Abstract
Oxalyl chloride is one of the most versatile reagents used in organic synthesis. Oxalyl chloride was employed in the convenient one-pot, two-step synthesis of the fungus-derived naturally occurring lipoids: N,N’-dipalmitoleyl urea (C16:1) and N,N’-dioleyl urea (C18:1). The two symmetrical diacyl urea-based natural products were previously identified as fungus-specific pathogen-associated molecules (PAMs), which act as inflammatory mediators during fungal infection. The highly lipophilic natural lipoids were efficiently synthesized from commercially available reagents in yields ranging from good to very good.
Keywords: oxalyl chloride, diacyl urea (DAU), lipoid, pathogen-associated molecules (PAMs), fungal natural products, one-pot reaction
1. Introduction
The synthesis of symmetrical and unsymmetrical diacyl ureas is well established in the literature. The first compound representing this scaffold, the symmetrical diacetyl urea (ureide) was prepared (Schmidt 1872) by the reaction of two equivalents of acetamide with phosgene (Fig. 1a). On the other hand, condensation of urea with carboxylic acids in the presence of a metal catalyst (iron or zinc oxide) (Dean 2002) (Fig. 1b), or alternatively with the excess of acyl chlorides in a DMAP–pyridine mixture (Kanji Kubo and Moriy 2005) (Fig. 1c) afforded symmetrical diacyl urea derivatives. Reacting urea with various esters in the presence of a base such as sodium ethoxide (Clemmensen and Heitman 1908, 1909) or sodium hydride (Wolfe and Trimitsis 1969) provided access to a diverse set of diacyl ureas (Fig 1d). A stepwise approach to unsymmetrical diacyl ureas was developed by reacting either monoacyl ureas with acyl chlorides and sulfuric acid as the catalyst (Werner 1916; Bayer 1919; Stoughton 1938) (Fig. 1e), or various amides with acyl isocyanates (Scholl 1890; Meyer and Jacobson 1893; Bayer 1913; ACS 1916; Micich 1982) (Fig. 1f). Use of aromatic carbodiimides and certain carboxylic acids in pyridine as well provided diacyl ureas (Zetzsche and Lindler 1938; Kurzer and Douraghi-Zadeh 1967) (Fig. 1g). The reaction of amides with oxalyl chloride to give symmetrical diaroyl ureas was first reported Bornwater in 1912, and recently this method was extensively elaborated (Garcia Hernandez et al. 2017) to afford a wide range of symmetrical and unsymmetrical diacyl ureas and related compounds in a convenient one-pot protocol (Garcia Hernandez et al. 2017) (Fig. 1h). The diacyl urea skeleton was also obtained from imidazolin-2-ones via dye-induced photooxygenation (Chawla and Pathak 1990) (Fig. 1i).
Figure 1.

Reported synthetic routes to obtain diacyl ureas.
Diverse bioactivity of various diacyl ureas was reported in the literature, including hypnotic properties (aliphatic diacyl ureas bearing 8 – 10 carbon atoms) (Stoughton 1938), plant growth enhancers (N,N’-diformylurea) (Dean 2002), inhibitors of fatty acid amide hydrolase (N-benzoyl-N’-oleyl ureas) (Cramer et al. 2019), and presumably bacteriostatic agents (Micich 1982). Dibenzoyl urea analog of the known insecticide, diflubenzuron was also reported (Garcia Hernandez et al. 2017). More recently, several unusual lipoids possessing the diacyl urea linker and fatty acid chains were identified (Schröder et al. 2002; Schröder and Häsler 2003; Noverr et al. 2003) from fungi (Fig. 2). These compounds were characterized as pathogen-associated molecules (PAMs) that display lipid-like leukocyte activator (LILA) properties thus inducing upon infection adherence and degranulation in human neutrophils and activation of leukocytes (Schröder et al. 2002; Schröder and Häsler 2003; Noverr et al. 2003). Compound 1, the N,N’-dipalmitoleyl urea (C16:1) turned out to be the most potent molecule, which induced the innate immune responses measured as neutrophil chemotactic activity at ED50 =140 nM. Its close analogs, compounds 2 and 3, were approximately 40-times less potent whereas the trans isomer of 1, compound 4 was inactive thus clearly implying the importance of a cis double bond in those PAMs (Schröder et al. 2002). Encouraged with their biological activity and interesting structure, we employed the one-pot, two-step reaction protocol developed previously in our labolatory (Garcia Hernandez et al. 2017) to synthesize N,N’-dipalmitoleyl urea (C16:1) and N,N’-dioleyl urea (C18:1) (Fig. 2) from commercially available starting materials.
