Abstract
Triglyceride glucose (TyG) index and inflammatory markers are reported to have a positive association with metabolic syndrome (MetS). However, no previous study has assessed the value of TyG index and inflammatory markers as predictors of metabolic syndrome in the same study. This study looks at the comparison of the triglyceride index and blood leukocyte indices as predictors of metabolic syndrome in the Chinese population. The study cohort involved 1542 Chinese population without metabolic syndrome. The subjects underwent comprehensive routine health examination in 2011 and returned for a follow-up examination in 2016. Metabolic syndrome was defined according to Chinese Diabetes Society criteria, using body mass index for the replacement of waist circumference. TyG index, total leukocytes, neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio (NLR) were measured. Adjust d logistic models were used to assess the relationship between TyG index, blood leukocyte indices, and incident MetS. Receiver operating characteristic (ROC) curves were performed to determine the predictive value of TyG index and blood leukocyte indices for MetS. Results from multivariate logistic regression analysis showed that, in the adjusted model, the subjects with the highest quartile of TyG index and neutrophils had a 3.894- and 1.663-fold increased incidence of MetS (P < 0.0001 and P = 0.027), respectively. No significant association was observed between total leukocytes, lymphocytes, NLR with incident MetS. ROC analysis showed that the AUC of TyG index and neutrophils were 0.674 and 0.568 for incident MetS, respectively. TyG index rather than blood leukocyte indices may have the strongest predictive value in MetS development over a 5-year period.
Subject terms: Predictive markers, Endocrine system and metabolic diseases
Introduction
Metabolic syndrome (MetS), a cluster of metabolic abnormalities characterized by obesity, hypertension, dyslipidemia, and glucose dysregulation, is associated with the risk of diabetes, cardiovascular disease, and overall mortality1,2. Insulin resistance plays a major role in the progression of Metabolic syndrome3. Chronic inflammation and oxidative stress also have been implicated in the underlying pathogenesis4,5.
The triglyceride glucose (TyG) index, which is calculated from fasting measurements of triglyceride and glucose, has been suggested as a reliable surrogate marker of IR6–8. Previous studies have found that a high baseline TyG index was related to the development of diabetes and coronary atherosclerosis9–11. To our knowledge, although a cross-sectional study has recently shown that TyG index was positively correlated with MetS12, the relationship between TyG and MetS development has not been evaluated in the longitudinal study.
Leukocytes are common, inexpensive, and broadly utilized marker of inflammation. Numerous studies have demonstrated increased total leukocytes, neutrophils, lymphocytes, and neutrophil to lymphocyte ratio (NLR) were significantly associated with MetS13–16. However, conclusions from some studies are inconsistent15,17,18. For example, Meng et al.15 reported that total leukocytes, neutrophils, and lymphocytes, but not NLR were associated with MetS, whereas Vahit et al.17 and Liu et al.18 reported that NLR had a significant association with MetS. However, compared with the control group, there were no changes in the level of total leukocytes and neutrophils except lymphocytes were significantly higher in the MetS group. Therefore, it is worthwhile to further evaluate the temporal association between the blood leukocyte indices and the risk of MetS. In addition, although the blood leukocyte indices and TyG index are associated with MetS, few studies have compared their predictive values in MetS development within one population.
Therefore, in this prospective study, as a primary outcome, we aimed to analyze the potential predictor value of the blood leukocyte indices in MetS development. Furthermore, the secondary outcome of this study was to compare the clinical utility of TyG index and the blood leukocyte indices in predicting incident MetS among healthy Chinese population during the 5 years of the follow-up period.
Methods
Study subjects
The study comprised 2585 Chinese population who had undergone routine health examination at Health Management Center of Shandong Provincial Hospital affiliated to Shandong First Medical University, China in 2011 and who had returned for a follow-up examination in 2016. Among them, the individuals (n = 628) who had an acute inflammatory disease or recent infection, renal dysfunction, liver problems, or all types of cancer, and those who (n = 241) with missing data on the questionnaire or smoking history questionnaire, anthropometric measurements, or biochemical parameters were excluded. In addition, subjects (n = 178) with MetS were further excluded at baseline. Therefore, a total of 1542 subjects (1056 men and 486 women) with a mean age of 44.8 ± 12.6 years (range: 22–85 years) remained. The study was approved by the institutional review board of Shandong Provincial Hospital affiliated to Shandong First Medical University. Written informed consents were obtained from all subjects prior to the health check-up when they visited the Health Management Center.
