Abstract
Technologies capable of cell separation based on cell images provide powerful tools enabling cell selection criteria that rely on spatially or temporally varying properties. Image-based cell sorting (IBCS) systems utilize microfluidic or microarray platforms each having unique characteristics and applications. The advent of IBCS marks a new paradigm in which cell phenotype and behavior can be explored with high resolution and tied to cellular physiological and omics data providing a deeper understanding of single-cell physiology and the creation of cell lines with unique properties. Cell sorting guided by high-content image information has far reaching implications in biomedical research, clinical medicine, and pharmaceutical development.
Keywords: Cell sorting, imaging, cytometry, microdevice, microfluidics, microarrays
Single-Cell Analysis and Sorting Paves the Way for Biomedical Breakthroughs
The ability to analyze individual cells has revolutionized biomedical research and clinical medicine leading to innovations that encompass genetic engineering, regenerative medicine, and cancer immunotherapies. While cell population data provide a wealth of information, there is an inherent loss of information from bulk cell samples. Small variations between adjacent cells are known to lead to profound physiologic differences, for example, immune cells vary in their ability to combat infection, neighboring hematopoietic stem cells differentiate into distinct cell lineages, and cancer cells display diverse responses to chemotherapeutic agents [1–3]. Single-cell analysis techniques are particularly powerful in oncology where subtle differences in single-cell gene expression signatures forecast resistance to anticancer drugs [4], and single-cell protein content predicts patient responses to checkpoint inhibitor immunotherapy [5]. Application of these analytical assays often demands that the cell of interest be isolated from a mixture of cells for efficient and reliable measurements. To address this need, a variety of cell-separation platforms have been developed as front-end single-cell isolation systems for these assays [6,7]. While advancing our knowledge of single-cell physiology, pre-processing cell separation methods have in the past employed single-timepoint, whole-cell metrics as selection criteria when making a sort decision; however, much of the richness that distinguishes cells within a population can only be observed at the subcellular level or as cell behavior changes over time.
In the past decade powerful strategies to sort cells utilizing high spatial and often time-resolved data have emerged, collectively called image-based cell sorting (IBCS)(see Glossary). IBCS pairs high-content image acquisition with computational analyses to produce novel sort criteria. Cellular images provide rich spatial information at the cellular and subcellular level, greatly expanding the criteria used for sort-decisions, for example, organelle properties, localized intracellular or cell-surface protein expression, and cell morphology. Furthermore, image-based selection enables the mapping of phenotype to single cell genomics data to provide important tools for disease identification and treatment. With acquisition of sequential images, subcellular dynamics can be captured and time-varying attributes extracted to characterize cells for selection. Temporal imaging also permits the study of cell-to-cell interactions, such as cytotoxic T cells interfacing with leukemic cells, embryonic lung fibroblasts with carcinoma cells, and neuronal cells with glial cells [8]. These IBCS platforms address major limitations of commonly used technologies, such as fluorescence-activated cell sorting (FACS) and magnetic activated cell sorting (MACS).
IBCS platforms are largely based on microdevices due to the advantages presented by miniaturization. These devices permit flexible design, customization, and low-reagent volumes. The small feature sizes, liquid volumes, and laminar flow provide more precise tracking and/or control of cell motion during imaging and isolation compared to traditional macro-devices. Miniaturization has the potential to provide increased throughput as well as decrease cell loss by virtue of parallel processing and multiplexing. At microscale, surface tension and capillary forces are powerful forces harnessed for precise cell positional control [9]. Fluid dynamics and diffusion kinetics are highly predictable within microfabricated systems, enhancing their usefulness for IBCS applications [9]. Lastly, microarray and microfluidic devices can be quickly prototyped, or mass produced, in glass, polydimethylsiloxane (PDMS), silicon, and thermoplastics like PMMA or polycarbonate [10]. For these various reasons, microdevices have been used for the vast majority of IBCS platforms to date. This review discusses emerging IBCS technologies of three basic types: (1) microfluidic flow devices used to image moving cells; (2) microfluidic containment devices for imaging entrapped cells; and (3) microarrays used to image localized cells (Figure 1, Key Figure). In each category, the focus is on methods that yield living cells for downstream assays or cloning.
Figure 1 (Key Figure): Overview of Image Based Cell Sorting and Main Technologies.
