Abstract
Kinetochore-associated protein 1 (KNTC1) is a kind of kinetochore components that ensure the proper functioning of the spindle-assembly checkpoint. To date, the functional information of KNTC1 in colon cancer remains unknown. This study was aimed to investigate the role of KNTC1 in colon cancer. We found KNTC1 was significantly upregulated in the colon cancer compared to the normal tissues. ROC curve showed the area under the curve value of KNTC1 for the prediction of colon cancer was 0.93. Kaplan–Meier revealed highly expressed KNTC1 was associated with poor prognosis. KNTC1 was widely expressed in different colon cancer cell lines. Compared with the control lentiviral infected cells, KNTC1-shRNA cells exhibited significant reduction in cell growth rates and increase in the proportion of cells in the S phase, while decrease in the G1 and G2/M phase. Furthermore, knockdown of KNTC1 dramatically increased apoptosis in the colon cancer cells. Gene set enrichment analysis (GSEA) in gene ontology (GO) showed that KNTC1 is closely associated with cell mitosis-related components, such as nuclear chromatin, centrosome, and spindle. Moreover, upregulated KNTC1 is significantly enriched in the biological process of DNA repair, mRNA processing, microtubule cytoskeleton organization and the molecular function of helicase activity, protein heterodimerization activity and catalytic activity acting on DNA in molecular function. Our data reveal the important roles of KNTC1 in driving tumor progression in colon cancers.
Keywords: KNTC1, Colon cancer, Cell growth, GSEA
Introduction
Colon cancer is the fourth most incident and the fifth most deadly cancer in the world. In 2018, 1 million people were projected to be diagnosed with colon cancer, which is expected to cause about 551,000 deaths comprising 5.8% of all cancer deaths in 2018 (Bray et al. 2018; Rawla et al. 2019). In the Chinese population, the incidence of colon cancer is steadily rising in recent years. The death rate of colon and rectum cancer is 13.24/100,000, accounting for 1.79% of deaths in China in 2017 (Zheng et al. 2019; Yin et al. 2019). Many patients diagnosed with advanced or metastatic colon cancer may have poor survival rates and multidisciplinary treatment remains a huge challenge due to cancer resistance (Siegel et al. 2017). Despite ongoing advances in targeted therapies including epidermal growth factor receptor and vascular endothelial growth factor, there remains insufficient molecular markers or therapeutic targets for routine clinical application in colon cancer.
Increasing studies have found that several kinetochore genes are upregulated in multiple cancers, including breast, gastric, and lung cancers (Thiru et al. 2014; Zhang et al. 2021; Li et al. 2020; Qiu et al. 2020). Kinetochore proteins also contribute to chemotherapy resistance (Yin-Ju and Chiou 2017). Highly expressed kinetochore genes are enriched in the altered gene expression subnetworks characterizing unstable cancers with high chromosome instability (CIN) (Thapa et al. 2019), indicating that overexpression of kinetochore genes drives genomic instability and cancer progression. Kinetochore-associated protein 1 (KNTC1) is one of RZZ subunits (ROD, ZWILCH, and ZW10) which constitute important kinetochore components originally identified in Drosophila, but it is also expressed in multicellular eukaryotes (Scaerou et al. 2001; Civril et al. 2010). KNTC1 is required for the homogeneous separation of chromosomes in the mitotic division as a spindle-assembly checkpoint apparatus protein. The absent or defective of KNTC1 results in checkpoint failure and premature mitotic exit, inducing chromosome instability, which is considered an important event for tumor initiation and development (Forment et al. 2012; Bakhoum and Landau 2017; Bakhoum et al. 2018). Recent studies have found that the KNTC1 gene is overexpressed in lung, bladder and liver tumors (Perez de Castro et al. 2007; Huang et al. 2021). Liu et al. reported that knockdown of KNTC1 effectively inhibited cell viability and increased apoptosis in esophageal squamous cell carcinoma (Liu et al. 2019). The homozygous missense mutation in KNTC1 was observed in colorectal cancers (Wang et al. 2004). The expression pattern and function of KNTC1 in colorectal cancers remain largely unknown, and the underlying molecular mechanism of KNTC1 in tumor progression was poorly understood. This study was to investigate the role of KNTC1 in colon cancer proliferation.
