Abstract
Tryptophan and its catabolites (TRYCATs) have been suggested to link peripheral immune system activation and central neurotransmitter abnormalities with relevance to the etio-pathophysiology of schizophrenia (SZ) and major depressive disorder (MDD). The relationship to different psychopathological dimensions within these disorders however remains to be elucidated. We thus investigated potential group differences of tryptophan, kynurenine, kynurenic acid, 3-hydroxy kynurenine and quinolinic acid in the plasma of 19 healthy controls (HC), 45 patients with SZ and 43 patients with MDD and correlated plasma proteins with the “motivation and pleasure” dimension and cognition. After correcting for the covariates age, sex, body mass index, smoking and medication, patients with MDD showed lower kynurenine and 3-hydroxy kynurenine levels compared to HC. Quinolinic acid correlated negatively with composite cognitive score in patients with SZ, indicating that more severe cognitive impairments were associated with increased plasma levels of quinolinic acid. No correlations were found in patients with MDD. These results indicate that MDD and SZ are associated with dysregulation of the kynurenine pathway. Quinolinic acid might be specifically implicated in the pathophysiology of cognitive deficits in patients with SZ. Further studies are needed to determine whether TRYCATs are causally involved in the etiology of these neuropsychiatric disorders.
Subject terms: Cognitive control, Neuroscience, Neuroimmunology
Introduction
Schizophrenia (SZ) and major depressive disorder (MDD) are prevalent neuropsychiatric disorders with high socioeconomic and individual burden1,2. Despite their significant contribution to the global burden of disabilities3 our knowledge of the underlying etio-pathophysiological mechanisms are sparse and as a result, treatment options remain limited4.
Both SZ and MDD are heterogeneous syndromes consisting of several symptom dimensions5,6. While for a long time considered as separate disorders, there is increasing evidence from clinical psychopathology, neuroimaging and genetic studies that there are both different and overlapping features that characterize these disorders7. Therefore, detailed psychopathological characterization and comparison of symptom dimensions with potential biological markers is important. Motivational deficits are symptoms of several psychiatric disorders, including SZ and MDD8. Apathy, a reduction of motivation and goal-directed behaviours, has been shown to be prevalent in both patients with MDD9 and SZ10. Further, cognitive dysfunction has been consistently described in both patients with SZ and MDD11,12.
There is increasing evidence that the immune system plays a major role in neuropsychiatric disorders, including being causally involved in the pathogenesis of the above discussed symptom dimensions13,14. One candidate pathway linking peripheral immune system activation and central neurotransmitter abnormalities involves tryptophan (TRP) and its catabolites (TRYCATs)15. The essential amino acid TRP is catabolized into several bioactive molecules, including serotonin and products of the kynurenine pathway15. Two enzymes are mainly involved in catabolizing tryptophan to kynurenines: Indoleamine 2,3-dioxygenase (IDO) (which can be indirectly assessed by the tryptophan / kynurenine (KYN) ratio, see below), which in the periphery is mainly present in myeloid cells, and the predominantly hepatic enzyme tryptophan 2,3-dioxygenase (TDO). Upon activation by pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β and interferon-γ16,17, TRP is catabolized to kynurenine. Kynurenine can then be catabolized along two pathways: Kynurenine aminotransferases produce kynureninc acid (KYNA) and kynurenine monooxygenase (KMO) converts KYN to 3-hydroxykynurenine (3-OHK), which is further catabolized to quinolinic acid (QUIN). Importantly, KYNA, 3-OHK and QUIN were shown to exhibit neuroactive properties, including affecting glutamatergic, dopaminergic and nicotinergic neurotransmission18.
Evidence for a causal role of kynurenine pathway dysregulation leading to behavioural alterations in several neuropsychiatric disorders comes from animal models, in which it was shown that pharmacologically blocking IDO prevents depression-like behaviours induced by immune stimulation using the bacterial endotoxin Lipopolysaccharide (LPS) or social defeat stress19,20.
Several studies have so far investigated group differences in TRYCATs in the circulation of patients with SZ and MDD, respectively, but have reported inconclusive findings. Recent meta-analyses have shown that KYNA and KYN blood levels were lower in patients with MDD in comparison to healthy controls (HC), while QUIN levels did not differ between the two groups21,22. In SZ, the findings are even more inconclusive: A recent meta-analysis has not found any differences in peripheral KYNA23. One large study has found decreased plasma concentrations of 3-OHK in patients with SZ compared to HC, but not other TRYCATs24. However, most studies up to date have focused on differences between diagnostic groups rather than correlating TRYCATs with various symptom dimensions across different diagnoses.
The overall hypothesis of the present study was that both MDD and SZ are associated with dysregulation of the kynurenine pathway. Specifically, based on the above discussed literature, we hypothesized that compared to HC, patients with MDD would display lower levels of KYN and KYNA, and patients with SZ would show lower levels of 3-OHK. Based on findings from pre-clinical animal models, we further hypothesized that in both patients with MDD and SZ, motivational deficits would correlate with the KYN/TRP ratio, as an indicator of IDO activity and the neurotoxic TRYCAT QUIN would be associated with cognitive deficits. Thus, we first investigated group differences in TRYCATs in the plasma of HC, SZ and MDD subjects and then correlated TRYCAT levels with the psychopathological dimensions “motivation and pleasure” and cognition.
Methods
Participants
45 patients meeting the DSM-IV (American Psychiatric Association, 2000) criteria for SZ, 44 patients with MDD and 19 HC were recruited. One patient with MDD was excluded from the study because of technical problems. Patients with other SZ spectrum disorder were not included. More patients (SZ and MDD) than HC were recruited to have adequate power for the correlational analyses. Patients were recruited from outpatient and inpatient units of the Psychiatric Hospital of the University of Zurich and affiliated institutions. Diagnoses were confirmed by conducting the Mini-International Neuropsychiatric Interview25. All patients were clinically stable and under a stable dose of medication for at least two weeks prior to testing. Inpatients were at the end of their hospitalization and engaged in a multimodal therapy program and activities outside the hospital. The average duration of hospitalization for patients with SZ and MDD in Swiss psychiatric hospitals is longer than in most other countries, so the majority of inpatients would have been treated as outpatients in other health care systems. HC were recruited from the community via advertisement. All participants gave written informed consent and the project was approved by the Ethics Committee of the Canton of Zurich. Assessment of psychopathology and cognition, and blood draw were performed on the same day and in accordance with relevant guidelines and regulations. The inclusion age was between 18 and 65 years.
