Abstract
Objective:
Ovarian cancer (OC) ranks one of the most prevalent fatal tumors of female genital organs. Aberrant promoter methylation triggers changes of microRNA (miR)-375 in OC. Our study aimed to evaluate the mechanism of methylated miR-375 promoter region in OC cell malignancy and to seek the possible treatment for OC.
Methods:
miR-375 promoter methylation level in OC tissues and cells was detected. miR-375 expression in OC tissues and cell lines was compared with that in demethylated cells. Role of miR-375 in OC progression was measured. Dual-luciferase reporter gene assay was utilized to verify the targeting relationship between miR-375 and Yes-associated protein 1 (YAP1). Then, Wnt/β-catenin pathway-related protein expression was tested. Moreover, xenograft transplantation was applied to confirm the in vitro experiments.
Results:
Highly methylated miR-375 was seen in OC tissues and cell lines, while its expression was decreased as the promoter methylation increased. Demethylation in OC cells brought miR-375 back to normal level, with obviously declined cell invasion, migration and viability and improved apoptosis. Additionally, miR-375 targeted YAP1 to regulate the Wnt/β-catenin pathway protein expression. Overexpressed YAP1 reversed the protein expression, promoted cell invasion, migration and viability while reduced cell apoptosis. Overexpressed miR-375 in vivo inhibited OC progression.
Conclusion:
Our study demonstrated that demethylated miR-375 inhibited OC growth by targeting YAP1 and downregulating the Wnt/β-catenin pathway. This investigation may offer novel insight for OC treatment.
Keywords: ovarian cancer, microRNA-375, promoter methylation, yes-associated protein 1, Wnt/β-catenin pathway
Introduction
Ovarian cancer (OC) is a heterogeneous tumor that occurs in malignant tubal epithelium.1, 2 Moreover, OC represents the seventh most prevalent cancer in female and also the eighth leading cause of cancer mortality worldwide, with almost 4% of new female cancer patients.3 For its lack of early symptoms and diagnostic treatment, there is no method to perform accurately diagnosis about OC.4 Now the effective ways to measure OC malignancy are ultrasound score, serum cancer antigen 125 and menopausal status.5 However, in most cases, OC is diagnosed at advanced stage, with 5-year survival rates below 30%.6 Surgery and chemotherapy are practical treatments for OC.7 First line chemotherapy of platinum compounds and taxanes is effective to OC patients, but chemo-resistance and recurrence often occur.2 What’s more, in developing countries, age-standardized rates and number of patients are increasing as life-expectancy improves.8 In this context, novel therapeutic strategies for OC are in urgent need. Toward this, we undertook a microRNA (miR)-based approach to understand the underlying mechanism in OC development, in order to develop novel intervention strategies.
Dysregulated miR is observed in many malignancies indicating tumor suppressive or oncogenic role.9 A previous study suggested that miR-375 functions as a potential biomarker to promote OC diagnosis.10 It has also been demonstrated that miR-375 promoter methylation downregulates its expression.11 In our research, targeting relationship between Yes-associated protein 1 (YAP1) and miR-375 was found. It has been proven that the pivotal significance of activated YAP1 which reprograms cancer cells and incites cancer cell biological behaviors in cancer making it a main target to possible treatment for OC.12, 13 YAP1, the downstream transcription coactivator in the Hippo pathway, was already proven to be an impetus of OC growth, suggesting a poor prognostic indicator.14 Evidence has shown that declined YAP1 leads to highly expressed miR-375 while overexpressed YAP1 results in knockdown of miR-375.15 Besides, our research found multiple relations between YAP1 and Wnt/β-catenin axis. As a typical Wnt-signaling pathway, Wnt/β-catenin pathway is of great importance in preeclampsia by regulating preeclampsia cell biological behaviors.16 YAP1 is revealed to be vital for Wnt/β-catenin signaling activation in various cancer types, and targeted YAP1 suppresses the Wnt/β-catenin pathway.17 The YAP1 and Wnt/β-catenin axis play active roles in maintaining cell homeostasis and controlling colorectal cancer progression.18 From all above, it is reasonable to hypothesize that there may be interactions among miR-375, YAP1 and Wnt/β-catenin axis in OC cell progression. Thus, we conducted a series of experiments to verify the hypothesis.
Materials and Methods
Ethics Statement
This study was approved and supervised by the ethics committee of The Third Affiliated Hospital of Nanchang University(Approval number: 000011). All the subjects have signed the informed consents. Significant efforts were made in order to minimize both the number of animal death and their suffering.
Sample Collection
From September 2016 to September 2018, 50 OC patients treated in The Third Affiliated Hospital of Nanchang University were enlisted in this experiment for gathering of OC tissues and non-tumor tissues. Exclusion criteria were: (1) patients with incomplete clinical data; (2) patients complicated with mental disease or consciousness disorder; (3) patients complicated with other primary malignant tumors, autoimmune diseases, severe organic diseases, dysfunction of important organs and coagulation; (4) juveniles and pregnant or lactating women; (5) allergic constitution or allergic to relevant examination; (6) patients with unqualified magnetic resonance imaging. All the OC patients were diagnosed by pathological examination.