Figure 2.

Diacyl urea-based pathogen-associated molecules (PAMs) isolated from fungi.
2. Results and Discussion
The target compounds 1 and 2 [N,N’-dipalmitoleyl urea (C16:1) and N,N’-dioleyl urea (C18:1), respectively] were synthesized from the commercially available starting amides (5): (Z)-hexadec-9-enamide and oleamide, respectively by following the protocol reported previously (Garcia Hernandez et al. 2017) (Scheme 1). Oxalyl chloride, a highly versatile chemical reagent (Mohammadkhani and Heravi 2019), was used in this transformation as a coupling agent and donor of the carbonyl group according to the well-established reaction mechanism (Speziale and Smith 1962) (Scheme 1). The crude materials were readily isolated followed by standard purification on chromatography column to give the natural products 1 and 2 in the yield of 62% and 84%, respectively. This reaction yield was substantially higher and the one-pot protocol much more convenient when compared to the natural products synthesized by the modified carbodiimide method (Schröder et al. 2002). The final products were easily distinguishable from their respective starting materials by 1H and 13C NMR spectroscopy as they lacked the characteristic signals pertinent to the amide protons (two broad peaks in the range of 5.64–5.39 ppm observed by 1H NMR in CDCl3). The signals pertinent to the imide NH in the final products were not visible by 1H NMR when deuterated chloroform (CDCl3) was used as the solvent. However, changing the solvent to deuterated dimethyl sulfoxide (DMSO-d6) allowed to discern the imide NH by 1H NMR at 10.86 ppm for compound 1. On the other hand, a characteristic signals were observed at 150.07 and 149.66 ppm by 13C NMR in CDCl3 that were pertinent to the central carbonyl groups present in the final products 1 and 2, respectively. The exact mass of the final products was confirmed by high resolution mass spectrometry (HRMS). The final products, however, were accompanied with an inseparable impurities (~3% for 1 and ~ 7% for 2), which were also present in the starting materials, as determined by 1H and 13C NMR. Based on the close proximity and even occasional overlap of the NMR signals associated with the impurity and the desired final product, it could be suspected that the impurity might be the E-isomers of the starting amides 5, which were then converted into the E-isomers of the final products 1 and 2, respectively. It is apparent that the lipoids 3 and 4 (Fig. 2) can be readily synthesized by using this synthetic method and the appropriate starting materials [i.e. (9Z, 12Z)-octadeca-9, 12-dienamide and (E)-hexadec-9-enamide, respectively], which are also commercially available.
