 (68) |
| Black | 106 (14) | 22 (17) | 58 (13) | 10 (14) | 5 (23) |
| Hispanic | 49 (7) | 5 (4) | 27 (6) | 5 (7) | 1 (5) |
| Other/unknown | 61 (8) | 15 (12) | 28 (6) | 6 (9) | 1 (5) |
| Echocardiography | |||||
| LV ejection fraction, % (n = 693) | 55 (38–60) | 47 (35–56) | 55 (43–63) | 46 (31–57) | 50 (30–60) |
| IVS, mm (n = 736) | 13 (11–15) | 15 (13–18) | 12 (10–13) | 16 (15–19) | 11 (10–14) |
| LVPWT, mm (n = 735) | 12 (10–14) | 15 (13–16) | 11 (9–12) | 16 (14–18) | 10 (9–13) |
| Left atrial volume index, ml/m2 (n = 556) | 50 (40–63) | 51 (43–62) | 50 (39–65) | 46 (41–52) | 51 (46–78) |
| PYP visual score | |||||
| Grade 0 | 307 (41) | 2* (1) | 278 (64) | 0 (0) | 12 (55) |
| Grade 1 | 177 (24) | 1* (1) | 153 (35) | 4 (6) | 7 (32) |
| Grade 2 | 37 (5) | 5 (3) | 3 (1) | 2 (3) | 3 (14) |
| Grade 3 | 232 (31) | 119 (94) | 0 (0) | 63 (91) | 0 (0) |
| PYP H:CL (n = 751) | |||||
| <1.5 | 502 (67) | 5 (4) | 430 (100) | 3 (4) | 21 (95) |
| ≥1.5 | 249 (33) | 122 (96) | 2 (0) | 66 (96) | 1 (5) |
Values are mean ± SD, n (%), or median (interquartile range). Patient characteristics in the total cohort, patients diagnosed or negative for ATTR-CA by imaging, and patients diagnosed or negative for ATTR-CA by cardiac biopsy are shown.
Three patients with grade 0 or 1 scans met criteria for CA on the basis of typical echocardiographic features, Phe64Leu mutation, and extracardiac biopsies showing ATTR.
ATTR-CA = amyloid transthyretin cardiac amyloidosis; CA = cardiac amyloidosis; H:CL = heart to contralateral chest ratio; IVS = interventricular septal thickness on echo; PYP = technetium Tc 99m pyrophosphate; LV = left ventricular; LVPWT = left ventricular posterior wall thickness on echo.
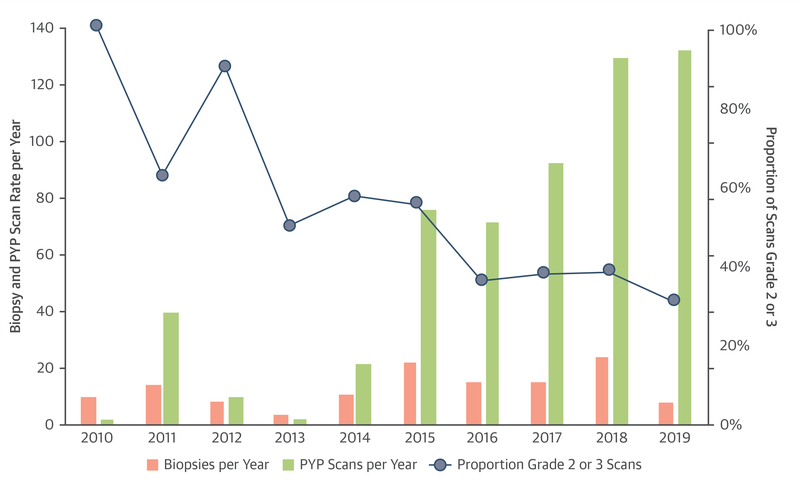
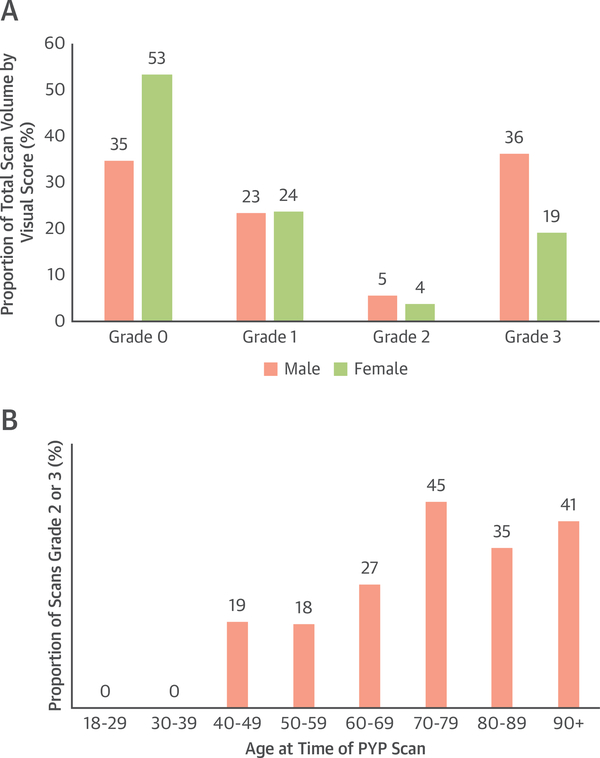
Of the 753 patients, 307 had a visual score of 0 (41%), 177 had a visual score of 1 (23%), 37 of 2 (5%), and 232 of 3 (31%). Overall clinical scan volume per year increased over the decade (p < 0.001) reaching a peak of 132 scans in 2019 (Figure 1). The proportion of scans that were visual grade 2 or 3 decreased over the study period but remained >30%. The H:CL could be calculated in 751 patients, with a median ratio of 1.26 (interquartile range: 1.10 to 1.64). Of those 751 patients, 249 had an H:CL ≥1.5 (33%). There was a high concordance between visual score and H:CL, with 479 of 482 