Abstract
Efficient immune responses rely on heterogeneity, which in CD8+ T cells, amongst other mechanisms, is achieved by asymmetric cell division (ACD). Here we find that ageing, known to negatively impact immune responses, impairs ACD in murine CD8+ T cells, and that this phenotype can be rescued by transient mTOR inhibition. Increased ACD rates in mitotic cells from aged mice restore the expansion and memory potential of their cellular progenies. Further characterization of the composition of CD8+ T cells reveals that virtual memory cells (TVM cells), which accumulate during ageing, have a unique proliferation and metabolic profile, and retain their ability to divide asymmetrically, which correlates with increased memory potential. The opposite is observed for naive CD8+ T cells from aged mice. Our data provide evidence on how ACD modulation contributes to long-term survival and function of T cells during ageing, offering new insights into how the immune system adapts to ageing.
Subject terms: Adaptive immunity, Immunological memory, Lymphocyte differentiation, T cells
Asymmetrical cell division helps to maintain cellular heterogeneity in the T cell compartment. Here the authors examine the differential immune responses built by naive and virtual memory T cells from young and aged individuals, and explore the effect of mTOR inhibition on asymmetrical cell division and memory formation.
Introduction
Efficient T-cell responses rely on heterogeneity. In the context of CD8+ T cells, this is achieved both by a diverse naive T-cell repertoire and by the ability of single cells to generate effector and memory progenies, responsible for both clearance of intracellular pathogens and long-term protection against reinfections. Asymmetric cell division (ACD) is an important mechanism to generate T-cell diversity, and its loss contributes to impaired immune responses, particularly with regard to the formation and maintenance of a memory T-cell pool1–4. Upon ACD, a single T cell can give rise to daughter cells inheriting distinct contents of fate determinants through the polarized segregation of cell cargoes, which culminates in different potential fates. We reported previously a correlation between stemness and the ability of a CD8+ T cell to undergo ACD by showing that short-lived effector and exhausted CD8+ T cells divide mainly symmetrically, while naive and central memory pluripotent CD8+ T cells are capable to divide asymmetrically3.
The ageing of the immune system, a multifaceted phenomenon also known as immunosenescence, threatens T cell diversity, as a result of thymic involution and antigen exposure history. This culminates in both inefficient immune responses and increased susceptibility to autoimmunity5–7. In other cell types, such as haematopoietic stem cells (HSCs), the loss of diversity observed during ageing has been linked to their impaired ability to undergo ACD. In this scenario, the loss of asymmetric fates in HSCs is clearly detrimental for haematopoiesis and maintenance of stemness8.
The decline of thymic functions observed in aged individuals results in a substantial change in T cell composition as fewer naive T cells emigrate to the periphery, and memory-phenotype CD8+ T cells start to play a more dominant role as they accumulate due to cytokine-driven homoeostatic proliferation9–11. Not being previously exposed to foreign antigen, memory-phenotype CD8+ T cells are usually termed virtual memory T cells (TVM cells)12–19. How TVM cells are generated has been a long-standing question, especially concerning whether this is a TCR-independent or TCR-instructed process. While TVM cells can be found in TCR-transgenic Rag deficient hosts20 and in both pathogen-free and germ-free mice21, a recent report identified a group of thymic precursors with self-reactive TCRs that give rise to memory-phenotype CD8+ T cells22. In contrast to cognate antigen-experienced memory T cells, TVM cells show consistent low expression of the α4 integrin CD49d. Therefore, CD49d can be used as a suitable marker to discriminate between true and virtual memory lymphocytes15,23,24.
TVM cells show paradoxical characteristics: their increased expression of CD122, a feature of memory cells, endows them with a competitive advantage in sensing IL-15, hence facilitating their long-term survival14,25, but they are also efficient producers of IFNγ, a feature of effector cells, resulting in their major protective role against infections in aged individuals26 despite their lower proliferative potential27. Indeed, TVM cells can readily produce cytokines in the absence of antigenic exposure upon stimulation with IL-12, IL-15 and IL-1813,14,19,27, and constitutively express T-bet (a transcription factor that promotes the expression of effector genes) as well as effector molecules such as granzyme B and NKG2D (usually found in NK cells and activated T lymphocytes), features that facilitate their effector and bystander function28.
As ageing drives a profound change in the composition of T cells, particularly due to the drop of naive T cells with a concommitant rise of TVM cells, it remains to be addressed whether the ability of CD8+ T cells to undergo ACD is impacted by ageing. In this study, we found that ageing leads to an overall decline in the ability of CD8+ T cells to undergo ACD. This phenomenon was due to the lower ACD rates found in naive CD8+ T cells from aged animals, as CD8+ TVM cells had this feature unaltered by ageing. As opposed to truly naive CD8+ T cells from aged mice, TVM cells were unresponsive to mTOR inhibition-mediated enforcement of ACD, a strategy shown to promote ACD and memory potential in naive and memory CD8+ T cells from young mice3. Functionally, CD8+ TVM showed better re-expansion potential in adoptive transfer experiments compared to naive CD8+ T cells from aged mice, providing new evidence of a correlation between the ability to divide asymmetrically and memory potential. Our data suggest that ACD might play an important role to counteract immunosenescence, in that it is retained in TVM cells but lost in aged naive cells, thereby preserving the ability to adopt differential fates in TVM cells.
Results
CD8+ T cells from aged mice are less able to undergo ACD
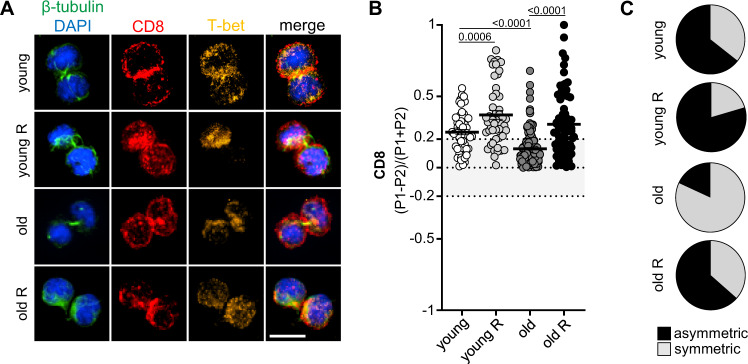
Considering the reduced capability of naive T cells to form memory populations during immunoscenescence, we investigated whether the ability of CD8+ T cells to undergo ACD is affected by ageing. To this end, we used bulk CD8+ T cells isolated from naive young (8–16 weeks old), middle-aged (>40 weeks old) and old (>70 weeks old) P14 mice (carrying a transgenic T-cell receptor specific for the lymphocytic choriomeningitis virus (LCMV) glycoprotein residues 33-41 (GP33) presented on H-2Db). The CD8+ T cells were stimulated on plates coated with human Fc-ICAM-1, α-CD3 and α-CD28 for 36 h, allowing them to enter the first round of cell division, identifiable as different stages of mitosis by confocal microscopy. CD8 polarization on mitotic cells was used as a reliable readout of ACD4. Comparison of the ACD rates from naive CD8+ T cells isolated from young or middle-aged/old P14 mice revealed that ageing correlated with an impaired ability to divide asymmetrically. We have shown previously that transient inhibition of the mTOR pathway by rapamycin is an efficient strategy to increase or restore the ability of CD8+ T cells to divide asymmetrically3. Interestingly, ACD rates, comparable to young CD8+ T cells, were restored when middle-aged/aged CD8+ T cells were submitted to transient mTOR inhibition (Fig. 1). We observed a similar trend when measuring the polarized inheritance of both T-bet and phosphorylated S629–31 (Supplementary Fig. 1). Together, our data provide evidence that ageing impairs the ability of CD8+ T cells to undergo ACD, and that transient mTOR inhibition can restore ACD in cells from aged mice. As ACD is correlated with stemness and memory potential3,4,30,31, these results reveal a possible strategy to reinvigorate CD8+ memory T-cell responses in the elderly.