Materials and methods
Cell lines and cell culture techniques
The human colon cancer cell lines RKO, SW480, SW620 and HCT116 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 (Invitrogen, Shanghai, China), containing 10% fetal bovine serum (Gibco, Logan, UT, USA) and 100 U/ml penicillin and 0.1 mg/ml (Sangon Co., Ltd., Shanghai, China) at 37 ℃ in a 5% CO2 incubator.
Western blot analysis
The cells were lysed with cell lysis buffer containing phosphatase inhibitor and protease inhibitor (Sigma-Aldrich). The lysates were centrifuged at 10,000g for 10 min at 4 ℃. The supernatants were collected and measured using a BCA protein assay kit (KeyGEN BioTECH, Nanjing, China). Equal amounts of proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Membranes were incubated with anti-KNTC1 (Santa Cruz, CA, USA) or anti-GAPDH primary antibody (Cell Signaling Technology, USA). After horseradish peroxidase-conjugated IgG goat anti-mouse secondary antibody (Santa Cruz Biotechnology) incubation, the protein band was visualized using enhanced chemiluminescence (KeyGEN BioTECH).
Lentiviral vector construction and cell infection
The pGCSIL-GFP (GeneChem Co. Ltd.) lentiviral constructs were prepared to generate the KNTC1 knockdown vector. The complementary DNA sequence (TGAGTTTATGGGATATTTA) of human KNTC1 short-hairpin RNA (shRNA) was designed from Shanghai GeneChem Co., Ltd. (Shanghai, China). Lentivirus expressing shKNTC1 or negative control-shRNA (shNC) was produced in HEK293T cells (from American Type Culture Collection) and packaged by pHelper 1.0 and pHelper 2.0. Subsequently, cells were infected with a mixture of viral supernatant and fresh medium at a 1:1 ratio. Transduction effects were determined by detecting green fluorescent protein (GFP) expression after 72 h infection under a fluorescence microscope (Olympus, Tokyo, Japan). Knockdown efficiency was confirmed with RT-PCR and western blot analysis.
Detection of cell apoptosis and cell cycle distribution by flow cytometry
Apoptotic incidence was measured with annexin V apoptosis detection kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, shKNTC1 or shNC cells were plated in 6-well plates at a concentration of 2.0 × 104 cells/ml. At the indicated time point, the cells were harvested and washed twice with PBS, and added with annexin V-APC solution (5 μl) at 37 °C for 30 min. Cell apoptosis was detected using BD FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA). To determine the cell cycle distribution, the harvested cells were stained with propidium iodide (PI; 50 µg/ml, Sigma-Aldrich® Co. LLC., St. Louis, MO, USA) in the presence of RNase A (100 µg/ml; Fermentas, Shanghai, China), and analyzed by flow cytometry.
Celigo cytometer cell growth tracking assay and MTT cell viability assay
Briefly, the cells following lentiviral transduction were seeded at 2000 cells/well in 96-well plates and scanned using Celigo Image Cytometer (Nexcelom Bioscience LLC, Lawrence, MA, USA) each day. The Celigo instrument could identify fluorescent cells, and calculate the number of cells in the 96-well plate by software analysis. Cell growth curves were displayed according to the cell proliferation at indicated time point. Cell viability was measured by MTT assay. The cells were seeded in 96-well plates, 20 μl of 5 mg/ml MTT (Genview, Australia) was added to each well and cultured for 4 h. Then the cell culture medium was replaced by 100 μl of dimethyl sulfoxide. The number of viable cells was estimated by measuring absorption at 490 nm wavelength and cell growth curves were determined according to the optical density value. All experiments were performed in triplicate and repeated at least three times.
RT-PCR analysis of KNTC1 mRNA
Total RNA of cell lines was extracted using 1 ml TRIzol reagent (Invitrogen, Shanghai, China), and reverse-transcribed into the single-strand cDNA using a Takara RNA PCR kit (Takara Bio, Inc.) based on the manufacturer’s instructions. Primers were obtained from Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China), and the sequences for the primers are as follows: 5′-GCAACAACTTGTAGACGACGCT-3′ (sense) and 5′-TCAATCCAAGAACTGCCACTG-3′ (antisense) for KNTC1; and 5′-TGACTTCAACAGCGACACCCA-3′ (sense) and 5′-CACCCTGTTGCTGTAGCCAAA-3′ (antisense) for GAPDH. Real-time quantitative PCR was performed using a standard SYBR Green PCR kit (Thermo) in LightCycler480 system (Roche Diagnostics). The reactions consisted of an initial denaturation at 95 ℃ for 15 s, then 45 cycles at 95 ℃ for 5 s, and 60 ℃ for 30 s. The gene expression was analyzed by the ΔΔCt calculation. All samples were examined in triplicates.
