Abstract
Introduction:
Almost 50% to 70% of patients who undergo axillary lymph node dissection (ALND) because of a single metastatic sentinel lymph node (SLN) have no further metastatic nodes at the axillary histology. On these grounds, the one-step nucleic acid amplification (OSNA) nomogram was designed and validated. As a mathematical model, calculated through tumor size (expressed in millimeters) and CK19 mRNA copy number, it is thought to predict nonsentinel lymph node (NSLN) status. The aim of the study is to verify the diagnostic accuracy of the OSNA nomogram in a group of patients with macrometastatic SLN, with a retrospective analysis.
Methods:
The OSNA nomogram was retrospectively applied to a group of 66 patients with macrometastatic SLN who underwent ALND. The result of the final histology of the axillary cavity was compared to the nomogram prediction. We calculated the prevalence of NSLN metastasis in patients who underwent ALND, sensitivity and specificity, negative and positive predictive value of the nomogram.
Results:
In patients with macrometastasis in SLN, the prevalence of patients with metastatic NSLN was 45%. The sensitivity of the nomogram was excellent (90%). The specificity was low (36%). Positive predictive value amounted to 54%, while negative predictive value was good (81%).
Conclusions:
These results suggest that the OSNA nomogram is a valid instrument that can help choose the best surgical strategy for the treatment of axillary cavity. The mathematical model is useful to avoid surgery in a selected group of patients because it accurately predicts NSLN status.
Keywords: Breast neoplasms, axilla, sentinel lymph node, lymph node excision, nucleic acid amplification techniques, nomograms
Introduction
To date, in patients who undergo sentinel lymph node (SLN) biopsy with one-step nucleic acid amplification (OSNA) analysis, the copy number of cytokeratin (CK)19 mRNA is the only parameter that is considered to decide whether to perform or not complete axillary lymph node dissection (ALND). The CK19 mRNA copy number determines the classification of the SLN in negative, micrometastatic, or macrometastatic.
Complete ALND is performed only if the SLN is macrometastatic. In cases when 2 or more SLN are excised, the lymph node that expresses the highest number of copies is considered for the choice. Almost 50% to 70% of patients, who undergo ALND because of a metastatic SLN, have no further metastatic lymph nodes at the axillary histology, and some authors describe that only 7.9% of them have more than 2 nodes with macrometastasis.1 Therefore, the hypothesis is that, for a group of selected patients, ALND could be safely avoided.2,3 Having a mathematical model that can give an appropriate estimation of the probability of having nonsentinel lymph node (NSLN) metastasis, could be a useful contribution in deciding intraoperatively whether to perform or not ALND. On these grounds, “European OSNA Committee” (a group of 22 European centers where OSNA technique is routinely used), developed a nomogram aimed to predict NSLN status, thus helping to offer the best treatment for each patient.4 OSNA nomogram was designed and validated through a clinical trial involving around 4000 patients having their SLN processed with OSNA.4,5
It appears that predictive models that have a good performance in one population, may not have the same results when applied to other population. The aim of the study was to verify diagnostic accuracy of OSNA nomogram through its retrospective application in a group of patients who already underwent sentinel node biopsy and subsequent ALND, from a population different from the one the model was derived.
Patients and Methods
The aim of the study was to verify diagnostic accuracy of OSNA nomogram in a selected population. One-step nucleic acid amplification nomogram is calculated with 2 parameters. In fact, a multivariate analysis showed that only the CK19 mRNA number (P < .0001) and the size of T (primary tumor) (P < 0.0001) are associated with NSLN metastasis.4 One-step nucleic acid amplification nomogram can be calculated entering the tumor size expressed in millimeters and the CK19 mRNA copy-number in the online calculator on the website www.osnauser.it. The calculation is immediate, and the result is expressed as the probability of having NSLN metastasis (patient-specific class of risk).
During the evaluation phase of the OSNA nomogram, the cutoff of 31% was established as the best compromise between false positive and false negative. Complete ALND could be avoided in patients with nomogram lower than 31% while, when the nomogram is higher than 31%, it should still be recommended.4,5
Our study population included 141 patients who underwent surgery in the service of Breast Surgical Oncology of Modena University Hospital (Italy) in the period from April 2015 to March 2018. The patients enrolled in the study fulfilled the following criteria, which are also resumed in Table 1:
Table 1.