Cell Cultivation
Human OC cell lines SKOV3, PEO1, OVCAR3, A2780 and human ovarian surface epithelial cell (HOSEpiC) purchased from American Type Culture Collection (ATCC, Manassas, Virginia, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM) consisting of 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin (Gibco, ThermoFisher Scientific, Inc., Waltham, Massachusetts, USA). Afterward, these cells were cultured in a group of 3 μmol/L 5-aza-2′-deoxycytidine (Sigma, St Louis, Missouri, USA) for 72 h and a control group of dimethyl sulfoxide (DMSO) to conduct demethylation.19
Methylation-Sensitive High-Resolution Melting (MS-HRM)
The extracted total DNA from cells or tissues was transformed by EpiTect fast DNA Bisulfite kit (Qiagen, Dusseldorf, Germany). Additionally, 1000 mg DNA, 35 µL DNA protective liquid and 85 µL conversion reagent was included in the reaction system. The reaction conditions were 5 min at 95°C, 20 min at 60°C, 5 min at 95°C and 20 min at 60°C. After reaction, the products were purified and recovered with the spin column in the kit and stored at −20°C for further use. MS-HRM was performed on Rotor-gene 6000 (Corbett, Sydney, Australia). Each sample was in duplicate. In every operation, 100%, 50%, 10% and 3% methylation and unmethylated standards were employed to detect the methylation level in every sample. MS-HRM primers were made and synthesized in Sangon Biotechnology Co., Ltd. (Shanghai, China) (Table 1). The 20 µL reaction system was composed by 1 × polymerase chain reaction buffer solution, 1.5-3.0 magnesiun chloride (MgCl2), 200 μM deoxy-ribonucleoside triphosphates mixture, 200-400 nM forward and reverse primers, 1 × SYTO9 intercalation dye (Thermo Fisher Scientific), 0.5 U Hotstar Taq DNA polymerase and 10 ng DNA transformed from bisulfite. Methylation levels were presented by percentage.
Table 1.
Primers Sequence.
| Gene | Primers |
|---|---|
| miR-375 promoter | F: 5’-TATAGTCTGCAAAGTCTTGTA-3’ |
| R: 5’-CGCTCAGGTCCGGTTTGTGCG-3’ |
Note: miR: microRNA.
Cell Transfection
The pcDNA3.1 vector (Invitrogen, Carlsbad, California, USA) was digested with restriction enzyme (EcoR I and Xba I) for linearization. After agarose gel electrophoresis, the DNA fragment was recovered. Then YAP1 cDNA (GenBank: NM_001130145.3) covered the whole genome of normal human ovarian epithelial cells (HOSEPiC) was cloned, sequenced and then connected into the linearized pcDNA3.1 to construct the overexpressed YAP1 vector pcDNA3.1-YAP1. miR-375 mimics, miR-negative control (NC), small interfere (si)-YAP1-1, si-YAP1-2, si-NC (ThermoFisher, Shanghai, China) were designed and synthesized. Lipofectamine 2000 (Invitrogen) was used with a firm compliance to its instructions to conduct transfection.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
TRIzol (Invitrogen) one-step method was applied to extract total RNA of cells and tissues. Next, the extracted qualified RNA was tested by ultraviolet analysis and formaldehyde denaturation electrophoresis. The fluorescent quantitative PCR was conduct based on instructions of RT-qPCR kit (ThermoFisher). PCR primers were made and synthesized in Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China) (Table 2). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized as an internal references of YAP1, and U6 as an internal reference of miR-375. After reaction, amplification curves and dissolution curve were identified. The relative expression was calculated by 2−ΔΔCt method.
Table 2.
List of Primers.
| Gene | Primer sequence |
|---|---|
| YAP1 | F: 5′-GCCATGGATCCCGGGCAGCAG-3′ |
| R: 5′-GGGCTCTATAACCATGTAAGA-3′ | |
| GAPDH | F: 5′-GGGAGCCAAAAGGGTCAT-3′ |
| R: 5′-GAGTCCTTCCACGATACCAA-3′ | |
| miR-375 | F: 5′-CCCCGCGACGAGCCCCTCGCAC-3′ |
| R: 5′-GCCTCACGCGAGCCGAACGAA-3′ | |
| U6 | F: 5′-CGCTTCGGCAGCACATATAC-3′ |
| R: 5′-AATATGGAACGCTTCACGA-3′ |
Note: YAP1: Yes-associated protein 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; miR: microRNA.
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) Assay
Differentially treated cells in exponential phase were prepared at 1 × 105 cells/mL for single-cell suspension. Then, these cells were inoculated into a 96-well plate, 200 µL per well. Cell viability was detected by 20 µL MTT solution (5 mg/mL) (Sigma, St. Louis, Missouri, USA) after 48 h. Each well was added with 150 µL DMSO dissolved crystallization for 15 min. Each well’s absorptivity ability was tested under 490 nm wavelength by a microplate reader. Cell proliferation rate was calculated by (experimental group A490-blank control group A490)/(NC group A490-blank control group A490) ×100%.
Transwell Assay and Scratch Test
Differentially treated SKOV3 or PEO1 cells were collected to be mixed with serum-free medium consisting of 0.1% albumin bovine serum (BSA) for resuspension to 1 × 105 cell/mL on the upper chamber. The lower chamber was added with DMEM with 10% FBS. Transwell was placed in a 37°C incubator with 5% CO2 for 24 h. The filter membrane was taken out, washed by phosphate buffered saline (PBS), and fixed with 0.5% glutaraldehyde. After that, the filter membrane was added into a 37°C crystal violet staining solution with 5% CO2 for 24 h. Under the microscope, 5 visual fields (200 ×) were randomly chosen to be counted.
