Abstract
Plasmodium vivax is the most geographically widespread cause of human malaria and is responsible for the majority of cases outside of the African continent. While great progress has been made towards eliminating human malaria, drug resistant parasite strains pose a threat towards continued progress. Resistance has arisen to multiple antimalarials in P. vivax, including to chloroquine, which is currently the first line therapy for P. vivax in most regions. Despite its importance, an understanding of the molecular mechanisms of drug resistance in this species remains elusive, in large part due to the complex biology of P. vivax and the lack of in vitro culture. In this review, we will cover the extent and challenges of measuring clinical and in vitro drug resistance in P. vivax. We will consider the roles of candidate drug resistance genes. We will highlight the development of molecular approaches for studying P. vivax biology that provide the opportunity to validate the role of putative drug resistance mutations as well as identify novel mechanisms of drug resistance in this understudied parasite. Validated molecular determinants and markers of drug resistance are essential for the rapid and cost-effective monitoring of drug resistance in P. vivax, and will be useful for optimizing drug regimens and for informing drug policy in control and elimination settings.
Keywords: Plasmodium vivax. Malaria, Drug resistance
Graphical abstract
Highlights
-
•
Drug resistance is emerging in Plasmodium vivax, an important cause of malaria.
-
•
The complex biology of P. vivax and the limited range of research tools make it difficult to identify drug resistance.
-
•
The molecular mechanisms of drug resistance in P. vivax remain elusive.
-
•
This review highlights the extent of drug resistance, the putative mechanisms of resistance and new technologies for the study of P. vivax drug resistance.
1. Introduction
Plasmodium vivax and Plasmodium falciparum are responsible for the vast majority of malaria cases globally, with P. vivax causing most malaria cases outside of the African continent (Price et al., 2014; WHO, 2019b). The significant progress that has been made towards reducing malaria transmission is threatened by the emergence of drug resistance in P. falciparum to all clinically used antimalarials (WHO, 2019a). Several recent reviews have summed up the state of knowledge around P. falciparum drug resistance (Haldar et al., 2018; Wicht et al., 2020). P. vivax resistance to antimalarial drugs, including chloroquine (CQ), mefloquine (MQ), sulfadoxine, and pyrimethamine (SP), has also been reported in many regions of the world (Baird et al., 1997; Fryauff et al., 1998; Hastings et al., 2005; Hastings and Sibley, 2002; Price et al., 2014). High-grade CQ-resistance (CQR) in P. vivax has emerged in Indonesia, Malaysia, and Papua New Guinea, and has been associated with severe and fatal malaria due to treatment failure (Asih et al., 2011; Grigg et al., 2016). However, the extent and public health consequences of drug resistance in P. vivax remains poorly defined in most regions of the world.
The standard treatment regimen for P. vivax in most regions of the world is a combination of CQ, which targets the blood stage, with either primaquine (PQ) or tafenoquine (TQ), which are used to clear dormant liver stage forms, known as hypnozoites, for radical cure. Hypnozoites can remain dormant for weeks to years before reactivating and initiating a new blood stage infection, known as a relapse (Fig. 1) (Hill et al., 2006; White, 2011). In several regions, the first-line treatment is now artemisinin combination therapies (ACTs) + PQ/TQ due to CQR or a high frequency of mixed infections with CQR P. falciparum (Douglas et al., 2010; Price et al., 2014; WHO, 2019a). The requirement to treat patients with PQ or TQ for radical cure is complicated by their propensity for causing hemolysis in patients with G6PD deficiency, preventing their use in a significant subset of patients (5% of global population according some estimates) (Luzzatto et al., 2020; Nkhoma et al., 2009). Another host genetic factor that can affect drug efficacy is polymorphisms in CYP2D6, that result in the reduced metabolism of PQ into its active metabolites for the clearance of hypnozoites (Baird et al., 2018; Bennett et al., 2013; Camarda et al., 2019).
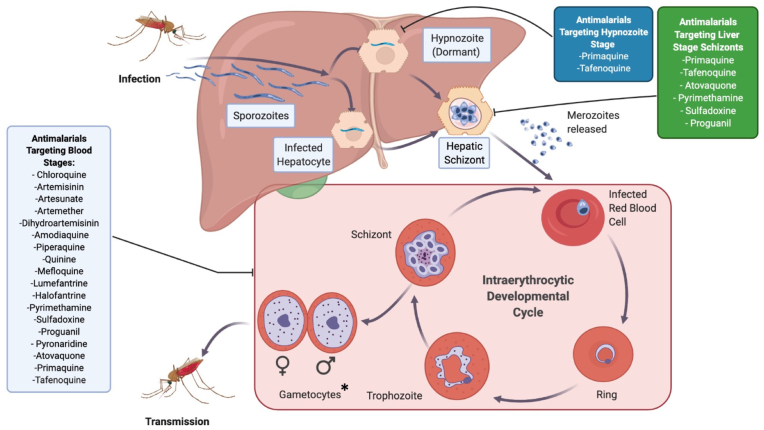
Fig. 1.
Phases of the P. vivax life cycle targeted by various antimalarial compounds. Infected mosquitoes take up a human blood meal and inject sporozoites into the bloodstream. Sporozoites then migrate to the liver, where they either become hepatic schizonts which release merozoites into the bloodstream, or become dormant hypnozoites. Merozoites in the blood infect RBCs, where they develop from rings, to trophozoites and finally to mature schizonts. The majority of antimalarials target this intraerythrocytic stage. *Antimalarial compounds are generally thought to be more active against P. vivax gametocytes compared to P. falciparum, however, this may in part be an indirect effect through targeting asexual stage parasites and requires further study. A subset of intraerythrocytic parasites will develop into sexual stage gametocytes which are then taken up by the mosquito during a blood meal where they eventually develop into sporozoites to begin the cycle anew. Most antimalarials target the blood stage, while primaquine (PQ) and tafenoquine (TQ) also target the liver stage, including hypnozoites. PQ and TQ are required to achieve “radical cure” to completely eliminate the parasite. Adapted from “P. vivax Replication Cycle”, by BioRender.com (2020).
In comparison to P. falciparum, CQR has spread relatively slowly in P. vivax. It has been hypothesized that there is reduced selection pressure in P. vivax compared to P. falciparum due to its lower parasite biomass, its ability to produce gametocytes early in infection, and to relapse from liver hypnozoites, which allows the parasite to transmit before drug treatment or after drug concentration has waned (Schneider and Escalante, 2013). The blood stage activity of PQ/TQ could also reduce the transmission of CQR parasites.
Despite more than two decades of research into drug-resistant P. vivax, and as molecular markers of drug resistance in P. falciparum have been identified, molecular markers of resistance in this species remain elusive (Price et al., 2014; WHO, 2019a). This is in large part due to the lack of in vitro culture and transgenic systems for P. vivax, which has been essential for studying P. falciparum drug resistance. The literature often refers to the presence of polymorphisms in putative resistance genes as evidence of drug resistance in that region, however, the current evidence for the role of many of these mutations in drug resistance is relatively weak, potentially leading to false conclusions that could impact drug policy. Validated drug resistance mutations could be used to rapidly and cost effectively survey drug resistance in an area over time. This information is essential for identifying the most effective treatment policy in a region and determining when it is appropriate to switch between first line antimalarials. Furthermore, an understanding of the molecular mechanisms of drug resistance, as well as the development of heterologous model systems to study them will be useful for the development of future antimalarials.
Despite substantial technical challenges towards interrogating the molecular mechanisms of drug resistance in P. vivax, significant progress has been made in recent years. In this review, we will summarize the current knowledge pertaining to the molecular basis of P. vivax drug resistance. We will consider the usefulness of polymorphisms in putative resistance genes as predictors of in vivo and in vitro drug resistance, and describe the identification of novel putative resistance markers. We will also highlight recent technological breakthroughs in studying P. vivax, and discuss the potential of these new techniques to identify and validate drug resistance markers.
2. Assessing in vivo and in vitro drug resistance in P. vivax: a lack of robust phenotypes
A large number of polymorphisms have been identified in putative drug resistance genes, including multidrug resistance gene 1 (pvmdr1), chloroquine resistance transporter (pvcrt), kelch 12 (pvk12), dihydrofolate reductase-thymidylate synthase (pvdhfr-ts), and dihydropteroate synthase (pvdhps). However, it is likely that many of these mutations do not affect drug sensitivity. Linking P. vivax mutations to a drug sensitivity phenotype, in vivo and ex vivo, is essential for narrowing the number of polymorphisms to validate as resistance markers. However, identification of resistance mutations in P. vivax remains difficult for some drugs, including CQ, in large part because defining drug-resistant P. vivax phenotypes is itself is difficult. We will discuss the strengths and limitations of currently available methods to measure drug sensitivity in P. vivax to the different antimalarials used clinically, and the current evidence of drug resistance globally.
2.1. Measuring in vivo drug resistance
Identifying resistance to CQ and ACTs has remained difficult in P. vivax for multiple reasons. The most clear definition of CQR in P. vivax is the ability to grow in CQ concentrations that would normally kill (CQ concentration >100 ng/ml), although it should be noted that this cutoff for resistance is somewhat arbitrary and may not identify low-grade resistance (Baird et al., 1997; Price et al., 2014). A high rate of treatment failure by day 28 is often used as a measure of resistance in the populations, however, the majority of recurrences that occur after day 21, and as early as day 14, are associated with low blood concentrations of CQ (<100 ng/ml) at the time of treatment failure (Añez et al., 2012, 2015; Baird et al., 1995; Congpuon et al., 2011; Getachew et al., 2015; Guthmann et al., 2008; Hwang et al., 2013; Phong et al., 2019; Phyo et al., 2011; Yohannes et al., 2011). Use of different definitions of clinical resistance can lead to vastly different conclusions about the degree, or even the presence, of CQR in an area (Ferreira et al., 2021). For these reasons, clinical studies that include measurement of blood CQ at the day of treatment failure, or with a high frequency of early treatment failures (≤14 days), provide a clearer picture of resistance, which has been demonstrated in Myanmar, Thailand, Ethiopia, Bolivia, and Brazil, ranging from ~0.5% to 10% treatment failure (Añez et al., 2012; Congpuon et al., 2011; Guthmann et al., 2008; Hwang et al., 2013; Ladeia-Andrade et al., 2019; Yohannes et al., 2011). In contrast, regions of Indonesia and Papua New Guinea have significantly higher rates of CQR in P. vivax populations, where treatment failure occurs in 20–97% of patients, with blood CQ levels >100 ng/ml, often including a high frequency of early treatment failures (Asih et al., 2011; Baird et al., 1995; Fryauff et al., 1998, 2002; Ratcliff et al., 2007; Sumawinata et al., 2003; Sutanto et al., 2010). Treatment failures in such regions has made it necessary to switch to ACTs as the first line treatment.