Study measurement
The physical examination comprised blood pressure (BP) and anthropometric measurements, including height, weight, and body mass index (BMI). BMI was calculated as weight (kg) divided by height (m)2. The BP was measured by well-trained nurses two times in the left arm of seated participants using an automated electronic device (OMRON model HEM-752 Fuzzy, Omron Company, Dalian, China) after 5-min sitting. The average of the two readings was calculated to determine the reported BP. Blood samples were collected from subjects who had fasted for ≥ 12 h. The levels of fasting blood glucose (FBG), lipids including triglyceride (TG), total cholesterol (CH), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) were measured using an Olympus AU5400 system. The TyG index was calculated as ln[fasting triglycerides (mg/dl) x fasting glucose (mg/dl)/2]19. Total leukocytes, including neutrophils and lymphocytes count analysis, were performed in the hematology laboratory of our hospital, and NLR values were calculated. Smoking habit was defined by having ≥ 5 cigarettes per day.
According to the guidelines for Type 2 Diabetes in China by Diabetes Branch of the Chinese Medical Association20, using BMI for the replacement of waist circumference, the MetS was defined as having any three or more of the following factors: (a) overweight and/or obesity: BMI ≥ 25.0 kg/m2; (b) raised FPG: ≥ 6.1 mmol/L or 2-h plasma glucose (2hPG) level ≥ 7.8 mmol/L or previously diagnosed as type 2 diabetes and taking anti glycemic medication; (c) raised BP: ≥ 130/85 mmHg, or previously diagnosed as hypertension and taking antihypertensive medication; (d) raised TG: ≥ 1.7 mmol/L; and/or (e) reduced HDL-C: < 1.04 mmol/L.
Statistical analysis
SPSS19.0 was used for data analysis, and PASS11 was used to estimate the sample size and analyze the statistical power of the results. A value of less than 0.05 was deemed statistically significant. The distribution of the different variables was examined for normality by the Kolmogotov-Smirnov test. Categorical variables were expressed in percentages and continuous variables in mean (SD) or geometric mean (95% confidence interval).
Between-group differences with respect to categorical variables were assessed using a Chi-square test. Between-group differences with respect to continuous variables of normal distribution were assessed using Student test or one-way ANOVA, and continuous variables of non-normal distribution were assessed using Mann–Whitney U test or Kruskal–Wallis test.
Multivariate logistic regression analysis was used to calculate the odds ratios (ORs) and 95% confidence intervals (CI) of incident MetS for each investigated indicator quartile compared with the lowest quartile, with adjustment for baseline age, sex, smoking status (yes, no), BMI, SBP, DBP. The age, BMI, SBP, DBP were treated as continuous variables. A receiver operating characteristic (ROC) curve analysis, which was quantified by the area under the ROC curve (AUC), was used to assess the value of indicators for predicting incident MetS. The area under ROC curve was compared by the nonparametric Z test. Youden’s index (sensitivity + specificity − 1) was used to determine the optimal cutoff point of each indicator.
All above methods were carried out in accordance with relevant guidelines and regulations.
Ethical approval
The study was approved by the institutional review board of Shandong Provincial Hospital affiliated to Shandong First Medical University.
Results
A total of 1542 metabolic syndrome-free participants at baseline were enrolled in this study. Of these, 179 (11.4%) subjects who developed MetS during the 5 years follow-up period were categorized as positive for MetS (MS), while 1363 (88.6%) subjects free of MetS were categorized as NMS. The baseline demographic characteristics and laboratory parameters of the population are shown in Table 1. A significant between-group difference in TyG index was observed in the subjects with MetS compared to subjects free of MetS (8.75 (8.68, 8.82) vs 8.45 (8.43, 8.48); P < 0.0001). Compared with subjects free of MetS, those with MetS were tend to have higher levels of total leukocytes, neutrophils, and lymphocytes. In addition, a higher BMI, SBP, DBP, CH, TG, LDL-C, and fasting blood glucose, the proportion of male subjects smokers, along with a lower level of HDL-C were observed in subjects with MetS (P < 0.01 for all). No significant differences were observed on NLR (P = 0.736) and age (P = 0.655) between MS and NMS groups.