patients (99.4%) with a visual score of 0 or 1 having an H:CL <1.5 and 246 of 269 patients (91.4%) with a visual score of 2 or 3 having an H:CL of ≥1.5 (Supplemental Figure 1). There was a significant difference in the proportion of men and women having a positive 99mTc-PYP scan, with 214 men (42%) and 55 women (23%) having a grade 2 or grade 3 visual score (p < 0.001) (Figure 2A). The proportion of grade 2 or grade 3 scans in each age bracket generally increased with increasing age up to age older than 70 years (Figure 2B). A total of 196 patients who underwent testing met criteria for ATTR-CA (26% of the total cohort). In the total cohort of patients who underwent 99mTc-PYP scanning, 28 patients were eventually diagnosed with AL amyloidosis. Of these patients, 12 (43%) had semiquantitative visual score grade 0 scans, 12 (43%) had grade 1 scans, 2 (7%) had grade 2 scans, and 2 (7%) had grade 3 scans.
FIGURE 1. Yearly Trends in Biopsy and Scan Volume and Positivity Rate in Patients Undergoing Clinical 99mTc-PYP Scans.
Scan and biopsy volume are plotted on the y-axis on the left side of the graph and the proportion of scans that are grade 2 and 3 for each year is plotted on the y-axis on the right side of the graph. From 2010 to 2019, a total of 578 clinically indicated technetium Tc 99m pyrophosphate (99mTc-PYP) scans were obtained after exclusion of 175 patients enrolled in the UNVEIL (Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement) study of patients with severe aortic stenosis. From 2015 to 2019, the mean number of clinical 99mTc-PYP scans per year was 101. Despite an increase in 99mTc-PYP scan volume over the study period, the absolute biopsy rate in this cohort did not significantly change and 99mTc-PYP scan positivity rate remained >30%. PYP = technetium Tc 99m pyrophosphate.
FIGURE 2. Sex, Age, and 99mTc-PYP Scan Results.
Male sex (A) and older age up to >70 years (B) are both highly associated with a grade 2 or 3 99mTc-PYP scan (p < 0.001). Abbreviations as in Figure 1.
In this cohort, 143 patients (73% of 196 patients with confirmed ATTR-CA) had genetic testing results available for review, with 65 having TTR mutations and 78 having a normal TTR genotype. Of the 65 patients with known TTR mutations, the most common mutations included the following: Val122Ile (33 patients), Thr60Ala (14), Phe64Leu (5), and Ser23Asn (2).