Inclusion criteria.
| Inclusion criteria |
|---|
| • T1-T3 with indication to surgery |
| • N0 clinical and radiological |
| • No prior chemotherapy or hormonal therapy |
| • Intraoperative OSNA analysis of SLN |
| • OSNA with micrometastasis or macrometastasis |
Abbreviations: OSNA, one-step nucleic acid amplification; SLN, sentinel lymph node.
Diagnosis of invasive carcinoma of the breast in stage T1-T3 with indication to surgery as primary therapeutic procedure.
Preoperative clinical and radiological negativity of axillary lymph nodes or suspect lymph nodes but with negative fine needle aspiration cytology (FNAC).
No prior chemotherapy or hormonal therapy.
Intraoperative identification and excision of SLN and OSNA analysis.
Micro or macrometastasis in the OSNA analysis of the SLN.
Each patient was identified with a numerical code, and the following parameters were recorded:
Date of birth.
Histological type of the primary tumor on the definitive histology (ductal infiltrating carcinoma, lobular infiltrating carcinoma, ductal in situ carcinoma).
Biomolecular assessment of the primary tumor (estrogen [ERs] and progesterone receptors [PgRs] expression, human epidermal growth factor receptor 2 [HER2] expression, and proliferation index [MIB1]).
Histological grading of the tumor (G1, G2, and G3).
Tumor size (T) expressed in millimeters on the definitive histology.
Result of the OSNA analysis of the SLN, classified as (+) in case of macrometastasis, (−) in case of micrometastasis. When more than one SLN was excised, we considered the one with the highest number of CK19 mRNA copies.
Nomogram calculation through the website www.osnauser.it and classification in (+) if higher than 31%, (–) if lower than 31%.
State of axillary NSLN in patients who underwent ALND after SLN biopsy, classified as (POSITIVE) when at least one NSLN was metastatic at the final histology, (NEGATIVE) when no further lymph node was metastatic. The axillary NSLN were examined using classical histologic technique by hematoxylin-eosin stain.
Ethical considerations
Patients’ data were collected anonymously and retrospective analysis did not influence the treatment. Thus, no written consent was required from the institution.
Identification and excision of SLN
SLNs were identified through 2 techniques that are alternatively used in our service.
The first one is lymphoscintigraphy with technetium-99m-labeled nanosized human serum albumin colloids, performed within 24 hr before surgical intervention. The second technique is based on periareolar injection of indocyanine green a few minutes before surgical incision. The techniques are used one at a time and the choice depends exclusively on the operating room scheduling because indocyanine green does not require an anticipated hospitalization while lymphoscintigraphy depends on the availability of the nuclear medicine service.
Once the SLN was identified and excised, it was deprived from the perinodal fat tissue and sent on ice to the Pathology Department. If 2 or more SLNs were found, they were sent separately in sterile boxes for OSNA.
One-step nucleic acid amplification
Each SLN was weighed, measured, and then processed. Sentinel lymph node weighing lower than 50 mg were excluded from OSNA analysis. Sentinel lymph node weighing higher than 600 mg were cut into 2 or more pieces and processed separately. If the weight of the lymph node is either above or below this specified range, accurate results may not be obtained.
The OSNA assay was performed according to the manufacturer’s instructions (Sysmex, Kobe, Japan). A lysate with CK19 mRNA copy-number/μL lower than 250 was regarded as negative; from 250 to 5000 as micrometastatic and higher than 5000 as macrometastatic.6 The result was immediately communicated to the surgeon. The whole process needed about 40 minutes to be completed. For statistical analysis, in case of 2 or more SLNs, the SLN with the greatest CK19 mRNA copies was chosen.
Only in case of macrometastasis in the SLN, the patient underwent an immediate ALND. When the SLN was negative or micrometastatic no further surgical procedure was performed.
Statistical analysis
One-step nucleic acid amplification nomogram was retrospectively applied in the population of patients with macrometastasis on the SLN. In patients with micrometastases, we routinely do not perform ALND, meaning that the histology of NSLN was a missing data, and the outcome could not be verified.