Table 1.
Participant baseline demographic characteristics and laboratory parameters by metabolic syndrome status (n = 1542).
| Characteristic | NMS | MS | P-Value |
|---|---|---|---|
| Subjects | 1363 | 179 | |
| Age (years) | 44.9 (44.2, 45.6) | 45.0 (44.3, 46.7) | 0.655 |
| Gender (male-n-%) | 902(67.0%) | 154 (86.0%) | < 0.0001 |
| Smoking (n-%) | 232 (17.2%) | 44 (24.6%) | 0.01 |
| Body mass index (kg/m2) | 24.0 (23.9, 24.2) | 26.2 (25.8, 26.6) | < 0.0001 |
| Systolic blood pressure (mmHg) | 119.5 (118.7, 120.4) | 125.8 (123.7, 128.0) | < 0.0001 |
| Diastolic blood pressure (mmHg) | 70.2 (69.6, 70.7) | 75.4 (73.8, 77.0) | < 0.0001 |
| Leukocytes (× 109/L) | 5.21 (6.14, 6.28) | 6.60 (6.39 6.81) | 0.001 |
| Neutrophils (× 109/L) | 3.39 (3.33, 3.44) | 3.60 (3.46, 3.75) | 0.003 |
| Lymphocytes (× 109/L) | 2.25 (2.22, 2.28) | 2.39 (2.30, 2.49) | 0.003 |
| NLR | 1.59 (1.56, 1.62) | 1.57 (1.50, 1.65) | 0.736 |
| High-density lipoprotein (mmol/L) | 1.38 (1.36, 1.39) | 1.22 (1.19, 1.25) | < 0.0001 |
| Low-density lipoprotein (mmol/L) | 3.28 ± 0.02 | 3.44 ± 0.82 | < 0.0001 |
| Total cholesterol (mmol/L) | 5.13 ± 0.25 | 5.21 ± 0.78 | < 0.0001 |
| Triglyceride (mmol/L) | 1.24 (1.20, 1.28) | 1.62 (1.48, 1.75) | < 0.0001 |
| Fasting blood glucose (mmol/L) | 5.41 (5.37, 5.45) | 5.53 (5.43, 5.64) | < 0.0001 |
| TyG index | 8.45 (8.43, 8.48) | 8.75 (8.68, 8.82) | < 0.0001 |
To determine independent variables for the incidence of MetS, multivariate logistic regression analysis was performed and results showed in Table 2. Subjects with the higher TyG index, total leukocytes, neutrophils, and lymphocytes significantly increased the incidence of MetS (P < 0.05 for all). After adjusting for age, sex, and smoking habits, total leukocytes and lymphocytes were no longer independent predictors of MetS (model 1), whereas the associations between TyG index, neutrophils, and incident MetS had no change, and continued to maintain even after further adjustments were made for BMI and blood pressure levels (model 2). In the final model, the subjects with the highest quartile of TyG index and neutrophils had a 3.894- and 1.663-fold increased incidence of MetS (P < 0.0001 and P = 0.027), respectively.
Table 2.