Fig. 1. CD8+ T cells from aged mice exhibit decreased ability to undergo asymmetric cell division.
A Confocal images of murine CD8+ T cells fixed 36–40 h after in vitro stimulation on α-CD3, α-CD28 and human Fc-ICAM-1 coated wells (young, n = 59; young R, n = 49; middle-aged/old, n = 171; middle-aged/old R, n = 74). Transient mTOR inhibition was achieved by treatment with rapamycin (R) (20 nM). Cells were exposed to drug treatment from 12 h post activation until fixation for confocal microscopy analysis. Scale bar = 10 µm. B ACD rates observed in CD8+ T cells from middle-aged/aged and young mice upon transient mTOR inhibition with rapamycin. Data are represented as mean ± SEM. C Pie charts indicating frequencies of asymmetrically dividing cells from B. Level of CD8 asymmetric inheritance is depicted in black and level of CD8 symmetric inheritance in grey. Pooled data from three independent experiments. Age of the animals: experiments 1 and 2 (40–43 weeks) and experiment 3 (71 weeks). Statistical analysis was performed using the unpaired two-tailed Student’s t test. Exact P values are depicted in the figure (see also Supplementary Fig. 1).
Re-establishment of ACD restores memory potential
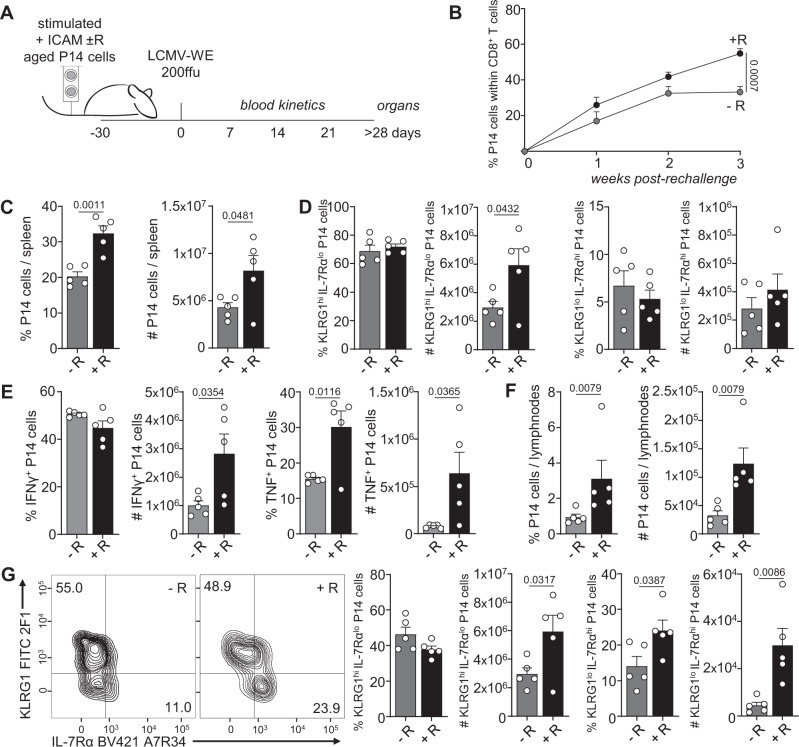
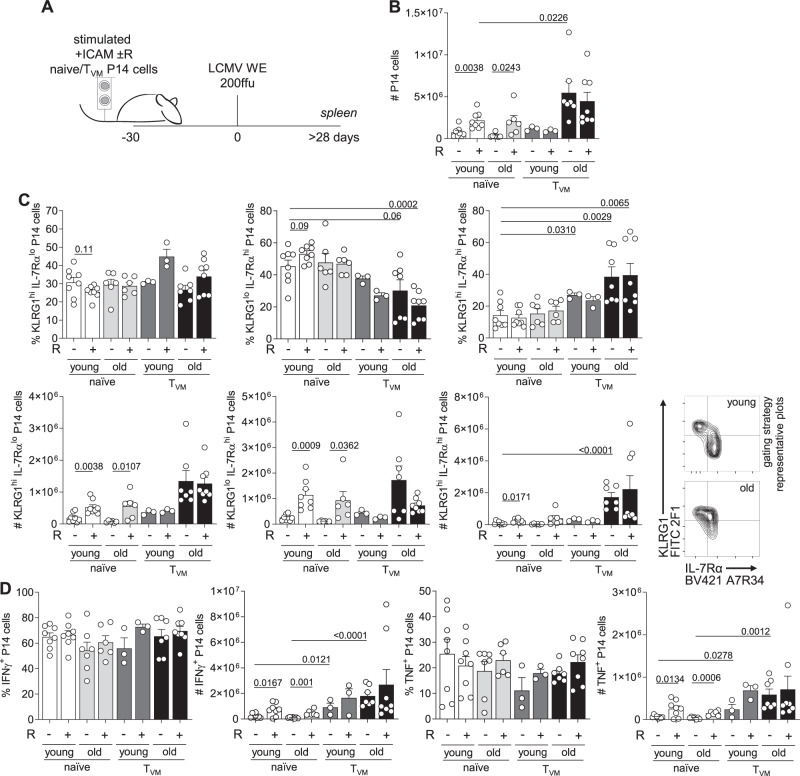
An increased ability to divide asymmetrically has been previously reported to promote memory potential of emerging CD8+ T cell progeny3. Thus, we decided to test whether this would also hold true for cells from aged mice in which transient mTOR inhibition increased ACD rates in vitro. CD8+ T cells isolated from aged P14 mice (>70 weeks) were stimulated under ACD conditions (+ICAM) in presence or absence of transient rapamycin treatment, and their progenies were adoptively transferred into naive and young wild-type recipient mice (Fig. 2A). Recipients were then acutely infected with 200 ffu LCMV-WE > 30 days after adoptive transfer, favoring maintenance of long-lived cells that can survive in absence of antigen, a feature of memory cells. We monitored the expansion of the adoptively transferred P14 cells in the blood, spleen and lymph nodes (LNs) of recipient mice (Fig. 2B, C, F). We observed higher frequencies and numbers of progenies derived from stimulation conditions in which mTOR was transiently inhibited with rapamycin. Phenotypic changes were only observed in P14 cells in the LNs, where transient rapamycin treatment during in vitro stimulation gave rise to progenies with higher frequencies and numbers of cells committed to a memory fate, defined as KLRG1lo IL-7Rαhi expressing cells (Fig. 2D, G). Furthermore, progenies originating from higher asymmetry rate conditions, imposed by transient mTOR inhibition, exhibited increased cytokine production (TNF; Fig. 2E). As total P14 cell numbers were higher in progenies of cells transiently treated with rapamycin, we could also observe a significantly higher number of both IFNγ and TNF producing cells in this group. Together, these data provide further evidence regarding a correlation between increased ACD rates and progenies with increased memory potential, as determined by their enhanced survival and recall response upon re-challenge.
Fig. 2. Re-establishment of asymmetric cell division in CD8+ T cells from aged mice correlates with increased memory potential.