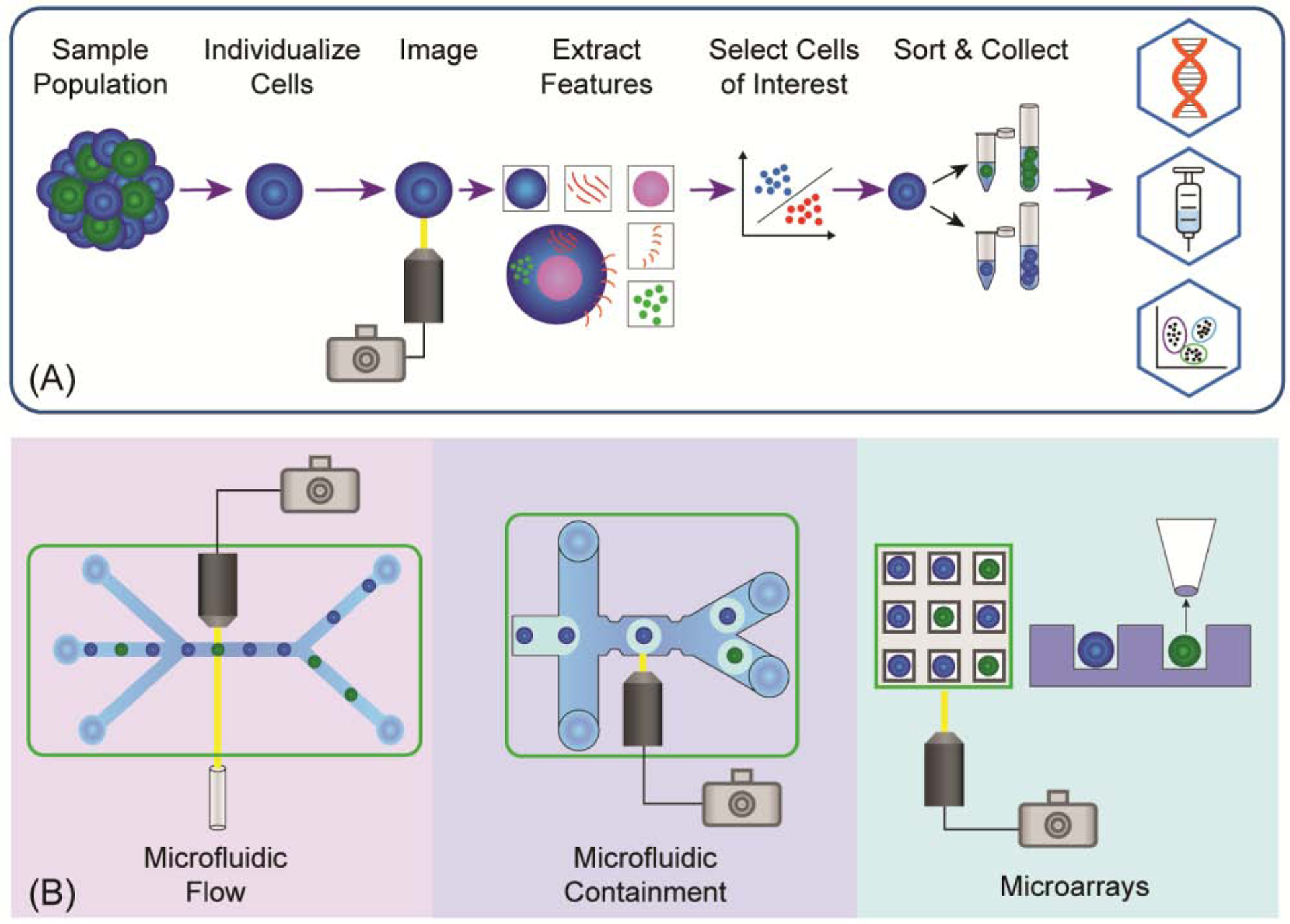
(A) IBCS platforms typically follow a process in which a sample of cells is collected, the sample is separated into either single cells or small colonies, each cell or colony is imaged, features are extracted using ML or traditional image analysis techniques, cells of interest are selected for isolation, and the cells are sorted into collection containers. The cells are often then used in additional assays, sequencing pipelines, or therapies. (B) The three principle strategies used in IBCS are microfluidic flow devices, microfluidic containment devices, and microarrays. In microfluidic flow (left), cells rapidly move within a fluid stream and are imaged with specialized imaging techniques, and then sorted into collection containers in a high-throughput manner. Using microfluidic containment (center), cells are individualized and captured within droplets or stationary traps for conventional imaging and medium-throughput sorting using fluid flow. Alternatively, elements in microarrays (right) are loaded with single or multiple cells, imaged with conventional imaging techniques—often temporally—and cells are isolated using low-throughput mechanical or optical retrieval methods.
Microfluidic Flow IBCS Platforms
Microfluidic flow IBCS platforms image moving cells within a flow stream and of the IBCS methods described here is most closely related to FACS. Although powerful, FACS is limited to single timepoint measurements of light scatter or fluorescence emission collected from an entire cell. A FACS-like sorting system paired with cell image acquisition has proven quite challenging due to: i) the limited time the cell spends in the interrogation beam (~10 μs) and ii) the very short transit time (~200 μs) between interrogation and the droplet-forming nozzle [11]. The short-duration exposure creates challenges in collection of sufficient photons over the cell body for image reconstruction. The brief interval between cell illumination and sorting, known as the drop delay, leaves little time for the image reconstruction and data processing needed to inform the sort decision. Advances in imaging detectors, electronics and computation have enabled the combination of imaging with flow cytometry (imaging flow cytometry), but without the ability to perform cell sorting [12–15]. These innovations permit a high rate of data acquisition while maintaining sufficient sensitivity. While they permit high quality image formation in flow cytometers, they have not proven sufficient to enable pairing of classical FACS platforms with IBCS.