Scheme 1.
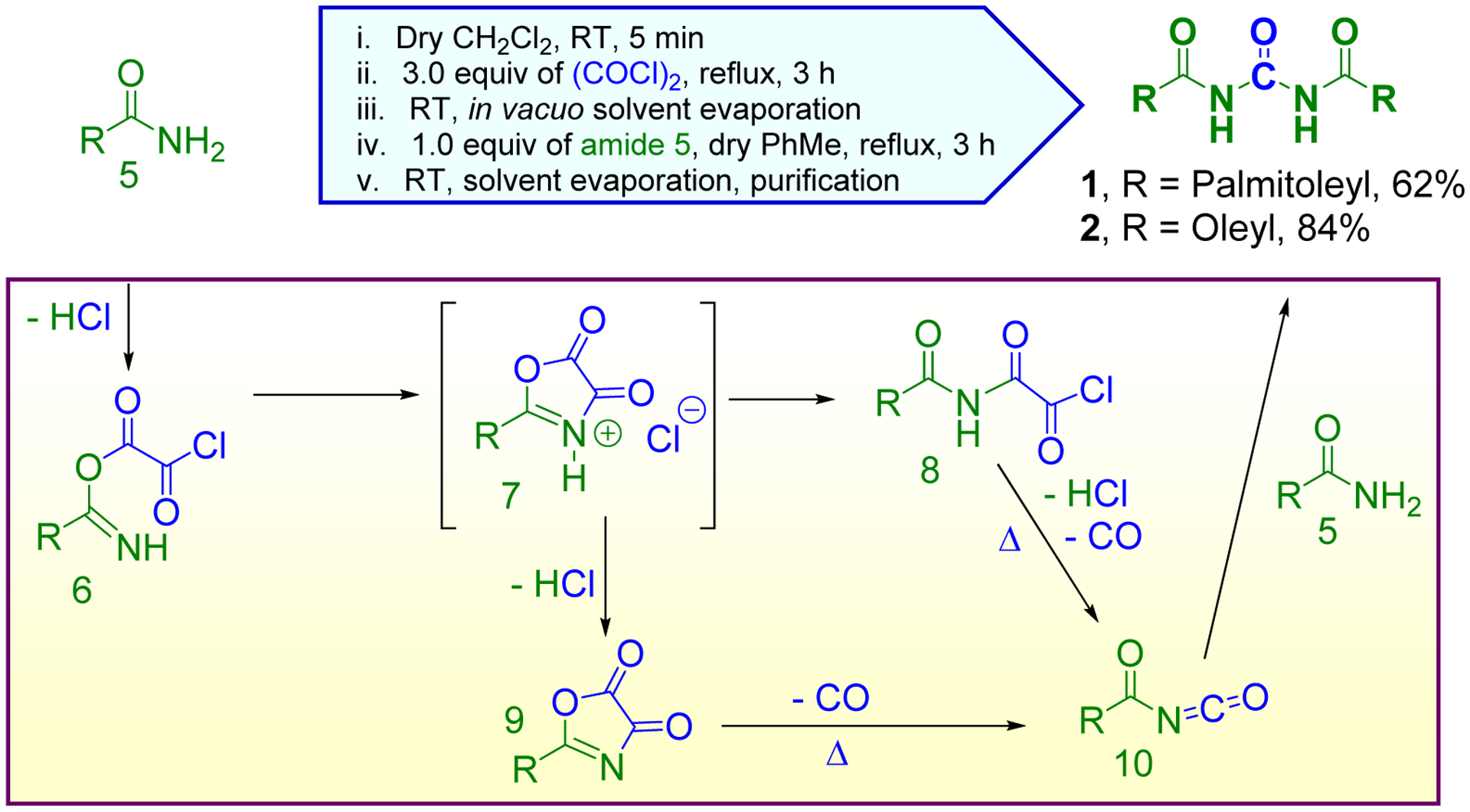
Convenient one-pot, two-step conversion of fatty acid amides into naturally occurring lipoids 1 and 2.
Due to the diacyl urea motif, lipophilic nature, and structural similarity of the natural products to the previously reported oleamide-derived diacyl urea FAAH inhibitors (Cramer et al. 2019), compounds 1 and 2 were tested in vitro in search for inhibitory activity of this enzyme. Sadly, neither of those compounds showed any inhibition of the human FAAH enzyme.
3. Experimental
3.1. General Information
(Z)-hexadec-9-enamide (95% purity) was purchased from Accel Pharmtech LLC whereas oleamide (95% purity) was purchased from AK Scientific. Oxalyl chloride (reagent plus grade) and anhydrous toluene (PhCH3) were purchased from Sigma-Aldrich whereas dichloromethane (CH2Cl2) was purchased from Fisher Scientific and was dried by distillation over calcium hydride. All other solvents were purchased from Fisher Scientific and used without further purification. 1H NMR and 13C NMR spectra were recorded on Bruker Avance spectrometer at 400 and 100 MHz, respectively. NMR spectra were reprocessed by using the ACD Labs 2019 Spectrus Processor software. Standard abbreviations indicating multiplicity were used as follows: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quadruplet, quin = quintet, m = multiplet, and br = broad. HRMS experiments were performed on an ultra performance liquid chromatography mass spectrometer (UPLC-MS, Thermo Scientific) with the ultimate 3000 binary pump system. The UPLC-MS system is a Q Exactive Focus Orbitrap. The column used for analysis was a Phenomenex Luna Omega 1.6 μm polar C18 100 mm × 21 mm column at a flow rate of 0.4 mL/min and column temperature of 40 °C. Heated electrospray ionization (HESI) in the positive mode was utilized. Melting points were measured with a MEL-TEMP II melting point apparatus and are reported uncorrected. TLC was performed on Merck 60 F254 silica gel plates. Traditional flash chromatography was done using silica gel and gradient of ethyl acetate in hexanes as the eluent.