Differentially treated SKOV3 or PEO1 cells was collected to be detached by 0.25% trypsin and was resuspended by complete medium with serum to 2 × 105 cell/mL. Then, these cells were inoculated into a 37°C 6-well plate, 2 mL per well with 5% CO2 for 12 h. Pipette tip was applied to draw a straight line perpendicular to the center on the cell surface after cell adherence. When the floating cells were washed by PBS, cells were cultured for another 24 h and photographed under the microscope. Image J software (National Institutes of Health, Bethesda, Maryland, USA) was used to analyze the width of cell scratch in different groups at different time points and calculate cell migration rate, which was equivalent to 0 h scratch width—24 h scratch width)/0 h scratch width ×100%
Cell Apoptosis Assay
The collected cells were then resuspended to 1 × 106 cells/mL and mixed and cultured with 500 µL cell suspension, 5 µL Annexin V-fluorescein isothiocyanate and 10 µL propidium iodide (Invitrogen) for 10 min in the dark before verified with flow cytometry.
Immunocytochemistry (ICC)
OC cells were placed on a dish with a cover glass to cultivate slides. When the cover glass was full of cells, the culture medium was discarded. After washed by PBS, the cover glass was fixed by 4% formaldehyde for 15 min and incubated in 0.3% Triton X-100 for 20 min. After a PBS wash, the cover glass was incubated in 1% BSA for 30 min. After that, the cover glass was incubated with primary antibodies at 37°C for 1 h. After washed by PBS, the cover glass was incubated with secondary antibody in a wet box for 30 min. The cover glass was washed by PBS again, visualized with normal 2,4-diaminobutyric acid, counterstained with hematoxylin and sealed for observation after dehydration and clearing. The antibodies used were E-cadherin (1:100, ab194982, Abcam, Cambridge, Massachusetts, USA), and IgG (1:200, ab97196, Abcam).
Western Blot Analysis
Protein from each sample was counted compliance with the instructions of bicinchoninic acid kit (ThermoFisher Scientific). With the loading buffer added, the extracted protein was boiled at 95°C for 10 min. Next, 30 µg of protein was separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% (w/v) when the voltage changed from 80 v to 120 v). The proteins were gradually changed to the polyvinylidene fluoride membrane by wet transformation at 100 mv for 45-70 min. The membrane was sealed for 1 h in 5% BSA, then cultured with primary antibodies at 4°C overnight. Afterward, the membrane was washed 3 times in tris-buffered saline tween (TBST), and then it was incubated with horseradish peroxidase-conjectured secondary antibody IgG for 1 h. After fully TBST washes, the membrane was visualized by electrochemiluminescence regent and the Bio-Rad Gec EZ imager (Bio-Rad, California, USA). The targeting relationship was verified by Image J software (National Institutes of Health) for gray value analysis. The antibodies used and the concentration of incubation were YAP1 (1:5000, ab52771, Abcam), E-cadherin (1:1000, ab76055, Abcam), IgG (1:200, ab97196, Abcam), Wnt1 (1:1000, ab15251, Abcam), β-catenin (1:1000, ab16051, Abcam), and β-actin (1:1500, ab8227, Abcam).
Xenograft Tumors in Nude Mice
Eighteen female BALB/c nude mice (20 ± 2 g) (Jingdi biomedicine Co., Ltd, Shanghai, China) were free to food and water, a d were numbered with body weight and randomly split into the SKOV3 group, SKOV + miR-NC group and SKOV3 + miR-375 mimics group, with 6 mice in each group. A collection of 200 µL untreated SKOV3 cells were subcutaneously injected into mice in the SKOV3 group, with 1 × 107 cells in each mouse. A total of 200 µL SKOV3 cells transfected with miR-NC were subcutaneously injected into mice in the SKOV3 + miR-NC group, with 1 × 107cells in each mouse for the first 7 days, after that, miR-NC was injected into mice intratumorally 7 days, with 2 mg in each mouse. A total of 200 µL SKOV3 cells transfected with miR-375 mimics were subcutaneously injected into mice in the SKOV3 + miR-375 mimics group, with 1 × 107 cells in each mice for the first 7 days, after that, miR-375 mimics was injected into mice intratumorally every 7 days, with 2 mg in each mouse. Methods of intratumoral injection refer to a previous literature.20 From the seventh day of SKOV3 injection, the volume of the tumors came from the formula (length × width)2 × 0.5 every 3 days. The tumors were selected and weighed after 28 days. Three mice tumors in each group were chosen to be paraffin-embedded and sliced. Ki67 (1:200, ab15580, Abcam) expression in tumor tissues was detected by immunohistochemistry. The remaining 3 tumors were grinded into homogenate for further detection.
Statistical Analysis
SPSS 21.0 (IBM Corp. Armonk, NY, USA) was employed to get data analysis. Kolmogorov-Smirnov test indicated whether the data were in normal distribution. The results were showed in mean ± standard deviation. The t test was used for analyzing comparisons between 2 groups, one-way analysis of variance (ANOVA) or two-way ANOVA for comparing different groups, and Tukey’s multiple comparisons test for pairwise comparisons after ANOVA. P was attained using a 2-tailed test and p < 0.05 indicated a significant difference.