A Experimental setup (illustration by MB). Naive P14 cells from aged mice were stimulated in vitro on α-CD3, α-CD28 and human Fc-ICAM-1 coated wells in presence or absence of transient rapamycin (R) treatment, harvested after 36 h, and adoptively transferred into young and naive wild-type recipients. Each recipient mouse received 1 × 104 cells and was infected intravenously with LCMV-WE > 30 days later. B Frequencies of P14 cells within the CD8+ T-cell population in the blood. C Frequency and numbers of adoptively transferred P14 cells within CD8+ T cells in the spleens of recipient mice. D Frequencies and numbers of KLRG1hi IL-7Rαlo or KLRG1lo IL-7Rαhi P14 cells in the spleens of recipient mice. E Frequencies and numbers of splenic IFNγ and TNF P14 producing cells. F Frequencies and numbers of adoptively transferred P14 cells within CD8+ T cells in the inguinal lymph nodes of recipient mice. G Representative flow cytometry plots of KLRG1 and IL-7Rα expression in P14 cells in the lymph nodes of recipient mice 30 days after LCMV-WE challenge (left panel); percentages and numbers of KLRG1hi IL-7Rαlo and KLRG1lo IL-7Rαhi P14 cells in the lymph nodes of recipient mice (right panel). Representative data (n = 5 per group) from one out of two experiments. Aged P14 animals used for adoptive cell transfer: 95 weeks (experiment 1); 71 weeks (experiment 2). Data are depicted as mean + SEM. Statistical analysis was performed using the unpaired two-tailed Student’s t test (B–E, G, week 3) or when data did not pass the normality test, the unpaired two-tailed Mann–Whitney U test (F, G, #KLRG1hi IL-7Rαlo). Exact P values are depicted in the figure.
TVM cells have a unique proliferative and metabolic phenotype
Our analysis of CD8+ T cells isolated from young and old P14 mice revealed an impaired ability of CD8+ T cells from aged mice to undergo ACD. In view of the heterogeneity of CD8+ T cells present in “naive” mice, we decided to assess phenotypical differences between cells derived from young or old mice. To that end, we analyzed the expression of CD44 in CD8+ T cells from the blood of young (<16 weeks old) and aged (>70 weeks) P14 mice, as previous work has shown that ageing leads to an increased CD44hi/CD44lo T-cell ratio32–34. The frequencies of CD44hi cells were significantly higher within the CD8+ T cell pool in aged mice (Fig. 3A). A previous study provided evidence of a correlation between a higher expression of CD44 and an exhausted signature during ageing32. Considering the lower asymmetry rates observed in CD8+ T cells from aged mice and the negative effect of T cell exhaustion on the ability of CD8+ T cells to divide asymmetrically3, we characterized whether CD44hi cells would exhibit an exhausted phenotype. We stained peripheral blood CD8+ T cells from young and old P14 mice for the expression of both the co-inhibitory molecule PD-135–37, and the exhaustion marker CD3938. In agreement with more recent studies27, despite a trend towards an increase in PD-1hi and CD39+ cells amongst CD44hi CD8+ T cells in aged animals, these cells were still a minority in the CD44hi compartment (Supplementary Fig. 2). These data exclude exhaustion as a direct or major cause for the lower ACD rates observed in CD8+ T cells from aged mice.
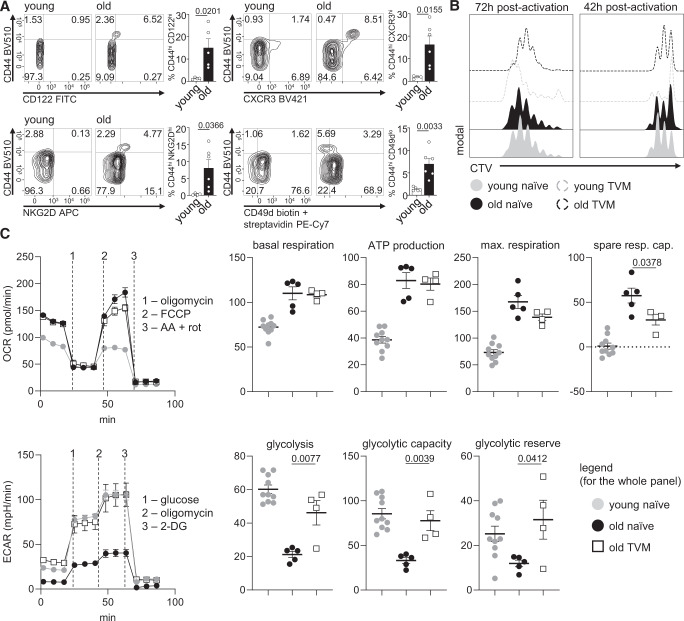
Fig. 3. TVM cells have a unique proliferative and metabolic phenotype.
A Representative flow cytometry plots showing CD122, CXCR3, NKG2D and CD49d expression on CD8+ T cells in the blood of young and old P14 mice. Frequencies of CD44lo and CD44hi CD8+ T cells expressing high CD122, CXCR3 and NKG2D levels and low CD49d levels are depicted in the bar graphs. Pooled data from two independent experiments (young, n = 4: old, n = 5; aged mice: 70, 90 or 100 weeks old). B Histograms depicting proliferation of purified naive or TVM CD8+ T cells from young or aged mice (CTV-labelled, stimulated on α-CD3, α-CD28 and human Fc-ICAM-1 coated wells). After 42 h, the proliferation profile was analyzed by CTV dilution (left panel) or cells were transferred to uncoated wells for further 30 h in medium containing IL-2, IL-7 and IL-15, until their proliferation kinetics was assessed 72 h post stimulation (right panel). Representative data from one out of two experiments (aged mice: 70 or 82 weeks old). C Oxygen consumption rate (OCR) (upper panel) and extracellular acidification rate (ECAR) (lower panel) of activated CD8+ T cells was measured under basal conditions and in response to indicated drugs. Representative data from one out of two experiments, where data points are technical replicates (young naive, n = 10; old naive, n = 5, old TVM, n = 4; aged mice > 100 weeks old in all experiments). Data are depicted as mean ± SEM. Statistical analysis was performed using the unpaired two-tailed Student’s t test. Exact P values are depicted in the figure.
CD44hi CD8+ T cells are known to accumulate with ageing39, and several reports have identified a large proportion of these cells as virtual memory cells (TVM)14,18,19,26,27. To investigate whether the CD44hi cells that were found at higher frequencies in aged P14 mice were indeed TVM cells, we first performed a phenotypic characterization16. We collected blood from both young and old unimmunized P14 mice and analyzed the expression of CD44, CD122, CXCR3, NKG2D and CD49d in CD8+ T cells. Confirming the TVM cell phenotype, CD44 expression on CD8+ T cells was accompanied by high expression of CD122, CXCR3 and NKG2D, and low expression of CD49d (Fig. 3A). Furthermore, as the CD44hi CD8+ T cells were not CD49dhi in either young or aged unimmunized P14 mice, we excluded them being antigen-driven memory cells. Other studies have shown an accumulation of true memory CD8+ T cells upon ageing in wild-type animals with a polyclonal lymphocyte repertoire (Quinn et al.27). The absence of this population in naive P14 mice, independent of their age, corroborated that TVM cells derive from naive cells in a mechanism distinct from the differentiation of “true” memory cells by cognate antigen recognition.