Gene expression and survival analysis
The Cancer Genome Atlas Colon Adenocarcinoma (TCGA-COAD) RNAseq datasets were downloaded from UCSC Xena (https://xenabrowser.net/datapages/). Tumor vs. normal log2 gene expression ratios was first calculated. Those samples with KNTC1 expression (fpkm value) less than 0.01 were excluded. The expression differences of KNTC1 between tumor and adjacent normal tissues were analyzed. ROC curve was applied to examine the diagnostic capability of KNTC1 in colon cancer. Kaplan–Meier curves were generated from data sets obtained through “Gene_Outcome” module of TIMER2 (Tumor immune estimation resource, version 2, http://timer.cistrome.org/), and the log-rank test was performed. Cutoff-high (30%) and cutoff-low (30%) values were used as the thresholds for splitting the high and low expression cohorts.
Gene set enrichment analysis (GSEA) in gene ontology (GO) terms
To evaluate the correlation between KNTC1 expression and other molecular or biological gene sets, the gene set enrichment analysis (GSEA) (Subramanian et al. 2005; Yu et al. 2015) in gene ontology (GO) terms were carried out using clusterprofiler package (Yu et al. 2012) from bioconductor (https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html). The enrichment score (ES) and p value of the ES was calculated and given in the table. The p < 0.05 was considered statistically significant.
Statistical analysis
Statistical analysis was performed using the R software program. The statistical data for each group were presented as the mean ± standard error of at least three independent experiments. Differences between the two groups were analyzed using Student’s t test, whereas differences in cell viability at several points in time were analyzed by 2-way ANOVA with pairwise multiple comparisons post hoc tests. The value of p < 0.05 was accepted as statistically significant.
Results
KNTC1 is upregulated in colon cancer tissues
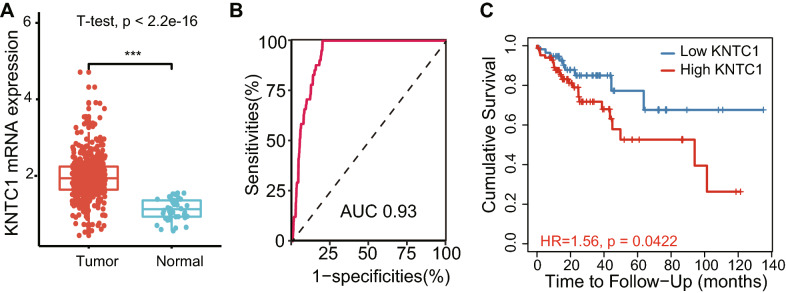
To identify potential KNTC1 involvement in colon cancer progression, the differential expression of KNTC1 between colon cancer and normal tissues was detected using TCGA database. Based on TCGA RNAseq data, KNTC1 was remarkably increased in colon tumors compared with normal tissues (p < 0.0001, Fig. 1a). ROC curve revealed the area under the curve (AUC) value of KNTC1 for the prediction of colon cancer was 0.93 (Fig. 1b). The likelihood ratio was 34.57, with 82.30% and 97.62% of sensitivity and specificity, respectively. Kaplan–Meier analysis indicated that the patients with high expression of KNTC1 had poor prognosis compared to those expressed low KNTC1 (p < 0.05, Fig. 1c).
Fig.1.