Innovations in microfluidics and computation have permitted imaging flow cytometry techniques to be paired with microfluidic devices to create the equivalent of imaging-FACS systems (Figure 2, Table 1). The move to a microfluidic platform enables longer times between imaging and the sort point, providing sufficient time for image processing and highly accurate cell velocity and trajectory tracking prior to the sort step. A plethora of sort mechanisms adds greater versatility compared to the droplet-in-air formation required by FACS. Further advances include implementation of advanced machine learning strategies to reduce computational analysis time, sorting based on virtual cell images, and combined improvements in hardware and digital image processing.
Figure 2: Microfluidic Flow IBCS Platforms.

(A) These IBCS platforms often use spatial and temporal excitation waveforms to form images, enabling information-rich signal collection. Machine learning strategies are then applied to the images to classify cells and the individual cells are sorted in a high-throughput manner. (B) Intelligent image-activated cell sorting system and images of Jurkat cells captured by the system. Reproduced from [21] with permission. (C) Machine learning based real-time image-guided cell sorting and classification system, using a spatial filter to produce excitation waveforms that are transformed into 2-D images for analysis. Reproduced from [19] with permission. (D) Ghost cytometry cell sorting platform which employs an optical filter to produce 1-D waveforms used for cell classification. Reprinted from [18] with permission.
Table 1:
Typical Attributes of IBCS Platforms.
| Type | Sorting Throughput (cells/s) | Post-Sort Cell viability | Repeated Imaging | Spatial resolution | Starting Sample Size | Sorting Purity | Sorting Yield | References |
|---|---|---|---|---|---|---|---|---|
| Microfluidic Flow | High (0.24 – 3,000) | Medium-Low | − | Low-Medium | 105 – 107 | ++ | +++ | [16,18–24] |
| Microfluidic Containment | Medium (0.2 – 5) | Medium | + | Medium | 103 −106 | ++ | ++ | [25,31–38] |
| Microarrays | Low (0.02 – 1) | High | ++ | High | 102 −105 | +++ | + | [44,45,47,48, 50,51,55,57] |
Imaging of rapidly moving cells is particularly challenging in microfluidic devices due to the cells’ high velocities (> 1 m/s) along the flow path, leading to blurred images when using traditional microscopes and CMOS or CCD cameras. To overcome this obstacle, several non-traditional methods of imaging are employed in flow-based IBCS platforms. For example, cell images can be reconstructed from 1-D temporal and/or spatial excitation waveforms enabling information-rich signal collection for reconstruction into 2-D images [16,17]. Raw high-content, 1-D data obtained by random structured illumination can also be analyzed directly without image reconstruction to maintain high throughput (3,000 cells/s) (Figure 2D) [18]. Alternatively, cell interrogation by multiple light beams coupled with spatial filtering of the emitted light can be combined to yield a light scan across the cell which is then transformed into 2-D images (Figure 2C) [19]. Cells can also be imaged using stimulated Raman scattering (SRS) microscopy. For example, after illuminating cells with a modulated pulsed elliptical light beam, a series of 1-D SRS lines are generated and then transformed into 2D SRS images [20]. Though imaging cells in the form of transformable waveforms appears to be the trend in flow IBCS, producing images with a camera is also possible. Integration of a polygon mirror image scanner along the emission light path cancels the motion of a cell and provides deblurring when imaged with a CMOS camera, supporting true 2-D images of cells acquired at high speed (Figure 2B) [21]. High-speed CMOS cameras coupled with pulsed illumination can also minimize motion artifacts [22]. More standard light detection systems (CCD cameras and video cameras) can be employed to image at low flow rates, but at a cost of decreased spatial resolution and/or slower sort rates of 0.24–200 cells/s [23,24] compared to other flow-based methods.
Recent advances in computational algorithms and imaging hardware are key to enabling ultrafast image reconstruction and decision making for cell sorting. Amongst the most important innovation is the application of machine learning, including support vector machines and deep neural networks, for image feature extraction and subsequent cell categorization [16,18,19,21,25,26]. Deep neural networks were proposed in the 1960’s [27]; however, within the past decade deep networks have made great progress due to advances in hardware, particularly of graphical processing units (GPUs) [25,28]. GPUs can now rapidly perform complex matrix operations, providing the means to apply deep neural networks to image classification and feature extraction in real-time [29]. Microfluidic flow systems are particularly suited to machine learning classifiers due to the large training data sets that can be produced in a short time. These platforms can incorporate long microchannels (up to 3 cm) between the interrogation site and sort point to provide sufficient time for image analysis. Other improvements include the use of sheathed fluid flow or cell focusing strategies, e.g. acoustic focusing to maintain cell velocity and position, so that sorting is accurate despite the long distance travelled by the cell within the microchannel [16]. The trained cell classification algorithms identify cells and send a signal to sorting hardware, culminating in typical sort rates of 100 to 3,000 cells/s [16,18–22]. Physical separation of cells into collection containers is accomplished using piezoelectrics, push-pull dual-membranes, dielectrophoresis (DEP), and standing acoustic waves [16,18–22,24].