Exclusion criteria were similar to our previous studies26–28. We excluded patients with any other than the above mentioned DSM-IV Axis I disorders, benzodiazepines (except less than 1 mg lorazepam per day) and acute psychotic symptoms. Participants with any alcohol use disorder based on lifetime criteria and participants with a current abuse or dependency of cannabis or any other substance abuse were excluded. HC were excluded if any psychiatric diagnosis was present in the structured Mini-International Neuropsychiatric Interview. In both groups, participants were excluded, if they had a history of head-injury or any autoimmune or chronic inflammatory disorder or if they took any pain-medication or anti-inflammatory drugs at least one week prior to testing (assessed by detailed questionnaire and medical records where available). Furthermore, participants were not included in the study if they had a history of any known acute inflammation two weeks prior to testing. Chlorpromazine equivalents were calculated according to29 and imipramine equivalents according to30.
Assessment of psychopathology and cognition
Motivation was assessed using the “motivation and pleasure” dimension (sum score of the subscales anhedonia (items 1–3), asociality (items 5, 6) and avolition (items 7, 8)) of the Brief Negative Symptom Scale (BNSS)31,32. Cognition was assessed with the Brief Neurocognitive Assessment (BNA)33. With the BNA, a cognitive score is computed for each participant by combining results from the Letter-Number-Span Test and the Symbol Coding Test. The BNA has been shown to be highly correlated with the MATRICS Consensus Cognitive Battery (MCCB)34 and has similarly good validity criteria35. Positive symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS)36,37. Depressive symptoms were assessed using the Beck Depression Inventory (BDI)38 and Calgary Depression Scale for Schizophrenia (CDSS)39. Global level of functioning was assessed with the adapted version of the Personal and Social Performance Scale (PSP)40,41.
Blood draw and processing
Blood was drawn between 8 and 10 am on the day of the psychopathological assessment. Study participants were instructed to fasten for at least 8 h prior to the blood draw which was verified by a questionnaire on the day of testing. To obtain plasma, blood was drawn into ethylenediaminetetraacetic acid (EDTA) tubes (Sarstedt, Switzerland), centrifuged for 15 min at 1500×g and plasma was frozen at − 80 °C.
TRYCATs
TRYCATs were measured by Brains Online, LLC42,43. Plasma samples were prepared for analysis of KYN, KYNA, 3-OH-Kynurenine 3-OHK, and TRP by mixing 5 µL of sample with 40 µL of internal standard solution (containing KYN-D4, KYNA-D5, 3-OH-KYN-13C6 and TRP-D5). Plasma samples were prepared for analysis of Quinolinic acid (QUIN) by mixing 10 µL of sample with 40 µL of internal standard solution (containing QUIN-D3). All samples were incubated at room temperature and centrifuged at 13,000 rpm for 5 min. The resultant supernatant was used for liquid tandem mass spectrometry (LC–MS/MS) analysis. Calibrators, QC’s and blanks were prepared following the same procedures using blank plasma. Concentrations of KYN, KYNA, 3-OH-KYN, AA and TRP were determined by high performance liquid chromatography (HPLC) with tandem mass spectrometry (MS/MS) detection. Of each sample, an aliquot was injected onto the HPLC column by an automated sample injector (SIL10-AD, Shimadzu, Japan). Chromatographic separation was performed on a reversed phase analytical column (150 × 2.1 mm, 3.0 µm) held at a temperature of 25 °C. Components were separated using a two-step linear gradient of ultrapurified H2O with 0.1% formic acid (FA) and acetonitrile with 0.1% FA at a flow rate of 0.2 mL/min 1, 2. The MS analyses were performed using an API 4000 MS/MS system, equipped with a Turbo Ion Spray interface (both from Sciex). The instrument was operated in multiple-reaction-monitoring (MRM) mode. The acquisitions were performed in positive ionization mode, with optimized settings for the analytes. Concentrations of QUIN were determined by HPLC with tandem mass spectrometry (MS/MS) detection. QUIN analysis was carried out in a single analyte assay. Chromatographic separation was performed on a reversed phase analytical column (100 × 3.0 mm, 2.5 µm) held at a temperature of 25 °C. Component was separated using a linear gradient of ultrapurified H2O with 0.2% tri-fluoric acid (TFA) and acetonitrile with 0.2% TFA (flow rate 0.3 mL/min) 2. The MS analyses were performed using an API 4000 MS/MS system, equipped with a Turbo Ion Spray interface (both from Sciex). The instrument was operated in multiple-reaction-monitoring mode. The acquisitions were performed in positive ionization mode, with optimized settings for QUIN. Suitable in-run calibration curves were fitted using weighted (1/x) regression, and the sample concentrations were determined using these calibration curves. Accuracy was verified by quality control samples after each sample series. Data were acquired and processed using the Analyst data system (software version 1.6.2, Sciex). One QUIN sample from a HC had a value below limit of quantification, otherwise, all values were above lower limit of detection and passed quality control.
Data analysis
First, normal distribution was tested using the Shapiro–Wilk test. Non-normally distributed data were log-transformed. If normal distribution was achieved, parametric tests were used. If normal distribution was not achieved, parametric tests were performed for log-transformed values. Comparisons between HC, patients with SZ and patients with MDD were performed using one-way ANOVAs with Tukey’s test for post-hoc comparisons (for normal distributed data) and Kruskal–Wallis test with Dunn’s test for post-hoc comparisons (for non-normally distributed data). General linear models (post-hoc test: least significant difference) were used to test for the effects of covariates [(age, sex, BMI, smoking and medication (chlorpromazine equivalents, imipramine equivalents, lorazepam medication)]. For correlations between TRYCATs and symptom dimensions Pearson correlation coefficients (normal distribution) or Spearman correlation coefficients (non-normally distributed data) were calculated. Partial correlations were calculated when controlling for covariates (age, sex, BMI, smoking, disease severity (i.e. illness onset and duration), medication (chlorpromazine equivalents, imipramine equivalents, lorazepam medication) and total years of education) using partial correlations. No correlations were calculated for the HC group, based on the low level of psychopathological symptoms. The level of statistical significance was set at p < 0.05. Statistical analyses were computed with SPSS version 25 (IBM Corp., SPSS Inc., Chicago IL, USA) and GraphPad Prism software 8 (GraphPad Software Inc.).