Associations of TyG index, inflammatory markers and risk of metabolic syndrome incidence (odds rations and 95% confidence intervals).
| Quartiles | P for trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| TyG index | |||||
| Range | ≤ 8.14 | 8.15–8.48 | 8.49–8.81 | > 8.82 | |
| MS cases/total number of each quartile (%) | 12/386 | 39/387 | 55/394 | 73/375 | |
| No adjusted | 1 | 3.496 (1.799–6.780) | 5.057 (2.662–9.604) | 7.534 (4.016–14.132) | < 0.0001 |
| Model 1 | 1 | 3.015 (1.542–5.896) | 4.127 (2.146–7.936) | 5.924 (3.113–11.275) | < 0.0001 |
| Model 2 | 1 | 2.316 (1.169–4.588) | 2.713 (1.389–5.300) | 3.894 (2.011–7.537) | < 0.0001 |
| Total leukocytes | |||||
| Range | ≤ 5.30 | 5.31–6.12 | 6.13–7.03 | > 7.04 | |
| MS cases/total number of each quartile (%) | 32/389 | 43/384 | 45/385 | 59/384 | |
| No adjusted | 1 | 1.407 (0.870–2.276) | 1.477 (0.916–2.379) | 2.025 (1.284–3.195) | 0.021 |
| Model 1 | 1 | 1.252 (0.769–2.038) | 1.255 (0.773–2.037) | 1.692 (1.064–2.689) | 0.147 |
| Model 2 | 1 | 1.187 (0.721–1.956) | 1.102 (0.671–1.809) | 1.298 (0.806–2.092) | 0.730 |
| Neutrophils | |||||
| Range | ≤ 2.72 | 2.73–3.28 | 3.28—3.95 | > 3.96 | |
| MS cases/total number of each quartile (%) | 31/387 | 50/384 | 36/387 | 62/384 | |
| No adjusted | 1 | 1.719 (1.072–2.757) | 1.178 (0.713–1.946) | 2.211 (1.400–3.491) | 0.002 |
| Model 1 | 1 | 1.707 (1.059–2.752) | 1.044 (0.628–1.735) | 2.090 (1.317–3.317) | 0.002 |
| Model 2 | 1 | 1.606 (0.985–2.618) | 0.971 (0.579–1.630) | 1.663 (1.034–2.672) | 0.027 |
| Lymphocytes | |||||
| Range | ≤ 1.85 | 1.86–2.20 | 2.21–2.62 | > 2.63 | |
| MS cases/total number of each quartile (%) | 33/390 | 46/383 | 40/387 | 60/382 | |
| No adjusted | 1 | 1.477 (0.922–2.366) | 1.247 (0.769–2.023) | 2.016 (1.284–3.164) | 0.015 |
| Model 1 | 1 | 1.378 (0.854–2.224) | 1.066 (0.650–1.750) | 1.663 (1.048–2.639) | 0.089 |
| Model 2 | 1 | 1.301 (0.796–2.126) | 0.959 (0.578–1.592) | 1.283 (0.797–2.064) | 0.421 |
| NLR | |||||
| Range | ≤ 1.17 | 1.18–1.47 | 1.48–1.88 | > 1.89 | |
| MS cases/total number of each quartile (%) | 42/390 | 48/388 | 45/386 | 44/378 | |
| No adjusted | 1 | 1.170 (0.753–1.817) | 1.093 (0.700–1.708) | 1.092 (0.697–1.710) | 0.762 |
| Model 1 | 1 | 1.155 (0.740–1.801) | 1.102 (0.702–1.730) | 1.186 (0.752–1.869) | 0.888 |
| Model 2 | 1 | 1.148 (0.728–1.810) | 1.094 (0.689–1.736) | 1.174 (0.736–1.873) | 0.908 |
Data are expressed as ORs (95% CI). Model 1: adjusted for age, sex, smoking; model 2: Model 1 adjusted further for body mass index, blood pressure.
The accuracy of TyG index and neutrophils and their sensitivity and specificity in predicting incident MetS were compared and the results showed in Fig. 1 and Table 3. The AUC of TyG index and neutrophils were 0.674 and 0.568 for incident MetS, respectively. Further Z test was performed to compare the area under ROC curve, results showed that the difference was statistically significant (Z = 3.56 P < 0.05). The statistical power for two ROC curves was 0.98 with β = 0.0165. The optimal cutoff value of TyG index, neutrophils based on Youden's index in predicting incident MetS was 8.52, 3.26 × 109/L, with a sensitivity and specificity of 70.4% and 55.9%, 55.3%, and 50.3%, respectively.