Advantages of flow IBCS systems include high analysis and sorting rates, and a wide range of applications in cell isolation, i.e. cloning, diagnostics, and others [16,18–22,24,30]. For example, a flow IBS platform has been used to detect and isolate activated platelet aggregates from human blood for diagnosis of atherothrombosis [16]. To purify the activated platelets, a system capable of rapidly separating cells from a large (>106) and heterogeneous population was required. Once separated, the platelets could be used for diagnostics or for transcriptomic and proteomic analysis, potentially leading to development of anti-platelet drugs. For fast cell enrichment processes and clinical diagnostic tests, flow IBCS platforms generally outperform other IBCS techniques. Furthermore, flow systems are capable of imaging cells using multiple fluorescence channels as well as using label-free methods [20,22]. Limitations include the requirement for complex hardware and software. Additional tradeoffs due to their high speed are relatively low spatial resolution in the XY plane and inability to form Z sections. While the computational power of these systems is impressive, machine learning is typically required to make the sub-second sort decisions for high throughput sorting. Additionally, repeated interrogation of the same cell prior to a sort decision is generally not supported by flow IBCS platforms. Other challenges for flow IBCS platforms are the requirement for expensive imaging equipment, powerful computing systems, microflow devices i.e. pumps, and microfabricated fluidic systems.
Microfluidic Containment for Imaging and Sorting
A second class of IBCS systems slows or stops cells using cell-sized, multifunctional containers such as droplets or microchambers to control cell motion and minimize image blur (Figure 3, Table 1). Cell containment is an especially useful strategy for IBCS when sample sizes are small, and efficient retrieval of viable cells is required, as these methods enable throughputs of 0.2–5 cells/sec with high sorting accuracies (often >98%). Unlike flow IBCS devices, which image cells that are moving at high velocity, these slower throughput systems work to obtain high quality, multi-dimensional images with traditional cameras and software. Hence, it is necessary to stop or significantly slow cell movement at the time of image formation. The gain in more precise positional control of the cells is often offset by increased complexity in device fabrication and operation. These systems confine cell movement using a variety of strategies often by entrapment within a microdroplet, microchannel, or other microstructure. A challenge that most of these devices face is their slow speed, although this allows users to employ standard electronics, imaging, and computational methods.
Figure 3. Microfluidic Containment IBCS.

(A) Schematic of the major steps or common components of containment IBCS. (B) Use of droplet microfluidics to separate interacting cells. Reproduced from [31] with permission. (C) Example of a cell containment strategy that relies on cell docking in a restricted channel location. A bead-based secretion assay is also integrated into this sorting platform. Reprinted from [37]. (D) IBCS device that relies on valve closure to entrap cells within a small region of a microchannel. Adapted from [35] under a Creative Commons license (http://creativecommons.org/licenses/by/4.0/).
Droplet IBCS platforms capture cells within nanoliter volumes of aqueous liquid surrounded by an immiscible medium that limit a cell’s location to a very small spatial region, i.e. 75–120 μm regions [31–33]. Immiscible phases include oil or a perfluorocarbon with surfactant [31–33]. A challenge with droplet-based IBSC, is that the immiscible phase restricts oxygen and nutrient supply to and waste removal from the cells limiting their viability over longer times (>9 h) [31]. To overcome this disadvantage, the aqueous medium can be gelled and the resulting cell-containing hydrogel bead moved into an aqueous medium for subsequent sorting and culture [34]. An advantage of the IBSC droplet strategy is the ability to place the droplets (>300) into a storage depot for repeated imaging without droplet intermixing [32]. Droplets can also be moved into arrayed microwells for storage at pre-determined addresses facilitating repeated imaging over time (Figure 3B) [31]. Finally, placement of cells within droplets permits low speed imaging under hydrodynamic flow, since all droplets move at identical speeds and cells within their droplet are readily tracked [32,33].
Other containment-based IBCS devices restrict cell movement by docking or attaching cells to stationary structures, dead-end compartments or controlling fluid flow with valves. For example, closing valves around a cell in a microchannel creates a reversible microcontainer for stationary cell imaging with the cell released immediately after valve opening and flow reinitiation (Figure 3D) [35]. Coated nanostructured substrates (NanoVelcro) capture selective cell types, typically circulating tumor cells (CTC), using affinity tags and microflow strategies to ensure cell contact with the surface. After imaging, cells are detached in bulk by competitive binders so capture of a single cell is not likely with this technology [36]. The limited velocity of cells supports higher spatial resolution images as well as the integration of other assays, such as measurement of secreted molecules [37]. While these devices are characterized by a greatly reduced throughput, the microdevice designs and inexpensive detection strategies lend themselves to highly parallelized operations that in the future will undoubtedly increase throughput [37]. An asset of these IBCS strategies is the ability to use pre-existing imaging hardware and software, including convolutional neural networks and machine-learning strategies [34,35]. Similar to microfluidic flow IBCS, computational algorithms rapidly classify cells into categories in preparation for sorting. Integration of deep learning with microfluidics will add greater potential for container-based IBCS in the future [25].