To further characterize the TVM cell population present in the blood of aged P14 animals, we separately analyzed the proliferation profiles of naive and TVM splenic CD8+ T cells isolated from young and aged mice 42 or 72 h post activation (Fig. 3B). At an early time point (42 h post activation), TVM cells from both young and aged animals had undergone fewer divisions in comparison to their naive counterparts. However, 72 h post activation, TVM cells isolated from young hosts exhibited the highest proliferative capacity among all tested cell types, while TVM cells from aged hosts were the ones showing the lowest proliferation speed, but still similar to the profile seen in naive CD8+ T cells from young or aged animals. Truly naive CD8+ T cells from young and aged animals showed very similar proliferation profiles at both timepoints analyzed. These data corroborate previous observations, showing that TVM cells from young hosts exhibit faster cell cycling in comparison to naive cells27, but also suggest a unique cell division kinetics of TVM cells, as the time for the first division seemed to be extended, especially in the ones originating from an ageing host. Cell cycling speed can be a result of distinct metabolic activities and plays a role in T cell differentiation40–42. It has been reported that resting TVM CD8+ T cells have a different metabolism in comparison to naive or true memory cells, exhibiting elevated spare respiratory capacity (SRC)25. We therefore investigated whether activated naive and TVM CD8+ T cells exhibited metabolic differences. Because frequencies of TVM cells were particularly rare in young P14 mice, these cells were not used for metabolic assays. We assessed the metabolic performance of naive CD8+ T cells from young and aged animals, and TVM cells from aged animals 36 h post activation (when cells either did not divide or underwent their first round of mitosis) using Seahorse assays. Both naive and TVM CD8+ T cells from aged animals outperformed naive cells from young individuals concerning their SRC (Fig. 3C, upper panel). TVM cells from aged individuals showed a similar glycolytic performance as young naive cells, both with respect to their glycolytic capacity and glycolytic reserve, features for which naive cells from aged hosts exhibited impaired function (Fig. 3C, lower panel). This could indicate that activated TVM cells from aged hosts are metabolically adapted to swiftly respond to environmental changes through their ability to use both glycolysis and oxidative phosphorylation (OXPHOS) in order to sustain their metabolic demands, while simultaneously being “slow-dividers”, a feature previously reported to benefit ACD3 and memory formation42.
TVM CD8+ T cells show intrinsically high ACD rates
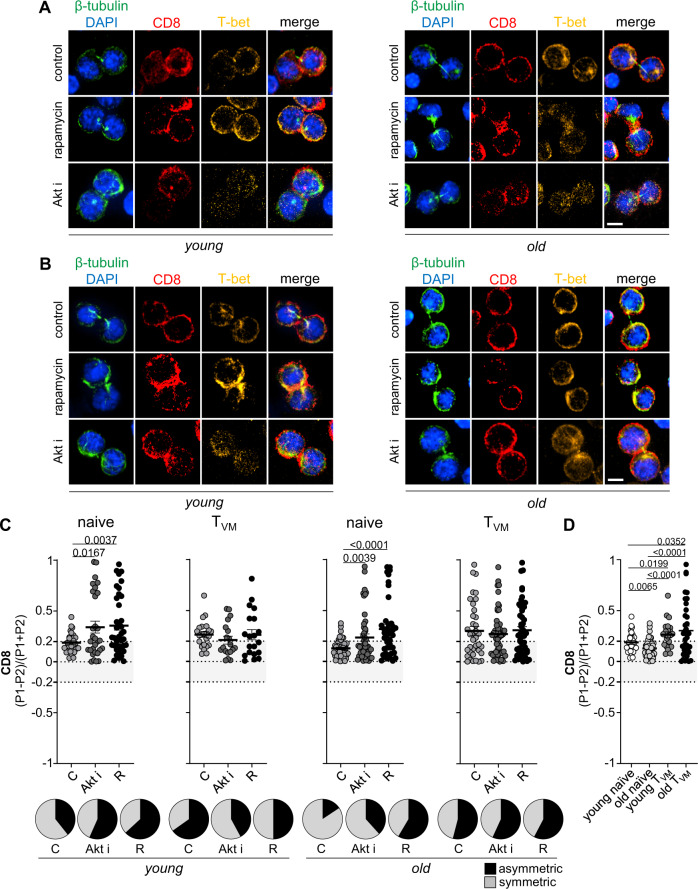
The observation of phenotypic, functional and metabolic differences between naive and TVM CD8+ T cells led us to investigate whether there would also be differences in their ability to divide asymmetrically. To that end, we sorted CD8+ T cells isolated from spleens and LNs of young and aged P14 mice into naive (CD44lo) and TVM (CD44hi, CD49dlo) populations. Cells were then stimulated in vitro under ACD conditions and submitted to transient mTOR inhibition with rapamycin or Akt-kinase inhibitor, known to increase ACD rates in naive and memory CD8+ T cells from young individuals3. The separation into naive and TVM cells prior to stimulation allowed us to directly compare their abilities to undergo ACD and to discriminate which cells were responsive to mTOR inhibition, evoking higher ACD rates and exhibiting improved memory potential after adoptive transfer (Figs. 1 and 2). TVM CD8+ T cells from both young and aged mice showed intrinsically high ACD rates, based on the levels of CD8 polarization (Fig. 4B, C). By contrast, naive CD8+ T cells from old mice had a markedly impaired capacity to undergo ACD in comparison to naive CD8+ T cells from young mice (Fig. 4A, C). As TVM cells take longer to undergo their first mitosis, it is likely that the frequencies of dividing TVM cells analysed amongst the bulk CD8+ T-cell population at 36 h post stimulation (Fig. 1) are under-represented. Furthermore, we cannot exclude that the analysed mitotic cells might represent a pool of TVM cells that retains their proliferation potential. These results, however, suggest that naive CD8+ T cells are responsible for the overall lower ACD rates observed in total CD8+ T cells isolated from aged P14 mice in comparison to their young counterparts (Fig. 1). The intrinsically high ACD rates in TVM CD8+ T cells could not be further increased by both rapamycin and Akt-kinase inhibitor treatment, while CD44lo naive CD8+ T cells from both young and old mice showed increased ACD rates under the same conditions (Fig. 4C). The responsiveness of naive CD8+ T cells from old mice to rapamycin treatment explains the re-established asymmetry observed when total CD8+ T cells from old P14 mice were exposed to rapamycin treatment (Fig. 1). We speculate that the failure in modulating ACD in TVM CD8+ T cells might result from both their higher mTOR activity in comparison to the their naive counterparts, and the inefficacy of the inhibitors, at least in the concentrations that they were administered, in downregulating this activity, especially what concerns downstream targets of mTORC2 (Supplementary Fig. 3). As ACD relies on the formation of a stable immune synapse, we analyzed the expression of CD3, CD28 and LFA-1 in naive and TVM CD8+ T cells, and observed that the latter exhibited significantly higher levels of LFA-1 on the surface, perhaps contributing to their intrinsically high ACD rates (Supplementary Fig. 4). We obtained similar results when performing experiments with polyclonal CD8+ T cells from wild-type C57BL/6 mice (Supplementary Fig. 5). Considering the superior memory potential exhibited by progenies of cells able to divide more asymmetrically, these results suggest that impaired immune responses in the elderly can be partially rejuvenated by re-establishing their ability to undergo ACD in naive CD8+ T cells through transient mTOR inhibition. In addition, as ACD proved to be beneficial for establishment of immune memory, the intrinsic ability of TVM CD8+ T cells to divide asymmetrically might be a compensatory mechanism to sustain efficient T-cell responses upon ageing.
Fig. 4. TVM CD8+ T cells show intrinsically high ACD rates.
A Confocal images from murine naive CD8+ T cells. B Confocal images from murine TVM CD8+ T cells. Cells were fixed 36–40 h after in vitro stimulation on α-CD3, α-CD28 and human Fc-ICAM-1 coated wells. Transient mTOR inhibition was achieved by treatment with rapamycin (20 nM) or Akt-kinase inhibitor (5 µM). Cells were exposed to drug treatment from 12 h post activation until fixation for confocal microscopy analysis. Scale bar = 5 µm. C CD8 asymmetry rates in naive and TVM CD8+ T cells isolated from young or old mice (young naive C, n = 28; young naive Akt i, n = 30; young naive R, n = 38; young TVM C, n = 23; young TVM Akt i, n = 19; young TVM R, n = 20; old naive C, n = 51; old naive Akt i, n = 47; old naive R, n = 42; old TVM C, n = 37; old TVM Akt i, n = 51; old TVM R, n = 52). Frequencies of asymmetrically dividing cells are depicted in pie charts on the right side of each graph. D Comparison of CD8 polarization amongst young naive, old naive, young TVM and old TVM CD8+ T cells (young naive, n = 28; old naive, n = 51; young TVM, n = 23; old TVM, n = 37). Data are shown as mean ± SEM. Pooled data from five independent experiments (aged mice: 71, 95, 97, 102 or 108 weeks old). For each experiment sorted cells from at least two animals were pooled. Statistical analysis was performed using the unpaired two-tailed Student’s t test. Exact P values are depicted in the figure.