Figure 1.
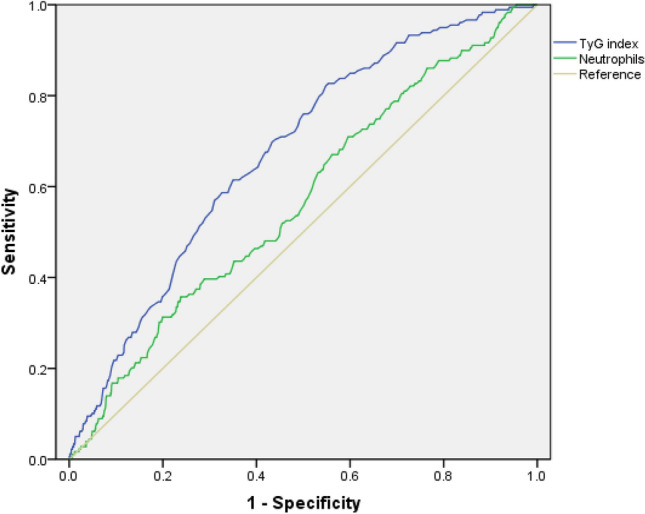
Receiver operating characteristic (ROC) curves of TyG index and neutrophils in predicting incident metabolic syndrome.
Table 3.
Areas under the ROC Curve (AUC), sensitivity and specificity by the optimized cutoff points for TyG index and neutrophils in predicting metabolic syndrome.
| AUC (95%CI) | Cutoff | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| TyG index | 0.674 (0.635, 0.713) | 8.52 | 70.4 | 55.9 |
| Neutrophils (× 109/L) | 0.586 (0.560, 0.612) | 3.26 | 55.3 | 50.3 |
Discussion
The current study examined associations between TyG index, blood leukocyte indices (total leukocytes, neutrophils, lymphocytes, NLR), and incident MetS, moreover compared their predicting values in MetS development in a normal check-up adult population during the 5 years of follow-up period. The results have demonstrated that, of the blood leukocyte indices, although total leukocytes, neutrophils, and lymphocytes were each associated with the incident MetS, neutrophils were the most strongly associated with MetS after adjusting for potential confounding factors (including age, sex, BMI, smoking status, and blood pressure). The most importantly, a ROC curve analysis in this study indicated that TyG index was more useful for predicting individuals who were most likely to develop MetS than neutrophils. To our knowledge, this study is the first prospective study to assess the predicting value of TyG index and blood leukocyte indices in incident MetS within one healthy population.
TyG index has been reported to be closely correlated with insulin resistance, the presence of coronary artery complications in type 2 diabetes and healthy adults. For example, previous cross-sectional studies suggested that TyG index was a useful marker for early identification of insulin resistance individuals6, and the predictive value of the TyG index for insulin resistance was even better than that of the HOMA-IR21,22. A 4-year retrospective longitudinal study in non-diabetic adults indicated that high baseline TyG index was related to diabetes development9. Moreover, it was confirmed that a high TyG index was associated with an increased risk of microalbuminuria and cerebrovascular disease among type 2 diabetes patients23. Previous studies have shown that TyG index was associated with coronary atherosclerosis10,11, and was also a useful marker for predicting subclinical coronary in healthy individuals with a low CV risk burden24. Results from the most current cross-sectional study showed that TyG index increased with the number of MetS components12. In our present longitudinal study, it is further confirmed that a high baseline TyG index is associated with the development of MetS in the healthy population.
Total leukocytes, neutrophils, and lymphocytes are common, inexpensive, and broadly utilized markers of inflammation. They activate major cell types involved in acute and chronic inflammation. Similar to previous studies15, increased total leukocytes, neutrophils, and lymphocytes were observed in the subjects of developed MetS in the present study. Previous studies presented that systemic inflammatory markers were associated with age, gender, smoking history, body weight, and disease of hypertension25–29. Therefore, confounding factors including age, sex, smoking status, BMI, and blood pressure were taken into account in our analysis. After adjusting for the multiple potential confounding factors, the associations between total leukocytes and lymphocytes with incident MetS no longer existed. However, neutrophils continued to have a strong association with incident MetS. These findings are consistent with previous findings that neutrophils is a useful biomarker of inflammation in nascent MetS30.