Several other factors can complicate the association of clinical failure with drug resistance. P. vivax treatment with CQ is usually combined with PQ, or more recently TQ, in order to clear hypnozoites. While many clinical trials delay the addition of PQ/TQ until day 28, those that do not will mask CQR due to the blood-stage activity of these drugs (Baird et al., 1995; Fukuda et al., 2017; Ladeia-Andrade et al., 2019; Wilairatana et al., 1999). High levels of immunity can also suppress parasitemia following treatment, therefore reducing the rate of recrudescence, which can mistakenly be classified as having lower rates of treatment failure, and drug resistance, compared to regions with lower immunity (McIntosh and Olliaro, 2000; Stepniewska et al., 2010; WWARN, 2015, WWARN et al., 2015). Genotyping P. vivax at the time of treatment and at recrudescence has been used to identify relapse, where parasite clones are observed at recrudescence that were not present in the initial population (Chiang et al., 2012; Imwong et al., 2007). However, the presence of a homologous clone before and after treatment can not differentiate between recrudescence and relapse, limiting its use to ruling out recrudescence but not relapse.
ACTs remain clinically active against CQR P. vivax, including artemisinin (ART) in combination with MQ, artemether in combination with lumefantrine (LM) (combination denoted as AL) or dihydroartemisinin (DHA) in combination with piperaquine (PPQ) (Abdallah et al., 2012; Abreha et al., 2017; Commons et al., 2019; Hwang et al., 2013; Karunajeewa et al., 2008; Ratcliff et al., 2007; Senn et al., 2013; Yohannes et al., 2011). A recent meta-analysis of AL compared to DHA-PPQ, demonstrated a higher recrudescence rate in patients treated with AL in the first 42 days (Commons et al., 2019). This is likely due to the longer half-life of PPQ compared to LM, which provides a longer lasting prophylactic effect against relapse from hypnozoites, which is supported by the more similar rates of recrudescence by day 63 between drugs, as well as significant effect of PQ in both treatments (Commons et al., 2019).
SP has not been used to treat P. vivax due to the concept that it was naturally resistant in earlier literature, which was most likely an artifact of hypnozoite relapse (Hawkins et al., 2007). SP has historically been used to treat P. falciparum and is still used for intermittent preventive treatment in pregnancy (IPTp), which is indiscriminate of species (WHO, 2019b). Interestingly, P. vivax SP resistance is relatively common even though it was never intentionally treated with SP, demonstrating strong pressure from inadvertent treatment of P. falciparum (Alam et al., 2007; Kaur et al., 2006).
2.2. Measuring ex vivo drug susceptibility
Given the limitations, cost and difficulty involved with in vivo studies for drug resistance, studies in P. falciparum heavily rely on in vitro drug sensitivity assays. In vitro assays are not affected by some of the confounding factors associated with in vivo studies, including differing levels of immunity, drug absorption and metabolism, allowing for more comparable data between isolates, regions and patient age. However, the lack of a continuous in vitro culture system for P. vivax has significantly hampered the ability to accurately measure drug susceptibility. Instead, measurement of P. vivax drug sensitivity has relied on short-term ex vivo assays that measure schizont maturation over a single replication cycle (ring to schizont). These assays have been useful in identifying drug resistance in P. vivax, however, require patient isolates with appropriate parasitemias, skilled microscopists, and are labor intensive, together limiting the number of studies that have used ex vivo drug sensitivity assays to measure resistance (Chaorattanakawee et al., 2017; Li et al., 2020; Suwanarusk et al., 2007, 2008). Using ex vivo assays has identified P. vivax trophozoites as being less sensitive to most clinically used drugs (Russell et al., 2008; Sharrock et al., 2008; Suwanarusk et al., 2007), complicating the determination of precise IC50 values between parasite isolates that vary in stage composition between individuals. Furthermore, the length of the assay, independent of the initial parasite age, also impacts the IC50 calculated for both P. vivax and P. falciparum in single cycle assays and exaggerates stage dependent differences if it is not carefully controlled for (Russell et al., 2008; Sharrock et al., 2008).
Significant improvements have been made in recent years to improve the quality and ease of ex vivo P. vivax drug assays. P. vivax patient samples typically contain low parasitemia (<0.1%). Several methods have been used to enrich P. vivax infected red blood cells (RBCs), including Percoll or Nycodenz density gradients, however, these methods typically purify mature schizonts, which are less suitable for drug sensitivity assays (Mons et al., 1988; Ribaut et al., 2008; Russell et al., 2011).
Novel methods using a potassium chloride Percoll gradient can enrich reticulocytes and therefore P. vivax parasites that are restricted to these cells (Rangel et al., 2018). Culture conditions have also been improved, with IMDM culture media supporting schizont maturation more than previously used media (Rangel et al., 2018). Optimized growth conditions and enrichment methods increase the ease and accuracy of ex vivo assays and makes the use of higher throughput assays achievable, including flow-cytometry and radiolabeled hypoxanthine uptake growth assays (Rangel et al., 2018; Wirjanata et al., 2015). However, these technologies require equipment that is not always present in resource-poor settings. Therefore, the use of cryopreserved isolates is advantageous as they can be transported to better equipped laboratories and used in large batches, which also improves consistency between samples as well as making it possible to perform multiple repeated biological assays with the same isolate. Cryopreservation also has the advantage of being selective for younger parasites, favoring synchronicity at ring stage parasites and thereby reducing the stage dependent differences between isolates (Rangel et al., 2018). However, freeze-thawing of isolates results in a loss of some parasites that may inadvertently select for a subset of the population with altered drug susceptibility. Ex vivo assays have been used to determine the activity of novel antimalarial compounds against P. vivax, which in some cases has highlighted significant differences between P. falciparum and P. vivax that could affect their clinical use for P. vivax (Kuhen et al., 2014; Leimanis et al., 2010; Lek-Uthai et al., 2008; Llanos-Cuentas et al., 2018; Marfurt et al., 2011; McNamara et al., 2013; Phillips et al., 2015; 2016; Price et al., 2010; Pukrittayakamee et al., 1994; Rottmann et al., 2010). For example, DSM265 was found to be less active against recombinant PvDHODH and subsequently demonstrated reduced in vivo effectiveness against P. vivax compared to P. falciparum, however, the newer DHODH inhibitor, DSM421, was found to have equal activity in ex vivo isolates between P. falciparum and P. vivax (Llanos-Cuentas et al., 2018; Phillips et al., 2015, 2016; Phillips et al., 2015). The use of high-throughput screening methods and cryopreserved isolates could ease the barrier to ex vivo drug screening and encourage testing lead compounds against P. vivax at an earlier stage of drug development. An increased number of studies that accurately measure in vivo and/or standardized ex vivo drug sensitivity assays, combined with genome sequencing will set the foundation for genotype-phenotype association studies to identify putative resistance mutations.
3. Candidate P. vivax drug resistance genes
Candidate P. vivax drug resistance genes have been identified based primarily on orthology from known P. falciparum drug resistance genes. Here we discuss what is known about each of the candidate P. vivax drug resistance genes, and current evidence for their role in mediating drug resistance (summarized in Supplementary Table 1).
3.1. Plasmodium vivax multidrug resistance gene 1 (PvMDR1)
pvmdr1 is the ortholog of the P. falciparum pfmdr1 gene, which mediates drug sensitivity to MQ, LM, and CQ in this species (Price et al., 1999). In both parasites, mdr1 encodes a transmembrane protein localized to the digestive vacuole (DV), where the parasite digests host cell proteins, and converts heme from hemoglobin into non-toxic hemozoin (Reiling and Rohrbach, 2015; Rohrbach et al., 2006; Sanchez et al., 2008). pvmdr1 was identified in 2005, and due to its strong sequence conservation with pfmdr1, has subsequently become one of the primary candidate genes assessed in P. vivax drug sensitivity studies (Barnadas et al., 2008; Brega et al., 2005; Orjuela-Sánchez et al., 2009). PvMDR1 localizes to the digestive vacuole when overexpressed in P. knowlesi, which is more closely related to P. vivax than P. falciparum is, suggesting that it plays a similar role to PfMDR1 in P. vivax (Verzier et al., 2019).
PfMDR1 is thought to transport drugs, including CQ and MQ into the DV (Reiling and Rohrbach, 2015; Rohrbach et al., 2006; Veiga et al., 2016). Several molecular epidemiological and functional studies have associated amino acid changes at positions 86, 184, 1034, 1042, and 1246 in PfMDR1 with drug resistance (Veiga et al., 2016; Wurtz et al., 2014). CQ primarily acts to inhibit hemozoin formation in the DV, which results in a toxic build up of heme, thus reduced CQ in the DV leads to CQR (Olafson et al., 2015; Veiga et al., 2016). Indeed, mutations in pfmdr1 are associated with CQR and with increased sensitivity to MQ and LM (Reed et al., 2000; Sidhu et al., 2005). MQ and LM are thought to primarily act on cytoplasmic proteins, although their mechanisms of action are less clear than CQ, and therefore reduced transport by PfMDR1 (and likely by PvMDR1 as well) into the DV, which sequesters it from its target, would lead to increased sensitivity to these compounds (Reiling and Rohrbach, 2015; Rohrbach et al., 2006; Sanchez et al., 2008).
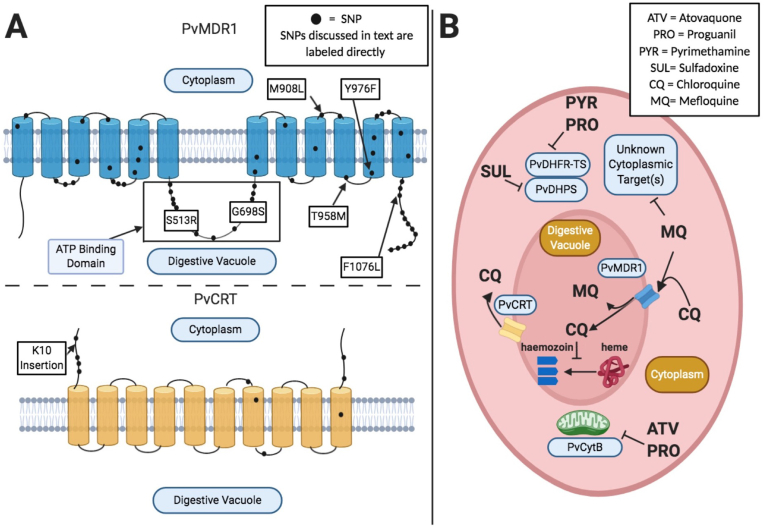
Sequencing of pvmdr1 across several regions of the world has revealed more than fifty polymorphisms in this gene, as well as copy number variants (CNVs). These single nucleotide polymorphisms (SNPs) correspond to amino acid changes throughout the protein sequence, including in the ATP binding domains and multiple transmembrane regions (Sá et al., 2005) (Fig. 2A). No single SNP, or set of SNPs, have emerged as definitive drug resistance markers. However, six SNPs have been reported at high frequency in multiple studies in regions with reported drug resistance; (relative to the SAL-1 reference) S513R, G698S, M908L, T958M, Y976F and F1076L (Brega et al., 2005; Huang et al., 2014; Joy et al., 2018; Orjuela-Sánchez et al., 2009; Vargas-Rodríguez et al., 2012). Amino acid changes at Y976F and F1076L in particular have been cited as possible markers of drug resistance (Brega et al., 2005; Spotin et al., 2020; Suwanarusk et al., 2008). However, these SNPs are also found in regions without reported CQR, making their association with drug resistance uncertain (Spotin et al., 2020). The T958M, Y976F, F1076L variants (and possibly others) in PvMDR1, have been shown to arise independently, even within the same population (Schousboe et al., 2015). This finding suggests that pvmdr1 mutations can arise on different genetic backgrounds and that they have not been subject to a selective sweep, possibly due to a lack of direct pressure from drug (Schousboe et al., 2015). If, and how, these SNPs play a role in mediating P. vivax drug resistance remains to be directly demonstrated in functional studies.