Results
miR-375 Promoter Is Methylated in OC
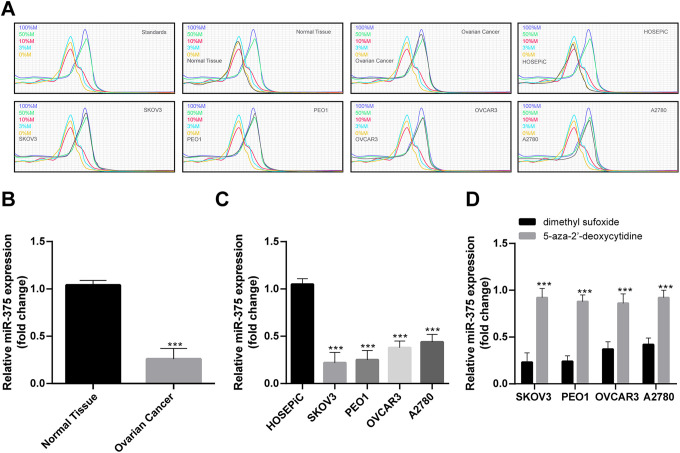
Abnormal expression and methylation are important indicators in the mechanism of genes encoding proteins or miRNAs in the progression of cancer as oncogenes or tumor suppressor genes. miR-375 represented one of the most uncontrollable miRNA in common cancers given its dual function.21 Since miR-375 shown significant association with hypermethylation of the genes in OC,22 50 pairs of cancerous tissues and non-cancerous tissues in OC patients were collected to measure miR-375 promoter methylation level in OC by MS-HRM. Meanwhile, miR-375 promoter methylation level in 4 kinds of human OC cell lines and normal HOSEPiC cell line were also determined. miR-375 promoter in OC tissues was higher methylated than that in non-cancerous tissues, what’s more, various levels of methylation in the 4 kinds of cell lines were observed, and SKOV3 and PEO1 cells were the ones with the highest methylation level (Figure 1A). RT-qPCR indicated that miR-375 was poorly expressed in tissues and cell lines where miR-375 promoter was hypermethylated, and miR-375 expression in OC cell lines was back to normal after being treated by demethylated 5-aza-2′-deoxycytidine (Figure 1B-D). Therefore, SKOV3 and PEO1 cells were chosen to conduct further experiments.
Figure 1.
miR-375 promoter is methylated in OC. (A) miR-375 promoter methylation in OC tissues, non-cancerous tissues and OC cell lines was detected by MS-HRM and the dissolution curve was represented by standards. In every sample, 0%, 3%, 10%, 50%, and 100% standards were included. Methylation levels were identified by comparison between sample dissolution and standards. miR-375 promoter methylation was obviously increased in OC tissues, SKOV3 and PEO1 cells were the ones with the highest methylation level in OC cell lines. (B-D) miR-375 expression in OC tissues, non-cancerous tissues and OC cell lines was detected by RT-qPCR. 5-aza-2′-deoxycytidine is a demethylation drug, dimethyl sufoxide is its control. (B) Compared with normal tissue, (C) compared with HOSEPiC, (D) compared with dimethyl sulfoxide. ***p < 0.001. (B) and (C) were analyzed by one-way ANOVA, (D) was analyzed by two-way ANOVA. Tukey’s multiple comparisons test was applied to determine statistical significance, N = 50 in OC tissues and non-cancerous tissues. Repetitions = 3 in cell line assay. OC, ovarian cancer; miR, microRNA; MS-HRM, methylation-sensitive high-resolution melting; RT-qPCR, reverse transcription quantitative polymerase chain reaction; HOSEPiC, human ovarian surface epithelial cell; ANOVA, analysis of variance.
Overexpressed MiR-375 Inhibits OC Cell Biological Behaviors, While Induces Its Apoptosis
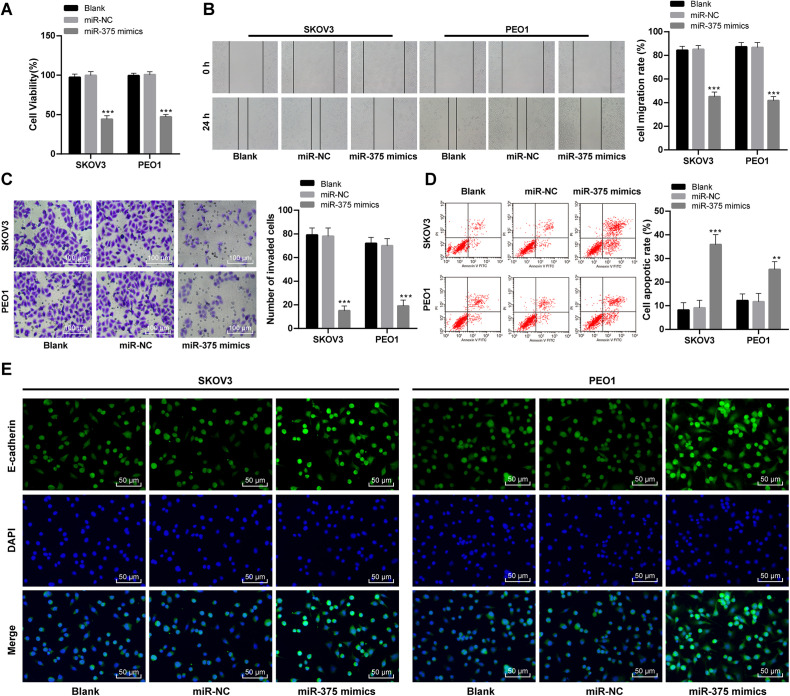
To find out the role of aberrantly methylated miR-375 promoter region in OC pathological processes, SKOV3 and PEO1 cells were transfected together with miR-375 mimics to overexpress miR-375. SKOV3 and PEO1 cell viability was significantly declined with overexpressed miR-375 as showed by MTT assay (p < 0.05; Figure 2A). Transwell assay and scratch test discovered that as miR-375 expressed higher, SKOV3 and PEO1 cell invasion and migration were remarkably decreased (Figure 2B-2C) while cell apoptosis was notably increased (Figure 2D). E-cadherin antibody was used to perform ICC on SKOV3 and PEO1 cells, as the result showed, E-cadherin expression in SKOV3 and PEO1 cells was greatly promoted when miR-375 was overexpressed (Figure 2E). These results revealed that OC cell malignancy was suppressed with overexpressed miR-375.
Figure 2.