We focused on the presence versus absence of one or more metastatic NSLNs in the axillary dissections following macrometastatic OSNA. The data were inserted in a contingency table together with the nomogram, classified as (+) ore (−) basing on the cutoff of 31%.
With the selected data, the following parameters were calculated:
Prevalence of metastatic NSLNs in the subgroup of patients with macrometastatic SLN.
Sensitivity of the nomogram (probability for a patient with one or more metastatic NSLN, of having a nomogram higher than 31%).
Specificity of the nomogram (probability for a patient without metastatic NSLN, of having a nomogram lower than 31%).
Positive predictive value (PPV) of the nomogram (probability for a patient with nomogram higher than 31%, of having one or more NSLN metastases).
Negative predictive value (NPV) of the nomogram (probability for a patient with nomogram lower than 31%, not to have metastatic NSLNs).
Results
The clinicopathologic variables of the examined population are summarized in Table 2.
Table 2.
Clinicopathologic variables of the examined population.
| Histopathologic features (n = 141) | N of patients | Percent | Mean value | Standard deviation |
|---|---|---|---|---|
| Histology | ||||
| Infiltrating ductal carcinoma (IDC) | 116 | 82.3% | ||
| Infiltrating lobular carcinoma (ILC) | 21 | 14.9% | ||
| Wide DCIS with microinfiltration | 4 | 2.8% | ||
| Grading | ||||
| G1 | 3 | 2.1% | ||
| G2 | 95 | 67.4% | ||
| G3 | 35 | 24.9% | ||
| Unknown | 8 | 5.7% | ||
| ER (%) | 87.5 | 27.3 | ||
| >20% (positive) | 129 | 91.5% | ||
| <20% (negative) | 12 | 8.5% | ||
| PgR (%) | 56.7 | 39 | ||
| >20% (positive) | 99 | 70.2% | ||
| <20% (negative) | 42 | 29.8% | ||
| HER2 | ||||
| Negative | 125 | 88.7% | ||
| Positive | 14 | 9.9% | ||
| Unknown | 2 | 1.4% | ||
| MIB1 (%) | 17.4 | 11.1 | ||
| <20% (low) | 100 | 70.9% | ||
| >20% (high) | 38 | 27.0% | ||
| Unknown | 3 | 2.1% | ||
| T (mm) | 21.1 | 14.3 | ||
| Unknown | 1 | |||
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; MIB1, proliferation index.
The mean age of the population was 59.7 years. Standard deviation was 12.8.
Most of the patients were affected by infiltrating ductal carcinoma (82.3%). The second most common histotype was infiltrating lobular carcinoma (14.9%). The remaining cases (2.8%) were wide clusters of ductal carcinoma in situ (DCIS) with focal microinfiltration.
In most cases, tumor grading was moderately differentiated G2 (67.4%) or poorly differentiated G3 (24.9%).
Regarding hormonal receptors expression, most tumors were hormone responsive, both for estrogen (>20% in 91.5% of patients) and for progesterone (>20% in 70.2% of patients). Human epidermal growth factor receptorHER2 expression was negative in most patients (88.7%) and MIB1 was high (>20%) only in a minority of cases (27.0%). Mean tumor size was 21.1 mm with standard deviation of 14.3 mm.
About the variables regarding OSNA analysis of SLN (Table 3), 67 out of 141 patients had macrometastasis in the SLN (47.5%) while the remaining 74 patients had micrometastasis.
Table 3.
OSNA variables in the study population.
| OSNA variables (n = 141) | N of patients | Percent |
|---|---|---|
| mRNA CK19 copy-number | ||
| >5000 (macrometastasis) | 67 | 47.5% |
| 250-5000 (micrometastasis) | 74 | 52,5% |
Abbreviations: OSNA, one-step nucleic acid amplification.
We considered the group of patients with macrometastatic SLN (n = 66) (Table 4). Out of the 67 initial cases, one was excluded because the nodular mass situated in the upper-external quadrant of the left breast, for which the preoperative diagnosis was C5 (cytological diagnosis of malignancy) on FNAC, turned out to be an intramammary metastatic lymph node at the final histology. So SLN technique with OSNA analysis was performed, but a posteriori, this case was classified as a “cancer of unknown primary origin (CUP syndrome)” because no primary cancer was found at the previous and following radiologic exams. The lack of the T size (Tx) made the calculation of the nomogram impossible.