After selected cells are identified for capture, a multitude of forces are available to direct cells to appropriate collection receptacles. Re-initiation of fluid flow in the absence or presence of a valve is commonly utilized [35,38]. Spatial modulation of electrical fields, e.g. DEP, efficiently directs droplet and cell movement into appropriate channels [33,34]. As mentioned above, release of cells captured by NanoVelcro is initiated by introduction of competitive binders or alternatively by temperature changes or laser-based substrate cutting, each strategy with a different efficiency and throughput [36]. Both optical and magnetic forces are readily utilized to push both cells and droplets, for example forces originating from pulsed microbeam cavitation or laser tweezers [31,39]. A clear advantage of containment IBCS is the plethora of sort modalities that can be integrated into the microdevices. Other advantages include the facile integration of other devices in series either prior to or after the sort point as well as the suitability of microfluidic containment IBCS for small sample sizes [37]. For instance, an automated microfluidic system was developed to study dynamic tumor necrosis factor (TNF) secretion from stimulated macrophages using time-lapse imaging (Figure 3C) [37]. Within microfluidic channels, secretion was measured from individual cells using an on-chip bead-based immunoassay demonstrating a powerful method of cell identification prior to sorting illustrating the potential for assay integration on microfluidic platforms. In general, the microfluidic containment IBCS platforms require less expensive imaging instrumentation than flow platforms; however microfluidic containment IBCS platforms often require additional microfluidic components leading to more complex devices and a need for proficiency in microfluidic design and fabrication to unlock the full potential of microfluidic containment IBCS.
Microarray IBCS Platforms
Cell-based microarrays enable high-throughput and high-content screening for a variety of single-cell applications. IBCS arrays employ wells, traps or patterning to array cells that are typically plated in a stochastic fashion followed by manual or automated assays [40,41]. Positioning cells at fixed addresses solves multiple imaging challenges. Very high-quality images using standard imaging hardware and algorithms along with facile data processing are a distinct advantage of imaging stationary cells (Figure 4, Table 1). Moreover, individual cells can be imaged repeatedly to produce temporal sequences of cellular attributes in response to stimuli, enabling kinetic studies of viable cells prior to a sort decision [42]. Conversely, a fundamental limitation has been the slow rate of cell collection often by manual picking [43]. Fortuitously, innovative approaches have been developed to collect viable cells at a significant rate from planar arrays into secondary vessels for analysis or cloning [44,45]. Integrating improved software algorithms for image cytometry and automated hardware for manipulation of target cells has advanced these platforms by increasing the throughput of image analysis, sort decision and cell collection, albeit at slower sort rates compared to that of flow and container IBCS [46–49].
Figure 4. Overview of Microarray-Based IBCS.

(A) Workflow for microarray platforms used in IBCS. (B) Sorting using confocal imaging on microraft arrays. Adapted from [58] with permission. (C) Microchip for DEP arraying and sorting of cells. Adapted from [51] with permission. (D) Sorting cells based on temporal imaging after placement into arrayed docking sites. Adapted from [54] with permission.
Four steps are employed when sorting cells from a microarray. Cells are plated, generally as single cells in suspension, on as many array address sites as possible. Once seeded, the cells are immobilized for imaging. After target cell identification, the cells of interest are collected. Efficiently seeding cells on the array, particularly at the single-cell level, is often challenging due to failing to fill all the addresses on the array. Loading methods relying on Poisson statistics provides the greatest number of sites with a single cell, but most addresses (~75%) remain empty or contain more than one cell [45,48,50]. A more deterministic approach has used DEP in a microfluidic device to load 35% of cell “cages” or array sites (Figure 4C) [51]. Arrayed weir-type traps can be loaded with excess cells to fill all array sites, although cells can be challenging to collect from the traps [52–54]. The efficiency of loading cell-based arrays remains an area that would benefit from technical advancement.
Array-based IBCS techniques have used a variety of strategies to maintain cells at known locations during imaging. The simplest strategy is cell attachment to the array surface often using a base layer of extracellular matrix, such as fibronectin [50], Matrigel™ [48] or collagen [49]. For non-adherent cells, a simple strategy that does not require a coating is the use of deep wells that act as physical containers with or without an overlaid hydrogel [45,55]. Alternative approaches to maintain nonadherent cell positioning during imaging include DEP [51,56], use of flow-based forces to hold cells in physical traps [54,55], and magnetic forces applied to immunomagnetically labeled cells [57]. For DEP, an integrated bipolar electrode immobilizes the arrayed cells [56]. A single pore membrane in which a cell is trapped by suction or a weir that physically traps cells loaded hydrodynamically are examples (Figure 4D) [54,55]. These methods typically require careful selection of the pore size or trap geometry for the particular cell type. Once immobilized, cells are imaged manually or via any number of automated modalities. The strength of array-based sorting strategies is the ease with which an extensive list of selection criteria can be utilized, including time varying attributes and properties measurable only at high spatial resolution, i.e. confocal images (Figure 4B) [49,58].