Overexpressed miR-375 in OC cell inhibits its malignancy. (A) OC cell viability before and after miR-375 overexpression was detected by MTT assay, (B) scratch test was employed to test cell migration, one scratch appeared after 0 h, and after 24 h, the scratch became narrower as cell migration improved. Cell migration rate equaled to (0 h scratch width—24 h scratch width)/0 h scratch width ×100%, (C) cell invasion was tested with Transwell assay. (D) Cell apoptosis was verified with flow cytometry. (E) ICC and DAPI was utilized to measure E-cadherin expression. Compared with the blank group, ***p < 0.001. Two-way ANOVA and Tukey’s multiple comparisons test were applied to determine statistical significance, repetitions = 3. OC, ovarian cancer; miR, microRNA; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; ICC, immunocytochemistry; DAPI, 4′, 6-diamidino-2-phenylindole; ANOVA, analysis of variance.
miR-375 Targets YAP1 to Regulate the Wnt/β-catenin Pathway-Related Protein Expression
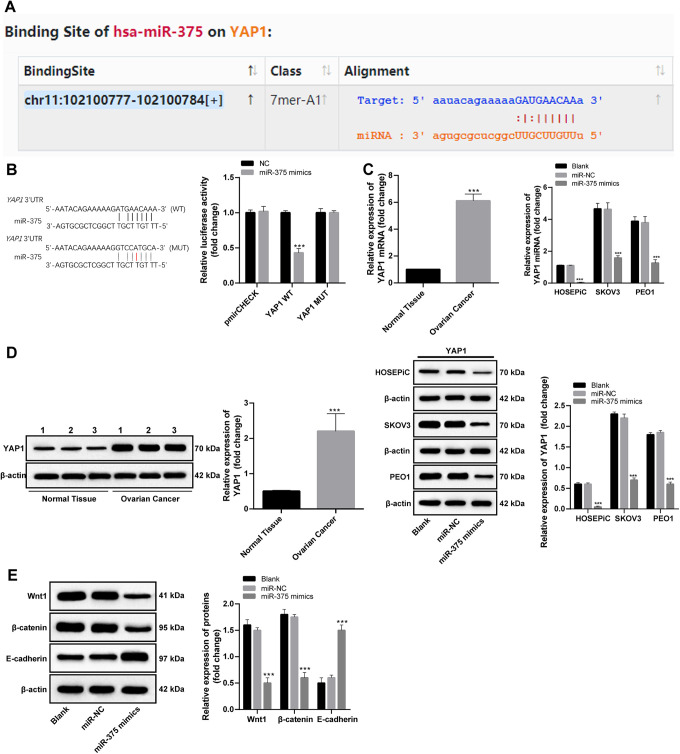
Targeting relationship between YAP1 and miR-375 was noticed during searching the targeting genes of miR-375 in database (http://starbase.sysu.edu.cn) (Figure 3A). The targeting relationship was proved by dual-luciferase reporter gene assay (Figure 3B). RT-qPCR and Western blot analysis found that YAP1 expression was noticeably increased in OC tissues (p < 0.05), SKOV3 and PEO1 cells (p < 0.05), and was expressly decreased as miR-375 was overexpressed (p < 0.05) (Figure 3C, D).
Figure 3.
Target relationship between miR-375 and YAP1 and expression of related proteins in the Wnt/β-catenin axis. (A) database (http://starbase.sysu.edu.cn) sought the targeting point of miR-375 and YAP1; (B) targeting relationship between miR-375 and YAP1 was verified by dual-luciferase reporter gene assay, compared with negative control group, ***p < 0.001; (C, D) YAP1 expression in OC tissues, non-cancerous tissues and OC cell lines was detected with RT-qPCR and Western blot analysis, compared with control group, ***p < 0.001; (E) Wnt/β-catenin signaling pathway-related protein after overexpressing miR-375 was measured with Western blot analysis, compared with control group, ***p < 0.001; two-way ANOVA and Tukey’s multiple comparisons test was applied to determine statistical significance, Repetitions = 3. miR: microRNA; YAP1: Yes-associated protein 1; OC: ovarian cancer; RT-qPCR: reverse transcription quantitative polymerase chain reaction; ANOVA: analysis of variance.
There were multiple associations between the Hippo axis and the Wnt/β-catenin axis.23 Detection on Wnt/β-catenin signaling pathway-related protein in SKOV3 cells revealed that with overexpressed miR-375 declined the expression of Wnt1 and β-catenin significantly (p < 0.05), while E-cadherin expression was evidently improved (p < 0.05; Figure 3E).