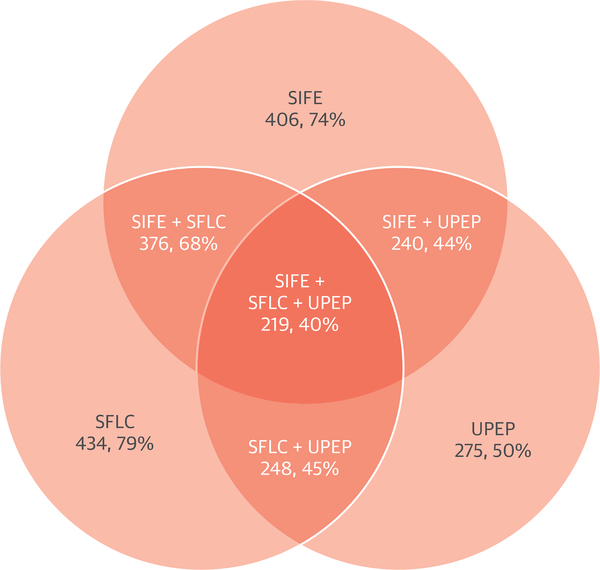
Monoclonal protein testing status was evaluated in 550 patients excluding 175 patients that were part of the UNVEIL study (7) and patients (n = 28) who had known TTR mutations and were being evaluated for early myocardial involvement with PYP imaging. Of these 550 patients, 464 (84%) underwent an SPEP, 406 (74%) had SPEP with immunofixation, 434 (79%) had serum light chains quantitated, 427 (78%) had both an SPEP and serum free light chain testing, and 376 (68%) had both an SPEP with immunofixation and serum free light chain testing (Figure 3). Among subjects seen in the CA program, the assessment for monoclonal proteins was higher (99.7%) than among subjects seen outside of the center (62%). Urine protein electrophoresis with or without immunofixation was performed in 275 patients (50%). In total, monoclonal protein testing with SPEP with immunofixation, serum free light chain testing, and urine protein electrophoresis was performed in 219 patients (40%).
FIGURE 3. Monoclonal Protein Testing.
The Venn diagram displays monoclonal protein testing rates in 550 patients in whom monoclonal protein testing status was assessed. A total of 406 patients (74%) had serum immunofixation (SIFE) test, 434 (79%) had serum free light chain (SFLC) test, and 275 (50%) had urine protein electrophoresis (UPEP) test. Only 219 patients (40%) had all 3 tests. Of note, in this cohort, UPEP was performed either with or without immunofixation, but consensus guidelines recommend that both serum protein electrophoresis and UPEP always be performed with immunofixation to avoid missing small monoclonal protein fractions.
BIOPSY AND IMAGING DIAGNOSIS.
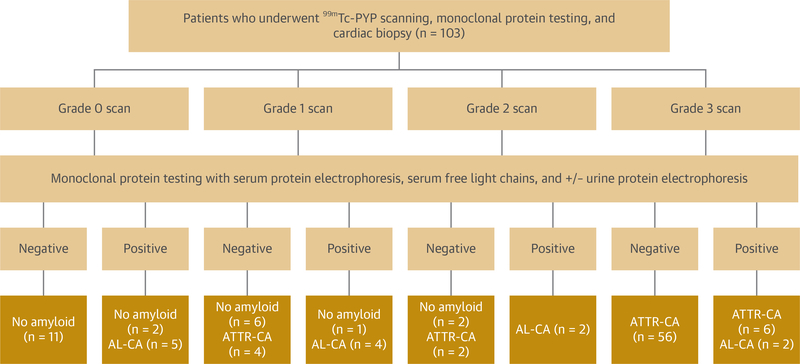
Of the total 753 patient cohort, 104 patients had cardiac biopsy results with staining for amyloidosis available in the medical record with 51 occurring prior to 2015 and 53 from 2015 to 2019. Of these biopsies, 69 showed ATTR, 13 AL, and 22 no amyloid. Over the period the study, biopsy rates remained largely constant over time with the ratio of 99mTc-PYP scans to cardiac biopsies increasing from 1.9 to 12.8 from 2010 to 2014 and 2015 to 2019, respectively. The test characteristics of the noninvasive imaging pathway combining 99mTc-PYP scanning and monoclonal protein assessment was analyzed using 103 patients who underwent cardiac biopsy, with 1 patient excluded who did not have documented monoclonal protein testing (Figure 4). This showed a sensitivity of 94% and specificity of 89% for the diagnosis of ATTR-CA. Common reasons for discrepancies between biopsy diagnosis and 99mTc-PYP scan result included false negative scans with Phe64Leu mutations and false positive grade 2 scans due to blood pooling confirmed on SPECT. There was 100% specificity with 99mTc-PYP scanning in the 56 patients with grade 3 scans and negative monoclonal proteins; all were found to have ATTR-CA on biopsy.