3.2. Synthesis of the Target Compounds 1 and 2
(Z)-Hexadec-9-enamide [or oleamide] (1.0 mmol, 1.0 equiv) was placed in a Schlenk Kjeldahl reaction flask and the flask was evacuated/Argon re-filled three times. Subsequently, anhydrous dichloromethane (25 mL) was added and the mixture was stirred at room temperature (RT) for 5 minutes before dropwise addition of oxalyl chloride (3.0 mmol, 3.0 equiv) followed. The reaction mixture was then stirred at reflux for 3.0 hours before cooling to RT and the solvent was removed under reduced pressure in an air-free manner. Subsequently, (Z)-hexadec-9-enamide [or oleamide] (1.0 mmol, 1.0 equiv) was rapidly added via regular powder funnel and the flask was again evacuated/Argon re-filled three times before dry toluene (12 mL) was added. The reaction mixture was then stirred at reflux for another 3 h before cooling to RT and concentration on rotary evaporator. The isolated crude material was purified by flash chromatography on silica with gradient of ethyl acetate in hexanes as the eluent.
3.3. Analytical Data of the Synthesized Natural Products
Dipalmitoleyl Urea (C16:1) (1):
Milky-white solid, 332 mg (62%). Melting point: 41 – 43 °C.
1H NMR (400 MHz, CDCl3) δ ppm 5.41–5.32 (m, 4H, CH=CH), 2.53 (br t, J=6.8 Hz, 4H), 2.03 (q, J=6.5 Hz, 8H), 1.69 (quin, J=7.3 Hz, 4H), 1.38–1.29 (m, 32H), 0.90 (t, J=6.9 Hz, 6H, CH3).
13C NMR (100 MHz, CDCl3) δ ppm 174.27 (br s, 2C, C=O), 150.07 (1C, C=O), 130.06 (2CH, CH=CH), 129.67 (2CH, CH=CH), 37.68 (2CH2), 31.78 (2CH2), 29.73 (2CH2), 29.68 (2CH2), 29.20 (2CH2), 29.07 (2CH2), 29.01 (2CH2), 28.99 (2CH2), 27.23 (2CH2), 27.16 (2CH2), 24.41 (2CH2), 22.66 (2CH2), 14.10 (2CH3).
1H NMR (400 MHz, DMSO-d6) δ ppm 10.86 (br s, 2H, NH), 5.32 (br t, J=4.7 Hz, 4H, CH=CH), 2.49 (br t overlapping with DMSO, J=7.3 Hz, 4H), 1.98 (q, J=6.0 Hz, 8H), 1.52 (quin, J=6.8 Hz, 4H), 1.31–1.25 (m, 32H), 0.85 (t, J=6.8 Hz, 6H, CH3).
13C NMR (100 MHz, DMSO-d6) δ ppm 175.34 (2C, C=O), 149.95 (1C, C=O), 130.12 (2CH, CH=CH), 130.07 (2CH, CH=CH), 36.88 (2CH2), 31.60 (2CH2), 29.56 (2CH2), 29.50 (2CH2), 29.01 (2CH2), 28.87 (2CH2), 28.81 (2CH2), 28.74 (2CH2), 27.06 (2CH2), 27.03 (2CH2), 24.36 (2CH2), 22.55 (2CH2), 14.37 (2CH3).
HRMS (HESI+): m/z [M+H]+ calculated for C33H61N2O3: 533.4677; found: 533.4678.
N,N’-Dioleyl Urea (C18:1) (2):
Milky-white solid, 492 mg (84%). Melting point: 76 – 78 °C.
1H NMR (400 MHz, CDCl3) δ ppm 5.41–5.32 (m, 4H, CH=CH), 2.53 (br t, J=6.0 Hz, 4H), 2.03 (q, J=6.0 Hz, 4H), 1.69 (quin, J=7.1 Hz, 4H), 1.34–1.27 (m, 44H), 0.90 (t, J=6.9 Hz, 6H, CH3).
13C NMR (100 MHz, CDCl3) δ ppm 174.22 (1C, C=O), 174.18 (1C, C=O), 149.66 (1C, C=O), 130.07 (2CH, CH=CH), 129.68 (2CH, CH=CH), 37.72 (2CH2), 31.93 (CH2), 31.92 (CH2), 29.77 (CH2), 29.71 (2CH2), 29.69 (2CH2), 29.66 (2CH2), 29.60 (CH2), 29.53 (CH2), 29.45 (CH2), 29.37 (CH2), 29.34 (CH2), 29.32 (CH2), 29.29 (CH2), 29.20 (CH2), 29.08 (CH2), 29.04 (CH2), 29.01 (CH2), 27.24 (CH2), 27.17(CH2), 24.44 (CH2), 24.41 (CH2), 22.69 (2CH2), 14.12 (2CH3).