Overexpressed YAP1 Enhances OC Cell Malignancy Inhibited by Overexpressed miR-375
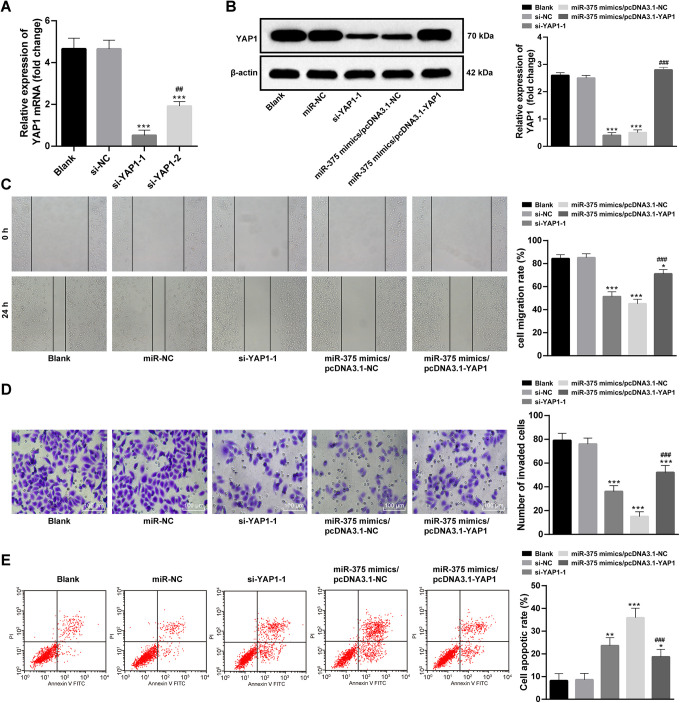
YAP1 was intervened by transfected with si-YAP1 in SKOV3 cells. Firstly, two si-YAP1 s were designed and synthesized, and si-YAP1-1 was found to have a more powerful intervention capacity in SKOV3 cells (Figure 4A), so it was utilized in further experiments. YAP1 expression in SKOV3 cells was dramatically declined after being intervened (Figure 4B), with an evident decrease of SKOV3 cell invasion and migration (Figure 4C, D) and a significant increase of cell apoptosis (Figure 4E). In the meantime, pcDNA3.1-YAP1 was transfected in SKOV3 cells with overexpressed miR-375, after that, YAP1 expression was remarkably promoted (p < 0.05; Figure 4B). Compared with controlled SKOV3 cells, SKOV3 cells with overexpressed YAP1 was more active in invasion and migration (Figure 4C, D), but was less powerful in apoptosis (Figure 4E).
Figure 4.
Alteration of OC cell malignancy after intervening YAP1 expression. (A) Effects of siRNA after being transfected were calculated by RT-qPCR, compared with control group, ***p < 0.001. Compared with si-YAP1-1,## p < 0.01; (B) Western blot analysis was used in detecting YAP1 expression in differentially treated SKOV3, compared with control group, ***p < 0.001. Compared with miR-375 mimics/pcDNA3.1-NC, ### p < 0.001; (C-E) scratch test, Transwell assay and flow cytometry were applied to determine biological behaviors in differentially treated cells respectively, compared with control group, *p < 0.05, **p < 0.01, ***p < 0.001. Compared with miR-375 mimics/pcDNA3.1-NC, ### p < 0.001; one-way ANOVA and Tukey’s multiple comparisons test was applied to determine statistical significance, Repetitions = 3. YAP1: Yes-associated protein 1; OC: ovarian cancer; si: small interfere; RT-qPCR: reverse transcription quantitative polymerase chain reaction; miR: microRNA; ANOVA: analysis of variance.
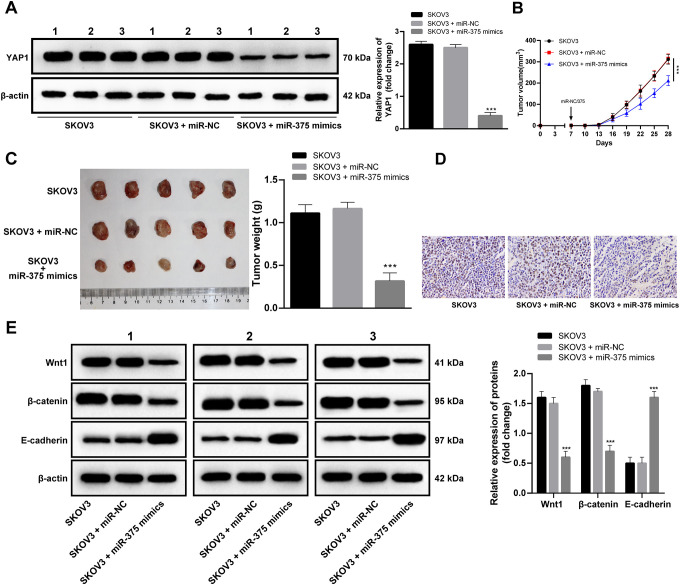
Overexpressed miR-375 In Vivo Inhibits OC Progression
Then, the subcutaneous tumorigenesis mouse models in OC was formed and miR-375 mimics was injected into mice. As miR-375 overexpressed, YAP1 expression in mice tumor tissues declined (Figure 5A), mice tumor growth slowed down significantly (Figure 5B, C), Ki67 positive rate reduced in mice tumor tissues (Figure 5D), Wnt1 and β-catenin expression decreased notably (p < 0.05), and E-cadherin expression increased noticeably (p < 0.05; Figure 5E). In a word, enhancement of miR-375 played an important role in inhibiting OC development.
Figure 5.
Overexpressed miR-375 in vivo inhibits OC progression. (A) Western blot analysis was employed to determine YAP1 expression in vivo after miR-375 mimic were injected into mice via tail vein. (B, C) Mice weight and volume measurement was obtained. Tumor volume (mm3) equaled to (length × width)2 × 0.5; (D) ki67 expression in tumor was tested with IHC; (E) Wnt/β-catenin signaling pathway-related protein expression in tumor tissues was verified by Western blot analysis. Compared with SKOV3 cells, ***p < 0.001. (A) (B) and (C) were analyzed by one-way ANOVA, (E) was analyzed by two-way ANOVA. Tukey’s multiple comparisons test was applied to determine statistical significance, N = 3. miR: microRNA; YAP1: Yes-associated protein 1; IHC: immunohistochemistry; ANOVA: analysis of variance.