ACD modulation targets naive CD8+ T cells from aged mice
Within CD8+ T cells from old P14 mice, the naive subset showed strongly impaired ability to undergo ACD, but simultaneously the best responsiveness to transient mTOR inhibition as a strategy to re-establish ACD. To investigate a potential improvement of immune responses by modulating ACD in these naive CD8+ T cells, we adoptively transferred progenies of stimulated naive and TVM P14 cells from young and old mice into naive wild-type recipients (Fig. 5A). Stimulation was performed under ACD conditions, with or without transient mTOR inhibition with rapamycin. After 30 days, recipients were infected with LCMV-WE and numbers of P14 cells were evaluated in the spleen 30 days post viral challenge (Fig. 5B, C). Progenies of rapamycin treated naive P14 cells from both young and old P14 mice were present in higher numbers than their untreated counterparts (Fig. 5B). Progenies of CD8+ TVM cells from both young and aged mice expanded to comparable frequencies or total numbers in presence or absence of mTOR inhibition during in vitro stimulation. As mTOR inhibition could not enhance their ability to undergo ACD, these data strengthen the correlation between the ability to undergo ACD and the potential to form a pool of memory cells. Rapamycin treatment did not lead to significant changes in the phenotypes of the resulting progenies, however, at least for naive cells from young mice, there was a trend towards higher frequencies of KLRG1lo IL-7Rαhi and lower frequencies of KLRG1hi IL-7Rαlo cells of the rapamycin treated group (Fig. 5C), as previously reported by us3. Interestingly, progenies of aged CD8+ TVM cells exhibited significantly lower frequencies of KLRG1lo IL-7Rαhi cells, with concomitant emergence of a double-positive population for KLRG1 and IL-7Rα, known to be effector-like memory cells that exhibit enhanced cytotoxic activity43 (Fig. 5C). Functionally, frequencies of IFNγ and TNF producing cells were not affected by cell type or ageing (Fig. 5D). As total P14 cell numbers were higher in progenies of naive CD8+ T cells transiently treated with rapamycin and in progenies of TVM cells (independent of previous rapamycin treatment), we also observed significantly elevated numbers of both IFNγ and TNF producing cells in these groups. Together, these results emphasize a link between ACD, memory potential and efficient immune responses, and provide new insights into how the ability to undergo ACD is affected by ageing. We propose that CD8+ TVM cells might represent an adaptation to immunosenescence and compensate for the loss of ACD potential in naive cells. Furthermore, as the memory potential of naive CD8+ T cells from aged hosts could be increased by transient mTOR inhibition and increased ACD rates, these data reinforce the potential of this intervention for translational purposes in regenerative medicine.
Fig. 5. Re-establishment of ACD by transient mTOR inhibition in naive CD8+ T cells from aged mice reflects in correlates with improved memory potential.
A Experimental setup (illustration by MB). Naive and TVM CD8+ T cells were sorted from young and old P14 mice spleens, stimulated under different conditions, harvested after 36 h and adoptively transferred into wild-type recipients. Each recipient mouse received 1 × 104 cells and was infected intravenously with LCMV-WE (200 ffu) 30 days later. B Numbers of adoptively transferred P14 cells within CD8+ T cells in the spleens of recipient mice. C Percentages and numbers of KLRG1hi IL-7Rαlo, KLRG1lo IL-7Rαhi and KLRG1hi IL-7Rαhi P14 cells in the spleens of recipient mice (left panel). Representative plots showing gating strategy used to access expression of KLRG1 and IL-7Rα (right panel). D Frequencies and numbers of IFNγ, TNF and IL-2 producing cells in the spleens of recipient mice. Pooled data from two independent experiments (young naive, n = 8; young naive R, n = 8; young TVM, n = 3; young TVM R, n = 3; old naive, n = 6; old naive R, n = 6; old TVM, n = 7; old TVM R, n = 8). Aged P14 animals used for adoptive cell transfer: 92 or 96 weeks (experiment 1); 70 or 75 weeks (experiment 2). Data are depicted as mean + SEM. Statistical analysis was performed using the unpaired two-tailed Student’s t test. Exact P values are depicted in the figure.
Discussion
Ageing is associated with a progressive decline in the efficiency of mounting effective and long-lasting immune responses and results in higher vulnerability to infections in both mice and humans44. Virtually all aspects of immunity are affected by ageing, from the initial contact with a pathogen to its clearance or coexistence with a persisting pathogen7. Concerning T cell responses, the most profound change observable upon ageing is thymic involution10,45, which leads to marked changes in peripheral T cell maintenance mechanisms. With the decrease of thymic output, T cells that are already in the periphery go through more rounds of homoeostatic proliferation. Amongst possible other factors yet to be discovered, this contributes to the naive-to-memory conversion of their phenotype, which is independent of cognate antigen encounter, hence these cells are referred to as virtual memory cells13–15,17–19,27. TVM CD8+ T cells accumulate with ageing and depend on homoeostatic cytokines for maintenance. Besides activation through the T-cell receptor to exert effector functions, TVM CD8+ T are also responsive to cytokine activation14,19,28.
In the present study, our aim was to investigate a new aspect of the ageing immune system and characterize the ability of CD8+ T cells to undergo ACD. Our results on bulk CD8+ T cells show that total naive cells from aged mice divide less asymmetrically than their young counterparts. We have previously developed a strategy to increase ACD rates by transient mTOR inhibition with either rapamycin or Akt-kinase inhibitor, which was based on the reported benefits of mTOR inhibition on memory formation46,47. This transient mTOR inhibition during in vitro stimulation proved to be efficient in enhancing memory potential of progenies of naive, memory and PD-1int Tcf1+ exhausted CD8+ T cells3. Of relevance, mTOR is a key regulator of ageing in different organisms, from yeast to mammals48, and its inhibition has been linked to extended life span and improved immune functions in old mice and humans49,50. Furthermore, the inhibition of the homologous pathway TOR in yeast contributes to rejuvenation of yeast cells through higher ACD rates51. Transient rapamycin treatment of CD8+ T cells from aged mice indeed led to increased ACD rates, confirming our initial hypothesis that ACD can be modulated in these cells. This remarkable result proved to be of functional relevance, as progenies originating from enforced ACD conditions exhibited improved memory potential in a viral challenge model.
In line with previous reports, we observed a marked increase in the CD44hi/CD44lo ratio with ageing32–34, and identified the emerging CD44hi population as TVM cells. Besides phenotypic differences with naive T cells in surface marker expression, TVM cells also exhibited different proliferation rates and metabolism. After stimulation in vitro, TVM cells from both young and aged animals required a longer time to undergo their first rounds of division in comparison to their naive counterparts. However, the opposite was observed at later timepoints, when progenies of TVM cells showed similar or higher proliferation rates. Activated TVM cells/progenies acquired a unique effector-memory mixed metabolic profile, being capable to rely on both glycolysis and OXPHOS.