Table 4.
Nomogram calculation in macrometastatic OSNA nodes.
| Nomogram in macrometastatic OSNA nodes (n = 66) | N of patients | Percent |
|---|---|---|
| >31% | 50 | 76% |
| <31% | 16 | 24% |
Abbreviations: OSNA, one-step nucleic acid amplification.
In a group of 66 patients, 24% had a nomogram lower than 31% (−), the remaining 76% had a nomogram higher than 31% (+).
These percentages were inserted in the contingency table together with the data regarding the axillary NSLN status (Table 5), to verify the diagnostic accuracy of the method.
Table 5.
Contingency table for nomogram accuracy calculation.
| Metastatic NSLNs in the axillary cavity | ||||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| Nomogram | <31% | 13 | 3 | 16 |
| >31% | 23 | 27 | 50 | |
| Total | 36 | 30 | 66 | |
Abbreviations: NSLNs, nonsentinel lymph node.
The prevalence of patients with metastatic NSLN in the group of patients with macrometastatic SLN was 45% (30 out of 66). In the remaining 36 patients (55%), NSLN were all negative.
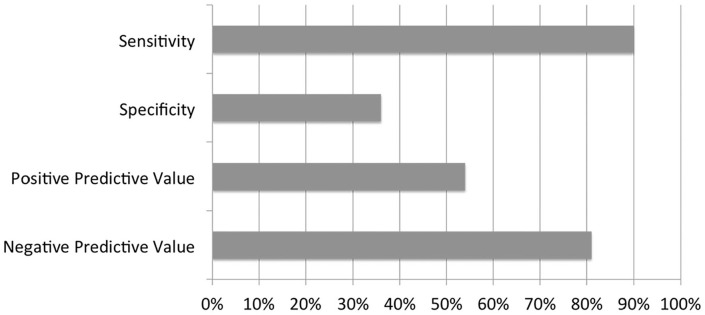
Sensitivity of the method was 90% with a CI95% between 73% and 98%. Specificity was 36% with CI95% between 21% and 54%. Positive predictive value was 54% with CI95% between 39% and 68% while negative predictive value was 81% with CI95% between 54% and 96%. The test has an excellent sensitivity, so it is highly probable that a patient with one or more macrometastatic NSLN will have a nomogram higher than 31%. Thus, the risk of undertreating patients by not performing ALND when NSLN are positive is low.
The specificity of the test is low (36%). This means that a patient with negative axillary cavity has an unsatisfying probability to have a nomogram lower than 31%.
This trend is confirmed by the positive predictive value (51%). It shows that the test is not able to accurately predict the probability that a patient with a nomogram higher than 31% has actual metastatic NSLN. Negative predictive value is high (81%) because the test has a good ability to predict the probability that a patient with nomogram lower than 31% has actually a negative axilla. The results are displayed in Figure 1.
Figure 1.
Diagnostic accuracy of OSNA nomogram.
OSNA indicates one-step nucleic acid amplification.