KNTC1 is upregulated and predicts poor prognosis in colon cancer. a The differential expression of KNTC1 between colon cancer and normal tissues was detected using TCGA database (***p < 0.001). b ROC curve of KNTC1 expression was analyzed to examine the validity of KNTC1 gene expression in discriminating tumor and non-tumor states of the TCGA-COAD samples. c Effect of KNTC1 expression on the overall survival of colon cancer patients in TCGA database (p < 0.05)
Lentivirus-mediated KNTC1 knockdown in RKO cells
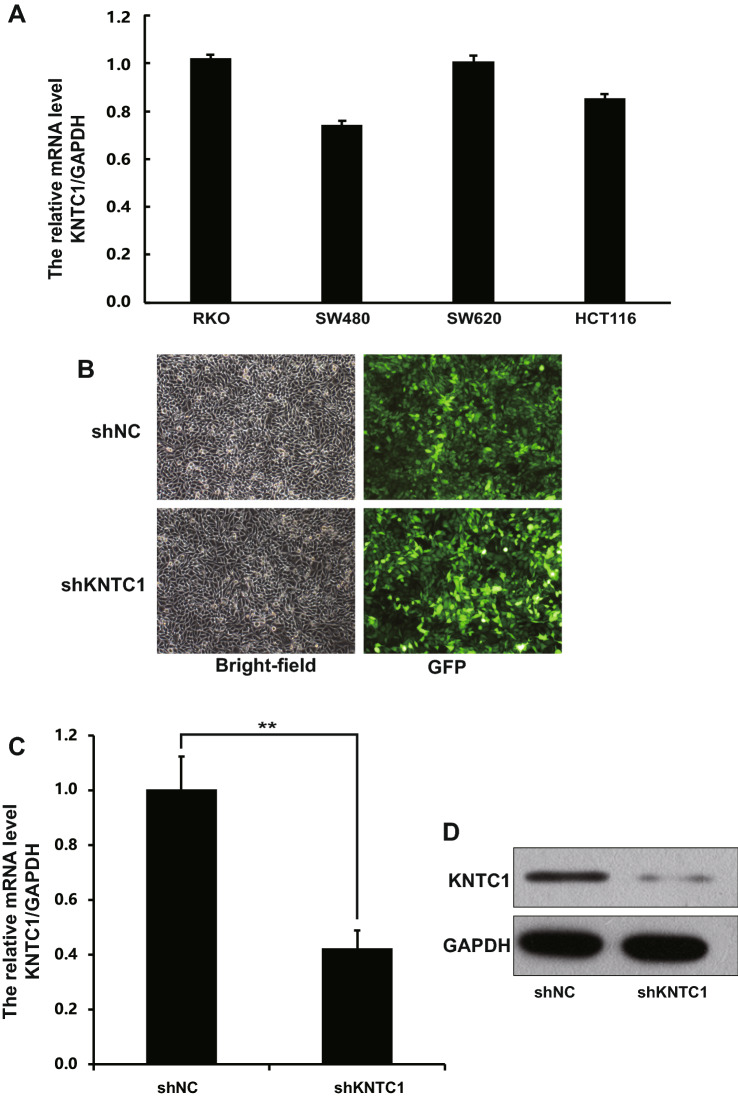
We next determined the expression of KNTC1 mRNA in four colon cancer cell lines, including RKO, SW480, SW620 and HCT116. The results showed that KNTC1 mRNA was expressed in all cell lines, and the highest expression of KNTC1 was found in RKO cells (Fig. 2a). To determine the role of KNTC1 in colon carcinoma, KNTC1 was knocked down in the RKO cell line. RKO cells were infected by recombinant KNTC1-shRNA lentivirus or control-shRNA (NC) lentivirus. Infection was efficient as over 80% of cells were observed to be GFP positive in both shNC and shKNTC1 groups 72 h after transfection (Fig. 2b). To evaluate knockdown efficiency, KNTC1 mRNA and protein expression was assessed in KNTC1-shRNA and NC RKO cells. There were significantly lower mRNA levels in the KNTC1-shRNA group at day 5 following transduction (shNC, 1.005 ± 0.119; shKNTC1, 0.423 ± 0.065, Fig. 2c), and greatly reduced KNTC1 protein expression compared to those in the NC group (Fig. 2d).
Fig.2.
Lentivirus-mediated KNTC1-shRNA knockdown in colon cancer cell. a KNTC1 mRNA levels in four colon cancer cell lines was measured by RT-PCR. b Visualization of GFP positive cells. The left photographs were bright-field micrographs, and the right photographs were fluorescence micrographs. c Confirmation of RPL34 knockdown in RKO cell by RT-PCR (**p < 0.01). d KNTC1 protein expression was analyzed by western blotting in shNC- and shKNTC1-infected RKO cells
Knockdown of KNTC1 suppresses growth in colon cancer cells
After shRNA lentivirus transfection, the cell growth rate was calculated based on the cell count value performed by Celigo Cell Cytometer for 5 days. As illustrated in Fig. 3a and confirmed by quantification in Fig. 3b, the growth of RKO cells in the KNTC1-shRNA group was significantly decreased compared with that in the control-shRNA group. A similar result was also observed by MTT assay, which showed KNTC1 deficiency dramatically inhibited the proliferation of RKO cells on day 4 and day 5 (Fig. 3c). These data demonstrate that KNTC1 might promote the viability of RKO cells.