Ethics approval and consent to participate
All participants gave written informed consent and the project was approved by the Ethics Committee of the Canton of Zurich.
Results
Sociodemographic data of the sample and statistics are summarized in Table 1. While there were no differences in sex between HC and patient groups, there were more female participants in the MDD group compared to the SZ group. Patients with SZ had experienced on average 5.3 psychotic episodes, while patients with MDD had 3.6 depressive episode. Body Mass Index (BMI) was higher in patients with SZ than HC or patients with MDD, with no difference between MDD and HC. Patients with SZ and MDD had similar motivational deficits [assessed by the “motivation and pleasure” dimension (BNSS)] compared to HC, but patients with MDD showed higher levels of depressive symptoms. Compared to HC and patients with MDD, patients with SZ showed significant deficits in the cognitive dimension (assessed by the BNA), while there were no differences between patients with MDD and HC. Patients with SZ showed more positive symptoms (assessed by the PANSS positive symptom subscale) than patients with MDD. Both, patients with SZ and MDD showed similar levels of functional impairment compared to HC, indicated by lower levels on the PSP. Patients with SZ showed fewer years of education compared to patients with MDD and HC.
Table 1.
Sociodemographic and clinical data.
| HC (n = 19) | SZ (n = 45) | MDD (n = 43) | Statistics | |||
|---|---|---|---|---|---|---|
| Test statistics | Post-hoc | |||||
| Age (years) | 32.53 ± 9.45 | 34.00 ± 10.47 | 35.79 ± 10.88 | KWS = 1.37 | p = 0.5032 | |
| Sex (male/female) | 9/10 | 31/14 | 17/26 | X2 = 7.94 | p = 0.0193 |
p = 0.312a p = 1.000b p = 0.018c |
| Smoking (no/yes) | 13/6 | 16/29 | 29/14 | X2 = 10.887 | p = 0.0043 |
p = 0.048a p = 1.000b p = 0.009c |
| Education (years)d | 14.39 ± 2.23 | 12.26 ± 3.88 | 14.57 ± 3.17 | KWS = 15.25 | p < 0.0012 |
p = 0.013a p > 0.999b p = 0.001c |
| Body mass index | 23.40 ± 4.32 | 26.40 ± 4.66 | 22.88 ± 4.30 | KWS = 15.44 | p < 0.0012 |
p = 0.025a p > 0.999b p < 0.001c |
| Number of psychotic episodes | 0 | 5.31 ± 5.14 | 0 | KWS = 93.33 | p < 0.0012 |
p < 0.001a p > 0.999b p < 0.001c |
| Number of depressive episodes | 0 | 0.09 ± 0.29 | 3.60 ± 3.19 | KWS = 86.75 | p < 0.0012 |
p > 0.999a p < 0.001b p < 0.001c |
| Age at illness onset (years) | – | 23.91 ± 6.88 | 28.77 ± 10.89 | U = 722 | p = 0.0414 | |
| Illness duration (months) | – | 121.07 ± 101.28 | 84.26 ± 95.46 | U = 736 | p = 0.0544 | |
| Antipsychotic medication (yes/no) | – | 44/1 | 2/41 | X2 = 76.44 | p < 0.0013 | |
| Chlorpromazine equivalents (mg/day) | – | 514.67 ± 444.82 | 2.34 ± 11.31 | U = 23.5 | p < 0.0014 | |
| Antidepressant medication (yes/no) | – | 6/39 | 33/10 | X2 = 10.89 | p < 0.0013 | |
| Imipramine equivalents | – | 21.43 ± 60.86 | 106.10 ± 93.57 | U = 376.5 | p < 0.0014 | |
| Lorazepam (yes/no) | – | 3/42 | 3/40 | X2 = 0.003 | p = 0.9543 | |
| CDSS (total) | – | 3.00 ± 3.65 | 12.33 ± 4.95 | U = 118.5 | p < 0.0014 | |
| BDI (total) | – | 14.16 ± 9.84 | 26.07 ± 12.54 | U = 433 | p < 0.0014 | |
| PANSS positive factor | – | 5.96 ± 2.70 | 4.37 ± 1.07 | U = 574 | p < 0.0014 | |
| Motivation and pleasure dimension (BNSS) | 0.74 ± 1.10 | 18.40 ± 9.46 | 20.58 ± 7.19 | KWS = 44.97 | p < 0.0012 |
p < 0.001a p < 0.001b p > 0.999c |
| Cognitive dimension (BNA) | 0.00 ± 0.68 | − 0.88 ± 0.70 | − 0.12 ± 0.85 | F(2, 100) = 13.83 | p < 0.0011 |
p < 0.001a p = 0.848b p < 0.001c |
| Global functioning (PSP total) | 97.05 ± 4.70 | 51.78 ± 14.42 | 57.26 ± 16.77 | KWS = 47.67 | p < 0.0012 |
p < 0.001a p < 0.001b p = 0.580c |
Statistics: 1One-way analysis of variance (ANOVA), 2Kruskal-Wallis test, 3Chi-square test, 4Mann-Whitney U test. Post-hoc. Tukey’s test for post-hoc comparisons of ANOVAs and Dunn’s test for post-hoc comparison of Kruskal–Wallis test: aHC vs. SZ, bHC vs. MDD, cSZ vs. MDD. BDI Beck Depression Inventory, BNA Brief Neurocognitive Assessment, BNSS Brief Negative Symptom Scale, CDSS Calgary Depression Scale for Schizophrenia, HC Healthy controls, MDD Major depressive disorder, PANSS Positive and Negative Syndrome Scale, PSP Personal and Social Performance Scale, SZ Schizophrenia. Data is presented as mean ± standard deviation. The level of significance was set at p < 0.05 (significant p-values are indicated in bold). dCompulsory education in Switzerland is 9 years.