Discussion
Sentinel lymph node technique has been routinely used for 15 years as the standard treatment for axillary staging in patients affected by breast cancer with clinical negativity of the axillary cavity at the time of first diagnosis.7,8 Among the patients who undergo SLN biopsy, complete ALND is nowadays recommended only when macrometastasis are found in the SLN.9
The evolution of breast surgery toward a more conservative approach pushed the surgeons to suppose that complete ALND could be avoided in a group of selected patients, thus reducing potential morbidities linked to radical surgery of the axillary cavity.10,11 This consideration also comes from the fact that only in 50% of patients treated with complete ALND, metastatic NSLN are found.12
Numerous mathematical models have been designed and tested with the goal to predict the presence of NSLN metastasis to overcome the risk of overtreating patients. Several nomograms that have been created on the basis of a standard pathology examination of the SLN are available. van la Parra et al13 performed a meta-analysis of predictive factors for non-SLN metastasis in breast cancer patients with a positive SLN. Eight predictive factors were defined: method of detection (hematoxilyn-eosin stain), SLN metastasis > 2 mm in size, extracapsular extension in the SLN, >1 positive SLN, 1 negative SLN, ratio of positive SLN >50%, tumor size >2 cm, and lymphovascular invasion (LVI) in the primary tumor. The MD Anderson group has validated a predictive nomogram that incorporates the size of the SLN metastasis by conventional histology.14 The Memorial Sloan Kettering Cancer Center calculator provides a risk estimate of NSLN metastasis based on 9 histopathologic variables. The MSKCC nomogram was proved reliable in identifying patients with a high risk of NSLN metastasis and its online availability makes it widely accessible. However, the need of 9 different parameters for the calculation makes this nomogram poorly applicable if the goal is to obtain an intraoperative rapid result.15 Another validated tool, the Stanford nomogram, requires 3 histopathologic variables.16 The validation phase of the Stanford nomogram was made through its application on a subset of patients with isolated tumor cells (ITC) or micrometastasis in the SLN, so no data are available on its accuracy on the population of patients with macrometastatic SLN.15 Despite their accuracy, the previously described nomograms are poorly applicable in an intraoperative setting because their calculation requires parameters that are known only after all the pathology of the tumor and SLN is finalized. If complete ALND would be recommended from these nomograms, it should necessarily be performed during a second surgery, which can be associated with patient distress, higher costs and delay in adjuvant therapies.
The ability of OSNA assay to provide a rapid and semiquantitative analysis of the whole SLN has been used in multiple studies trying to define an ideal mathematical model to predict nonsentinel node status. Many authors explored the possibility to consider OSNA total tumor load (TTL, defined as the amount of CK19 mRNA copies in all positive SLNs) as a predictive factor for additional NSLN metastasis in the complete ALND and which cutoff could provide the best correlation with the axillary tumor burden.17,18 The results describing accuracy parameters are resumed in Table 6. The common feature of these predictors, as confirmed in our study, is to maintain a high sensitivity and a high NPV, lacking in specificity and PPV. These findings may indicate that OSNA-based models are reliable and accurate in sparing surgery in patients without any NSLN metastasis but their use would still permit a high number of complete ALND in patients without further nodal metastasis. Determination of NSLN status might be complicated because a greater number of dissected SLNs results in less remaining NSLN and the number of dissected SLNs directly affects TTL. This leads to a wide range of TTL assessed by OSNA, especially in the patients with a higher number of dissected SLNs and with a high copy-number.19 The OSNA user nomogram was tested in our study only on the highest number of CK19 mRNA copies found among the SLNs dissected, overcoming the problem of multiple sentinel nodes dissected. Overall, the simultaneous calculation of TTL and OSNA user nomogram for each patient could corroborate the choice to avoid ALND in a selected population. Moreover, the standardization of OSNA technique may help to achieve a wide reproducibility and to offer a homogeneous surgical planning to all patients that undergo axillary surgery for breast cancer, no matter if in different centers. Some authors,20 aimed at incorporating the TTL obtained by OSNA in a predictive nomogram together with tumor size, number of affected SLNs, HER2 overexpression, LVI. Results show that, although the TTL alone is a good predictor of NSLN status, the prediction is better when the TTL is associated to the former 4 variables. Despite that, the main advantage of TTL alone and OSNA user nomogram remains the immediate usability during surgical interventions.
Table 6.
Comparison of OSNA-based models.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| OSNA USER (present study results) | 90 (CI95% 73.0-98.0) | 36 (CI95% 21.0-54.0) | 54 (CI95%39.0-68.0) | 81 (CI95%54.0-96.0) |
| TTL 15 000 (Peg et al)17 | 76.7 (95%CI 70.4-82.0) | 55.2 (95%CI50.7-59.5) | 41.1 (CI95%36.3-46.1) | 85.5 (CI95% 81.0-88.8) |
| TTL 1.2 × 105 (Espinosa-Bravo et al)18 | 47 | 85 | 56 | 80 |
Abbreviations: CI, confidence interval; NPV, negative predictive value; OSNA, one-step nucleic acid amplification; PPV, positive predictive value; TTL, total tumor load.