Fig. 2.
A) Depicts the putative structures of PvMDR1 and PvCRT including their predicted transmembrane domains and where observed polymorphisms are positioned within the proteins. Specific SNPs discussed in text are labeled, while other observed mutations are shown as a black dot. Transmembrane domain locations are adapted from Sá et al., 2005 for PvMDR1, and Nomura et al., 2001 for PvCRT. SNP locations are approximate and inferred based on location in protein sequence. B) Depicts putative mechanisms of antimalarial compound action and suspected molecular resistance mechanisms. PvMDR1 and PvCRT are digestive vacuole (DV) transmembrane proteins. Based on orthology to P. falciparum, it is thought that PvMDR1 mutants confer CQ resistance by reducing transport of CQ into the digestive vacuole. PvCRT is thought to act as an efflux pump; actively transporting CQ out of the DV and away from its target (the process of converting heme into hemozoin). PvMDR1 is thought to confer resistance to MQ and possibly other compounds, by transporting them into the DV, thereby sequestering them away from their putative cytoplasmic targets.
Studies exploring the relationship between these SNPs and drug sensitivity paint a mixed picture. A survey of isolates from China that associated sequencing of pvmdr1 with ex vivo measurements of drug susceptibility found an association between M908L and reduced susceptibility to CQ, MQ, pyronaridine, PPQ, quinine, ART, and DHA (Li et al., 2020). A study in Cambodia found a correlation between Y976F and F1076L mutations and resistance to MQ and PPQ, but not CQ (Chaorattanakawee et al., 2017). Similarly Suwanarusk et al. found an association between Y976F and reduced susceptibility to CQ (Suwanarusk et al., 2008). Yet multiple other studies have found no relationship between polymorphisms in pvmdr1 and drug resistance (Faway et al., 2016; Li et al., 2020; Nyunt et al., 2017; Orjuela-Sánchez et al., 2009). This in part could stem from methodological differences in ex vivo phenotyping, and the fact that several studies are conducted in regions with low rates of CQR. Differences between studies may simply reflect real geographical differences. It will be critical to evaluate these polymorphisms in an in vitro culture system to assess their impact on drug resistance. Two pvmdr1 haplotypes were episomally overexpressed in a P. knowlesi model system, both of which encoded the 698S, 908L and 958T mutations, but did not alter sensitivity to either CQ or MQ (Verzier et al., 2019), suggesting that at least in this genetic background and expression system, these mutations did not confer resistance (discussed further in section 4.1). Another limitation is that many studies sequence only part of the gene, thus limiting and biasing our understanding of mutations to these regions of the gene.
Copy number variation (CNV) of pvmdr1 is distributed globally at variable frequencies (7–31.6%), including Thailand, Cambodia, Laos, India, Brazil, French Guinea, and other countries (Costa et al., 2017; Faway et al., 2016; Imwong et al., 2008; Joy et al., 2018; Khim et al., 2014; Suwanarusk et al., 2008; Vargas-Rodríguez et al., 2012). CNVs in pvmdr1 have more clearly been implicated as a potential cause of P. vivax drug resistance (Costa et al., 2017; Imwong et al., 2008; Suwanarusk et al., 2008). Sequencing of isolates in Thailand and Indonesia found a correlation between increases in IC50 of amodiaquine (AQ), ART, and MQ, measured by ex vivo drug susceptibility assays, and pvmdr1 amplification (Auburn et al., 2016; Suwanarusk et al., 2008). Notably this mirrors the similar effect of pfmdr1 amplification on MQ resistance in P. falciparum (Preechapornkul et al., 2009).
3.2. Plasmodium vivax chloroquine resistance transporter (PvCRT)
The pvcrt gene (also referred to as pvcrt-o) emerged as a candidate drug resistance gene due to its orthology with the pfcrt gene that mediates CQR in P. falciparum (Fig. 2A). The pfcrt gene was first identified as a determinant of CQR in a genetic cross between a CQ-sensitive parasites line and CQR parasites (Su et al., 1997). Numerous pfcrt polymorphisms have been implicated in causing CQ drug resistance (Ecker et al., 2012; Fidock et al., 2000,; Johnson et al., 2004; Pulcini et al., 2015; Summers et al., 2014). Additionally, polymorphisms in pfcrt are associated with PPQ resistance, and conversely, increased sensitivity to MQ and ART (Johnson et al., 2004; Phyo et al., 2011; Pulcini et al., 2015; van der Pluijm et al., 2019).
Unlike pfcrt, and in contrast to pvmdr1, very few SNPs (~10) in pvcrt have been reported, however, most occur at very low frequency (Barnadas et al., 2008; Joy et al., 2018; Noisang et al., 2020). The most common PvCRT polymorphism is a lysine insertion at position 10 (K10) (Barnadas et al., 2008; Joy et al., 2018; Noisang et al., 2020; Silva et al., 2018a). The K10 insertion has been observed in both Southeast Asian and South American parasites (Noisang et al., 2020; Silva et al., 2018b.; Zhao et al., 2020). However, no association between the K10 insertion and in vitro P. vivax drug resistance has been found (Silva et al., 2018a; Suwanarusk et al., 2007). It is currently unknown if pvcrt variants that may be associated with CQR have a deleterious impact on parasite fitness, which is the case for P. falciparum and could explain the low frequency of pvcrt mutations (Ord et al., 2007; Petersen et al., 2015).
There is evidence that increased expression of pvcrt potentially mediates CQR (Melo et al., 2014; Sá et al., 2019; Silva et al., 2018b). Sa and colleagues recently performed a P. vivax genetic cross between the CQR P. vivax NIH-1993-R line and the CQS NIH-1993-S line. Bulk segregant analysis of blood stage progeny implicated a 76 KB region on chromosome one, that includes pvcrt, as having a role in CQR. The study found no SNPs in pvcrt, but did identify a TGAAGH motif with an increased number of repeats both upstream of the 5’ UTR of pvcrt, as well as a deletion within intron nine of the gene, and that CQR progeny had increased expression of pvcrt.
Evidence that overexpression of pvcrt, resulting from either increased gene copy numbers, or enhanced transcription levels, is linked to drug resistance has been reported in several studies of patient isolates from Brazil (Costa et al., 2017; Melo et al., 2014; Silva et al., 2018a). However, a study of pvcrt expression in parasites from Indonesia, where there is high-grade drug resistance, found no relationship between pvcrt expression levels and ex vivo susceptibility to CQ, PPQ, MQ and Artesunate (AS) (Pava et al., 2015). As with pvmdr1, whether or not these discrepancies are due to differences in the genetic background, such as pvmdr1 polymorphisms and CNVs, or whether they are due to technical differences will require further investigation.
3.3. P. vivax dihydrofolate reductase-thymidylate synthase (DHFR-TS) and dihydropteroate synthase (DHPS)
The essential enzymes dihydrofolate reductase-thymidylate synthase (PvDHFR-TS) and dihydropteroate synthase (PvDHPS) are involved in folate synthesis, and are the targets of pyrimethamine and sulfadoxine, respectively (Asih et al., 2015; Auliff et al., 2006; 2010; Barnadas et al., 2008; Hastings et al., 2005; Hastings and Sibley, 2002). SP has long been used to treat P. falciparum, but is now primarily only used for IPTp due to widespread resistance (WHO, 2007). PfDHPS and PfDHFR-TS are well understood in P. falciparum and highly conserved in P. vivax (Hawkins et al., 2007). While SP has not been intentionally used for P. vivax treatment, mutations in PvDHPS and PvDHFR-TS, that are conserved with mutations known to confer resistance in P. falciparum, are widespread in P. vivax populations around the world, resulting in treatment failure in more than 50% of patients (Maguire et al., 2006; Pukrittayakamee et al., 1994; Tjitra et al., 2002). It has been suggested that genomic surveillance of pvdhfr-ts and pvdhps could provide an understanding of which regions these drugs could be used to cheaply and safely treat P. vivax infection, although to date SP has not be recommended for P. vivax (Hawkins et al., 2007). pvdhfr-ts and pvdhps also represent a key example of how molecular studies could be performed to characterize other resistance alleles and monitor the spread of resistance.
Amino acid changes in PvDHFR-TS at positions N50I, S58R, S117N and I173L exist as double, triple, and quadruple mutants at frequencies ranging from as 20–90% in Malaysia, Thailand, Papua New Guinea, and Indonesia (Auburn et al., 2019; Imwong et al., 2001; Marfurt et al., 2008). These mutations align with mutations N51I, C59R, S108N and I164L in PfDHFR-TS, which are associated with pyrimethamine resistance (Briolant et al., 2012; Hastings and Sibley, 2002). An additional F57L mutation in PvDHFR-TS, which has no P. falciparum equivalent, has also been reported at high frequency in combination as a double mutant with 58R or 117N, and a quadruple mutant with 58R/61M/117N (Asih et al., 2015; Auliff et al., 2006; Hastings et al., 2005; Hastings and Sibley, 2002).