3-OHK and KYN plasma levels differ between disorders
We first investigated group differences in TRYCATs (see Table 2 for detailed test statistics). There was a significant reduction of 3-OHK in patients with MDD compared to HC (uncorrected: p = 0.026, corrected for covariates age, sex, BMI, smoking and medication: p = 0.019) (Fig. 1), but no differences between SZ and HC (uncorrected: p = 0.122, corrected: p = 0.068) or SZ and MDD (uncorrected: p = 0.660, corrected: p = 0.661). There was a significant reduction of KYN specifically in patients with MDD compared to HC only after correcting for covariates (MDD vs. HC: uncorrected: p = 0.181, corrected: p = 0.016; SZ vs. HC: uncorrected: p = 0.427, corrected: 0.067; SZ vs. MDD: uncorrected: p = 0.764, corrected: p = 0.622). We also observed a reduction of KYNA in patients with MDD compared to HC (p = 0.046) but this reduction was no longer significant after introducing covariates (p = 0.336). No differences in KYNA were present between patients with SZ and HC (uncorrected: p > 0.999, corrected: p = 0.167) or patients with SZ and patients with MDD (uncorrected: p = 0.098, corrected: p = 0.620).
Table 2.
Group differences in tryptophan and its catabolites between healthy controls (HC), patients with schizophrenia (SZ) or major depressive disorder (MDD).
| HC (n = 19) | SZ (n = 45) | MDD (n = 43) | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test statistics (uncorrected) | Post-hoc (uncorrected) | Test statistics (corrected6) | Post-hoc (corrected6) | ||||||
| Tryptophan3 (nM) | 58,539.56 ± 11,511.48 | 57,624.27 ± 9738.86 | 56,094.68 ± 9774.15 | F(2,97) = 0.44 | p = 0.6481 | F(2, 97) = 0.313 | 6p = 0.732 | ||
| Kynurenine (nM) | 2258.92 ± 736.61 | 2089.81 ± 450.33 | 2015.96 ± 401.99 | F(2,97) = 1.59 | p = 0.2091 | F(2, 97) = 3.24 | p = 0.0436 |
p = 0.067a p = 0.016b p = 0.622c |
|
| Tryptophan / Kynurenine | 28.16 ± 8.57 | 28.89 ± 8.10 | 28.57 ± 6.00 | F(2,97) = 0.068 | p = 0.9341 | F(2, 97) = 1.13 | p = 0.3276 | ||
| Kynurenic Acid4 (nM) | 55.48 ± 24.56 | 50.55 ± 20.93 | 46.06 ± 28.87 | KWS = 7.53 | p = 0.0232 |
p > 0.999a p = 0.046b p = 0.098c |
F(2, 97) = 1.01 | p = 0.3686 | |
| 3-Hydroxy Kynurenine (nM) | 37.09 ± 13.58 | 31.99 ± 9.25 | 30.24 ± 7.17 | F(2,97) = 3.50 | p = 0.0341 |
p = 0.122a p = 0.026b p = 0.660c |
F(2, 97) = 3.11 | p = 0.0496 |
p = 0.068a p = 0.019b p = 0.661c |
| Kynurenine / 3-Hydroxy Kynurenine | 62.54 ± 11.46 | 68.43 ± 15.91 | 68.99 ± 15.84 | F(2,97) = 1.30 | 1p = 0.277 | F(2, 97) = 0.12 | p = 0.8896 | ||
| Kynurenic Acid / 3-Hydroxy Kynurenine4,5 | 1.48 ± 0.32 | 1.63 ± 0.58 | 1.50 ± 0.75 | F(2,97) = 0.38 | p = 0.6841 | F(2, 97) = 0.074 | p = 0.9296 | ||
| Quinolinic Acid (nM) 4,5 | 378.93 ± 478.46 | 341.38 ± 177.23 | 270.98 ± 105.48 | KWS = 2.47 | p = 0.2902 | F(2, 97) = 0.31 | p = 0.7326 | ||
| Kynurenic Acid / Quinolinic Acid4,5 | 0.23 ± 0.11 | 0.18 ± 0.10 | 0.18 ± 0.10 | KWS = 4.63 | p = 0.102 | F(2, 97) = 0.1 | p = 0.9086 | ||
Statistics: 1One-way analysis of variance, 2Kruskal-Wallis test, Post-hoc (Tukey’s test for post-hoc comparisons of ANOVAs and Dunn’s test for post-hoc comparison of Kruskal–Wallis tests): aHC vs. SZ, bHC vs. MDD, cSZ vs. MDD. 3log10-transformation achieved normal distribution in HC, 4log10-transformation achieved normal distribution in patients with SZ. 5log10-transformation achieved normal distribution in patients with MDD. 6General linear model with age, sex, BMI, smoking and medication (chlorpromazine equivalents, imipramine equivalents, lorazepam medication) as covariates (post-hoc comparison: least significant difference). Data is presented as mean ± standard deviation. The level of significance was set at p < 0.05 (significant p-values are indicated in bold).
Figure 1.
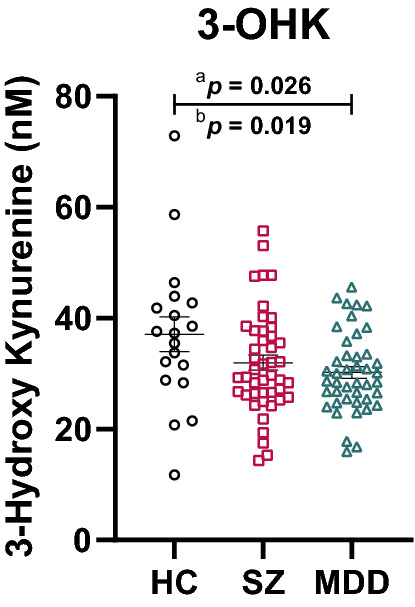
Group differences of 3-hydroxy kynurenine (3-OHK). Compared to healthy controls (HC), patients with major depressive disorder (MDD) but not schizophrenia (SZ) display a reduction in 3-OHK. aWithout correcting for covariates, bAfter correction for the covariates age, sex, BMI, smoking and medication (chlorpromazine equivalents, imipramine equivalents, lorazepam medication). The level of significance was set at p < 0.05 (significant p-values are indicated in bold).