Fig.3.
Knockdown of KNTC1 suppresses growth in RKO cells. a Representative cell images were taken by Celigo cell cytometer for 5 days. b Quantitative cell count analysis shows that cell growth rates of the RKO cells transduced with shKNTC1 were significantly decreased compared to shNC cells (***p < 0.001). Cell count/fold refers to fold change of cell count compared to day 1. c MTT assay shows that KNTC1 knockdown significantly inhibited proliferation and growth of RKO cells (***p < 0.001). OD490/fold refers to fold change of OD490 compared to day 1
Silencing of KNTC1 induces apoptosis in colon cancer cells
To evaluate the effect of KNTC1 on apoptosis, apoptosis rate was detected by flow cytometry in KNTC1-shRNA and control-shRNA transfected RKO cells. The cell apoptosis rate was significantly increased in the KNTC1-shRNA group (13.79% ± 0.285) compared to the control group (4.57% ± 0.0749; p < 0.001) as shown in Fig. 4a, b. These results indicate that KNTC1 might inhibit cell apoptosis in RKO cells.
Fig.4.
Knockdown of KNTC1 promotes apoptosis in RKO cells. a Cell apoptosis was analyzed by flow cytometry. The histogram shows a comparison of the distribution of annexin V staining positive cells (R3) between shNC and shKNTC1 transduced RKO cells. b Quantification of results showed the significant increase in apoptosis in the KNTC1-shRNA group to 13.79% ± 0.285% compared to the control group 4.57% ± 0.0749% (***p < 0.001)
KNTC1 deficiency induces S phase arrest
To determine whether KNTC1 mediates cell viability by affecting the cell cycle progression, the changes in cell cycle distribution following KNTC1 knockdown were analyzed by flow cytometry. Compared to the NC group, the results revealed KNTC1 deficiency led to a dramatical promotion in the percentage of cells in the S phase from 44.23% ± 0.81 to 49.48% ± 1.259 (p < 0.01, Fig. 5). Moreover, with KNTC1 silencing, a considerable decrease in the percentage of cells in the G1 (31.53% ± 1.19) and G2/M (18.99% ± 0.17) of RKO cells with respect to 34.98% ± 0.35 and 20.79% ± 0.62 of that in the NC group, respectively. Therefore, downregulation of KNTC1 inhibits the cellular proliferation via S phase cell cycle arrest, suggesting KNTC1 probably play a pivotal role in cell cycle progression and cell growth.
Fig.5.
The effect of KNTC1 on cell cycle of RKO cells. a Comparison of percentage of cells in G1, S, and G2/M cell cycle phases in RKO cells treated with shNC and shKNTC1 lentivirus. b The shKNTC1 group showed significant decrease in the proportion of cells in the G1 and G2/M phase (**p < 0.01), and significant increase in the S phase (**p < 0.01) compared with the NC group
The analysis of gene set enrichment analysis (GSEA) in gene ontology (GO) terms
GO terms GSEA were done to further investigate the molecular significance of KNTC1 in colon cancer (Fig. 6a). The result in cellular component (CC) GSEA (Fig. 6b) showed that KNTC1 is closely associated with cell mitosis-related components, such as nuclear chromatin, centrosome, and spindle. Besides, upregulated KNTC1 were significantly enriched in DNA repair, mRNA processing, microtubule cytoskeleton organization, and others in biological process (BP) analysis, and helicase activity, protein heterodimerization activity, catalytic activity acting on DNA in molecular function (MF) analysis (Table 1).
Fig.6.
Gene set enrichment analysis (GSEA) in gene ontology (GO) terms. a Representative dotplot for enriched GO terms from GSEA analysis. b GSEA enrichment score curves of representative GO terms. Normalized enrichment score (NES) of the related GO functional or molecular terms has statistical significance, indicating that KNTC1 expression was positively correlated with indicated pathways
Table 1.