Discussion
As a fatal disease in female, OC was always diagnosed at its advanced stages for its symptomless condition and lacking of practical treatments and diagnostic target markers.2 Differentially expressed miRs have been demonstrated to be oncogenes or suppressors in many different cancers.24 As a kind of exosomal miRNA, miR-375 acted as a potential biomarker of OC and was expected to promote OC diagnosis.10 Evidence showed that miR-375 was often downregulated in various kinds of cancer.25 However, miR-375 promoter methylation brought about a knockdown of miR-375.11 In this study, we hypothesized that there can be mechanism of miR-375 promoter methylation in OC cell malignancy by targeting YAP1 and regulating the Wnt/β-catenin pathway. Consequently, our data showed that demethylated miR-375 discouraged OC cell invasion and migration and its overexpression functioned as a suppressor to OC growth.
Firstly, the results of MS-HRM shown that miR-375 promoter was methylated in OC. Prior research has already proven that abnormal promoter methylation obviously downregulated miR-375 expression in cancers.26 Promoter methylation resulted in homologous recombination deficiency in almost half of OC patients.27 Hypermethylation was associated with cisplatin resistance, which would result in a bad treatment outcome.28 Moreover, miR-375 hypermethylation in OC was closely relevant to lymph node and peritoneum metastasis of OC.22 Our study also found that SKOV3 and PEO1 cells were the most methylated cells in OC cells. Recently, a research discovered that SKOV3 cells overexpression was found to be able to enhance tumorigenesis.29 And PEO1 cell was a kind of glycolytic phenotype, which propelled ovarian tumor to conduct metabolic reprogramming and gain more energy.30 What’s more, it was observed that overexpressed miR-375 inhibited OC cell invasion and migration. In a previous study, it was tested that miR-375 overexpression made OC cell more susceptible to ruthenium derived compound, which encouraged OC cell death.31 Interestingly, E-cadherin was increased evidently as miR-375 overexpressed. E-cadherin was closely relative to OC development as the knockdown of E-cadherin demonstrated OC cell development.32 In a word, miR-375 overexpression was proved to greatly refrained OC cell malignancies.
Additionally, dual-luciferase reporter gene assay found a targeting link between miR-375 and YAP1, the effector in Hippo axis. Then, we focused on YAP1. By controlling various organ size, Hippo signaling pathway actively regulated many cancers, including OC.33 As a major component of the Hippo signaling pathway, highly expressed YAP was a sign of low OC survival rates and poor prognosis.34 YAP1 was negatively relevant to Hippo, serving as an oncoprotein to improve cancer cell activity.18 Functional assays implied that ectopic miR-375 suppressed YAP transcriptional viability and protein expression.35 Additionally, highly expressed YAP1 diminished miR-375 effects on inhibiting tumors.36 Importantly, the connection between YAP1 and the Wnt/β-catenin axis was also noticed. Wnt/β-catenin signaling pathway is able to regulate OC cell generation and development by controlling OC cell biological behaviors, making it a possible target in OC treatment.37 There were complex relations between YAP1 and the Wnt/β-catenin signaling pathway: methylated YAP1 contributed to loss of Wnt/β-catenin activation in destruction complex, at the same time, joint with Wnt/β-catenin, YAP1 played a role in its transcription progression.23 A study has revealed that Wnt/β-catenin signaling pathway was activated via suppressing the positive feedback loop of YAP.38 Besides, in our study, we found that overexpressed YAP1 enhanced OC cell malignancy induced by miR-375 overexpression. YAP1 was already proved to be oncogenes in OC malignancy.39 Finally, we drew a conclusion that overexpressed miR-375 in vivo refrained OC growth. Meanwhile, miR-375 expression in cervical cancer (CC) diminished cell progression, while improve cell apoptosis, discouraging CC progression.40 Generally speaking, highly expressed miR-375 was effective in blocking OC growth.
In summary, our study supported that miR-375 inhibited OC malignancy by targeting YAP1 and downregulating the Wnt/β-catenin signaling pathway. These results unveiled a brand-new way to OC treatment. More attention will be paid to seeking reliable therapeutic targets of OC. Although our findings provide therapeutic implication in OC treatment, the experiment results and effective application into clinical practice need further validation.
Footnotes
Authors’ Note: This study was approved and supervised by the ethics committee of The Third Affiliated Hospital of Nanchang University (SYXK2018-0011). All the subjects have signed the informed consents. Significant efforts were made in order to minimize both the number of animal death and their suffering. All the data generated or analyzed during this study are included in this published article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Junjun Shu, MD  https://orcid.org/0000-0002-0654-3650
https://orcid.org/0000-0002-0654-3650
References
- 1. Grunewald T, Ledermann JA. Targeted therapies for ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:139–152. [DOI] [PubMed] [Google Scholar]
- 2. Kujawa KA, Lisowska KM. Ovarian cancer—from biology to clinic [in Polish]. Postepy Hig Med Dosw (Online). 2015;69:1275–1290. [DOI] [PubMed] [Google Scholar]
- 3. Tew WP. Ovarian cancer in the older woman. J Geriatr Oncol. 2016;7(5):354–361. [DOI] [PubMed] [Google Scholar]
- 4. Dong X, Men X, Zhang W, Lei P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer. 2014;51(Suppl 3):e72–e76. [DOI] [PubMed] [Google Scholar]
- 5. Rooth C. Ovarian cancer: risk factors, treatment and management. Br J Nurs. 2013;22(17):S23–S30. [DOI] [PubMed] [Google Scholar]