The differences in observed phenotype, proliferation kinetics and metabolism between naive and TVM CD8+ T cells led us to investigate which cell type is responsible for the lower ACD rates found in total CD8+ T cells originating from aged mice. Surprisingly, the ability of CD44lo naive CD8+ T cells to undergo ACD was strongly affected by ageing, as CD8+ T cells from old mice exhibited low ACD rates compared to their young counterparts. This defect could be reversed by transient mTOR inhibition. As expected, increased ACD rates observed in vitro led to improved function and correlated with a higher re-expansion potential upon antigenic challenge in vivo. By contrast, TVM cells exhibited intrinsically high ACD rates, which could not be further increased by transient mTOR inhibition. As ACD is thought to be beneficial for diversified T-cell responses, in particular to impart memory potential, we propose that TVM cells are an adaptation of the immune system to immunesenescence. Indeed, our results might explain how TVM cells end up comprising the majority of the CD44hiCD62Lhi TCM cell population in LCMV infected mice25, or how these cells are able to compose the majority of the central memory CD8+ T cell (TCM) compartment after LM-OVA antigenic challenge, while also being able to generate effector responses19.
The better expansion potential of TVM CD8+ T-cell progenies in comparison to their naive counterparts in adoptive transfer experiments followed by antigenic challenge contradicts previous findings, where CD44lo naive CD8+ T cells were described as superior in that context27,32. However, the divergent results may be explained by the different experimental setups. In our study, adoptively transferred cells were progenies of TVM cells that underwent ACD and were required to survive for over 30 days before re-challenge. In previous studies27,32, recipient mice received unstimulated cells and were either immediately immunized with cognate antigen or left untreated to assess the differential survival of naive CD8+ T cells and TVM CD8+ T cells.
Taken together, our results suggest that the extended time taken by TVM cells to undergo their first rounds of division are beneficial for ACD, and thus for the establishment of asymmetric fates, which potentially also explains their exclusive proliferation and mixed metabolic phenotypes. ACD contributes to the simultaneous rise of (1) a pool of memory cells reliant on OXPHOS and exhibiting elevated SRC, and (2) a pool of effector cells that relies on glycolysis, and undergoes rapid proliferation at later timepoints42. We observed that TVM cells from aged animals, which exhibit higher expression of CD122 than their naive counterparts, are better survivors under limiting IL-15 concentrations in vitro, highlighting a potential role of survival in the observed increased recovery of adoptively transferred cells upon re-challenge/re-expansion (Supplementary Fig. 6). Thus, the virtual memory pool of CD8+ T cells might present an adaptation of the ageing immune system to maintain a memory-like pool of cells in absence of previous antigen encounter without compromising effector responses.
A question that remains unresolved is the reason for the refractoriness of TVM CD8+ T cells to mTOR inhibition when measured by modulation of ACD rates. CD44 engagement is known to lead to downstream phosphorylation of Akt, resulting in overall higher mTOR activity52. When we assessed mTORC1 and mTORC2 activities, measured by phosphorylation levels of S6 and NFκB53, our data indicated that TVM cells have higher basal mTOR activity in comparison to their naive CD44lo counterparts and are refractory to mTORC2 inhibition (Supplementary Fig. 3). As there is evidence on the role of mTORC2 inhibition as an efficient strategy for ACD modulation3, these data suggest that constitutive mTORC2 activity might be the reason for both the lack of ACD modulation in TVM cells and their efficient glycolytic metabolism. Furthermore, the inability to modulate ACD in TVM CD8+ T cells, by either rapamycin or Akt-kinase inhibitor treatment, could be caused by the altered role of mTOR during CD8+ T cell senescence, which potentially leads to differential activation of its downstream targets54–56. However, the reason for their higher intrinsic ability to divide asymmetrically remains unresolved and needs to be further investigated.
Transient mTOR inhibition efficiently restored the ability of naive CD8+ T cells from aged mice to undergo ACD. This correlated with an increased re-expansion of transferred cells upon LCMV challenge, which is evidence of their better survival capacity and memory potential. Re-establishment of ACD might therefore represent a strategy for rejuvenation of senescent naive CD8+ T cells found in aged individuals. Conversely, TVM cells exhibited intrinsically high ACD rates which could not be further increased by transient mTOR inhibition. Functionally, these properties culminated in increased memory potential shown by adoptive transfer experiments, providing a further link between ACD and efficient immunity. The accumulation of TVM cells with ageing might therefore, at least to some extent, compensate for the diminished memory potential of naive cells. Reinforcing ACD in these naive cells in aged individuals might offer new perspectives for improvement of immune functions in the elderly, and brings new insights on how the immune system adapts to ageing.
Methods
Ethics statement
Animal experiments were performed in accordance with institutional policies, Swiss and British federal regulations, and were approved by the veterinary office of the Canton of Zürich (animal experimental permissions: 147/2014 and 115/2017) or by a local ethical review committee in UK (project license PPL 30/3388).
Mice
P14 mice (Rag sufficient) expressing a transgenic T-cell receptor specific for the glycoprotein 33-41 (gp33) epitope of the LCMV presented on H-2Db57 on a congenic CD45.1 background were bred and maintained at the ETH Phenomics Center. CD45.2 C57BL/6 were bred at the ETH Phenomics Center or purchased from Janvier Elevage (Saint Berthevin, France) or Charles River (UK). Six-to-sixteen-week-old male or female mice were considered young, mice > 50 weeks of age were considered middle-aged and mice > 70 weeks were considered aged. For all experiments where adoptive cell transfer was performed, aged mice were >70 weeks old. All mice were bred and maintained under specific pathogen-free conditions at 24 °C and 50% humidity, exposed to a 12:12 h light/dark cycle and kept in individually ventilated cages with autoclaved water and irradiated pellet feed provided ad libitum.
Virus, viral peptides and infections
LCMV peptide gp33-41 (gp33; KAVYNFATM) was purchased from EMC microcollections GmbH (Tübingen, Germany). LCMV-WE was originally provided by R.M. Zinkernagel (University Hospital, Zurich, Switzerland) and was propagated on BHK-21 cells58. Acute infections were performed by intravenously injecting mice into the tail vein with 200 ffu of LCMV-WE.
CD8+ T-cell isolation
CD8+ T cells were isolated from spleens of CD45.1 P14 mice using the EasySepTM Mouse CD8+ T cell Isolation Kit (Stemcell, Grenoble, France) or the MojoSortTM Mouse CD8+ T cell Isolation Kit (BioLegend, Lucerna Chem AG, Luzern, Switzerland), following manufacturer’s instructions. For analysis of specific cell subsets and CD8+ T-cell enrichment, erythrocytes were lysed and remaining splenocytes were incubated with α-B220 biotin (RA3-6B2, BioLegend, 1/200), and α-CD4 biotin (GK1.5, BioLegend, 1/200) antibodies for 20 min at room temperature, followed by incubation with MojoSortTM streptavidin magnetic beads (BioLegend) for 5 min at room temperature and further magnetic separation. After enrichment, cells were then stained for phenotypical markers, and subpopulations of interest were sorted on a FACS Aria cell sorter (BD Biosciences).
CD8+ T-cell in vitro stimulation and adoptive transfer
For transfer of daughter cells, when confocal microscopy analysis of mitotic cells was performed or when cell proliferation was assessed, cells were cultured in T-cell medium (RPMI 1640 (Bioconcept), 2 mM L-Glutamine (Bioconcept), 2% penicillin-streptomycin (Sigma-Aldrich), 10% foetal bovine serum (Omnilab), 25 mM HEPES (Gibco, Life Technologies, Zug, Switzerland), 1× non-essential amino acids (Sigma-Aldrich), 50 µM β-Mercaptoethanol (Gibco), 1 mM sodium pyruvate (Gibco) supplemented with self-made human IL-2, and stimulated on plate-bound human Fc-ICAM-1 (50 µg/ml) (R&D Biosciences, Bio-Techne AG, Zug, Switzerland), α-CD3 (5 µg/ml) (145-2C11, BioLegend) and α-CD28 (5 µg/ml) (37.51, BioLegend) for 30–36 h. mTOR modulation was done by adding 20 nM of rapamycin (Santa Cruz, LabForce AG, Muttenz, Switzerland) or 1–2 µM of Akt-kinase inhibitor (Sigma-Aldrich) 12 h post stimulation. For adoptive transfer, 1 × 104 purified CD45.1 P14 cells (stimulated as indicated) were intravenously injected into naive C57BL/6 CD45.2 recipient mice. For cell proliferation assays, cells were stained with CellTrace VioletTM, CellTrace YellowTM or CellTrace VioletTM (Life Technologies) following manufacturer’s instructions prior to stimulation.