The neutrophil-to-lymphocyte ratio (NLR), calculated by dividing the neutrophil by the lymphocyte, has been found to be a reliable indicator of inflammation and correlates with the presence and severity of MetS18,31. It is interesting that the previous cross-sectional studies have reported a positive association between NLR and MetS, in contrast, our study did not find this association. A most recent study showing a no-significant increase in NLR in patients with nascent MetS30, which may explain why our findings did not show a positive correlation between NLR and the development of MetS.
It is noteworthy that the present study provides beneficial data for comparing the value of TyG index and leukocyte indices in predicting incident MetS in one report. A high AUC of the TyG index was expected, as the TyG index includes triglyceride and glucose, the main components of the metabolic syndrome. As determined based on the AUC of the ROC curve and a further Z test, TyG index had a greater predictive power for incident MetS than neutrophils.
The present study showed the optimal TyG index cutoff for incident MetS in Chinese adults was 8.52. Shin et al12 reported the optimal cutoff of TyG index to be 8.81 for MetS in middle-aged and older Korean populations. Based on the findings, it is suggested there might be a standardized cut-off value for TyG index in estimating the development of MetS, as well as initial evaluation12. Due to the importance of early lifestyle intervention in the treatment of MetS, elevated TyG index levels can provide a significant clue for the assessment of the high-risk MetS population. Given the variability of triglyceride levels according to ethnicity, further studies are needed to assess the TyG index in other populations.
There are several limitations of the present study that need to be noted. First, the objects of study and analysis were those who visited the health management center and were annually follow-up examination, therefore, the findings of this study might not be representative of the general population. Second, the diagnostic criteria for MetS in this study were not the most commonly used International Diabetes Federation and the National Cholesterol Education Adult Treatment Panel III-R, but the Diabetes Branch of the Chinese Medical Association based on the study of MetS in China. However, which is more consistent with the Chinese characteristics. Third, instead of waist circumference, BMI was used in the definition of MetS due to a lack of corresponding data. This lack of data might have led to a miss estimation of the actual prevalence of MetS. Fourth, our study population was relatively small, therefore the statistical power may be limited due to the small number of incident cases.
In conclusion, compared with the total leukocytes, lymphocytes, and NLR, neutrophils appear to be a robust cost-effective marker of inflammation in predicting the incidence of MetS in healthy people. Furthermore, TyG index appears to be superior to the neutrophils in predicting the risk of incident MetS in this group. Additional large and long-term prospective studies are required to establish the role of TyG index in the MetS development.
Acknowledgements
We thank all doctors, nurses, and other staff members of the Health Management Center, Shandong. Provincial Hospital affiliated to Shandong First Medical University for their involvement in the project.