Expression of mutant PvDHFR-TS in a yeast system demonstrated that these mutations confer high levels of pyrimethamine resistance. Single mutations at positions 57L and 117N, and double or triple mutants, 58R/117N and 117N/173L, 58R/117N/173L resulted in a 50-, 87-, 460-, 700- and 500-fold increase in resistance to pyrimethamine, respectively (Hastings and Sibley, 2002). Using an episomally expressed copy of PvDHFR-TS in P. falciparum, Auliff et al. found that a single mutation, 117N, resulted in 46-, 6-, 2- and 6-fold increases in resistance, relative to wild-type, to pyrimethamine, clociguanil, WR99210, and cycloguanil, respectively. Additionally, double PvDHFR-TS mutant haplotypes of 57L/117T and 58R/117T, resulted in 67- and 114-fold increases in pyrimethamine resistance, 6- and 4-fold increase in cycloguanil resistance, 10- and 26-fold increase in clociguanil resistance, and 22- and 10-fold increases in WR99210 resistance for each haplotype, respectively. They also found that a PvDHFR-TS quadruple mutant (57L/58R/61M/117T) resulted in high resistance to pyrimethamine, cycloguanil, chlorcycloguanil, and WR99210, with resistance increases of 8497-, 746-, 2565-, and 44-fold, respectively. Interestingly, expression of a triple PvDHFR-TS mutant (58R/61M/117T) resulted in susceptibilities similar to wild-type for all drugs with the exception of pyrimethamine, for which this haplotype conferred a 58-fold increase in resistance. The authors hypothesized that 61M may be a compensatory mutation to offset possible fitness costs with carrying other resistance mutations. Studies assessing PvDHFR-TS mutants and SP treatment outcomes have confirmed that these results correlate with in vivo efficacy, with treatment failure 23 times more likely to occur when infected with P. vivax parasites containing the 57L/58R/61M/117T haplotype (Auliff et al., 2006; Hastings et al., 2004). A structure analysis of PvDHFR-TS showed that a mutation at position 117 leads to steric conflict with pyrimethamine binding, resulting in resistance, similar to the equivalent mutation at position 108 in PfDHFR-TS (Kongsaeree et al., 2005). These data suggest that P. vivax resistance to antifolates arises from molecular changes and is highly conserved with P. falciparum.
Mutations at amino acids 383 and 553 in PvDHPS, correspond with known drug resistance mutations 437 and 581 In PfDHPS (Auliff et al., 2006; Imwong et al., 2005). Similar to SP resistance in P. falciparum, mutations in PvDHPS alone is thought to not be sufficient to provide resistance to SP (Imwong et al., 2005). Furthermore, the wild-type PvDHPS allele at position 585 is a Valine, which aligns with position 613 in PfDHPS, and is thought to reduce the binding to sulfadoxine (Korsinczky et al., 2004). This V585 allele is found commonly in isolates, suggesting possible innate resistance to sulfadoxine in this species (Auliff et al., 2006; Korsinczky et al., 2004). Structure analysis of PvDHPS mutants support the role of these mutations in providing resistance to sulfadoxine by reducing binding affinity to the mutant enzyme (Yogavel et al., 2018). Particularly, strains containing both PvDHPS 383G and 553G mutations and mutant PvDHFR-TS alleles, have been implicated in SP treatment failure (Imwong et al., 2005).
Studies looking at the distribution of pvdhfr-ts alleles in natural populations have found a high prevalence (80–90%) of double, triple, and quadruple mutations in Malaysia, Thailand, India, Indonesia, Madagascar, and China, which all have high SP treatment failure rates (Alam et al., 2007; Auburn et al., 2019; Barnadas et al., 2011; Hastings et al., 2005; Huang et al., 2014; Imwong et al., 2001). Global and regional population genetic studies of P. vivax have found pvdhfr-ts and pvdhps in regions of the genome with strong evidence of selection by linkage disequilibrium and integrated haplotype score metrics (Auburn et al., 2018; Hupalo et al., 2016). Strong signals of genetic differentiation were found between Ethiopian populations, which have a low prevalence of pvdhfr-ts mutants, compared to Indonesian or Thai populations, which have high prevalence of pvdhfr-ts mutants, providing evidence of region-specific drug pressure (Auburn et al., 2019). Furthermore, a study comparing P. vivax isolates in Indonesia, Malaysia, and Thailand, found signals of genetic differentiation of pvdhfr-ts and pvdhps between regions, which all have a high prevalence of pvdhfr-ts mutants, suggesting that mutant haplotypes can arise on different genetic backgrounds (Auburn et al., 2018).
Two studies in India found a higher prevalence of pvdhfr-ts mutations in regions where P. falciparum and P. vivax are co-endemic, than regions where P. vivax is the dominant parasite (Alam et al., 2007; Kaur et al., 2006). These results suggest that pressure of SP treatment of P. falciparum can lead to co-selection of pvdhfr-ts mutant alleles, which could also occur for other drugs, including ACT partner drugs. Hence, the authors suggest that future studies should consider not only the drugs used to treat P. vivax, but also those for used P. falciparum in co-endemic regions.
3.4. P. vivax kelch 12 (PvK12): ortholog of the P. falciparum kelch 13 (PfK13) determinant of reduced ART susceptibility
ACTs are the recommended frontline treatment for P. falciparum, due to widespread resistance to older antimalarials, as well as for P. vivax in areas of high CQR and in the case of mixed infections. ACTs combines ART, which rapidly kills parasites but has a short elimination half-life, with a longer-lasting partner drug, usually MQ, LM, SP, PPQ or AQ (Commons et al., 2019). P. falciparum ART resistance, which first arose in the Mekong Subregion (GMS) in 2008, is mediated by the pfkelch13 gene (Ariey et al., 2014; Ménard et al., 2016; Miotto et al., 2015; Takala-Harrison et al., 2015; Tun et al., 2015). P. vivax treatment with ACTs is recommended by the WHO mainly in Africa (with the exception of Ethiopia), The Western Pacific, and Eastern Mediterranean regions (WHO, 2019a).
To date, no cases of P. vivax resistance to ACTs have been reported. However, several studies have monitored the P. vivax ortholog of pfkelch13, pvkelch12, for polymorphisms that may lead to ART resistance (Brazeau et al., 2016; Deng et al., 2016; Gresty et al., 2019). While several polymorphisms have been found in pvkelch12, they do not align to the respective mutations in pfkelch13 that mediate artemisinin resistance (Brazeau et al., 2016; Deng et al., 2016; Gresty et al., 2019). These results suggest a lack or reduced selection pressure from ART on pvkelch12 to date. Notably there is a lack of persistence of pvkech12 SNPs over time in the GMS, and no polymorphism in pvkelch12 in Papua New Guinea, where one might expect selection pressure from ART indirectly from P. falciparum treatment, or directly as the first-line therapy in this region (Brazeau et al., 2016; Gresty et al., 2019). These results suggest a lack of strong selection pressure from ART on pvkelch12 at this time (Brazeau et al., 2016; Gresty et al., 2019). Recently, an alternative gene, pfcoronin, has been identified to confer resistance to ART in P. falciparum but the ortholog has not been studied in P. vivax (Demas et al., 2018). Should ART resistance in P. vivax arise, it could be mediated by one of these genes or through other P. vivax-specific mechanisms. Regardless, continued monitoring of both clinical ART resistance and possible mutations in pvkelch12 and pvcoronin orthologs is warranted.
3.5. Plasmodium vivax multidrug resistance protein 1 (PvMRP1)
P. vivax multidrug resistance protein 1 (pvmrp1) has emerged as a candidate drug resistance gene because of signals of selection in population genetic studies (Dharia et al., 2010; Flannery et al., 2015). pvmrp1 is an ortholog of the pfmrp1 gene, which transports glutathione adducts out of the parasite, a process that is thought to help the parasite regulate oxidative stress (Müller, 2015; Raj et al., 2009). pfmrp1 knockouts in P. falciparum have increased susceptibility to quinine, CQ, ART, and PQ, as well a reduced parasite growth rate in vitro (Raj et al., 2009). SNPs in pfmrp1 have also been associated with AL resistance, where the amino acid change, I876V, was found in recurrent infections after treatment with AL (Dahlström et al., 2009).
Genetic sequencing of P. vivax samples in South America has found strong signals of molecular evolution in this gene, with a high ratio of nonsynonymous substitutions per synonymous site relative to the number of synonymous substitutions per synonymous sites (dN/dS) (Dharia et al., 2010; Flannery et al., 2015). Sequencing of a Peruvian isolate also demonstrated evidence of pvmrp1 gene amplification (Flannery et al., 2015). Studies in Sudan, Thailand, Indonesia, and Cambodia found pvmrp1 is situated in a long region of homozygosity, which indicates recent selection (Auburn et al., 2018, 2019; Bright et al., 2013,; Parobek et al., 2016).
Sequencing of a P. vivax isolate from a patient, with no mutations in CYP genes, that experienced PQ failure found several SNPs in pvmrp1 (Bright et al., 2013). PQ and TQ are the only approved drugs that can eliminate hypnozoites, which is required to achieve radical cure of P. vivax (Chiang et al., 2012; Hill et al., 2006; Llanos-Cuentas et al., 2019; Taylor et al., 2019). PQ is oxidized by CYPD26 into hydroxyl-metabolites whose oxidation, in turn, generates quinoneimine and subsequently generates hydrogen peroxide (H2O2) (Camarda et al., 2019). Qinoneimine is also a substrate for CPR, CYPD26's redox partner, which leads to accumulation of H2O2 and subsequent antimalarial activity through oxidative stress in the parasite. PQ tolerance, leading to relapse of infection is rare but has been reported in cases following the correct treatment regimen (Chiang et al., 2012; Townell et al., 2012). Knockouts of pfmrp1 have increased sensitivity to PQ in P. falciparum, further supporting that this protein plays a role in mediating tolerance to PQ, although the active metabolites were not tested (Raj et al., 2009). The experimental evidence of pfmrp1 mediating PQ tolerance and evidence of selection pressure on pvmrp1 have led to this gene as a candidate for mediating PQ tolerance in P. vivax (Bright et al., 2013, 2014; Dharia et al., 2010; Flannery et al., 2015).
Dharia and colleagues proposed a model of pvmrp1 mediating PQ tolerance, where pvmrp1 mutations improve the transport of glutathione adducts resulting from PQ treatment and thus allow for PQ tolerance by mitigating oxidative damage (Dharia et al., 2010). In light of this evidence, pvmrp1 is a compelling research target as a putative P. vivax drug resistance gene, and elucidating if and how it contributes to P. vivax drug resistance warrants further investigation.
3.6. Other putative resistance genes
Population genetic studies looking for evidence of positive selection via increased polymorphism or selective sweeps of drug resistance-associated loci have identified several putative P. vivax drug resistance genes (Auburn et al., 2018, 2019; Brazeau et al., 2016; Dharia et al., 2010; Flannery et al., 2015; Parobek et al., 2016). These include the candidate genes described above (pvmdr1, pvcrt, pvdhfr-ts, pvdhps, and pvmrp1), as well as several additional genes of interest.
Several studies of parasites in the GMS found evidence of selective sweeps via extended haplotype homozygosity (XP-EHH) and iHS tests around the pvmdr2 and pvmrp2 genes in Cambodia (Auburn et al., 2018; Brashear et al., 2020; Parobek et al., 2016). Another study in the Peruvian Amazon found a higher number of SNPs in pvmrp2, though this study did not evaluate any measures of selection on this gene (Cowell et al., 2018). The P. falciparum ortholog of pvmrp2 has been implicated in conferring resistance to CQ, MQ and PPQ (Mok et al., 2014; Nogueira et al., 2008; Veiga et al., 2014). Similarly, pfmdr2, the P. falciparum ortholog of pvmdr2, has been implicated in pyrimethamine resistance (Briolant et al., 2012).