FIGURE 4. Diagnostic Pathway Assessment.
The chart shows the noninvasive diagnosis pathway results in 103 patients that underwent a cardiac biopsy who had 99mTc-PYP scans and monoclonal protein testing available. When grade 0 and 1 scans are considered negative tests and grade 2 and 3 scans are considered positive tests, the sensitivity of 99mTc-PYP scanning is 94% and specificity is 89% with all false positive tests occurring in patients with grade 2 scans. AL-CA = amyloid light chain cardiac amyloidosis; ATTR-CA = amyloid transthyretin cardiac amyloidosis; other abbreviations as in Figure 1.
Of the 127 patients diagnosed with ATTR-CA with cardiac scintigraphy without undergoing biopsy, 96 (76%) were men and 31 (24%) were women. Of men diagnosed with ATTR-CA by cardiac scintigraphy, 12 (9%) were Black as compared to 9 of 31 women (29%) with higher rates of variant TTR genotype seen in Black women (6 of 9, 67%) and Black men (4 of 12, 33%) as compared to in White women (1 of 15, 7%) and White men (7 of 78, 9%).
SPECT ANALYSIS.
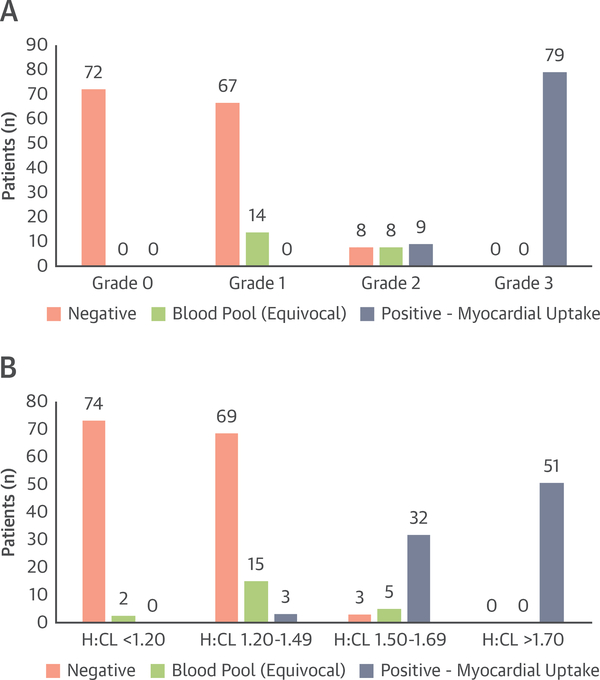
The use of SPECT scanning increased over time in this series, and by 2018, 74 of 76 patients (97%) with visual grade 1 to 3 scans underwent SPECT imaging. A subset of 257 patients who underwent SPECT scanning after 1-h planar imaging was reanalyzed with rereading of planar imaging followed by grading of myocardial uptake and blood pooling on SPECT. This subset included patients with grade 0 (n = 72), grade 1 (n = 81), grade 2 (n = 25), and grade 3 (n = 79) (Figure 5). Of the 72 patients with grade 0 1-h planar scans, all 72 (100%) had negative SPECTs with no blood pooling or myocardial uptake, none had blood pooling, and none had myocardial uptake. Of the 81 patients with grade 1 scans, 67 (83%) had negative SPECTs, 14 (17%) had blood pooling, and none had myocardial uptake. Of the 25 patients with grade 2 scans, 8 (32%) had negative SPECTs, 8 (32%) had blood pooling, and 9 (36%) had myocardial uptake. Of the 79 patients with grade 3 scans, none had negative SPECTs, none had blood pooling, and all 79 (100%) had myocardial uptake.
FIGURE 5. SPECT Scanning Results in Patients Undergoing 99mTc-PYP Scanning.
Single-photon emission computed tomography (SPECT) scanning results are shown as a function of planar visual grading score (n = 257) (A) and heart to contralateral chest ratio (H:CL) (n = 254) (B). SPECT analysis did not change the test outcome in any of the patients with grade 0 or 3 scans, none of whom had significant blood pooling. In contrast, there was a discrepancy in testing in patients with grade 1 scans (17% blood pooling) and grade 2 scans (32% entirely negative, 32% blood pooling), resulting in markedly decreased test accuracy. Analysis of SPECT results by H:CL shows similar results, with intermediate H:CLs of 1.20 to 1.49 resulting in some false negative planar reads and 1.50 to 1.69 resulting in some false positive planar reads. Other abbreviations as in Figure 1.