Enriched GO terms from GSEA analysis of differentially expressed KNTC1 in colon cancers
| GO | ID | Description | Set size | Enrichment score | NES | p value |
|---|---|---|---|---|---|---|
| BP | GO:0000226 | Microtubule cytoskeleton organization | 419 | 0.41 | 1.432 | 9.99E−04 |
| GO:0006281 | DNA repair | 476 | 0.476 | 1.671 | 9.99E−04 | |
| GO:0006397 | mRNA processing | 452 | 0.481 | 1.687 | 9.99E−04 | |
| GO:0008380 | RNA splicing | 401 | 0.488 | 1.702 | 0.001 | |
| GO:0007059 | Chromosome segregation | 245 | 0.486 | 1.676 | 0.001 | |
| GO:0031047 | Gene silencing by RNA | 231 | 0.656 | 2.253 | 0.001 | |
| GO:0006302 | Double-strand break repair | 196 | 0.574 | 1.958 | 0.001 | |
| GO:0000819 | Sister chromatid segregation | 149 | 0.52 | 1.752 | 0.001 | |
| GO:0006323 | DNA packaging | 160 | 0.698 | 2.362 | 0.001 | |
| GO:0006333 | Chromatin assembly or disassembly | 153 | 0.711 | 2.396 | 0.001 | |
| GO:0051321 | Meiotic cell cycle | 144 | 0.528 | 1.773 | 0.001 | |
| GO:0140014 | Mitotic nuclear division | 226 | 0.439 | 1.505 | 0.002 | |
| GO:0000075 | Cell cycle checkpoint | 189 | 0.438 | 1.493 | 0.002 | |
| CC | GO:0000228 | Nuclear chromosome | 455 | 0.494 | 1.731 | 9.99E−04 |
| GO:0000785 | Chromatin | 454 | 0.542 | 1.902 | 9.99E−04 | |
| GO:0005813 | Centrosome | 459 | 0.42 | 1.474 | 9.99E−04 | |
| GO:0044454 | Nuclear chromosome part | 420 | 0.489 | 1.707 | 9.99E−04 | |
| GO:0005819 | Spindle | 312 | 0.424 | 1.466 | 0.001 | |
| GO:0000776 | Kinetochore | 127 | 0.505 | 1.682 | 0.001 | |
| GO:0005814 | Centriole | 115 | 0.555 | 1.838 | 0.001 | |
| MF | GO:0046982 | Protein heterodimerization activity | 412 | 0.508 | 1.772 | 0.001 |
| GO:0004386 | Helicase activity | 121 | 0.553 | 1.84 | 0.001 | |
| GO:0140097 | Catalytic activity, acting on DNA | 139 | 0.488 | 1.635 | 0.002 |
Discussion
Colon cancer is a very common cancer worldwide with high morbidity and mortality. Several kinetochore proteins have been identified as a novel regulator of carcinogenesis in colorectal cancer (Dang et al. 2020; Bai et al. 2019; Zhou et al. 2020; Zhang et al. 2021). In the present study, KNTC1, a key kinetochore component, was shown to be overexpressed in colon cancer tissues. Kaplan–Meier survival analysis presented a correlation between high expression KNTC1 and poor overall survival for colon cancer. We employed siRNA to knockdown KNTC1 expression in colon cells, and demonstrated that KNTC1 deficiency significantly reduced cell growth rate along with an increase in the percentage of cells in S phase and a decrease in the G1 and G2/M phase. Moreover, the knockdown of KNTC1 increased apoptosis in the RKO cells. Taken together, these results suggest that KNTC1 might promote RKO cell growth and viability.
To explore the molecular mechanisms of KNTC1 in colon cancers, gene ontology (GO) set enrichment analysis was conducted using COAD RNAseq datasets from TCGA. The results showed that KNTC1 was positively correlated with cell mitosis-related components, such as nuclear chromatin, centrosome and spindle, which reflected the knowledge that KNTC1 was widely involved in the molecular process of mitotic division. Furthermore, the hallmark of gene sets revealed KNTC1 was positively associated with the biological process of DNA repair, mRNA processing, and microtubule cytoskeleton organization, and the molecular function of helicase activity, protein heterodimerization activity, and catalytic activity acting on DNA. The DNA helicases were upregulated in many types of cancer, and were essential for cancer proliferation. Several missense mutations in helicase genes were linked to chromosomal instability (Brosh 2013; Datta and Brosh 2018). Additionally, protein heterodimerization was widely involved in tumorigenesis. The most common and relevant heterodimers in colorectal carcinogenesis were MLH1/PMS2 and MSH2/MSH6 which represented a mismatch repair mechanism (Chen et al. 2017). BRAF/CRAF was another heterodimer that is involved in cell proliferation in colorectal cancer (Lee et al. 2019). Sustained activation of the ERK pathway by mutated BRAF heterodimer contributed to tumorigenesis (Cope et al. 2018). In a word, it was inferred that KNTC1 might induce genomic instability through augmented helicase activity and protein heterodimerization activity, which led to the initiation and development of colon cancer.