Author contributions
X.-H.L. designed the study. H.-Y.L., X.-J.Z., Y.-M.L., L.-Y.G. and L.-Y.G. collected the data. Y.-M.L. and L.-Y.G. performed the analyses, H.-Y.L. and X.-J.Z. wrote the first draft of the manuscript. X.-H.L. provided language help and writing assistance. All authors contributed to and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laaksonen DE, et al. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am. J. Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 2.Isomaa B, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 3.Gluvic Z, et al. Link between metabolic syndrome and insulin resistance. Curr. Vasc. Pharmacol. 2017;15:30–39. doi: 10.2174/1570161114666161007164510. [DOI] [PubMed] [Google Scholar]
- 4.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 5.Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014;395:203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 6.Du T, et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerrero-Romero F, et al. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch. Med. Res. 2016;47:382–387. doi: 10.1016/j.arcmed.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Van der Walt A, Baatjies R, Singh T, Jeebhay MF. Environmental factors associated with baseline and serial changes in fractional exhaled nitric oxide (FeNO) in spice mill workers. Occup. Environ. Med. 2016;73:614–620. doi: 10.1136/oemed-2015-103005. [DOI] [PubMed] [Google Scholar]
- 9.Lee DY, et al. Predictive value of triglyceride glucose index for the risk of incident diabetes: a 4-year retrospective longitudinal study. PLoS ONE. 2016;11:e0163465. doi: 10.1371/journal.pone.0163465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MK, et al. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc. Diabetol. 2017;16:108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Won KB, et al. The relationship of insulin resistance estimated by triglyceride glucose index and coronary plaque characteristics. Medicine. 2018;97:e10726. doi: 10.1097/MD.0000000000010726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin KA, Kim YJ. Usefulness of surrogate markers of body fat distribution for predicting metabolic syndrome in middle-aged and older Korean populations. Diabetes Metab. Syndr. Obes. Targets Ther. 2019;12:2251–2259. doi: 10.2147/DMSO.S217628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babio N, et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the PREDIMED study. PLoS ONE. 2013;8:e58354. doi: 10.1371/journal.pone.0058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, et al. Positive association between the metabolic syndrome and white blood cell counts in Chinese. Asia Pac. J. Clin. Nutr. 2017;26:141–147. doi: 10.6133/apjcn.102015.13. [DOI] [PubMed] [Google Scholar]
- 15.Meng G, et al. Comparing the diagnostic ability of inflammatory markers in metabolic syndrome. Clin. Chim. Acta. 2017;475:1–6. doi: 10.1016/j.cca.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Sun S, et al. Subnormal peripheral blood leukocyte counts are related to the lowest prevalence and incidence of metabolic syndrome: Tianjin chronic low-grade systemic inflammation and health cohort study. Med. Inflamm. 2014;2014:412386. doi: 10.1155/2014/412386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vahit D, Akboga MK, Samet Y, Huseyin E. Assessment of monocyte to high density lipoprotein cholesterol ratio and lymphocyte-to-monocyte ratio in patients with metabolic syndrome. Biomark Med. 2017;11:535–540. doi: 10.2217/bmm-2016-0380. [DOI] [PubMed] [Google Scholar]
- 18.Liu CC, et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Med. (Baltimore) 2019;98:e17537. doi: 10.1097/MD.0000000000017537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrero-Romero F, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 20.Fan L, et al. Non-linear relationship between sleep duration and metabolic syndrome: a population-based study. Medicine. 2020;99:e18753. doi: 10.1097/MD.0000000000018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasques AC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju metabolic disease cohort (CMC) study. PLoS ONE. 2014;9:e90430. doi: 10.1371/journal.pone.0090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu H, et al. Associations between triglyceride-glucose index and micro- and macro-angiopathies in type 2 diabetes mellitus. Nutrients. 2020 doi: 10.3390/nu12020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park GM, et al. Triglyceride glucose index is a useful marker for predicting subclinical coronary artery disease in the absence of traditional risk factors. Lipids Health Dis. 2020;19:7. doi: 10.1186/s12944-020-1187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 26.Cartier A, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am. J. Clin. Nutr. 2009;89:1307–1314. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- 27.Strohacker K, Wing RR, McCaffery JM. Contributions of body mass index and exercise habits on inflammatory markers: a cohort study of middle-aged adults living in the USA. BMJ Open. 2013 doi: 10.1136/bmjopen-2013-002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frohlich M, et al. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) Eur. Heart J. 2003;24:1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 29.Dunkley AJ, Taub NA, Davies MJ, Stone MA, Khunti K. Is having a family history of type 2 diabetes or cardiovascular disease a predictive factor for metabolic syndrome? Prim Care Diabetes. 2009;3:49–56. doi: 10.1016/j.pcd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Adams-Huet B, Jialal I. The neutrophil count is superior to the neutrophil/lymphocyte ratio as a biomarker of inflammation in nascent metabolic syndrome. Ann. Clin. Biochem. 2019;56:715–716. doi: 10.1177/0004563219866221. [DOI] [PubMed] [Google Scholar]
- 31.Buyukkaya E, et al. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin. Appl. Thromb. Hemost. 2014;20:159–163. doi: 10.1177/1076029612459675. [DOI] [PubMed] [Google Scholar]