1H NMR (400 MHz, DMSO-d6) δ ppm 10.86 (br s, 2H, NH), 5.38 – 5.26 (m, 4H, CH=CH), 2.30 (t, J=7.6 Hz, 1H), 2.05 – 1.91 (m, 7H), 1.58 – 1.43 (m, 6H), 1.38 – 1.15 (m, 42H), 0.85 (t, J=6.4 Hz, 6H, CH3).
13C NMR (100 MHz, DMSO-d6) δ ppm 175.38 (1C, C=O), 175.34 (1C, C=O), 149.94 (1C, C=O), 130.13 (2CH, CH=CH), 130.08 (2CH, CH=CH), 36.88 (2CH2), 31.74 (2CH2), 29.56 (2CH2), 29.49 (2CH2), 29.29 (2CH2), 29.15 (2CH2), 29.04 (2CH2), 28.99 (2CH2), 28.86 (2CH2), 28.80 (2CH2), 27.08 (2CH2), 27.03 (2CH2), 24.36 (2CH2), 22.55 (2CH2), 14.40 (2CH3).
HRMS (HESI+): m/z [M+Na]+ calculated for C37H68N2NaO3: 611.5122; found: 611.5121.
3.4. hFAAH Enzyme Screening Assay
Human recombinant FAAH enzyme (Item No. 100101183, Batch No. 0523867) (Item No. 10011669) was obtained from Cayman Chemical. Measurement of FAAH potency was performed using the substrate N-(6-methoxypyridin-3-yl) octanamide (OMP) ([S]final=50 μM) in sodium phosphate buffer (0.1 M, pH = 8, 0.1 mg/mL BSA). Progress of the reaction was measured by fluorescence detection of 6-methoxypyridin-3-amine at an excitation wavelength of 303 nm and an emission wavelength of 394 nm at 37 °C by the use of microplate reader (Molecular Devices., CA, USA). All experiments were run in triplicate, and values reported as average +/− SD. The substrate OMP was synthesized following a previously reported synthetic procedure and reaction conditions (Wilt et al. 2020). Quantification of inhibitor potencies were performed following the previously published procedure for FAAH enzyme (Kodani et al. 2016).
4. Conclusions
Herein we report a convenient and high-yielding synthesis of the symmetrical fatty acid-derived diacyl ureas N,N’-dipalmitoleyl urea (C16:1) and N,N’-dioleyl urea (C18:1). Those compounds were initially isolated from fungi and defined as pathogen-associated molecules (PAMs), which act as pro-inflammatory factors. The presented herein quick access to those molecules and their derivatives might spark an interest in investigating further their immunomodulatory function. Additionally, those unusual natural products could be used for detection of fungi in biological samples. The natural products were screened in vitro in FAAH enzyme assay and unfortunately, they turned out to be inactive.
Acknowledgments
MBKU COP is greatly acknowledged for startup funds and faculty travel stipend. The unlimited access to the NMR facility (Bruker Avance 400) in the Department of Chemistry and Biochemistry at California State University Fullerton (CSUF) is acknowledged. We thank Dr. Paula Hudson (CSUF) for obtaining the HRMS data. Research reported in this publication was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number SC2GM135020. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Instrumentation support was provided by the National Science Foundation MRI (CHE1726903) for acquisition of an UPLC-MS.
Footnotes
Declaration of Interest
The authors have no conflict of interest to declare.
References
- American Chemical Society. 1916. Acylbromodiethylacetylcarbamides. Chem Abstr. 10:1579. [Google Scholar]
- Bayer & Co. 1913. German Patent 286 760.
- Bayer & Co. 1919. British Patent 132,795.