Former research, through which the OSNA nomogram was validated,4 considered a population of patients for whom complete ALND was performed even if micrometastasis were found in the SLN. Our study, instead, could not assess the accuracy of OSNA nomogram for the population of patients with micrometastasis in the SLN, because the histologic data on NSLN was missing. Anyway, the use of OSNA nomogram in this subgroup of patients can be considered redundant because the lack of prognostic advantage in performing ALND in patients with micrometastatic SLN has already been proved.9
After excluding the micrometastatic group, 66 patients were left to calculate the nomogram accuracy. From a clinical point of view, the low specificity showed by the nomogram means that, if we would use the OSNA nomogram in clinical practice, we would keep on performing a high number of useless ALND, in patients with a nomogram higher than 31% but with no NSLN metastasis. Nonetheless, complete ALND would have correctly been avoided in 13 of the 66 patients, if we applied the nomogram to choose the surgical strategy.
Talking about the sample of patients examined in this study, we must consider that it did not include all the patients that we treat with SLN biopsy. In fact, the OSNA assay and the related technicians are not always available in our center. A mean of 5 OSNA assays per week are performed out of a mean of 12 SLN biopsies. Therefore, a preliminary selection of which patient can have a major advantage from OSNA analysis is done before surgery. This method is mainly dedicated to those patients who have a more aggressive histology (triple-negative breast cancer, HER2-positive, luminal-B like) or a big size luminal A-like tumor or when the radiologic staging describes suspected axillary lymph node even if a FNAC was performed with negative result.
The consequence is that patients with a low probability to have SLN metastasis and consequently an even lower probability of having NSLN metastasis are excluded from the use of OSNA assay and from this study, representing a potential selection bias and a limitation of our study.
Another subgroup of patients that was excluded a priori from this study is the group of women that undergo surgery after neo-adjuvant chemotherapy because our Breast Unit follows the Emilia Romagna Regional protocol that still recommends not using OSNA technique after chemotherapy. Furthermore, the optimal surgical approach of the axilla in patients who undergo chemotherapy is still an extremely discussed topic among breast surgeons and oncologists.21 Therefore, a probabilistic method as the nomogram should be applied only after a uniform standardized treatment is reached.
Conclusions
Among published and validated nomograms, the OSNA one is rapid and easy to use. In the sample of patients considered in the study, OSNA nomogram resulted to have a high sensibility and a high negative predictive value. These features confirm that the risk of undertreating patients with macrometastatic SLN is low and that the method could be helpful to avoid complete ALND to those patients with an extremely low risk of having NSLN metastasis. This is fundamental for a test that is thought to guide a therapeutic decision.
To pursue the creation of an even more immediate tool, further studies could explore the possibility to validate the OSNA nomogram using the preoperative radiological T size, which is a datum already available even before the time of surgical treatment.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: FC and GT conceived the presented study. FC wrote the manuscript with support from AA, AG, EP and SP. AB and SZ performed data collection. GF contributed to the analysis of the results. GT supervised the project and all authors discussed the results and commented on the manuscript.