Stimulation of lymphocytes and flow cytometry
Blood samples used for kinetics analysis were obtained from the tail vein. Spleens were obtained from PBS-perfused mice. Single-cell splenocytes were prepared by meshing whole spleens through 70 µm strainers (BD Biosciences) using a syringe plunger. When cytokine production was assessed, CD8+ T cells within splenocytes were stimulated with 1 µg/ml of gp33 peptide in the presence of 10 µg/ml of brefeldin A (Sigma-Aldrich) for 6 h at 37 °C. Fluorophore-conjugated antibodies used for flow cytometry staining were purchased from BD Biosciences (α-CD44 FITC IM7 (1/200); α-IL-2 APC JES6-5H4 (1/100); streptavidin APC (1/500); streptavidin PE-Cy7 (1/500)), eBioscience (Lucerna Chem AG, Luzern, Switzerland) (α-IFNγ PE XMG1.2 (1/100); α-KLRG1 PE-Cy7 2F1 (1/200); α-PD-1 FITC J43 (1/100)), Cell Signalling (Bioconcept, Allschwil, Switzerland) (α-phospho-Akt PE D9E (1/50); α-phospho-NFκB PE 93H1 (1/50); α-phospho-S6 PE D57.2.2E (1/50)) or BioLegend (α-CD28 APC 37.51 (1/200); α-CD122 FITC TM-β1 (1/100); α-CD3ε APC-Cy7 145-2C11 (1/100); α-CD39 AF647 Duha59 (1/100); α-CD44 BV510 IM7 (1/200); α-CD44 PE IM7 (1/200); α-CD45.1 APC A20 (1/200); α-CD45.1 PerCP A20 (1/200); α-CD8 APC-Cy7 53-6.7 (1/100); α-CD8 BV510 53-6.7 (1/200); α-CD8 PerCP 53-6.7 (1/200); α-CD49d biotin R1-2 (1/200); α-CD62L PerCP MEL-14 (1/100); α-CXCR3 BV421 CXCR3-173 (1/100); α-KLRG1 FITC 2F1 (1/200); α-IL-7Rα BV421 A7R34 (1/100); α-LFA-1 PE 2D7 (1/200); α-NKG2D APC CX5 (1/100); α-PD-1 PE-Cy7 29F.1A12 (1/100); α-TNF FITC MP6-XT22 (1/100); streptavidin APC-Cy7 (1/500)). Identification of viable cells was done by fixable near-IR dead cell staining (Life Technologies). Erythrocytes were lysed by ACK lysis buffer treatment for 5 min at room temperature. Surface stainings were performed for 20 min at 4 °C. For cytokine production analysis, cells were further fixed and permeabilized in 2× FACS Lysis Solution (BD Biosciences) with 0.08% Tween 20 (National Diagnostics, Chemie Brunschwig AG, Basel, Switzerland) for 10 min at room temperature, followed by intracellular staining for 30 min at room temperature in the dark. For the assessment of phosphoprotein expression from in vitro activated CD8+ T lymphocytes, surface staining was followed by fixation with pre-heated 4% paraformaldehyde (Sigma-Aldrich) for 10 min at 37 °C, permeabilization with 90% of pre-cooled methanol for 30 min on ice and staining for 40 min at room temperature in the dark. All samples were washed and stored in PBS containing 2% FBS (Omnilab) and 5 mM of EDTA (Sigma-Aldrich) before acquisition. Stained samples were acquired on a FACS LSR II flow cytometer (BD Biosciences) with FACSDiva software. Data analysis was done using FlowJo software (FlowJo Enterprise, version 10.7.1, BD Biosciences). A representative gating strategy is shown in Supplementary Fig. 7.
Immunofluorescence staining and confocal microscopy
CD8+ T-cell lymphocytes, previously stimulated in vitro, were washed in PBS and transferred on Poly-L-Lysine (Sigma-Aldrich) treated coverslips, followed by incubation for 30 min at 37 °C. Cells were then fixed with 2% paraformaldehyde (Sigma-Aldrich) for 10 min, permeabilized with 0.1% Triton X (Sigma-Aldrich) for 10 min and blocked in PBS containing 2% bovine serum albumin (GE Healthcare) and 0.01% Tween 20 (National Diagnostics) for 1 h at room temperature. The following antibodies were used to perform immunofluorescence stainings in murine cells: mouse α-β-tubulin (Sigma-Aldrich); α-mouse IgG AF488 (Abcam, Lucerna Chem AG, Luzern, Switzerland); α-CD8 APC (53-6.7, BioLegend); α-T-bet PE (4B10, BioLegend); α-phospho-S6 PE (D57.2.2E, Cell Signalling); goat α-rabbit IgG (H + L) AF568 (Life Technologies). DAPI (Sigma-Aldrich) was used to detect DNA. Mowiol (Calbiochem, Merck-Millipore, Schaffhausen, Germany) was used as mounting medium. Mitotic cells (late anaphase to cytokinesis) were identified by nuclear morphology, presence of two microtubule organizing centres (MTOCs) and a clear tubulin bridge between two daughter cells. Twenty to thirty Z-stacks were acquired with a Visitron Confocal System or a Nikon A1R confocal system with 100× magnification. Data were analyzed using Volocity software (version 6.3., PerkinElmer). Thresholds for quantification were setup individually for each fluorophore. Asymmetry rates were calculated based on CD8 quantification (volume and fluorescence intensity) in each hemisphere of mitotic cells. Mitoses were considered asymmetric when CD8 enrichment was 1.5-fold greater in one daughter cell in comparison to the other.
Metabolic flux analysis
The real-time extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were measured using a XF 96 extracellular flux analyser (Seahorse Bioscience). 1–2 × 106 activated CD8+ T cells were washed in RPMI 1640 without sodium bicarbonate, 20 mM glucose, 1% FCS, 2 mM pyruvate and seeded in a XF plate coated with poly-L-lysine (Sigma-Aldrich) at equal densities in corresponding assay medium (for ECAR—XF Assay Medium, pH 7.4; for OCR—XF Assay Medium, pH 7.4 supplemented with 25 mM glucose, 1 mM sodium pyruvate and 2 mM L-glutamine). For measurement of OCR, test compounds were sequentially injected to obtain the following concentrations: 0.75 µM oligomycin, 1 µM FCCP, 1 µM rotenone and 1 µM antimycin A. For quantification of ECAR, 11.1 mM D-glucose, 0.75 µM oligomycin and 100 mM 2-deoxy-D-glucose were used. Two independent experiments were performed with at least three technical replicates per group.