Correlation between cognition and QUIN plasma levels in patients with SZ
Lastly, we correlated TRYCATS with the assessed psychopathological dimensions within each disorder (Table 3).
Table 3.
Correlations between TRYCATs and psychopathological dimensions.
| Motivation and pleasure dimension (BNSS) | Cognitive symptom dimension (BNA) | |
|---|---|---|
| Tryptophan (nM) |
SZ: r(p) = − 0.043, p = 0.781 MDD: r(p) = 0.070, p = 0.657 |
SZ: r(p) = − 0.022, p = 0.887 MDD: r(p) = 0.080, p = 0.616 |
| Kynurenine (nM) |
SZ: r(p) = − 0.092, p = 0.49 MDD: r(p) = − 0.217, p = 0.162 |
SZ: r(p) = − 0.209, p = 0.179 MDD: r(p) = 0.052, p = 0.741 |
| Tryptophan/kynurenine (log1) |
SZ: r(p) = − 0.013, p = 0.933 MDD: r(p) = − 0.199, p = 0.200 |
SZ: r(p) = − 0.146, p = 0.349 MDD: r(p) = − 0.016, p = 0.919 |
| Kynurenic acid (nM) (log1) |
SZ: r(p) = − 0.099, p = 0.519 MDD: r(s) = − 0.211, p = 0.174 |
SZ: r(p) = − 0.186, p = 0.233 MDD: r(s) = − 0.059, p = 0.711 |
| 3-Hydroxy kynurenine (nM) |
SZ: r(p) = − 0.025, p = 0.870 MDD: r(p) = − 0.202, p = 0.195 |
SZ: r(p) = − 0.089, p = 0.571 MDD: r(p) = − 0.126, p = 0.425 |
| Kynurenine/3-hydroxy kynurenine |
SZ: r(p) = 0.034, p = 0.823 MDD: r(p) = 0.057, p = 0.715 |
SZ: r(p) = − 0.061, p = 0.698 MDD: r(p) = 0.159, p = 0.316 |
| Kynurenic acid/3-hydroxy kynurenine (log1,2) |
SZ: r(p) = − 0.067, p = 0.663 MDD: r(p) = − 0.155, p = 0.321 |
SZ: r(p) = − 0.131, p = 0.401 MDD: r(p) = 0.019, p = 0.905 |
| Quinolinic acid (nM) (log1,2) |
SZ: r(p) = 0.104, p = 0.495 MDD: r(p) = − 0.177, p = 0.256 |
SZ: r(p) = − 0.324, p = 0.034 MDD: r(p) = − 0.056, p = 0.723 |
| Kynurenine acid/quinolinic acid (log1,2) |
SZ: r(p) = − 0.167, p = 0.273 MDD: r(p) = − 0.116, p = 0.459 |
SZ: r(p) = 0.160, p = 0.306 MDD: r(p) = 0.012, p = 0.941 |
Statistics: 1log10-transformation achieved normal distribution in patients with SZ, 2log10-transformation achieved normal distribution in patients with MDD. BNA Brief Neurocognitive Assessment, BNSS Brief Negative Symptom Scale, HC Healthy controls, MDD Major depressive disorder, SZ Schizophrenia. Data is presented as mean ± standard deviation. The level of significance was set at p < 0.05 (significant p-values are indicated in bold).
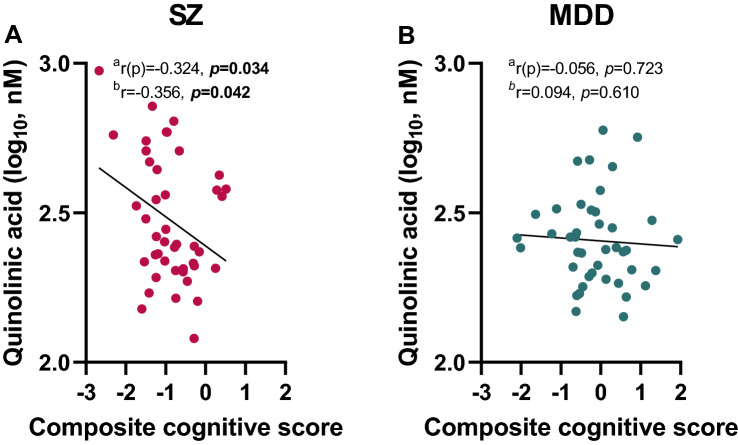
Interestingly, there was a negative correlation between QUIN (log) and composite cognitive score in patients with SZ (Fig. 2A) but not MDD (Fig. 2B) (SZ: r(p) = − 0.324, p = 0.034; MDD: r(p) = − 0.056, p = 0.723), indicating that increased plasma levels of QUIN in patients with SZ are associated with increased cognitive impairment. This association remained significant after correcting for the covariates age, sex, BMI, smoking, disease severity (i.e. illness onset and duration), medication (chlorpromazine equivalents, imipramine equivalents, lorazepam medication) and total years of education (r = − 0.356, p = 0.042). There were no other significant correlations between TRYCATs and symptom dimensions in patients with SZ or MDD.
Figure 2.
Correlations between quinolinic acid and composite cognitive score in patients with schizophrenia (SZ) and major depressive disorder (MDD). Significant negative correlations between quinolinic acid and composite cognitive score in patients with (A) SZ but not (B) MDD. Higher cognitive scores indicate better cognitive performance. aWithout correcting for covariates, bAfter correction for the covariates age, sex, BMI, smoking, disease severity (i.e. illness onset and duration), medication (chlorpromazine equivalents, imipramine equivalents, lorazepam medication) and total years of education. The level of significance was set at p < 0.05 (significant p-values are indicated in bold).
Discussion
In the present study we have investigated differences in tryptophan and its catabolites between patients with SZ or MDD, and HC, and correlated the TRYCATs with the symptom dimensions “motivation and pleasure” and cognition. We show that plasma levels of 3-OHK are lower in patients with MDD compared to HC. Dimensional assessment revealed a negative correlation between plasma QUIN and composite cognitive score in patients with SZ but not in patients with MDD. In other words, cognitive impairment was related to higher plasma QUIN levels.