DISCUSSION
In summary, we present here a single-center study of 753 consecutive patients who underwent 99mTc-PYP scanning at 1 h for the diagnosis of ATTR-CA. The principal findings of this study include: 1) noninvasive testing is increasingly used for the initial work-up of ATTR-CA and has favorable test characteristics compared with biopsy results; 2) a significant proportion of patients in this cohort did not have recommended monoclonal protein evaluation to rule out AL amyloidosis nor genetic testing for TTR mutations; and 3) grade 1 and 2 results by planar scanning alone with 1-h scanning protocols have low diagnostic accuracy that is improved by SPECT (Central Illustration).
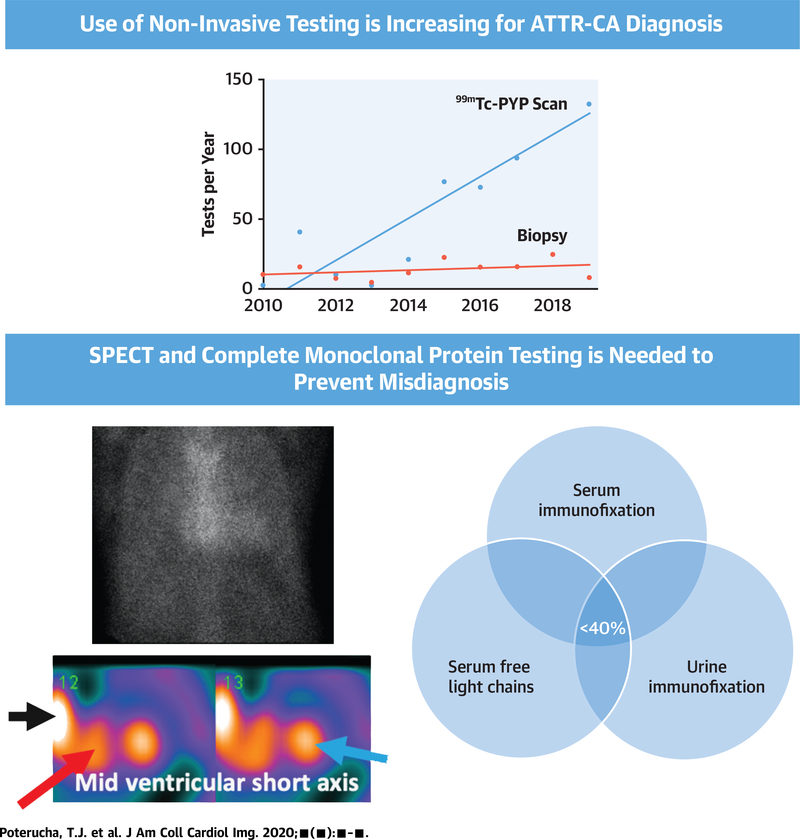
CENTRAL ILLUSTRATION. Noninvasive Testing for ATTR-CA Is Increasing and the Use of SPECT and Monoclonal Protein Testing Is Crucial for Accurate Diagnosis.
Noninvasive testing has been increasingly used for amyloid transthyretin cardiac amyloidosis (ATTR-CA) diagnosis with increasing technetium Tc 99m pyrophosphate (99mTc-PYP) scanning rates and flat biopsy rates. Whereas planar scanning has traditionally been the test of choice for 99mTc-PYP scanning, we find a high rate of false positive grade 2 planar scans with no myocardial uptake seen on single-photon emission computed tomography (SPECT) imaging. In this Figure, there is a grade 2 planar scan with apparent cardiac uptake equal to ribs. When SPECT imaging is performed, only right ventricular (red arrow) and left ventricular (blue arrow) blood pooling is seen without myocardial uptake, indicating that the planar scan was falsely positive. Of note, bony structures such as ribs (solid arrow) show up as “hot spots” that can be mistaken for ventricular uptake or blood pooling and result in SPECT misinterpretation. Complete monoclonal protein testing with serum immunofixation, serum free light chain analysis, and urine immunofixation is needed for all suspected cases of cardiac amyloidosis to assess for light chain amyloidosis and is frequently missed in clinical practice, with <40% of our cohort undergoing complete testing. PYP = technetium Tc 99m pyrophosphate.