In vitro survival upon limiting cytokine availability
Isolated CD8+ T cells (naive or TVM) from aged P14 mice were cultured in T-cell medium supplemented with human IL-2, and stimulated on α-CD3 (5 µg/ml) (BioLegend), α-CD28 (5 µg/ml) (BioLegend) and human Fc-ICAM-1 (50 µg/ml) (R&D Biosciences) coated plates for 36 h. When transient mTOR inhibition was conducted, at 12 h post stimulation 20 nM of rapamycin (Santa Cruz) or 1–2 µM of Akt-kinase inhibitor (Sigma-Aldrich) was added to the cell culture. After plate-bound stimulation, cells were harvested, washed in PBS and transferred to uncoated new wells containing limited concentrations of recombinant mouse IL-15 (<1 ng/ml) or a cocktail of recombinant mouse IL-2, IL-7 and IL-15 (<1 ng/ml). Cells were checked for viability by flow cytometry 7 days later.
Statistical analysis
To test if data point values were distributed in a Gaussian distribution, Shapiro–Wilk normality test was performed. For statistical analysis, two-tailed Student’s t test or non-parametrical analysis Mann–Whitney U tests were performed using GraphPad Prism Software (version 7.0 or 8.2.0). P values were considered significant when <0.05, and exact P values are provided in the figure legends. For animal experiments, sample size varied from 2 to 5 mice per group for each individual experiment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
These authors contributed equally: Isabel Barnstorf, Nicolas S. Baumann, Katharina Pallmer. The authors thank members of the Oxenius, Joller, Barral and Jessberger groups for helpful discussions. This work was supported by ETH Zurich, the Swiss National Science Foundation (grant no. 310030_166078 to A.O., grant no. P2EZP3-188074 to M.Bo.), the Novartis Foundation for Biomedical Research (to A.O. and M.Bo.), the European Union’s Horizon 2020 (under the Marie Sklodowska–Curie grant agreement 893676 to M.Bo.) and the Wellcome Trust (103830/Z/14/Z to A.K.S.).
Source data
Author contributions
M.Bo., A.K.S., N.J., Y.B., R.S. and A.O. designed the experiments. M.Bo., N.B., F.G., I.B., N.S.B., K.P., S.B., D.S., M.Ba. and C.W. performed the experiments. N.O. and F.W. provided technical assistance. M.Bo., N.B., F.G. and A.O. analyzed the experiments. M.Bo. and A.O. wrote the manuscript.
Data availability
Additional flow cytometry and imaging data supporting the findings are available from the first/corresponding author upon request. Source data for Figs. 1B, 2B–G, 3A, C, 4C, D and 5B–D and Supplementary Figs. 1B, 2 and 4–6 are available in the Source Data file. Source Data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Kylie Quinn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Niculò Barandun, Fabienne Gräbnitz.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-22954-y.
References
- 1.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat. Rev. Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 2.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borsa, M. et al. Modulation of asymmetric cell division as a mechanism to boost CD8(+) T cell memory. Sci. Immunol.4, eaav1730 (2019). [DOI] [PubMed]
- 4.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells - a reflection of T cell differentiation, cell senescence and host environment. Semin. Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira BI, Akbar AN. Convergence of innate and adaptive immunity during human aging. Front. Immunol. 2016;7:445. doi: 10.3389/fimmu.2016.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolich-Zugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J. Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florian MC, et al. Aging alters the epigenetic asymmetry of HSC division. PLoS Biol. 2018;16:e2003389. doi: 10.1371/journal.pbio.2003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixit VD. Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence. Semin. Immunol. 2012;24:321–330. doi: 10.1016/j.smim.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc. Natl Acad. Sci. USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horai R, et al. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosinowski T, et al. CD8alpha+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J. Immunol. 2013;190:1936–1947. doi: 10.4049/jimmunol.1203149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White JT, et al. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat. Commun. 2016;7:11291. doi: 10.1038/ncomms11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldrath AW, Luckey CJ, Park R, Benoist C, Mathis D. The molecular program induced in T cells undergoing homeostatic proliferation. Proc. Natl Acad. Sci. USA. 2004;101:16885–16890. doi: 10.1073/pnas.0407417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White JT, Cross EW, Kedl RM. Antigen-inexperienced memory CD8(+) T cells: where they come from and why we need them. Nat. Rev. Immunol. 2017;17:391–400. doi: 10.1038/nri.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J. Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu BC, Martin BE, Stolberg VR, Chensue SW. Cutting edge: central memory CD8 T cells in aged mice are virtual memory cells. J. Immunol. 2013;191:5793–5796. doi: 10.4049/jimmunol.1302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc. Natl Acad. Sci. USA. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyss L, et al. Affinity for self antigen selects Treg cells with distinct functional properties. Nat. Immunol. 2016;17:1093–1101. doi: 10.1038/ni.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller CH, et al. Eomes identifies thymic precursors of self-specific memory-phenotype CD8(+) T cells. Nat. Immunol. 2020;21:567–577. doi: 10.1038/s41590-020-0653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc. Natl Acad. Sci. USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn KM, et al. Metabolic characteristics of CD8(+) T cell subsets in young and aged individuals are not predictive of functionality. Nat. Commun. 2020;11:2857. doi: 10.1038/s41467-020-16633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanzer KG, Cookenham T, Reiley WW, Blackman MA. Virtual memory cells make a major contribution to the response of aged influenza-naive mice to influenza virus infection. Immun. Ageing. 2018;15:17. doi: 10.1186/s12979-018-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn KM, et al. Age-related decline in primary CD8(+) T cell responses is associated with the development of senescence in virtual memory CD8(+) T cells. Cell Rep. 2018;23:3512–3524. doi: 10.1016/j.celrep.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 28.Chu T, et al. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang JT, et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollizzi KN, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat. Immunol. 2016;17:704–711. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbist KC, et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532:389–393. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decman V, et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J. Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naylor K, et al. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 34.Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–307. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 37.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Gupta PK, et al. CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog. 2015;11:e1005177. doi: 10.1371/journal.ppat.1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudd BD, et al. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc. Natl Acad. Sci. USA. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geltink RIK, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu. Rev. Immunol. 2018;36:461–488. doi: 10.1146/annurev-immunol-042617-053019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimeloe S, Burgener AV, Grahlert J, Hess C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology. 2017;150:35–44. doi: 10.1111/imm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kretschmer L, et al. Differential expansion of T central memory precursor and effector subsets is regulated by division speed. Nat. Commun. 2020;11:113. doi: 10.1038/s41467-019-13788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8(+) T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl. Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 45.Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol. Res. 2000;22:253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 46.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, et al. Mammalian target of rapamycin complex 2 controls CD8 T cell memory differentiation in a Foxo1-dependent manner. Cell Rep. 2016;14:1206–1217. doi: 10.1016/j.celrep.2015.12.095. [DOI] [PubMed] [Google Scholar]
- 48.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 51.Baldi, S., Bolognesi, A., Meinema, A.C. & Barral, Y. Heat stress promotes longevity in budding yeast by relaxing the confinement of age-promoting factors in the mother cell. Elife6, e28329 (2017). [DOI] [PMC free article] [PubMed]
- 52.Herishanu Y, et al. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk. Lymphoma. 2011;52:1758–1769. doi: 10.3109/10428194.2011.569962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henson SM. CD8+ T-cell senescence: no role for mTOR. Biochem. Soc. Trans. 2015;43:734–739. doi: 10.1042/BST20150092. [DOI] [PubMed] [Google Scholar]
- 55.Henson SM, et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J. Clin. Investig. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanna A, et al. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 2017;18:354–363. doi: 10.1038/ni.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pircher H, et al. T cell receptor (TcR) beta chain transgenic mice: studies on allelic exclusion and on the TcR+ gamma/delta population. Eur. J. Immunol. 1990;20:417–424. doi: 10.1002/eji.1830200227. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional flow cytometry and imaging data supporting the findings are available from the first/corresponding author upon request. Source data for Figs. 1B, 2B–G, 3A, C, 4C, D and 5B–D and Supplementary Figs. 1B, 2 and 4–6 are available in the Source Data file. Source Data are provided with this paper.