While cell-based arrays are well suited for image cytometry applications, the various approaches to immobilizing cells for image collection will impact the method needed for eventual cell isolation. The most common method is suction removal using a microcapillary pipet. This approach is low throughput and manually intensive, and for cloning the loss of cell viability can be a problem, particularly for retrieval of adherent cells. When combined with an automated micromanipulator, high yield and purity can be achieved, albeit still at low throughput in comparison to flow or container-based IBSC [54,57]. Use of DEP for cell retrieval from arrays can have recovery rates as high as 0.1 cell/s with excellent efficiency [51]. A unique cell-retrieval approach utilizes a motorized microneedle to dislodge magnetic cell carriers held within microwells followed by collection with a magnetic wand. This method has a demonstrated collection rate of 0.02–0.2 cells/s with a purity nearing 100%, and is compatible with confocal imaging [44,45,48,58]. The automated isolation of non-adherent cells has also been described with this technique [45,47]. Cells trapped in a pore at the base of a microwell can also be retrieved using a concave-tipped needle to punch out the bottom of the microwell and deposit the cell in a microcentrifuge tube at a rate of 1 cell/s and isolation efficiency ≥90% [55]. The retrieval techniques described above and the implementation of automation have made possible efficient and automated collection of target cells from planar arrays. This unique approach to IBCS enables facile correlation of diverse phenotypic criteria combined with downstream genetic analysis or cloning. In one example, T cells were assayed for antigen-specific killing of target cells on an array, isolated based on killing proficiency, and sequenced for their T cell receptor [46]. This application required the ability to monitor cell-to-cell interactions over time, which is not possible using microfluidic flow IBCS.
Microarray systems provide the advantage of low complexity and cost compared to their microfluidic flow and containment counterparts. Microarrays do not typically require fluidic components and can be imaged using traditional imaging setups, such as fluorescence microscopes and CMOS cameras, resulting in relatively low-cost imaging and computation. As with the other technologies, microfabrication infrastructure is required to produce the microarrays. However, fabrication complexity can range from low-to-high depending on the application requirements. The mechanical or optical methods utilized to retrieve cells from microarrays do add complexity to these systems.
Concluding Remarks and Future Directions
The platforms developed for IBCS come in many forms, including microfluidic flow, microfluidic containment, and microarray systems, each having unique performance characteristics (Table 1). Microfluidic flow IBCS are attractive for quickly sorting large input samples, with an emphasis on throughput and speed. Microfluidic containment IBCS analyze smaller sample sizes with intermediate throughput but provide high flexibility in imaging strategies and sample manipulation. Microarray IBCS allow cells to be imaged over time at exceptionally high spatial resolution to dissect cell-cell interactions and cellular dynamics and are compatible with a vast number of standard imaging modalities, including confocal microscopy. The ability to collect cells based on image-derived information provides the opportunity to tie physiologic and morphologic attributes to the powerful pipelines developed for omics data such as DNA sequencing, RNA expression, protein quantification and lipid assays. Collected viable cells have the potential to be expanded for personalized diagnostic/toxicologic assays, cell-based therapeutics, and large-scale biomedical hypothesis testing.
While IBCS platforms have made significant progress in recent years, hurdles must be overcome for widespread adoption (see Outstanding Questions). The biological applications presented in IBCS research have primarily focused on proof-of-concept experiments and many still require additional development for widespread research, clinical or industrial adoption. For instance, IBCS systems will require full automation with easy-to-use interfaces along with cost effective consumable microfluidic and microarray components for incorporation into clinical or drug discovery workflows. An attractive aspect of the presented systems is their potential for scalability through fabrication of arrays of microdevices with the attendant enhancements in throughput. Finally, systems that rely on ML algorithms will require simple, automated training to appeal to the widest range of users. The development of such turnkey systems with robust and automated hardware and workflows will enable these technologies to reach beyond the research bench, including into medical diagnostic labs and pharmaceutical development pipelines.
Outstanding Questions.
How can IBCS throughput be increased without jeopardizing spatial resolution, sort purity or yield?
How can microfluidic and microarray IBCS platforms be scaled and validated for clinical and industrial applications?
End-to-end documentation and accessible user-interfaces must be integrated in IBCS systems for adoption in clinical settings. What other enhancements will these platforms need before they are useful, everyday diagnostic tools?
How will commercial systems use deep neural networks and how will non-specialists train networks for cell classification?
How will standardized online training data repositories be developed so that they become critical aspects of high-throughput IBCS systems?
Will microdevices continue to be the primary platform for IBCS or will forms of macro-devices emerge as promising alternatives?
What innovations are needed to combine single cell sorting and sequencing in a single instrument?
Highlights.
Advancements in imaging and computational decision-making have given rise to a new method for classifying and isolating single cells from samples which addresses major limitations of commonly used technologies, for example, fluorescence activated cell sorting (FACS)
Microfluidic flow, microfluidic containment, and microarray-based systems have been utilized to separate cells utilizing high-spatial resolution and real-time kinetic information
From inexpensive and flexible in-lab devices to precise clinical tools, image-based cell sorting (IBCS) systems have broad potential
Machine learning and deep neural networks can be combined with IBCS for intelligent cell selection and isolation
Microfluidics and microarrays can be combined in single IBCS platforms, taking advantage of both technologies
Acknowledgements
This work was supported by the National Cancer Institute awards CA224763 and CA233811.