DIAGNOSIS PATTERNS AND OPPORTUNITIES FOR IMPROVEMENTS IN TESTING.
There has been a marked shift over the last decade in how ATTR-CA is being diagnosed at our institution with increasing use of noninvasive testing in preference to biopsy. Even with increases in the total amount of testing for ATTR-CA, 99mTc-PYP scan positivity persists above 30% within the last 5 years. With the advantages of improvements in patient experience, safety, and cost, noninvasive testing offers the opportunity to diagnose thousands of new patients with ATTR-CA within the coming years. However, we identified 3 significant gaps in deployment of this approach. First, required monoclonal protein testing to rule out AL amyloidosis was not performed a significant percentage of subjects being imaged. In our overall cohort, 32% of patients did not undergo complete serum monoclonal protein testing and 50% did not have urine protein electrophoresis performed with or without immunofixation. Given the rapid progression of cardiac AL amyloidosis in part due to the toxic infiltrative nature of the condition, delaying AL amyloidosis can be lethal for affected patients. In addition, up to 21% of patients with AL amyloidosis have been shown to have false positive planar cardiac scintigraphy (3). Due to concerns about misdiagnosis, our institution is moving toward creation of order sets that include monoclonal protein testing at the time of 99mTc-PYP order entry in a manner similar to diagnostic workflows established at other centers (9).
Second, among subjects who had evidence of myocardial retention of 99mTc-PYP consistent with ATTR-CA, 126 of the 269 patients had TTR gene sequencing. Genetic counseling and testing in cases of ATTR is recommended by multiple societies, and failure to test results in missed opportunities to identify and counsel family members at risk and to offer certain therapies that are only approved for patients with variant ATTR (5,6,10–12). Genetic testing may be of particular importance with certain genotypes such as Phe64Leu, which has been identified as a mutation that results in truncated amyloid fibril expression that is associated with false negative 99mTc-labeled 3,3-diphos-phono-1,2-propanodicarboxylic acid scintigraphy scans (13).
Finally, the addition of SPECT to planar imaging may be the most important addition to the noninvasive testing pathway in the last several years. Whereas grade 2 planar scans were considered positive in early papers evaluating the test (3,4,8), we note that nearly two-thirds of grade 2 scans in our analysis were either entirely negative or equivocal with blood pooling when evaluated by SPECT. In a cohort evaluated at another institution, similar findings were found with grade 2 planar scans at 1 h frequently being found to be false positive on SPECT (14) with additional studies confirming the importance of SPECT in making an accurate diagnosis (15,16). In the modern era of expensive therapeutics and lack of biopsy confirmation, misdiagnosis has serious medical and financial ramifications. Based on our data here, the rates of false positive testing using planar imaging alone with 1-h scanning protocols are unacceptably high and may be reduced with a combination of SPECT and imaging at 2 to 3 h. Our findings here support the multisocietal recommendations to perform SPECT in all positive planar scans to confirm myocardial uptake and rule out false positive tests caused by blood pooling.
Although noninvasive testing has substantial advantages over biopsy for patients, there may be long-term consequences to the field by reducing tissue availability for research. Cancer researchers have access to large, mature tissue banks such as the National Cancer Institute Cooperative Human Tissue Network or the Cancer and Leukemia Group B Leukemia Tissue Bank. Research using these shared resources has resulted in identification of risk factors and targetable mutations such as in patients with acute myeloid leukemia (17), and it is possible that similar advances in CA may only be possible with tissue banks.
MOVING THE NEEDLE TOWARD EARLIER DISEASE DETECTION.
Because the benefits of ATTR-stabilizing therapy are likely higher in patients with earlier stage disease before extensive end-organ damage has occurred, it is critical to develop systems that recognize patients at risk and diagnose them as early and accurately as possible. ATTR-CA was thought to be a rare disease, and work over the last decade has shown that it is far more common than was previously suspected (7,18–21). Most patients with CA see multiple physicians over a period of months to years before a correct diagnosis is made (22) with diagnosis being delayed >4 years after the onset of symptoms in up to 42% of patients (23–25). One barrier to testing that may be overcome by noninvasive testing includes reticence among clinicians to proceed with invasive endomyocardial biopsy. With the wide availability of cardiac scintigraphy and increasing recognition of the disease, it is likely that patient cohorts at increasingly lower pre-test probability will be selected for testing.