Several studies have investigated 3-OHK in MDD vs. HC but have not found any group differences42–47. Research regarding comparisons between SZ vs. HC is mixed, with some studies showing no differences48,49 some indicating higher50 and some lower24,51 3-OHK levels. The biological effects of 3-OHK are complex and studies have shown both antioxidant effects potentially resulting in neuroprotection and induction of reactive oxygen species leading to neurotoxicity52. In addition, 3-OHK is one of the TRYCATs that is examined least frequently: a recent meta-analysis showed that out of 59 studies comparing patients with MDD vs. HC only 6 studies included 3-OHK22. Therefore, further studies are needed to elucidate the role of 3-OHK in the pathogenesis of MDD and SZ. After correcting for the covariates age, sex, smoking, BMI and medication, we report a reduction of KYN specifically in patients with MDD. This reduction specifically in patients with MDD compared to HC is in line with recent meta-analyses21,22. We additionally found a reduction of KYNA in patients with MDD. However, this reduction was no longer observed after correcting for the above mentioned covariates. While the uncorrected KYNA finding is as in line with recent meta-analyses21,22, it is important to note, that to our knowledge a majority of the studies that reported significant differences in KYNA in MDD vs. HC did not control for the above mentioned covariates43,53–56, which are known to be casually linked to a pro-inflammatory state57,58. Our findings can therefore be viewed in the context of the overall hypothesis that MDD and SZ are associated with a dysbalance between neurotoxic and neuroprotective tryptophan catabolites59. In addition, the present study underlines the importance of rigorous control of covariates when investigating peripheral inflammatory markers.
An important and novel finding of the current study is the SZ specific negative correlation between composite cognitive score and plasma QUIN levels, an effect that remains significant after adjusting for several covariates. Cognitive deficits are a hallmark symptom of schizophrenia and have detrimental effects on functional outcome and quality of life60,61. While the underlying pathophysiological mechanisms are not well understood, cognitive dysfunctions have been associated with dysfunctional dopaminergic and glutamatergic neurotransmission in the prefrontal cortex and hippocampus62. QUIN was shown to be neurotoxic by inducing excitotoxicity (QUIN is a glutamatergic agonist acting on N-methyl-D-aspartate receptors [NMDARs]), promoting oxidative stress and disrupting energy homeostasis63. Direct evidence of a role of QUIN in eliciting cognitive deficits comes from pre-clinical rodent studies. Injections of QUIN into the medial prefrontal cortex of mice induced cognitive deficits and impairments in behavioural flexibility64. While increased QUIN and associated neurological and cognitive function have been associated with several neurodegenerative disorders such as Alzheimer’s disease65 or Huntington’s disease66, studies investigating its role in cognitive deficits in psychiatric disorders are scarce. One post-mortem study has demonstrated fewer QUIN-immunoreactive microglia cells in the hippocampus in patients with SZ compared to HC67. While these results might be contradictory to the current findings at first sight, it has to be noted that the reduced microglial expression of QUIN may represent compensatory mechanisms, attenuating the oxidative stress67. Given the function of QUIN as an NMDAR agonist it can be hypothesized that QUIN contributes to the glutamate theory of schizophrenia, which postulates that several symptom dimensions of schizophrenia are a result of hypofunctional NMDARs that affect the downstream mesolimbic and mesocortical pathways68. However, further studies are needed to elucidate the exact mechanisms of how dysregulation of QUIN contributes to glutamatergic hypofunction.
Interestingly, we did not find an association between cognitive deficits and TRYCATs in MDD. This is in contrast to a recent study, where Zhou et al. reported an association between cognitive impairment and elevated serum KYN levels and the KYN/TRP ratio. However, the authors found this association only in female but not male patients and further, QUIN was not studied69. Our study population was not large enough to investigate female and male patients separately and therefore future studies are needed to investigate potential sex differences in TRYCATs and cognitive deficits across neuropsychiatric disorders.
Contrary to our initial hypothesis, we did not observe an association between motivational deficits and TRYCATs in the SZ or MDD group. The rational for testing this hypothesis came from the observation that inflammatory processes in general and TRYCATs specifically can alter dopaminergic signalling, and the dopaminergic system plays an important role in negative symptoms and motivational deficits15,70. In addition, several pre-clinical studies have causally linked activation of the kynurenine pathway with several behaviours reminiscent of impairments in goal-directed behaviours19,71. However, the findings of the present study suggest that TRYCATs might be involved in other symptom dimensions than motivation.
There are strengths and limitations of the study that should be considered. The strengths are the well characterized patient population, the inclusion of both patients with SZ and MDD and the comprehensiveness of the TRYCATs measured. In addition, we only measured TRYCATs in the circulation but not in the central nervous system. Only KYN and 3-OHK can readily cross the BBB, while for example KYNA and QUIN access the brain via passive diffusion at a relatively low rate15,72. However, both pre-clinical studies in rodents and human neuroimaging studies provide some evidence that the BBB is impaired in neuropsychiatric disorders and thus certain neurotoxic substances that under physiological conditions do not cross the BBB could potentially access the brain in these conditions73–76. In addition, several studies comparing plasma with cerebrospinal fluid TRYCAT levels have revealed a high correlation, suggesting that peripheral TRYCAT levels indeed reflect levels in the central nervous system77,78. Further studies need to elucidate, if peripheral TRYCATs can access the brain facilitated by a disrupted BBB or if immune activation induces increased brain tryptophan catabolism independently from circulation, e.g. by infiltrating cytokines that activate microglia. One further limitation of the present study was the relatively small sample size of the control group resulting in limited power to detect small effects. In addition, motivational deficits were assessed using a clinician-rated scale and thus should in future studies be complemented by objective task based measures. Lastly, although the BNA is a well validated tool to assess cognitive function in patients with schizophrenia and has been shown to correlate well with other more comprehensive batteries of cognitive testing35, it will be important to follow up on the current findings with instruments that will allow for more detailed assessment of specific neurocognitive domains such as attention, working memory or executive function. Furthermore, although the two cognitive tests that constitute the BNA, the Letter-Number span (sequencing) test and the Symbol Coding Test are frequently used in depression research79,80, the BNA itself has not been validated in patients with MDD.