Glossary
- Confocal Imaging
An optical imaging device which scans highly focused light across an object to collect a 3-dimensional representation
- Deep Neural Networks
Form of artificial neural network with three or more hidden layers connecting the input and output layers, often used for classification and prediction modeling
- Dielectrophoresis (DEP)
A process in which a non-uniform electric field exerts a force on an object, guiding it to a specific location or holding the object in place.
- Fluorescence-Activated Cell Sorting (FACS)
Instrument that interrogates cells flowing through a sheath-flow cuvette based on emitted fluorescence and light scattering measurements followed by droplet formation and sorting into a collection chamber
- Image-Based Cell Sorting (IBCS)
A technique which images cells in 2 or greater dimensions and then utilizes these data to sort cells based on spatial and temporal attributes
- Machine Learning (ML)
Form of artificial intelligence that allows computers to learn from past information and improve without the need for user input and programming
- Microarray
Precisely organized wells, traps or coatings with micron-scale features on a 2-dimensional surface
- Microfluidics
Manipulation of a liquid within micron-scale features to gain precise control of an object or fluid
- Support Vector Machine (SVM)
Supervised machine learning technique for classification and regression analysis which separates data into two groups with a hyperplane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
N.L.A. and C.E.S. disclose a financial interest in Cell Microsystems, Inc. All other authors declare no conflicts.
References
- 1.Bossel Ben-Moshe N et al. (2019) Predicting bacterial infection outcomes using single cell RNA-sequencing analysis of human immune cells. Nat. Commun 10, 3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buenrostro JD et al. (2018) Integrated Single-Cell Analysis Maps the Continuous Regulatory Landscape of Human Hematopoietic Differentiation. Cell 173, 1535–1548.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C et al. (2018) Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 173, 879–893.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra AK et al. (2016) Single-cell analysis of targeted transcriptome predicts drug sensitivity of single cells within human myeloma tumors. Leukemia 30, 1094–1102 [DOI] [PubMed] [Google Scholar]
- 5.Krieg C et al. (2018) High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med 24, 144–153 [DOI] [PubMed] [Google Scholar]
- 6.Valihrach L et al. (2018) Platforms for single-cell collection and analysis. Int. J. Mol. Sci 19, 22–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalili A et al. (2019) A review of sorting, separation and isolation of cells and microbeads for biomedical applications: microfluidic approaches. Analyst 144, 87–113 [DOI] [PubMed] [Google Scholar]
- 8.Rothbauer M et al. (2018) Recent advances in microfluidic technologies for cell-to-cell interaction studies. Lab Chip 18, 249–270 [DOI] [PubMed] [Google Scholar]
- 9.Sackmann EK et al. (2014) The present and future role of microfluidics in biomedical research. Nature 507, 181–189 [DOI] [PubMed] [Google Scholar]
- 10.Convery N and Gadegaard N (2019) 30 years of microfluidics. Micro Nano Eng. 2, 76–91 [Google Scholar]
- 11.Cossarizza A et al. (2017) Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol 47, 1584–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Y et al. (2016) Review: imaging technologies for flow cytometry. Lab Chip 16, 4639–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goda K et al. (2019) In Flow Cytometry, Image Is Everything. Cytom. Part A 95, 475–477 [DOI] [PubMed] [Google Scholar]
- 14.Lei C et al. (2018) High-throughput imaging flow cytometry by optofluidic time-stretch microscopy. Nat. Protoc 13, 1603–1631 [DOI] [PubMed] [Google Scholar]
- 15.Han Y et al. (2019) Cameraless high-throughput three-dimensional imaging flow cytometry. Optica 6, 1297 [Google Scholar]
- 16.Nitta N et al. (2018) Intelligent Image-Activated Cell Sorting. Cell 175, 266–276.e13 [DOI] [PubMed] [Google Scholar]
- 17.Isozaki A et al. (2019) A practical guide to intelligent image-activated cell sorting. Nat. Protoc 14, 2370–2415 [DOI] [PubMed] [Google Scholar]
- 18.Ota S et al. (2018) Ghost cytometry. Science 360, 1246–1251 [DOI] [PubMed] [Google Scholar]
- 19.Gu Y et al. (2019) Machine Learning Based Real-Time Image-Guided Cell Sorting and Classification. Cytom. Part A 95, 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitta N et al. (2020) Raman image-activated cell sorting. Nat. Commun 11, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isozaki A et al. (2020) Intelligent image-activated cell sorting 2.0. Lab Chip 20, 2263–2273 [DOI] [PubMed] [Google Scholar]
- 22.Nawaz AA et al. (2020) Intelligent image-based deformation-assisted cell sorting with molecular specificity. Nat. Methods 17, 595–599 [DOI] [PubMed] [Google Scholar]
- 23.Odaka M et al. (2019) Size Distribution Analysis with On-Chip Multi-Imaging Cell Sorter for Unlabeled Identification of Circulating Tumor Cells in Blood. Micromachines 10, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas RSW et al. (2019) Image-based sorting and negative dielectrophoresis for high purity cell and particle separation. Electrophoresis 40, 2718–2727 [DOI] [PubMed] [Google Scholar]
- 25.Riordon J et al. (2019) Deep Learning with Microfluidics for Biotechnology. Trends Biotechnol. 37, 310–324 [DOI] [PubMed] [Google Scholar]
- 26.Gupta A et al. (2019) Deep Learning in Image Cytometry: A Review. Cytom. Part A 95, 366–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivakhnenko AG (1968) The Group Method of Data Handling-A Rival of the Method of Stochastic Approximation. Sov. Autom. Control 1, 43–55 [Google Scholar]