ORCID iD: Francesca Combi  https://orcid.org/0000-0001-7780-2851
https://orcid.org/0000-0001-7780-2851
References
- 1. Fung V, Kohlhardt S, Vergani P, Zardin GJ, Williams NR. Intraoperative prediction of the two axillary lymph node macrometastases threshold in patients with breast cancer using a one-step nucleic acid cytokeratin-19 amplification assay. Mol Clin Oncol. 2017;7:755-762. doi: 10.3892/mco.2017.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleming FJ. Factors affecting metastases to non-sentinel lymph nodes in breast cancer. J Clin Pathol. 2004;57:73-76. doi: 10.1136/jcp.57.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolster MJ, Peer PGM, Bult P, et al. Risk factors for non-sentinel lymph node metastases in patients with breast cancer. The outcome of a multi-institutional study. Ann Surg Oncol. 2007;14:181-189. doi: 10.1245/s10434-006-9065-1. [DOI] [PubMed] [Google Scholar]
- 4. Di Filippo F, Di Filippo S, Ferrari AM, et al. Elaboration of a nomogram to predict nonsentinel node status in breast cancer patients with positive sentinel node, intraoperatively assessed with one step nucleic amplification: retrospective and validation phase. J Exp Clin Cancer Res. 2016;35:193. doi: 10.1186/s13046-016-0460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Filippo F, Giannarelli D, Bouteille C, et al. Elaboration of a nomogram to predict non sentinel node status in breast cancer patients with positive sentinel node, intra-operatively assessed with one step nucleic acid amplification method. J Exp Clin Cancer Res. 2015;34:136. doi: 10.1186/s13046-015-0246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsujimoto M, Nakabayashi K, Yoshidome K, et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807-4816. doi: 10.1158/1078-0432.CCR-06-2512. [DOI] [PubMed] [Google Scholar]
- 7. Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. JNCI J Natl Cancer Inst. 1999;91:368-373. doi: 10.1093/jnci/91.4.368. [DOI] [PubMed] [Google Scholar]
- 8. Rahusen FD, Pijpers R, Van Diest PJ, Bleichrodt RP, Torrenga H, Meijer S. The implementation of the sentinel node biopsy as a routine procedure for patients with breast cancer. Surgery. 2000;128:6-12. doi: 10.1067/msy.2000.107229. [DOI] [PubMed] [Google Scholar]
- 9. Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23tases (IBCSG 23crandomised controlled trial. Lancet Oncol. 2013;14:297-305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279-293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 11. Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452-461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17:1720-1726. doi: 10.1200/JCO.1999.17.6.1720. [DOI] [PubMed] [Google Scholar]
- 13. van la Parra RF, Peer PG, Ernst MF, Bosscha K. Meta-analysis of predictive factors for non-sentinel lymph node metastases in breast cancer patients with a positive SLN. Eur J Surg Oncol. 2011;37:290-299. doi: 10.1016/j.ejso.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 14. Mittendorf E, Hunt K, Boughey J, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2012;255:109-115. doi: 10.1097/SLA.0b013e318238f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hessman CJ, Naik AM, Kearney NM, et al. Comparative validation of online nomograms for predicting nonsentinel lymph node status in sentinel lymph node-positive breast cancer. Arch Surg. 2011;146:1035-1040. doi: 10.1001/archsurg.2011.201. [DOI] [PubMed] [Google Scholar]
- 16. The Bay Area SLN, Study Kohrt HE, Olshen RA, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. doi: 10.1186/1471-2407-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peg V, Espinosa-Bravo M, Vieites B, et al. Intraoperative molecular analysis of total tumor load in sentinel lymph node: a new predictor of axillary status in early breast cancer patients. Breast Cancer Res Treat. 2013;139:87-93. doi: 10.1007/s10549-013-2524-z. [DOI] [PubMed] [Google Scholar]
- 18. Espinosa-Bravo M, Sansano I, Pérez-Hoyos S, et al. Prediction of non-sentinel lymph node metastasis in early breast cancer by assessing total tumoral load in the sentinel lymph node by molecular assay. Eur J Surg Oncol. 2013;39:766-773. doi: 10.1016/j.ejso.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 19. Sa-Nguanraksa D, O-Charoenrat E, Kulprom A, et al. Nomogram to predict non-sentinel lymph node status using total tumor load determined by one-step nucleic acid amplification: first report from Thailand. Breast Cancer. 2019;26:471-477. doi: 10.1007/s12282-019-00945-8. [DOI] [PubMed] [Google Scholar]
- 20. Rubio IT, Espinosa-Bravo M, Rodrigo M, et al. Nomogram including the total tumoral load in the sentinel nodes assessed by one-step nucleic acid amplification as a new factor for predicting nonsentinel lymph node metastasis in breast cancer patients. Breast Cancer Res Treat. 2014;147:371-380. doi: 10.1007/s10549-014-3108-2. [DOI] [PubMed] [Google Scholar]
- 21. Morigi C. Highlights from the 15th St Gallen International Breast Cancer Conference 15-18 March, 2017, Vienna: tailored treatments for patients with early breast cancer. E cancer medical science. 2017;11:732. doi: 10.3332/ecancer.2017.732. [DOI] [PMC free article] [PubMed] [Google Scholar]