In conclusion, our data reveal the important roles of KNTC1 in driving tumor progression in colon cancers. We found that knockdown of KNTC1 inhibited the proliferation of colon cancer cells, and induced cell cycle arrest at S phase and increased apoptosis. KNTC1 was upregulated in colon cancer compared with normal tissues. GSEA analysis revealed that KNTC1 might act as a mitotic checkpoint regulator, which is probably responsible for chromosome instability and results in tumor initiation and development of colon cancer. However, the detailed molecular mechanisms need to be further investigated, and the molecular pathways of KNTC1 involved in DNA helicase activity revealed by the present study represented an interesting candidate for future study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81602674), the Scientific Research Fund of Talents Introduction of Yijishan hospital (No. KY23960106) and Science Foundation for Young Scholars of Wannan medical college (No. WK2018F11).
Author contributions
ZZ and TY contributed equally to this work. ZZ and TY performed the experiments, contributed to data analysis, and wrote the paper. LZ analyzed the data. YZ made the study design and wrote the paper. All the authors approved the final manuscript and confirmed its accuracy.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest to this work.
References
- Bai T, Zhao Y, Liu Y, Cai B, Dong N, Li B. Effect of KNL1 on the proliferation and apoptosis of colorectal cancer cells. Technol Cancer Res Treat. 2019;18:1533033819858668. doi: 10.1177/1533033819858668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Landau DA. Chromosomal instability as a driver of tumor heterogeneity and evolution. Cold Spring HarbPerspect Med. 2017;7(6):a029611. doi: 10.1101/cshperspect.a029611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Ngo B, Laughney AM, Cavallo J-A, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TB, Taunk NK. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467–472. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Can J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brosh RM., Jr DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer. 2013;13(8):542–558. doi: 10.1038/nrc3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. DiagnPathol. 2017;12(1):24. doi: 10.1186/s13000-017-0613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civril F, Wehenkel A, Giorgi FM, Santaguida S, Di Fonzo A, Grigorean G, Ciccarelli FD, Musacchio A. Structural analysis of the RZZ complex reveals common ancestry with multisubunit vesicle tethering machinery. Structure. 2010;18(5):616–626. doi: 10.1016/j.str.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Cope N, Candelora C, Wong K, Kumar S, Nan H, Grasso M, Novak B, Li Y, Marmorstein R, Wang Z. Mechanism of BRAF activation through biochemical characterization of the recombinant full-length protein. ChemBioChem. 2018;19(18):1988–1997. doi: 10.1002/cbic.201800359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Hu D, Xu J, Li C, Tang Y, Yang Z, Liu Y, Zhou W, Zhang L, Xu H, Xu Y, Ji G. Comprehensive analysis of 5-hydroxymethylcytosine in zw10 kinetochore protein as a promising biomarker for screening and diagnosis of early colorectal cancer. ClinTransl Med. 2020;10(3):e125. doi: 10.1002/ctm2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Brosh RM. New insights into DNA helicases as druggable targets for cancer therapy. Front MolBiosci. 2018 doi: 10.3389/fmolb.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forment JV, Kaidi A, Jackson SP. Chromothripsis and cancer: causes and consequences of chromosome shattering. Nat Rev Cancer. 2012;12(10):663–670. doi: 10.1038/nrc3352. [DOI] [PubMed] [Google Scholar]
- Huang H, Fan X, Qiao Y, Yang M, Ji Z. Knockdown of KNTC1 inhibits the proliferation, migration and tumorigenesis of human bladder cancer cells and induces apoptosis. Crit Rev Eukaryot Gene Expr. 2021;31(1):49–60. doi: 10.1615/CritRevEukaryotGeneExpr.2021037301. [DOI] [PubMed] [Google Scholar]