A study of P. vivax parasites in the Peruvian Amazon uncovered possible evidence of increased copy number of the PVX_000585 gene. PVX_000585 is predicted to encode a homologue of the E. coli emrE gene, a multidrug transporter, but has not been evaluated for a role in drug resistance in any Plasmodium spp. (Flannery et al., 2015). Additionally, a study of P. vivax found evidence of selection and significant genetic differentiation of the I165V variant in the P. vivax plasmepsin IV gene, an ortholog of the P. falciparum plasmepsin II, between Malaysian parasite populations, where there is a higher reported prevalence of CQR, and Thailand, where there is reportedly a lower prevalence of CQR (Auburn et al., 2018). P. falciparum plasmepsin II has been implicated in mediating PPQ resistance in this species (Bopp et al., 2018). The study also found evidence of significant genetic differentiation of variants in the putative drug/metabolite transporters pvdmt1, and a CG2-related protein (PVP01_1450700) between Thailand and Malaysia (Auburn et al., 2018). While these genes have not previously been the subject of intense research focus, the evidence of recent selection or increased copy number variation at loci encompassing them suggests that further research effort is merited.
4. Experimental approaches for assessing drug resistance and drug resistance determinants
4.1. Culturable heterologous model systems and reverse genetics
Significant improvements have been made to P. vivax ex vivo drug susceptibility assays, however, they remain limited to short single cycle assays with relatively poor growth compared to P. falciparum as well as requiring patient isolates that can not be well controlled for parasite stage and parasitemia, limiting their accuracy. Improved growth conditions and the use of multiple-cycle assays would reduce the differences observed from starting parasite age and parasitemia, as well as making it possible to study slower-acting drugs, including TQ (Russell et al., 2003). Improved ex vivo assays will significantly improve the ability to determine drug susceptibility in natural isolates and link drug phenotypes to specific resistance loci. However, validation of putative resistance alleles will remain unobtainable in P. vivax until robust ex vivo short-term culture, or more ideal methods for a continuous culture system are developed. In lieu of a stable culture system, heterologous systems will be invaluable for validation studies in P. vivax.
P. falciparum has been used to episomally express P. vivax dhfr alleles, which demonstrated the role of polymorphisms observed in the field in mediating pyrimethamine resistance (Auliff et al., 2010). The role and protein sequence of DHFR-TS, and its resistance mutations, are relatively well conserved between Plasmodium spp., as well as to higher eukaryotes (Hastings and Sibley, 2002). In contrast, the sequence of other putative resistance genes, as well as mutations found within them, are more divergent between Plasmodium spp., which may impact their accuracy as model systems. For these reasons, the field has moved towards using Plasmodium spp. that are more closely related to P. vivax. P. knowlesi primarily infects primates, but can also cause outbreaks in humans, and is more closely related to P. vivax than P. falciparum is. P. knowlesi has long been used for research, but only recently has been adapted to grow in human RBCs, which opens up its use to the wider community (Lim et al., 2013; Moon et al., 2013). P. knowlesi is also genetically tractable and a CRISPR/Cas9 system has been developed, which was successfully used to replace pkdbp with its P. vivax ortholog for vaccine studies (Mohring et al., 2019). P. knowlesi has also been used to overexpress two PvMDR1 variants, which correctly localized to the digestive vacuole (Verzier et al., 2019). Expression of these variants did not alter sensitivity to CQ or MQ (Verzier et al., 2019). Whether or not the lack of an effect is due to PvMDR1, or the variants tested, not being involved in drug resistance or due to technical reasons, such as poor expression levels or the presence of the wild-type PkMDR1, will require further study. Allelic replacement using CRISPR/Cas9 could replace the native pkmdr1 with pvmdr1 variants, which would ensure it is expressed from the native promoter in the absence of pkmdr1. The same methods could also be used to study other putative resistance genes.
More recently, P. cynomolgi, another primate malaria which is even more closely related to P. vivax, has been adapted to in vitro culture in rhesus macaque RBCs (Chua et al., 2019). The closer evolutionary relationship between P. cynomolgi and P. vivax may make it a better model for drug resistance mechanisms. Transgenic methods have been developed for P. cynomolgi in vivo using a primate model, which was used to produce fluorescent or luminescent reporter lines for high-throughput screening of compounds active against hypnozoites (Voorberg-van der Wel et al., 2013; Voorberg-van der Wel et al., 2020). Development of hypnozoites is a significant advantage of using P. cynomolgi over P. knowlesi, and could be used to investigate putative resistance markers of PQ or TQ. The development of transfection for in vitro cultured P. cynomolgi, as well as a CRISPR/Cas9 system, would significantly advance the P. cynomolgi model for understanding P. vivax biology. Advances in these model systems will make it possible to definitively define the role of polymorphisms in P. vivax genes on drug susceptibility.
4.2. Genetic crosses for mapping resistance determinants
Genetic crosses are powerful tools to help map genetic loci to phenotypic characteristics (Sá et al., 2019; Wellems et al., 1991). Genetic crosses of P. falciparum lines have been used successfully to map loci involved in CQR and cell invasion ligands (Hayton et al., 2008; Wellems et al., 1991). P. falciparum genetic crosses are labor-intensive, as gametocytes from two different strains need to be cultured, mixed together, and fed to mosquitos as part of a blood meal. Once inside the mosquito, parasites undergo recombination (the only diploid stage of the life cycle). Furthermore, parent lines with desired phenotypes to be investigated may not yet exist, thereby limiting the number of phenotypes for which crosses will be informative. The infected mosquitos are used to infect splenectomized chimpanzees, and blood-stage parasites are isolated and grown in culture for further analysis (Walliker et al., 1987).
The lack of an in vitro culture system for P. vivax has complicated development of a genetic cross, however as described previously, Sa and colleagues used a bulk segregant approach on a cross of a CQS line and a CQR line to identify a region on P. vivax chromosome one, which includes pvcrt, that is linked to a CQR phenotype (Sá et al., 2019). In place of the in vitro culture steps used for P. falciparum, in vivo infection of Aotus and Saimiri monkeys was required to propagate P. vivax. It should be noted that in the United States, the NIH has stopped supporting research using chimpanzees, though research with other non-human primates (NHP) is still allowed (NIH, 2015).
More recently, P. falciparum crosses have been performed in human hepatocyte-liver chimeric mice, significantly reducing the cost and labor of generating crosses (Minkah et al., 2018; Vaughan et al., 2015). Another advantage of the mouse model is that multiple mice can be used for a cross, and subsequently more progeny from a cross can be collected, allowing for more powerful and fine-scale analysis linking genetic variation to phenotypes (Vendrely et al., 2020). Development of a P. vivax humanized mouse model would also open up new avenues to identify P. vivax loci involved in cell invasion, drug resistance, and other biological processes using genetic crosses. Furthermore, pairing genetic crosses with single-cell sequencing can provide powerful approaches towards understanding within host dynamics of genetic variation (Nair et al., 2014). With regards to drug resistance, single-cell analysis of genetic crosses could shed light on different genetic backgrounds upon which resistance can arise, within-host competition of different drug resistance haplotypes, and help understand the full range of genetic diversity at drug resistance loci (Nair et al., 2014). Ultimately, innovations in propagating P. vivax in vitro would ease the process of performing P. vivax genetic crosses.
4.3. Beyond candidate SNPs/CNVs: advances in genomics and transcriptomics
Advances in genomics and transcriptomics over the past decade have opened a range of possibilities for conducting population genetics and molecular epidemiological studies of P. vivax. Whole Genome Sequencing (WGS) of P. vivax directly from patient samples can be difficult because of the typically low parasitemia, and the subsequent difficulty of separating the P. vivax DNA from the background human genome (Cowell et al., 2017; Melnikov et al., 2011). Leukocyte depletion, which removes the background human DNA, and hybrid selection to enrich parasite DNA, has been used to successfully sequence P. vivax genomes from clinical samples (Hupalo et al., 2016; Melnikov et al., 2011; Pearson et al., 2016). Similarly, selective whole genome amplification approaches, which use a highly processive polymerase to amplify the parasite genome from the background human genome, for WGS is a low-cost and easily scalable method for conducting WGS on P. vivax clinical samples (Cowell et al., 2017).
These technologies have opened up the possibility of conducting large scale population molecular epidemiological studies in P. vivax and hold promise to help conduct genetic association studies highlighting selected loci that are under different selective pressures, (i.e. from drugs) between regions. Furthermore, whole-genome data can help illuminate how drug resistance alleles spread in a population, and be used to conduct molecular surveillance of drug resistance alleles to inform control efforts (Brazeau et al., 2016; Hupalo et al., 2016; Pearson et al., 2016).
However, whole genome approaches can miss rare variants due to lower sequence coverage, which limits the ability to uncover rare variants that could cause drug resistance (Gruenberg et al., 2019; Rao et al., 2016). Additionally, low sequencing coverage can affect the ability to differentiate between parasite lineages in a patient, which could be used to identify mixed infections and determine if persistent malaria is the result of recrudescence, relapse, or reinfection (Lin et al., 2015). Targeted sequencing approaches such as amplicon sequencing, where genes are amplified in a PCR reaction in parallel and then sequenced, allows for deep sequencing of select loci to uncover low frequency variants and uncover the full range of genetic variation in a population (Boyce et al., 2018; Gruenberg et al., 2019; Lin et al., 2015). P. vivax amplicon sequencing has been used to differentiate between recrudescence, reinfection, or relapse as a cause of recurrent infection, which could help determine if a patient has drug resistant P. vivax (Lin et al., 2015). Amplicon sequencing in P. falciparum has been used to identify low-frequency SNPs in the drug resistance genes pfcrt, pfkelch13, pfmdr1, pfmrp1, and pfdhfr-ts (Rao et al., 2016; Talundzic et al., 2017). Development of P. vivax amplicon panels for putative resistance genes could help identify SNPs associated with drug resistance (Lin et al., 2015; Rao et al., 2016; Talundzic et al., 2017).
The low parasitemia and lack of culture system in P. vivax has also hindered the use of transcriptomics to study gene expression. Recent advances in the ability to conduct transcriptomics from clinical samples and single-cell transcriptomics from monkey adapted lines demonstrate the feasibility to conduct transcriptomic analysis on P. vivax (Kim et al., 2019; Rangel et al., 2020; Sà et al., 2020). P. vivax transcriptomes from Cambodian patients treated with or without CQ, showed no differential expression of the candidate CQR genes pvmdr1 and pvcrt between the two treatment groups (Kim et al., 2019). The lack of an association could be due to a lack of drug resistance alleles in this sample set, or a lack of a change in gene expression in response to drug pressure (Kim et al., 2019). Comparative transcriptomics between CQR and CQS patient isolates could identify putative resistance loci that have elevated expression in CQR parasites, since increased expression is known to mediate P. falciparum drug resistance and there is evidence that the same is true in P. vivax (Kim et al., 2019; Sà et al., 2020; Sá et al., 2019).