Conclusions
In summary, the current study suggests that both MDD and SZ are associated with dysregulation of the kynurenine pathway. However, certain catabolites might contribute to different psychopathologies in specific disorders, e.g. QA might be involved in cognitive impairments in patients with SZ but not MDD. Further studies are needed to determine whether TRYCATs are causally involved in the etiology of these neuropsychiatric disorders and to elucidate the underlying mechanisms.
Acknowledgements
We thank Rebecca Schlegel for her help with patient recruitment and all the study participants.
Author contributions
F.C., K.G., F.K. and S.K. designed the study and analysed the data. F.C. and S.K. wrote the manuscript with inputs from all authors. F.C., K.G. and F.K. collected data. E.S. provided major contributions to the manuscript and supervised the project. All authors read and approved the final manuscript.
Funding
This work was supported by an Early.Postdoc Mobility Fellowship of the Swiss National Science Foundation (SNSF) (FK), a Postdoc.Mobility Fellowship of the SNSF (FC), a Walter and Gertrud Siegenthaler Postdoctoral Fellowship (FC), and project support by the Hartmann Mueller Foundation (FC) and Kurt und Senta Herrmann Stiftung (FC).
Data availability
The datasets of the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Flurin Cathomas, Karoline Guetter, Federica Klaus and Stefan Kaiser.
References
- 1.McGrath J, et al. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008;30(1):67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Whiteford HA, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 4.Cuijpers P. The challenges of improving treatments for depression. JAMA. 2018;320(24):2529–2530. doi: 10.1001/jama.2018.17824. [DOI] [PubMed] [Google Scholar]
- 5.Lux V, Kendler KS. Deconstructing major depression: A validation study of the DSM-IV symptomatic criteria. Psychol. Med. 2010;40(10):1679–1690. doi: 10.1017/S0033291709992157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan RW, Carpenter WT. Domains of psychopathology: An approach to the reduction of heterogeneity in schizophrenia. J. Nerv. Ment. Dis. 1994;182(4):193–204. doi: 10.1097/00005053-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, et al. Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am. J. Psychiatry. 2010;167(10):1254–1263. doi: 10.1176/appi.ajp.2010.09091335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain M, Roiser JP. Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nat. Rev. Neurosci. 2018;19(8):470–484. doi: 10.1038/s41583-018-0029-9. [DOI] [PubMed] [Google Scholar]
- 9.Yuen GS, et al. Apathy in late-life depression: Common, persistent, and disabling. Am. J. Geriatr. Psychiatry. 2015;23(5):488–494. doi: 10.1016/j.jagp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazbek H, et al. The Lille Apathy Rating Scale (LARS): Exploring its psychometric properties in schizophrenia. Schizophr. Res. 2014;157(1–3):278–284. doi: 10.1016/j.schres.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 11.Barch DM. Neuropsychological abnormalities in schizophrenia and major mood disorders: Similarities and differences. Curr. Psychiatry Rep. 2009;11(4):313–319. doi: 10.1007/s11920-009-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewandowski KE, et al. Relationship of neurocognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophr. Res. 2011;133(1–3):212–217. doi: 10.1016/j.schres.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cathomas F, et al. Neurobiology of resilience: Interface between mind and body. Biol. Psychiatry. 2019;86(6):410–420. doi: 10.1016/j.biopsych.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller N, Schwarz MJ. Immune system and schizophrenia. Curr. Immunol. Rev. 2010;6(3):213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savitz J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry. 2020;25(1):131–147. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erhardt S, et al. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2017;112(Pt B):297–306. doi: 10.1016/j.neuropharm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Schwarcz R, et al. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012;13(7):465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor JC, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuertig R, et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav. Immun. 2016;54:59–72. doi: 10.1016/j.bbi.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Ogyu K, et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018;90:16–25. doi: 10.1016/j.neubiorev.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Marx W, et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry. 2020;1:1–10. doi: 10.1038/s41380-020-00951-9. [DOI] [PubMed] [Google Scholar]
- 23.Plitman E, et al. Kynurenic acid in schizophrenia: A systematic review and meta-analysis. Schizophr. Bull. 2017;43(4):764–777. doi: 10.1093/schbul/sbw221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steen NE, et al. Metabolic dysfunctions in the kynurenine pathway, noradrenergic and purine metabolism in schizophrenia and bipolar disorders. Psychol. Med. 2019;1:1–12. doi: 10.1017/S0033291719000400. [DOI] [PubMed] [Google Scholar]
- 25.Lecrubier Y, Weiller E, Herugeta T. Mini International Neuropsychiatric Interview German Version 5.0.0. Psychiatrischen Universitätsklinik München; 1999. [Google Scholar]
- 26.Cathomas F, et al. Increased random exploration in schizophrenia is associated with inflammation. NPJ Schizophr. 2021;7(1):6. doi: 10.1038/s41537-020-00133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirschner M, et al. Shared and dissociable features of apathy and reward system dysfunction in bipolar I disorder and schizophrenia. Psychol. Med. 2020;50(6):936–947. doi: 10.1017/S0033291719000801. [DOI] [PubMed] [Google Scholar]
- 28.Klaus F, et al. Peripheral biopterin and neopterin in schizophrenia and depression. Psychiatry Res. 2021;297:113745. doi: 10.1016/j.psychres.2021.113745. [DOI] [PubMed] [Google Scholar]
- 29.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64(6):663–667. doi: 10.4088/JCP.v64n0607. [DOI] [PubMed] [Google Scholar]
- 30.Hayasaka Y, et al. Dose equivalents of antidepressants: Evidence-based recommendations from randomized controlled trials. J. Affect. Disord. 2015;180:179–184. doi: 10.1016/j.jad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick B, et al. The brief negative symptom scale: Psychometric properties. Schizophr. Bull. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bischof M, et al. The brief negative symptom scale: Validation of the German translation and convergent validity with self-rated anhedonia and observer-rated apathy. BMC Psychiatry. 2016;16(1):415–415. doi: 10.1186/s12888-016-1118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fervaha G, et al. Toward a more parsimonious assessment of neurocognition in schizophrenia: A 10-minute assessment tool. J. Psychiatr. Res. 2014;52:50–56. doi: 10.1016/j.jpsychires.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Nuechterlein KH, et al. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 35.Fervaha G, et al. Examination of the validity of the brief neurocognitive assessment (BNA) for schizophrenia. Schizophr. Res. 2015;166(1–3):304–309. doi: 10.1016/j.schres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Wallwork RS, et al. Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr. Res. 2012;137(1–3):246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. Psychol. Corp. 1996;78(2):490–498. [Google Scholar]