- Lee HM, Morris V, Napolitano S, Kopetz S. Evolving strategies for the management of BRAF-Mutant metastatic colorectal cancer. Oncology. 2019;33(6):206–211. [PubMed] [Google Scholar]
- Li HN, Zheng WH, Du YY, Wang G, Dong ML, Yang ZF, Li XR. ZW10 interacting kinetochore protein may serve as a prognostic biomarker for human breast cancer: an integrated bioinformatics analysis. OncolLett. 2020 doi: 10.3892/ol.2020.11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CT, Min L, Wang YJ, Li P, Wu YD, Zhang ST. shRNA mediated knockdown of KNTC1 suppresses cell viability and induces apoptosis in esophageal squamous cell carcinoma. Int J Oncol. 2019;54(3):1053–1060. doi: 10.3892/ijo.2019.4672. [DOI] [PubMed] [Google Scholar]
- Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28(5):899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- Qiu HZ, Huang J, Xiang CC, Li R, Zuo ED, Zhang Y, Shan L, Cheng X. Screening and discovery of new potential biomarkers and small molecule drugs for cervical cancer: a bioinformatics analysis. Technol Cancer Res Treat. 2020;19:1533033820980112. doi: 10.1177/1533033820980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Przgastroenterol. 2019;14(2):89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaerou F, Starr DA, Piano F, Papoulas O, Karess RE, Goldberg ML. The ZW10 and rough deal checkpoint proteins function together in a large, evolutionarily conserved complex targeted to the kinetochore. J Cell Sci. 2001;114(Pt 17):3103–3114. doi: 10.1242/jcs.114.17.3103. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics. CA Can J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. ProcNatlAcadSci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa R, Vasudevan S, Ashqar MA-A, Reich E, Kravchenko-Balasha N, Klutstein M. Meiosis and kinetochore genes are used by cancer cells as genome destabilizers and transformation catalysts. bioRxiv. 2019 doi: 10.1101/826081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiru P, Kern DM, McKinley KL, Monda JK, Rago F, Su KC, Tsinman T, Yarar D, Bell GW, Cheeseman IM. Kinetochore genes are coordinately up-regulated in human tumors as part of a FoxM1-related cell division program. MolBiol Cell. 2014;25(13):1983–1994. doi: 10.1091/mbc.E14-03-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cummins JM, Shen D, Cahill DP, Jallepalli PV, Wang TL, Parsons DW, Traverso G, Awad M, Silliman N, Ptak J, Szabo S, Willson JK, Markowitz SD, Goldberg ML, Karess R, Kinzler KW, Vogelstein B, Velculescu VE, Lengauer C. Three classes of genes mutated in colorectal cancers with chromosomal instability. Can Res. 2004;64(9):2998–3001. doi: 10.1158/0008-5472.CAN-04-0587. [DOI] [PubMed] [Google Scholar]
- Yin-Ju C, Chiou J-F. Abstract 99: identify potential kinetochore protein inhibitors to overcome cisplatin resistance. Can Res. 2017;77(13 Supplement):99–99. doi: 10.1158/1538-7445.am2017-99. [DOI] [Google Scholar]
- Yin J, Bai Z, Zhang J, Zheng Z, Yao H, Ye P, Li J, Gao X, Zhang Z. Burden of colorectal cancer in China, 1990–2017: findings from the global burden of disease study 2017. Chung-kuo yen cheng yen chiu. 2019;31(3):489–498. doi: 10.21147/j.issn.1000-9604.2019.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, Yan GR, He QY. DOSE: an R/bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics. 2015;31(4):608–609. doi: 10.1093/bioinformatics/btu684. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu Y, Pu S, He J, Zhou C. Spindle and kinetochore associated complex subunit 3 accelerates breast cancer cell proliferation and invasion through the regulation of Akt/Wnt/beta-catenin signaling. Breast Cancer Res Treat. 2021;186(1):247–258. doi: 10.1007/s10549-020-06078-3. [DOI] [PubMed] [Google Scholar]
- Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. Report of cancer epidemiology in China, 2015. ZhonghuaZhong Liu ZaZhi. 2019;41(1):19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Zhou H, Yang Z, Yue J, Chen Y, Chen T, Mu T, Liu H, Bi X. Identification of potential hub genes via bioinformatics analysis combined with experimental verification in colorectal cancer. MolCarcinog. 2020;59(4):425–438. doi: 10.1002/mc.23165. [DOI] [PubMed] [Google Scholar]