Virtually all of the research in cardiac scintigraphy for ATTR-CA, including this cohort here, has tested populations with a prevalence of disease of at least 30% to 40% (3,26). In these referral populations, the diagnostic accuracy of cardiac scintigraphy is excellent. However, there is a long history of diagnostic tests looking promising in early studies in selected cohorts that are later shown to be markedly less accurate in lower risk or more complex patients (27). Documented examples of this phenomenon include cardiac troponins for detection of myocardial infarction in medically complex patients (28), exercise radionucleotide ventriculography for obstructive coronary artery disease (29), and carcinoembryonic antigen testing for colorectal cancer screening (27). As cardiac scintigraphy is increasingly deployed in screening populations with minimal increase in left ventricular wall thickness, we suspect that testing results are going to be less favorable with high false positive rates. The combination of careful case selection for cardiac scintigraphy, delayed scanning protocols at 2 to 3 h after injection, confirmation of all positive planar imaging by SPECT, and a low threshold for biopsy in cases of clinical uncertainty may help to mitigate this and maintain diagnostic accuracy in the work-up of suspected ATTR-CA.
STUDY LIMITATIONS.
Limitations in this paper are principally due to the retrospective design of this study. First, this was a selected cohort in which there was a high pre-test probability for ATTR-CA and its relevance to populations with a lower pre-test probability is uncertain. Second, our protocol for 99mTc-PYP imaging has changed over time with historic use of 1-h scanning protocols with only intermittent SPECT imaging, and this likely caused variability in diagnostic test performance over the time period studied. Third, clinical information such as echocardiography was abstracted as close as possible to the 99mTc-PYP scan but would ideally be obtained on the same day. Fourth, an attempt was made to comprehensively review all available documentation for monoclonal protein testing including scanned outside records, but it is possible that some monoclonal protein testing performed outside our institution was incompletely captured.
CONCLUSIONS
Noninvasive testing pathways with nuclear scintigraphy using bone-seeking tracers such as 99mTc-PYP are increasingly used for the initial work-up of suspected ATTR-CA. Such pathways have the potential to identify more patients with ATTR-CA but adherence to consensus guidelines such as monoclonal protein testing and the performance of SPECT is essential. Further work is needed to optimize, prospectively validate, and deploy predictive models that can accurately identify early cases of CA where we can have the greatest impact on changing the natural history of this disease.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
99mTc-PYP scanning in combination with monoclonal protein testing is increasingly used for the diagnosis of ATTR-CA, and new therapies have been shown to improve prognosis in these patients.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS 1:
In clinical practice, patients frequently do not have indicated monoclonal protein testing to rule out AL amyloidosis. Given the rapid progression of AL amyloidosis, this error can be lethal.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS 2:
99mTc-PYP scanning that is visual score grade 2 with planar imaging alone is often a false positive result and the addition of SPECT to positive planar scanning is needed to prevent misdiagnosis.
TRANSLATIONAL OUTLOOK:
Additional research is needed to develop strategies to identify patients with early stage ATTR-CA prior to the development of irreversible end-organ damage.
ACKNOWLEDGMENTS
We thank nuclear technologists Lenhurst Leslie, CNMT, Ketan Bhatia, R.T. (N)(ARRT), Amsale Robi, R.T. (N)(ARRT), Rita Malkovskaya, R.T. (N)(CT)(ARRT), and April Holland R.T. (N)(CT)(ARRT) for their tireless work in caring for our patients.
ABBREVIATIONS AND ACRONYMS
- AL
amyloid light chain
- AL-CA
light chain cardiac amyloidosis
- ATTR
amyloid transthyretin
- ATTR-CA
amyloid transthyretin cardiac amyloidosis
- CA
cardiac amyloidosis
- H:CL
heart to contralateral chest ratio
- 99mTc-PYP
technetium Tc 99m pyrophosphate
- SPECT
single-photon emission computed tomography
- SPEP
serum protein electrophoresis
- TTR
transthyretin
Footnotes
APPENDIX For the supplemental figure, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Imaging author instructions page.