5. Looking forward
The complex biology of P. vivax, that includes dormant liver stage hypnozoites, the lack of in vitro culture, and the difficulty in performing ex vivo assays has made it hard to readily determine clinical resistance to CQ and other drugs in this species. Improved techniques for ex vivo drug assays will allow researchers to more readily screen larger numbers of P. vivax isolates and determine the frequency and level of drug resistance within different populations. If paired with WGS and transcriptomics, putative molecular markers of drug resistance could be determined with greater detail and accuracy (Fig. 3). Many of the resistance mutations in P. falciparum are not found in P. vivax, limiting the value of using orthology to infer resistance mutations in P. vivax. This could be due to biological differences at the molecular level, including a strong divergence of putative resistance alleles between species, where mutations occurring in similar regions of the gene, but different positions, could confer the same resistance, or the divergent genes may also have significantly different abilities to transport antimalarial compounds. Furthermore, biological differences in P. vivax likely significantly alter the selection pressure it faces compared to P. falciparum. For example, compared to P. falciparum, P. vivax has a reduced biomass, a different host cell preference (restriction to reticulocytes), produces transmissible gametocytes early in infection and can relapse from dormant hypnozoites. Using genomic tools that are not biased towards suspected resistance genes based on orthology to P. falciparum, may reveal novel P. vivax specific mechanisms of drug resistance that have remained largely unexplored. Putative resistance markers will need to be validated in a heterologous system, such as using transgenic systems in P. knowlesi or P. cynomolgi.
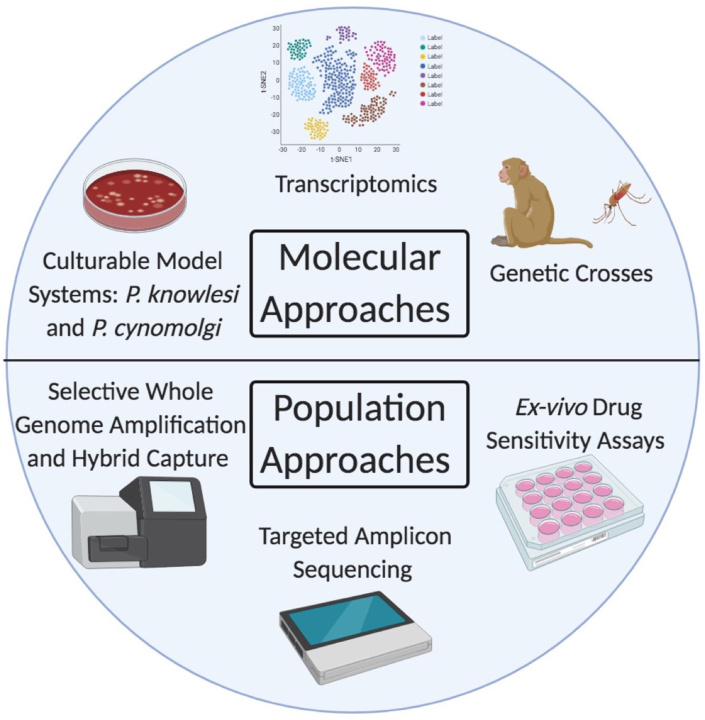
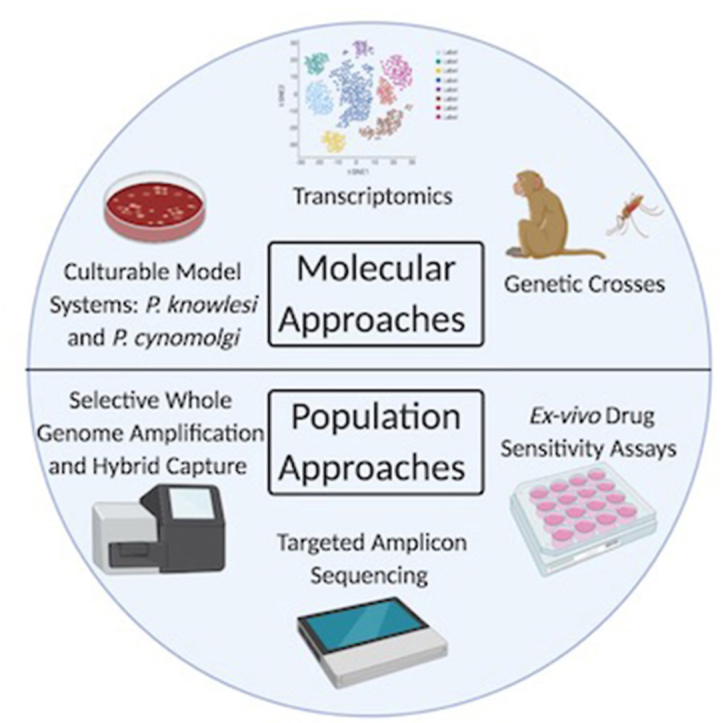
Fig. 3.
Summary of current and emerging techniques used to interrogate P. vivax drug resistance.
In the literature, putative resistance mutations are frequently referred to as markers of drug resistance in P. vivax. Given the relatively limited evidence supporting these putative resistance markers, care should be taken when using sequencing data to inform the levels of CQR or drug policy in a given geographical region. Instead, ex vivo assays and clinical treatment failure in the presence of adequate drug concentrations in the blood are the only reliable methods currently available for determining the level of drug resistance in a population. Verified genetic markers of drug resistance could be used to replace these laborious and costly approaches, allowing high-throughput and frequent sampling of parasite populations that can be used to inform drug policy in that region. Molecular surveillance for resistance markers would allow for treatment regimens to be changed upon the emergence of significant drug resistance in a population (Ehrlich et al., 2020; Nkhoma et al., 2007). Understanding of the evolutionary and population dynamics of drug resistance will be critical for molecular surveillance to both identify when these alleles arise and understand how they move throughout and between populations. Investigation of candidate P. vivax drug resistance genes using advances in in vitro culture, ex vivo assays, and sequencing will help make this possible and improve P. vivax control and elimination efforts.
Decaration of competing interest
The authors have no conflicts of interest.
Acknowledgements
MTD was supported by the National Institutes of Health grants R01AI140751 and U19AI089688.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2021.04.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdallah T.M., Ali A.A.A., Bakri M., Gasim G.I., Musa I.R., Adam I. Efficacy of artemether-lumefantrine as a treatment for uncomplicated Plasmodium vivax malaria in eastern Sudan. Malar. J. 2012;11:404. doi: 10.1186/1475-2875-11-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreha T., Hwang J., Thriemer K., Tadesse Y., Girma S., Melaku Z., Assef A., Kassa M., Chatfield M.D., Landman K.Z., Chenet S.M., Lucchi N.W., Udhayakumar V., Zhou Z., Shi Y.P., Kachur S.P., Jima D., Kebede A., Solomon H. Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax infection in Ethiopia: a randomized controlled trial. PLoS Med. 2017;14(5) doi: 10.1371/journal.pmed.1002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.T., Bora H., Bharti P.K., Saifi M.A., Das M.K., Dev V., Kumar A., Singh N., Dash A.P., Das B., Wajihullah null, Sharma Y.D. Similar trends of pyrimethamine resistance-associated mutations in Plasmodium vivax and P. falciparum. Antimicrobial Agents and Chemotherapy. 2007;51(3):857–863. doi: 10.1128/AAC.01200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Añez A., Navarro-Costa D., Yucra O., Garnica C., Melgar V., Moscoso M., Arteaga R., Nakao G. [Therapeutic response of Plasmodium vivax to chloroquine in Bolivia] Biomedica. 2012;32(4):527–535. doi: 10.1590/S0120-41572012000400008. Revista Del Instituto Nacional De Salud. [DOI] [PubMed] [Google Scholar]
- Ariey F., Witkowski B., Amaratunga C., Beghain J., Langlois A.-C., Khim N., Kim S., Duru V., Bouchier C., Ma L., Lim P., Leang R., Duong S., Sreng S., Suon S., Chuor C.M., Bout D.M., Ménard S., Rogers W.O. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asih P.B.S., Marantina S.S., Nababan R., Lobo N.F., Rozi I.E., Sumarto W., Dewi R.M., Tuti S., Taufik A.S., Mulyanto, Sauerwein R.W., Syafruddin D. Distribution of Plasmodium vivax pvdhfr and pvdhps alleles and their association with sulfadoxine–pyrimethamine treatment outcomes in Indonesia. Malar. J. 2015;14(1):365. doi: 10.1186/s12936-015-0903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asih P.B.S., Syafruddin D., Leake J., Sorontou Y., Sadikin M., Sauerwein R.W., Vinetz J., Baird J.K. Phenotyping clinical resistance to chloroquine in Plasmodium vivax in northeastern Papua, Indonesia. International Journal for Parasitology. Drugs and Drug Resistance. 2011;1(1):28–32. doi: 10.1016/j.ijpddr.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auburn S., Benavente E.D., Miotto O., Pearson R.D., Amato R., Grigg M.J., Barber B.E., William T., Handayuni I., Marfurt J., Trimarsanto H., Noviyanti R., Sriprawat K., Nosten F., Campino S., Clark T.G., Anstey N.M., Kwiatkowski D.P., Price R.N. Genomic analysis of a pre-elimination Malaysian Plasmodium vivax population reveals selective pressures and changing transmission dynamics. Nat. Commun. 2018;9(1):2585. doi: 10.1038/s41467-018-04965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auburn S., Getachew S., Pearson R.D., Amato R., Miotto O., Trimarsanto H., Zhu S.J., Rumaseb A., Marfurt J., Noviyanti R., Grigg M.J., Barber B., William T., Goncalves S.M., Drury E., Sriprawat K., Anstey N.M., Nosten F., Petros B. Genomic analysis of plasmodium vivax in southern Ethiopia reveals selective pressures in multiple parasite mechanisms. J. Infect. Dis. 2019;220(11):1738–1749. doi: 10.1093/infdis/jiz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auburn S., Serre D., Pearson R.D., Amato R., Sriprawat K., To S., Handayuni I., Suwanarusk R., Russell B., Drury E., Stalker J., Miotto O., Kwiatkowski D.P., Nosten F., Price R.N. Genomic analysis reveals a common breakpoint in amplifications of the plasmodium vivax multidrug resistance 1 locus in Thailand. J. Infect. Dis. 2016;214(8):1235–1242. doi: 10.1093/infdis/jiw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auliff, Adams J.H., O'Neil M.T., Cheng Q. Defining the role of mutations in plasmodium vivax dihydrofolate reductase-thymidylate synthase gene using an episomal plasmodium falciparum transfection system. Antimicrob. Agents Chemother. 2010;54(9):3927–3932. doi: 10.1128/AAC.00628-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auliff, Wilson D.W., Russell B., Gao Q., Chen N., Anh L.N., Maguire J., Bell D., O'Neil M.T., Cheng Q. Amino acid mutations in Plasmodium vivax DHFR and DHPS from several geographical regions and susceptibility to antifolate drugs. Am. J. Trop. Med. Hyg. 2006;75(4):617–621. [PubMed] [Google Scholar]
- Baird, Basri H., Subianto B., Fryauff D.J., McElroy P.D., Leksana B., Richie T.L., Masbar S., Wignall F.S., Hoffman S.L. Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J. Infect. Dis. 1995;171(6):1678–1682. doi: 10.1093/infdis/171.6.1678. [DOI] [PubMed] [Google Scholar]
- Baird J.K., Leksana B., Masbar S., Fryauff D.J., Sutanihardja M.A., Suradi null, Wignall F.S., Hoffman S.L. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 1997;56(6):621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- Baird J.K., Wiady I., Fryauff D.J., Sutanihardja M.A., Leksana B., Widjaya H., Kysdarmanto null, Subianto B. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 1997;56(6):627–631. doi: 10.4269/ajtmh.1997.56.627. [DOI] [PubMed] [Google Scholar]