- 6. Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: cell and molecular processes in cancer metastasis. Am J Physiol Cell Physiol. 2015;309(7) C444–C456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol. 2016;13(4):255–261. [DOI] [PubMed] [Google Scholar]
- 8. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. [DOI] [PubMed] [Google Scholar]
- 9. Khan S, Ayub H, Khan T, Wahid F. MicroRNA biogenesis, gene silencing mechanisms and role in breast, ovarian and prostate cancer. Biochimie. 2019;167:12–24. [DOI] [PubMed] [Google Scholar]
- 10. Su YY, Sun L, Guo ZR, et al. Upregulated expression of serum exosomal miR-375 and miR-1307 enhance the diagnostic power of CA125 for ovarian cancer. J Ovarian Res. 2019;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu SL, Sui YF, Lin MZ. MiR-375 is epigenetically downregulated due to promoter methylation and modulates multi-drug resistance in breast cancer cells via targeting YBX1. Eur Rev Med Pharmacol Sci. 2016;20(15):3223–3229. [PubMed] [Google Scholar]
- 12. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. [DOI] [PubMed] [Google Scholar]
- 13. Zanconato F, Battilana G, Cordenonsi M, Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol. 2016;29:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G, Xie J, Huang P, Yang Z. Overexpression of TAZ promotes cell proliferation, migration and epithelial-mesenchymal transition in ovarian cancer. Oncol Lett. 2016;12(3):1821–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Selth LA, Das R, Townley SL, et al. A ZEB1-miR-375-YAP1 pathway regulates epithelial plasticity in prostate cancer. Oncogene. 2017;36(1):24–34. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Z, Wang X, Zhang L, Shi Y, Wang J, Yan H. Wnt/beta-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review). Mol Med Rep. 2017;16(2):1007–1013. [DOI] [PubMed] [Google Scholar]
- 17. Mazzu YZ, Hu Y, Shen Y, Tuschl T, Singer S. miR-193b regulates tumorigenesis in liposarcoma cells via PDGFR, TGFbeta, and Wnt signaling. Sci Rep. 2019;9(1):3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wierzbicki PM, Rybarczyk A. The Hippo pathway in colorectal cancer. Folia Histochem Cytobiol. 2015;53(2):105–119. [DOI] [PubMed] [Google Scholar]
- 19. Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9(6):435–443. [DOI] [PubMed] [Google Scholar]
- 20. Sur S, Steele R, Shi X, Ray RB. . miRNA-29b inhibits prostate tumor growth and induces apoptosis by increasing bim expression. Cells. 2019;8(11):1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shajari E, Mollasalehi H. Ribonucleic-acid-biomarker candidates for early-phase group detection of common cancers. Genomics. 2020;112(1):163–168. [DOI] [PubMed] [Google Scholar]
- 22. Loginov VI, Pronina IV, Burdennyy AM, et al. Novel miRNA genes deregulated by aberrant methylation in ovarian carcinoma are involved in metastasis. Gene. 2018;662:28–36. [DOI] [PubMed] [Google Scholar]
- 23. Kriz V, Korinek V. Wnt, RSPO and Hippo signalling in the intestine and intestinal stem cells. Genes (Basel). 2018;9(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han RL, Wang FP, Zhang PA, Zhou XY, Li Y. miR-383 inhibits ovarian cancer cell proliferation, invasion and aerobic glycolysis by targeting LDHA. Neoplasma. 2017;64(2):244–252. [DOI] [PubMed] [Google Scholar]
- 25. Hu C, Lv L, Peng J, et al. MicroRNA-375 suppresses esophageal cancer cell growth and invasion by repressing metadherin expression. Oncol Lett. 2017;13(6):4769–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yan JW, Lin JS, He XX. The emerging role of miR-375 in cancer. Int J Cancer. 2014;135(5):1011–1018. [DOI] [PubMed] [Google Scholar]
- 27. Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27(8):1449–1455. [DOI] [PubMed] [Google Scholar]
- 28. Lund RJ, Huhtinen K, Salmi J, et al. DNA methylation and transcriptome changes associated with cisplatin resistance in ovarian cancer. Sci Rep. 2017;7(1):1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang M, Zhao L. CKAP2 promotes ovarian cancer proliferation and tumorigenesis through the FAK-ERK pathway. DNA Cell Biol. 2017;36(11):983–990. [DOI] [PubMed] [Google Scholar]
- 30. Dar S, Chhina J, Mert I, et al. Bioenergetic adaptations in chemoresistant ovarian cancer cells. Sci Rep. 2017;7(1):8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shao X, Mei W, Weng W, et al. Mir-375 enhances ruthenium-derived compound Rawq01 induced cell death in human ovarian cancer. Int J Clin Exp Pathol. 2013;6(6):1095–1102. [PMC free article] [PubMed] [Google Scholar]
- 32. Rosso M, Majem B, Devis L, et al. E-cadherin: a determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS One. 2017;12(9):e0184439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. [DOI] [PubMed] [Google Scholar]
- 34. Xia Y, Chang T, Wang Y, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9(3):e91770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu AM, Poon RT, Luk JM. MicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor properties. Biochem Biophys Res Commun. 2010;394(3):623–627. [DOI] [PubMed] [Google Scholar]
- 36. Kang W, Huang T, Zhou Y, et al. miR-375 is involved in Hippo pathway by targeting YAP1/TEAD4-CTGF axis in gastric carcinogenesis. Cell Death Dis. 2018;9(2):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arend RC, Londono-Joshi AI, Straughn JM, Jr, Buchsbaum DJ. The Wnt/beta-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013;131(3):772–779. [DOI] [PubMed] [Google Scholar]
- 38. Kim W, Khan SK, Gvozdenovic-Jeremic J, et al. Hippo signaling interactions with wnt/beta-catenin and notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127(1):137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fu D, Lv X, Hua G, et al. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer. 2014;21(2):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu X, Zhao W, Yang X, Wang Z, Hao M. . miR-375 affects the proliferation, invasion, and apoptosis of HPV16-positive human cervical cancer cells by targeting IGF-1 R. Int J Gynecol Cancer. 2016;26(5):851–858. [DOI] [PubMed] [Google Scholar]