REFERENCES
- 1.Maurer MS, Sultan MB, Rapezzi C. Tafamidis for transthyretin amyloid cardiomyopathy. N Engl J Med 2019;380:196–7. [DOI] [PubMed] [Google Scholar]
- 2.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:2872–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–12. [DOI] [PubMed] [Google Scholar]
- 4.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2—evidence base and standardized methods of imaging. J Card Fail 2019;25:e1–39. [DOI] [PubMed] [Google Scholar]
- 6.Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 2 of 2—diagnostic criteria and appropriate utilization. J Card Fail 2019;25:854–65. [DOI] [PubMed] [Google Scholar]
- 7.Castano A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokhari S, Morgenstern R, Weinberg R, et al. Standardization of (99m)technetium pyrophosphate imaging methodology to diagnose TTR cardiac amyloidosis. J Nucl Cardiol 2018;25: 181–90. [DOI] [PubMed] [Google Scholar]
- 9.Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol 2018;72:2040–50. [DOI] [PubMed] [Google Scholar]
- 10.Hershberger RE, Givertz MM, Ho CY, et al. genetic evaluation of cardiomyopathy—a Heart Failure Society of America practice guideline. J Card Fail 2018;24:281–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershberger RE, Givertz MM, Ho CY, et al. , for the ACMG Professional Practice and Guidelines Committee. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2018;20:899–909. [DOI] [PubMed] [Google Scholar]
- 12.Adams D, Suhr OB, Hund E, et al. , for the European Network for TTR-FAP. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol 2016;29 Suppl 1: S14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musumeci MB, Cappelli F, Russo D, et al. Low sensitivity of bone scintigraphy in detecting Phe64Leu mutation-related transthyretin cardiac amyloidosis. J Am Coll Cardiol Img 2020;13:1314–21. [DOI] [PubMed] [Google Scholar]
- 14.Masri A, Bukhari S, Ahmad S, et al. Efficient 1-hour technetium-99 m pyrophosphate imaging protocol for the diagnosis of transthyretin cardiac amyloidosis. Circ Cardiovasc Imaging 2020;13: e010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regis C, Harel F, Martineau P, et al. Tc-99m-pyrophosphate scintigraphy for the diagnosis of ATTR cardiac amyloidosis: Comparison of quantitative and semi-quantitative approaches. J Nucl Cardiol 2020. May 31 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Sperry BW, Burgett E, Bybee KA, et al. Technetium pyrophosphate nuclear scintigraphy for cardiac amyloidosis: imaging at 1 vs 3 hours and planar vs SPECT/CT. J Nucl Cardiol 2020. May 15 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Schilsky RL, Dressler LM, Bucci D, et al. Cooperative group tissue banks as research resources: the cancer and leukemia group B tissue repositories. Clin Cancer Res 2002;8:943–8. [PubMed] [Google Scholar]
- 18.Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med 2008;40:232–9. [DOI] [PubMed] [Google Scholar]
- 19.Dungu JN, Papadopoulou SA, Wykes K, et al. Afro-Caribbean heart failure in the United Kingdom: cause, outcomes, and ATTR V122I cardiac amyloidosis. Circ Heart Fail 2016;9:e003352. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed-Salem L, Santos-Mateo JJ, Sanchez-Serna J, et al. Prevalence of wild type ATTR assessed as myocardial uptake in bone scan in the elderly population. Int J Cardiol 2018;270:192–6. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–94. [DOI] [PubMed] [Google Scholar]
- 22.Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Light chain amyloidosis: patient experience survey from the Amyloidosis Research Consortium. Adv Ther 2015;32:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019;140:16–26. [DOI] [PubMed] [Google Scholar]
- 24.Bishop E, Brown EE, Fajardo J, Barouch LA, Judge DP, Halushka MK. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid 2018;25:174–9. [DOI] [PubMed] [Google Scholar]
- 25.Milandri A, Farioli A, Gagliardi C, et al. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail 2020; 22:507–15. [DOI] [PubMed] [Google Scholar]
- 26.Marume K, Takashio S, Nishi M, et al. Combination of commonly examined parameters is a useful predictor of positive (99 m)Tc-labeled pyrophosphate scintigraphy findings in elderly patients with suspected transthyretin cardiac amyloidosis. Circ J 2019;83: 1698–708. [DOI] [PubMed] [Google Scholar]
- 27.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 1978;299: 926–30. [DOI] [PubMed] [Google Scholar]
- 28.Shah ASV, Sandoval Y, Noaman A, et al. Patient selection for high sensitivity cardiac troponin testing and diagnosis of myocardial infarction: prospective cohort study. BMJ 2017; 359:j4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozanski A, Diamond GA, Berman D, Forrester JS, Morris D, Swan HJ. The declining specificity of exercise radionuclide ventriculography. N Engl J Med 1983;309:518–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.