- 39.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr. Res. 1990;3(4):247–251. doi: 10.1016/0920-9964(90)90005-R. [DOI] [PubMed] [Google Scholar]
- 40.Morosini PL, et al. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiatr. Scand. 2000;101(4):323–329. doi: 10.1111/j.1600-0447.2000.tb10933.x. [DOI] [PubMed] [Google Scholar]
- 41.Juckel G, et al. Validation of the personal and social performance (PSP) scale in a German sample of acutely ill patients with schizophrenia. Schizophr. Res. 2008;104(1–3):287–293. doi: 10.1016/j.schres.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 42.Meier TB, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav. Immun. 2016;53:39–48. doi: 10.1016/j.bbi.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wurfel BE, et al. Serum kynurenic acid is reduced in affective psychosis. Transl. Psychiatry. 2017;7(5):e1115–e1115. doi: 10.1038/tp.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho HJ, et al. Sleep disturbance and kynurenine metabolism in depression. J. Psychosom. Res. 2017;99:1–7. doi: 10.1016/j.jpsychores.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuwano N, et al. Tryptophan-kynurenine and lipid related metabolites as blood biomarkers for first-episode drug-naïve patients with major depressive disorder: An exploratory pilot case-control study. J. Affect. Disord. 2018;231:74–82. doi: 10.1016/j.jad.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Pan JX, et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Transl. Psychiatry. 2018;8(1):130. doi: 10.1038/s41398-018-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young KD, et al. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav. Immun. 2016;56:335–342. doi: 10.1016/j.bbi.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao JK, et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol.. Psychiatry. 2010;15(9):938–953. doi: 10.1038/mp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kindler J, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry. 2019;25:2860–2872. doi: 10.1038/s41380-019-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myint AM, et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain Behav. Immun. 2011;25(8):1576–1581. doi: 10.1016/j.bbi.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Fazio F, et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci. Rep. 2015;5(1):17799. doi: 10.1038/srep17799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colín-González AL, Maldonado PD, Santamaría A. 3-Hydroxykynurenine: An intriguing molecule exerting dual actions in the Central Nervous System. Neurotoxicology. 2013;34:189–204. doi: 10.1016/j.neuro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Schwieler L, et al. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J. Neuroinflamm. 2016;13(1):51. doi: 10.1186/s12974-016-0517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myint AM, et al. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J. Affect. Disord. 2007;98(1–2):143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Colle R, et al. Peripheral tryptophan, serotonin, kynurenine, and their metabolites in major depression: A case-control study. Psychiatry Clin. Neurosci. 2020;74(2):112–117. doi: 10.1111/pcn.12944. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, et al. The metabolic factor kynurenic acid of kynurenine pathway predicts major depressive disorder. Front. Psych. 2018;9:552. doi: 10.3389/fpsyt.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Vaart H, et al. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax. 2004;59(8):713–721. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Festa A, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int. J. Obes. Relat. Metab. Disord. 2001;25(10):1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 59.Myint AM, Kim YK. Cytokine–serotonin interaction through IDO: A neurodegeneration hypothesis of depression. Med. Hypotheses. 2003;61(5):519–525. doi: 10.1016/S0306-9877(03)00207-X. [DOI] [PubMed] [Google Scholar]
- 60.Halverson TF, et al. Pathways to functional outcomes in schizophrenia spectrum disorders: Meta-analysis of social cognitive and neurocognitive predictors. Neurosci. Biobehav. Rev. 2019;105:212–219. doi: 10.1016/j.neubiorev.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Alptekin K, et al. Is quality of life associated with cognitive impairment in schizophrenia? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(2):239–244. doi: 10.1016/j.pnpbp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J. Psychopharm. 2015;29(2):97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez DeLa Cruz V, Carrillo-Mora P, Santamaria A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012;5:1–8. doi: 10.4137/IJTR.S8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Latif-Hernandez A, et al. Quinolinic acid injection in mouse medial prefrontal cortex affects reversal learning abilities, cortical connectivity and hippocampal synaptic plasticity. Sci. Rep. 2016;6(1):36489. doi: 10.1038/srep36489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer's disease. Redox Rep. 2002;7(4):199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- 66.Schwarcz R, et al. Of mice, rats and men: Revisiting the quinolinic acid hypothesis of Huntington's disease. Prog. Neurobiol. 2010;90(2):230–245. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gos T, et al. Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain Behav. Immun. 2014;41:59–64. doi: 10.1016/j.bbi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Moghaddam B, Javitt D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Y, et al. Cross-sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology. 2019;101:72–79. doi: 10.1016/j.psyneuen.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Brown RG, Pluck G. Negative symptoms: The 'pathology' of motivation and goal-directed behaviour. Trends Neurosci. 2000;23(9):412–417. doi: 10.1016/S0166-2236(00)01626-X. [DOI] [PubMed] [Google Scholar]
- 71.O'Connor JC, et al. Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J. Immunol. 2009;182(5):3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukui S, et al. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 73.Najjar S, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: A theoretical integration of clinical and experimental evidence. Front. Psychiatry. 2017;8:83. doi: 10.3389/fpsyt.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pollak TA, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79–92. doi: 10.1016/S2215-0366(17)30293-6. [DOI] [PubMed] [Google Scholar]
- 75.Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl. Psychiatry. 2020;10(1):373. doi: 10.1038/s41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greene C, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatry. 2018;23(11):2156–2166. doi: 10.1038/mp.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raison CL, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haroon E, et al. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology. 2020;2:10. doi: 10.1038/s41386-020-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaeger J. Digit symbol substitution test: The case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol. 2018;38(5):513–519. doi: 10.1097/JCP.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lanza C, et al. Cognitive profiles in persons with depressive disorder and Alzheimer’s disease. Brain Commun. 2020;2:2. doi: 10.1093/braincomms/fcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the current study are available from the corresponding author on reasonable request.