- Bornwater JT. 1912. L’action du chlorure d’oxalyle avec des amines et des amides. Rec Trav Chim Pays-Bas. 31:105–141. [Google Scholar]
- Chawla HM, Pathak M. 1990. Dye sensitized photooxygenation of imidazolin-2-ones. Tetrahedron. 46:1331–1342. [Google Scholar]
- Clemmensen E, Heitman AHC. 1908. Ureides and cyanamides of the dialkyloxyacetic acids. Am Chem J. 40:280–302. [Google Scholar]
- Clemmensen E, Heitman AHC. 1909. Ureides and cyanamides of the oxyfatty acids. Am Chem J. 42:319–340. [Google Scholar]
- Cramer S, Johnson J, Ngo T, El-Alfy AT, Stec J. 2019. Modulation of the endocannabinoid system via inhibition of fatty acid amide hydrolase (FAAH) by novel urea and carbamate derivatives. ChemistrySelect. 4:11609–11614. [Google Scholar]
- Dean FW. 2002. Methods for preparing and using diacyl ureas. US Patent 6448440 B1.
- Garcia Hernandez A, Grooms GM, El-Alfy AT, Stec J. 2017. Convenient one-pot two-step synthesis of symmetrical and unsymmetrical diacyl ureas, acyl urea/carbamate/thiocarbamate derivatives, and related compounds. Synthesis. 49:2163–2176. [Google Scholar]
- Kanji Kubo K, Mori A. 2005. Synthesis and gelation properties of N,N’-bis(3,4,5-trialkoxy)benzoylurea: Terpene and perfume gels. Chem Lett. 34:1250–1251. [Google Scholar]
- Kodani SD, Overby HB, Morisseau C, Chen J, Zhao L, Hammock BD. 2016. Parabens inhibit fatty acid amide hydrolase: A potential role in paraben-enhanced 3T3-L1 adipocyte differentiation. Tox Lett. 262:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzer F, Douraghi-Zadeh K. 1967. Advances in the chemistry of carbodiimides. Chem Rev. 67:107–152. [DOI] [PubMed] [Google Scholar]
- Meyer V, Jacobson P. 1893. Lehrbuch der Organischen Chemie: Allgemeiner Theil; Veit & Comp: Leipzig. 1013–1014. [Google Scholar]
- Micich TJ. 1982. A rapid synthesis of fatty acyl urea derivatives. J Am Oil Chem Soc. 59:92–94. [Google Scholar]
- Mohammadkhani L, Heravi MM. 2019. Oxalyl chloride: A versatile reagent in organic transformations. ChemistrySelect. 4(20):6309–6337. [Google Scholar]
- Noverr MC, Erb-Downward JR, Huffnagle GB. 2003. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin Microbiol Rev. 16(3):517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E 1872. J Prakt Chem. 5:63. [Google Scholar]
- Scholl R 1890. Ber Dtsch Chem Ges. 23:3516. [Google Scholar]
- Schröder JM, Häsler R, Grabowsky J, Kahlke B, Mallet AI. 2002. Identification of diacylated ureas as a novel family of fungus-specific leukocyte-activating pathogen-associated molecules. J Biol Chem. 277(31):27887–27895. [DOI] [PubMed] [Google Scholar]
- Schröder JM, Häsler R. 2003. Bis-acyl ureas, derivatives thereof, and use thereof. Ger Offen. DE 10134445 A1 20030213.
- Speziale AJ, Smith LR. 1962. A new and convenient synthesis of acyl isocyanates. J Org Chem. 27:3742–3743. [Google Scholar]
- Stoughton RW. 1938. Diacylureas. I. Preparation and properties of diacylureas derived from normal aliphatic acids. J Org Chem. 2:514–521. [Google Scholar]
- Werner EA. 1916. LXXXI.—The constitution of carbamides. Part III. The reaction of urea and of thiourea with acetic anhydride. Note on potassium thiourea. J Chem Soc Trans. 109:1120–1130. [Google Scholar]
- Wilt SR, Rodriguez M, Le TNH, Baltodano EV, Salas A, Pecic S. 2020. Design, microwave‐assisted synthesis, biological evaluation and molecular modeling studies of 4‐phenylthiazoles as potent fatty acid amide hydrolase inhibitors. Chem Biol Drug Des. 95(5):534–547. [DOI] [PubMed] [Google Scholar]
- Wolfe JF, Trimitsis GB. 1969. Convenient new synthesis of 1,3-diaroylurea. Can J Chem. 47:2097–2099. [Google Scholar]
- Zetzsche F, Lindler H. 1938. Die Kennzeichnung von Carbonsäuren als Ureide mit Hilfe der Carbodiimide (III. Mitteil.). Chem Ber. 71:2095. [Google Scholar]