- 28.Sun J et al. (2020) Deep Learning-Based Single-Cell Optical Image Studies. Cytom. Part A 97, 226–240 [DOI] [PubMed] [Google Scholar]
- 29.Doan M et al. (2018) Diagnostic Potential of Imaging Flow Cytometry. Trends Biotechnol. 36, 649–652 [DOI] [PubMed] [Google Scholar]
- 30.Cossarizza A et al. (2017) Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol 47, 1584–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segaliny AI et al. (2018) Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip 18, 3733–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girault M et al. (2017) An on-chip imaging droplet-sorting system: A real-time shape recognition method to screen target cells in droplets with single cell resolution. Sci. Rep 7, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sesen M and Whyte G (2020) Image-Based Single Cell Sorting Automation in Droplet Microfluidics. Sci. Rep 10, 8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anagnostidis V et al. (2020) Deep learning guided image-based droplet sorting for on-demand selection and analysis of single cells and 3D cell cultures. Lab Chip 20, 889–900 [DOI] [PubMed] [Google Scholar]
- 35.Yu BY et al. (2018) An integrated microfluidic device for the sorting of yeast cells using image processing. Sci. Rep 8, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jan YJ et al. (2018) NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv. Drug Deliv. Rev 125, 78–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Junkin M et al. (2016) High-Content Quantification of Single-Cell Immune Dynamics. Cell Rep. 15, 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utharala R et al. (2018) A Versatile, Low-Cost, Multiway Microfluidic Sorter for Droplets, Cells, and Embryos. Anal. Chem 90, 5982–5988 [DOI] [PubMed] [Google Scholar]
- 39.Keloth A et al. (2018) Single cell isolation using optical tweezers. Micromachines 9, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindström S and Andersson-Svahn H (2010) Overview of single-cell analyses: microdevices and applications. Lab Chip 10, 3363. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z et al. (2019) Single-cell patterning technology for biological applications. Biomicrofluidics 13, 061502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y et al. (2007) Broadening cell selection criteria with micropallet arrays of adherent cells. Cytom. Part A 71, 866–874 [DOI] [PubMed] [Google Scholar]
- 43.Love JC et al. (2006) A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat. Biotechnol 24, 703–707 [DOI] [PubMed] [Google Scholar]
- 44.Smiddy NM et al. (2020) Microraft array-based platform for sorting of viable microcolonies based on cell-lethal immunoassay of intracellular proteins in microcolony biopsies. Analyst 145, 2649–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaBelle CA et al. (2020) Assay and Isolation of Single Proliferating CD4+ Lymphocytes Using an Automated Microraft Array Platform. IEEE Trans. Biomed. Eng 67, 2166–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attayek PJ et al. (2016) Identification and isolation of antigen-specific cytotoxic T lymphocytes with an automated microraft sorting system. Integr. Biol 8, 1208–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Attayek PJ et al. (2017) Automated microraft platform to identify and collect non-adherent cells successfully gene-edited with CRISPR-Cas9. Biosens. Bioelectron 91, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiSalvo M et al. (2019) Automated sensing and splitting of stem cell colonies on microraft arrays. APL Bioeng. 3, 036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowotarski HL et al. (2020) Automated platform for cell selection and separation based on four-dimensional motility and matrix degradation. Analyst 145, 2731–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westerhof TM et al. (2017) Highly efficient cellular cloning using Ferro-core Micropallet Arrays. Sci. Rep 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Trapani M et al. (2018) DEPArray™ system: An automatic image-based sorter for isolation of pure circulating tumor cells. Cytom. Part A 93, 1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y et al. (2018) Time Sequential Single-Cell Patterning with High Efficiency and High Density. Sensors 18, 3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X and Lee AP (2018) High-throughput microfluidic single-cell trapping arrays for biomolecular and imaging analysis. Methods Cell Biol. 148, 35–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dura B et al. (2016) Longitudinal multiparameter assay of lymphocyte interactions from onset by microfluidic cell pairing and culture. Proc. Natl. Acad. Sci. U. S. A 113, E3599–E3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens M et al. (2018) VyCAP’s puncher technology for single cell identification, isolation, and analysis. Cytom. Part A 93, 1255–1259 [DOI] [PubMed] [Google Scholar]
- 56.Cole RH et al. (2017) Printed droplet microfluidics for on demand dispensing of picoliter droplets and cells. Proc. Natl. Acad. Sci 114, 8728–8733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelep C and Eberhardt J (2018) Automated rare single cell picking with the ALS cellcelector™. Cytom. Part A 93, 1267–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheeler EC et al. (2020) Pooled CRISPR screens with imaging on microraft arrays reveals stress granule-regulatory factors. Nat. Methods 17, 636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]