- Baird, Louisa M., Noviyanti R., Ekawati L., Elyazar I., Subekti D., Chand K., Gayatri A., Instiaty, Soebianto S., Crenna-Darusallam C., Djoko D., Hasto B.D., Meriyenes D., Wesche D., Nelwan E.J., Sutanto I., Sudoyo H., Setiabudy R. Association of impaired cytochrome P450 2D6 activity genotype and phenotype with therapeutic efficacy of primaquine treatment for latent Plasmodium vivax malaria. JAMA Network Open. 2018;1(4) doi: 10.1001/jamanetworkopen.2018.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnadas, Kent D., Timinao L., Iga J., Gray L.R., Siba P., Mueller I., Thomas P.J., Zimmerman P.A. A new high-throughput method for simultaneous detection of drug resistance associated mutations in Plasmodium vivax dhfr, dhps and mdr1 genes. Malar. J. 2011;10:282. doi: 10.1186/1475-2875-10-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnadas, Ratsimbasoa A., Tichit M., Bouchier C., Jahevitra M., Picot S., Ménard D. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. Antimicrob. Agents Chemother. 2008;52(12):4233–4240. doi: 10.1128/AAC.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnadas, Tichit M., Bouchier C., Ratsimbasoa A., Randrianasolo L., Raherinjafy R., Jahevitra M., Picot S., Ménard D. Plasmodium vivax dhfr and dhps mutations in isolates from Madagascar and therapeutic response to sulphadoxine-pyrimethamine. Malar. J. 2008;7(1):35. doi: 10.1186/1475-2875-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.W., Pybus B.S., Yadava A., Tosh D., Sousa J.C., McCarthy W.F., Deye G., Melendez V., Ockenhouse C.F. Massachusetts Medical Society. 2013. Primaquine failure and cytochrome P-450 2D6 in plasmodium vivax malaria [letter] October 2. Http://dx.doi.org/10.1056/NEJMc1301936 (world) [DOI] [PubMed] [Google Scholar]
- Bopp S., Magistrado P., Wong W., Schaffner S.F., Mukherjee A., Lim P., Dhorda M., Amaratunga C., Woodrow C.J., Ashley E.A., White N.J., Dondorp A.M., Fairhurst R.M., Ariey F., Menard D., Wirth D.F., Volkman S.K. Plasmepsin II–III copy number accounts for bimodal piperaquine resistance among Cambodian Plasmodium falciparum. Nat. Commun. 2018;9(1):1769. doi: 10.1038/s41467-018-04104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R.M., Hathaway N., Fulton T., Reyes R., Matte M., Ntaro M., Mulogo E., Waltmann A., Bailey J.A., Siedner M.J., Juliano J.J. Reuse of malaria rapid diagnostic tests for amplicon deep sequencing to estimate Plasmodium falciparum transmission intensity in western Uganda. Sci. Rep. 2018;8(1):10159. doi: 10.1038/s41598-018-28534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear A.M., Fan Q., Hu Y., Li Y., Zhao Y., Wang Z., Cao Y., Miao J., Barry A., Cui L. Population genomics identifies a distinct Plasmodium vivax population on the China-Myanmar border of Southeast Asia. PLoS Neglected Trop. Dis. 2020;14(8) doi: 10.1371/journal.pntd.0008506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau N.F., Hathaway N., Parobek C.M., Lin J.T., Bailey J.A., Lon C., Saunders D.L., Juliano J.J. Longitudinal pooled deep sequencing of the plasmodium vivax K12 kelch gene in Cambodia reveals a lack of selection by artemisinin. Am. J. Trop. Med. Hyg. 2016;95(6):1409–1412. doi: 10.4269/ajtmh.16-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega S., Meslin B., de Monbrison F., Severini C., Gradoni L., Udomsangpetch R., Sutanto I., Peyron F., Picot S. Identification of the plasmodium vivax mdr-like gene (pvmdr1) and analysis of single-nucleotide polymorphisms among isolates from different areas of endemicity. J. Infect. Dis. 2005;191(2):272–277. doi: 10.1086/426830. [DOI] [PubMed] [Google Scholar]
- Bright, Alenazi T., Shokoples S., Tarning J., Paganotti G.M., White N.J., Houston S., Winzeler E.A., Yanow S.K. Genetic analysis of primaquine tolerance in a patient with relapsing vivax malaria. Emerg. Infect. Dis. 2013;19(5):802–805. doi: 10.3201/eid1905.121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright, Manary M.J., Tewhey R., Arango E.M., Wang T., Schork N.J., Yanow S.K., Winzeler E.A. A high resolution case study of a patient with recurrent plasmodium vivax infections shows that relapses were caused by meiotic siblings. PLoS Neglected Trop. Dis. 2014;8(6) doi: 10.1371/journal.pntd.0002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briolant S., Bogreau H., Gil M., Bouchiba H., Baret E., Amalvict R., Rogier C., Pradines B. The F423Y mutation in the pfmdr2 gene and mutations N51I, C59R, and S108N in the pfdhfr gene are independently associated with pyrimethamine resistance in plasmodium falciparum isolates. Antimicrob. Agents Chemother. 2012;56(5):2750–2752. doi: 10.1128/AAC.05618-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda G., Jirawatcharadech P., Priestley R.S., Saif A., March S., Wong M.H.L., Leung S., Miller A.B., Baker D.A., Alano P., Paine M.J.I., Bhatia S.N., O'Neill P.M., Ward S.A., Biagini G.A. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat. Commun. 2019;10(1):3226. doi: 10.1038/s41467-019-11239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaorattanakawee S., Lon C., Chann S., Thay K.H., Kong N., You Y., Sundrakes S., Thamnurak C., Chattrakarn S., Praditpol C., Yingyuen K., Wojnarski M., Huy R., Spring M.D., Walsh D.S., Patel J.C., Lin J., Juliano J.J., Lanteri C.A., Saunders D.L. Measuring ex vivo drug susceptibility in Plasmodium vivax isolates from Cambodia. Malar. J. 2017;16(1):392. doi: 10.1186/s12936-017-2034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T.-Y., Lin W.-C., Kuo M.-C., Ji D.-D., Fang C.-T. Relapse of imported vivax malaria despite standard-dose primaquine therapy: an investigation with molecular genotyping analyses. Clin. Microbiol. Infect.: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18(7):E232–E234. doi: 10.1111/j.1469-0691.2012.03820.x. [DOI] [PubMed] [Google Scholar]
- Chua A.C.Y., Ong J.J.Y., Malleret B., Suwanarusk R., Kosaisavee V., Zeeman A.-M., Cooper C.A., Tan K.S.W., Zhang R., Tan B.H., Abas S.N., Yip A., Elliot A., Joyner C.J., Cho J.S., Breyer K., Baran S., Lange A., Maher S.P. Robust continuous in vitro culture of the Plasmodium cynomolgi erythrocytic stages. Nat. Commun. 2019;10(1):3635. doi: 10.1038/s41467-019-11332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons R.J., Simpson J.A., Thriemer K., Abreha T., Adam I., Anstey N.M., Assefa A., Awab G.R., Baird J.K., Barber B.E., Chu C.S., Dahal P., Daher A., Davis T.M.E., Dondorp A.M., Grigg M.J., Humphreys G.S., Hwang J., Karunajeewa H. The efficacy of dihydroartemisinin-piperaquine and artemether-lumefantrine with and without primaquine on Plasmodium vivax recurrence: a systematic review and individual patient data meta-analysis. PLoS Med. 2019;16(10) doi: 10.1371/journal.pmed.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congpuon K., Satimai W., Sujariyakul A., Intanakom S., Harnpitakpong W., Pranuth Y., Cholpol S., Bualombai P. In vivo sensitivity monitoring of chloroquine for the treatment of uncomplicated vivax malaria in four bordered provinces of Thailand during 2009-2010. J. Vector Borne Dis. 2011;48(4):190–196. [PubMed] [Google Scholar]
- Costa G.L., Amaral L.C., Fontes C.J.F., Carvalho L.H., de Brito C.F.A., de Sousa T.N. Assessment of copy number variation in genes related to drug resistance in Plasmodium vivax and Plasmodium falciparum isolates from the Brazilian Amazon and a systematic review of the literature. Malar. J. 2017;16(1):152. doi: 10.1186/s12936-017-1806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell A.N., Loy D.E., Sundararaman S.A., Valdivia H., Fisch K., Lescano A.G., Baldeviano G.C., Durand S., Gerbasi V., Sutherland C.J., Nolder D., Vinetz J.M., Hahn B.H., Winzeler E.A. Selective whole-genome amplification is a robust method that enables scalable whole-genome sequencing of plasmodium vivax from unprocessed clinical samples. mBio. 2017;8(1) doi: 10.1128/mBio.02257-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell A.N., Valdivia H.O., Bishop D.K., Winzeler E.A. Exploration of Plasmodium vivax transmission dynamics and recurrent infections in the Peruvian Amazon using whole genome sequencing. Genome Med. 2018;10(1):52. doi: 10.1186/s13073-018-0563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström S., Ferreira P.E., Veiga M.I., Sedighi N., Wiklund L., Mårtensson A., Färnert A., Sisowath C., Osório L., Darban H., Andersson B., Kaneko A., Conseil G., Björkman A., Gil J.P. Plasmodium falciparum multidrug resistance protein 1 and artemisinin-based combination therapy in Africa. J. Infect. Dis. 2009;200(9):1456–1464. doi: 10.1086/606009. [DOI] [PubMed] [Google Scholar]
- Demas A.R., Sharma A.I., Wong W., Early A.M., Redmond S., Bopp S., Neafsey D.E., Volkman S.K., Hartl D.L., Wirth D.F. Mutations in Plasmodium falciparum actin-binding protein coronin confer reduced artemisinin susceptibility. Proc. Natl. Acad. Sci. U.S.A. 2018;115(50):12799–12804. doi: 10.1073/pnas.1812317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Ruan Y., Bai Y., Hu Y., Deng Z., He Y., Ruan R., Wu Y., Yang Z., Cui L. Genetic diversity of the Pvk12 gene in Plasmodium vivax from the China-Myanmar border area. Malar. J. 2016;15(1):528. doi: 10.1186/s12936-016-1592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharia N.V., Bright A.T., Westenberger S.J., Barnes S.W., Batalov S., Kuhen K., Borboa R., Federe G.C., McClean C.M., Vinetz J.M., Neyra V., Llanos-Cuentas A., Barnwell J.W., Walker J.R., Winzeler E.A. Whole-genome sequencing and microarray analysis of ex vivo Plasmodium vivax reveal selective pressure on putative drug resistance genes. Proc. Natl. Acad. Sci. U.S.A. 2010;107(46) doi: 10.1073/pnas.1003776107. 20045–20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas N.M., Anstey N.M., Angus B.J., Nosten F., Price R.N. Artemisinin combination therapy for vivax malaria? Lancet Infect. Dis. 2010;10(6):405–416. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker A., Lehane A.M., Clain J., Fidock D.A. PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 2012;28(11):504–514. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.Y., Jones J., Parikh S. Molecular surveillance of antimalarial partner drug resistance in sub-Saharan Africa: a spatial-temporal evidence mapping study. The Lancet Microbe. 2020;1(5):e209–e217. doi: 10.1016/S2666-5247(20)30094-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faway E., Musset L., Pelleau S., Volney B., Casteras J., Caro V., Menard D., Briolant S., Legrand E. Plasmodium vivax multidrug resistance-1 gene polymorphism in French Guiana. Malar. J. 2016;15(1):540. doi: 10.1186/s12936-016-1595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M.U., Nobrega de Sousa T., Rangel G.W., Johansen I.C., Corder R.M., Ladeia-Andrade S., Gil J.P. Monitoring Plasmodium vivax resistance to antimalarials: persisting challenges and future directions. Int. J. Parasitol.: Drugs and Drug Resistance. 2021;15:9–24. doi: 10.1016/j.ijpddr.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock D.A., Nomura T., Talley A.K., Cooper R.A., Dzekunov S.M., Ferdig M.T., Ursos L.M.B., Sidhu A., bir S., Naudé B., Deitsch K.W., Su X., Wootton J.C., Roepe P.D., Wellems T.E. Mutations in the P. Falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell. 2000;6(4):861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery E.L., Wang T., Akbari A., Corey V.C., Gunawan F., Bright A.T., Abraham M., Sanchez J.F., Santolalla M.L., Baldeviano G.C., Edgel K.A., Rosales L.A., Lescano A.G., Bafna V., Vinetz J.M., Winzeler E.A. Next-generation sequencing of plasmodium vivax patient samples shows evidence of direct evolution in drug-resistance genes. ACS Infect. Dis. 2015;1(8):367–379. doi: 10.1021/acsinfecdis.5b00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryauff D.J., Leksana B., Masbar S., Wiady I., Sismadi P., Susanti A.I., Nagesha H.S., Syafruddin null, Atmosoedjono S., Bangs M.J., Baird J.K. The drug sensitivity and transmission dynamics of human malaria on Nias Island, North Sumatra, Indonesia. Ann. Trop. Med. Parasitol. 2002;96(5):447–462. doi: 10.1179/000349802125001249. [DOI] [PubMed] [Google Scholar]
- Fryauff D.J., Tuti S., Mardi A., Masbar S., Patipelohi R., Leksana B., Kain K.C., Bangs M.J., Richie T.L., Baird J.K. Chloroquine-resistant plasmodium vivax in transmigration settlements of west Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 1998;59(4):513–518. doi: 10.4269/ajtmh.1998.59.513. [DOI] [PubMed] [Google Scholar]
- Fukuda M.M., Krudsood S., Mohamed K., Green J.A., Warrasak S., Noedl H., Euswas A., Ittiverakul M., Buathong N., Sriwichai S., Miller R.S., Ohrt C. A randomized, double-blind, active-control trial to evaluate the efficacy and safety of a three day course of tafenoquine monotherapy for the treatment of Plasmodium vivax malaria. PloS One. 2017;12(11) doi: 10.1371/journal.pone.0187376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresty K., Anderson K., Pasay C., Waters N.C., Cheng Q. Polymorphisms in plasmodium falciparum kelch 13 and P. Vivax kelch 12 genes in parasites collected from three South pacific countries prior to extensive exposure to artemisinin combination therapies. Antimicrob. Agents Chemother. 2019;63(7) doi: 10.1128/AAC.00536-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg M.J., William T., Menon J., Barber B.E., Wilkes C.S., Rajahram G.S., Edstein M.D., Auburn S., Price R.N., Yeo T.W., Anstey N.M. Efficacy of artesunate-mefloquine for chloroquine-resistant plasmodium vivax malaria in Malaysia: an open-label, randomized, controlled trial. Clin. Infect. Dis. 2016;62(11):1403–1411. doi: 10.1093/cid/ciw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg M., Lerch A., Beck H.-P., Felger I. Amplicon deep sequencing improves Plasmodium falciparum genotyping in clinical trials of antimalarial drugs. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-54203-0. 17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmann J.-P., Pittet A., Lesage A., Imwong M., Lindegardh N., Min Lwin M., Zaw T., Annerberg A., de Radiguès X., Nosten F. Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop. Med. Int. Health: TM & IH. 2008;13(1):91–98. doi: 10.1111/j.1365-3156.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- Haldar K., Bhattacharjee S., Safeukui I. Drug resistance in plasmodium. Nat. Rev. Microbiol. 2018;16(3):156–170. doi: 10.1038/nrmicro.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings M.D., Maguire J.D., Bangs M.J., Zimmerman P.A., Reeder J.C., Baird J.K., Sibley C.H. Novel Plasmodium vivax dhfr alleles from the Indonesian Archipelago and Papua New Guinea: association with pyrimethamine resistance determined by a Saccharomyces cerevisiae expression system. Antimicrob. Agents Chemother. 2005;49(2):733–740. doi: 10.1128/AAC.49.2.733-740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings M.D., Porter K.M., Maguire J.D., Susanti I., Kania W., Bangs M.J., Sibley C.H., Baird J.K. Dihydrofolate reductase mutations in Plasmodium vivax from Indonesia and therapeutic response to sulfadoxine plus pyrimethamine. J. Infect. Dis. 2004;189(4):744–750. doi: 10.1086/381397. [DOI] [PubMed] [Google Scholar]
- Hastings M.D., Sibley C.H. Pyrimethamine and WR99210 exert opposing selection on dihydrofolate reductase from Plasmodium vivax. Proc. Natl. Acad. Sci. Unit. States Am. 2002;99(20):13137–13141. doi: 10.1073/pnas.182295999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins V.N., Joshi H., Rungsihirunrat K., Na-Bangchang K., Sibley C.H. Antifolates can have a role in the treatment of Plasmodium vivax. Trends Parasitol. 2007;23(5):213–222. doi: 10.1016/j.pt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hayton K., Gaur D., Liu A., Takahashi J., Henschen B., Singh S., Lambert L., Furuya T., Bouttenot R., Doll M., Nawaz F., Mu J., Jiang L., Miller L.H., Wellems T.E. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of plasmodium falciparum invasion. Cell Host Microbe. 2008;4(1):40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D.R., Baird J.K., Parise M.E., Lewis L.S., Ryan E.T., Magill A.J. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am. J. Trop. Med. Hyg. 2006;75(3):402–415. [PubMed] [Google Scholar]
- Huang B., Huang S., Su X., Tong X., Yan J., Li H., Lu F. Molecular surveillance of pvdhfr, pvdhps, and pvmdr-1 mutations in Plasmodium vivax isolates from Yunnan and Anhui provinces of China. Malar. J. 2014;13(1):346. doi: 10.1186/1475-2875-13-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupalo D.N., Luo Z., Melnikov A., Sutton P.L., Rogov P., Escalante A., Vallejo A.F., Herrera S., Arévalo-Herrera M., Fan Q., Wang Y., Cui L., Lucas C.M., Durand S., Sanchez J.F., Baldeviano G.C., Lescano A.G., Laman M., Barnadas C. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat. Genet. 2016;48(8):953–958. doi: 10.1038/ng.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Alemayehu B.H., Reithinger R., Tekleyohannes S.G., Takele Teshi null, Birhanu S.G., Demeke L., Hoos D., Melaku Z., Kassa M., Jima D., Malone J.L., Nettey H., Green M., Poe A., Akinyi S., Udhayakumar V., Kachur S.P., Filler S. In vivo efficacy of artemether-lumefantrine and chloroquine against Plasmodium vivax: a randomized open label trial in central Ethiopia. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0063433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong, Pukrittakayamee S., Looareesuwan S., Pasvol G., Poirriez J., White N.J., Snounou G. Association of genetic mutations in plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob. Agents Chemother. 2001;11:7. doi: 10.1128/AAC.45.11.3122-3127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong, Pukrittayakamee S., Cheng Q., Moore C., Looareesuwan S., Snounou G., White N.J., Day N.P.J. Limited polymorphism in the dihydropteroate synthetase gene (dhps) of plasmodium vivax isolates from Thailand. Antimicrob. Agents Chemother. 2005;49(10):4393–4395. doi: 10.1128/AAC.49.10.4393-4395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong, Pukrittayakamee S., Pongtavornpinyo W., Nakeesathit S., Nair S., Newton P., Nosten F., Anderson T.J.C., Dondorp A., Day N.P.J., White N.J. Gene amplification of the multidrug resistance 1 gene of plasmodium vivax isolates from Thailand, Laos, and Myanmar. Antimicrob. Agents Chemother. 2008;52(7):2657–2659. doi: 10.1128/AAC.01459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong, Snounou G., Pukrittayakamee S., Tanomsing N., Kim J.R., Nandy A., Guthmann J.-P., Nosten F., Carlton J., Looareesuwan S., Nair S., Sudimack D., Day N.P.J., Anderson T.J.C., White N.J. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J. Infect. Dis. 2007;195(7):927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- Johnson D.J., Fidock D.A., Mungthin M., Lakshmanan V., Sidhu A.B.S., Bray P.G., Ward S.A. Evidence for a central role for PfCRT in conferring plasmodium falciparum resistance to diverse antimalarial agents. Mol. Cell. 2004;15(6):867–877. doi: 10.1016/j.molcel.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy S., Mukhi B., Ghosh S.K., Achur R.N., Gowda D.C., Surolia N. Drug resistance genes: pvcrt-o and pvmdr-1 polymorphism in patients from malaria endemic South Western Coastal Region of India. Malar. J. 2018;17(1):40. doi: 10.1186/s12936-018-2188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunajeewa H.A., Mueller I., Senn M., Lin E., Law I., Gomorrai P.S., Oa O., Griffin S., Kotab K., Suano P., Tarongka N., Ura A., Lautu D., Page-Sharp M., Wong R., Salman S., Siba P., Ilett K.F., Davis T.M.E. A trial of combination antimalarial therapies in children from Papua New Guinea. N. Engl. J. Med. 2008;359(24):2545–2557. doi: 10.1056/NEJMoa0804915. [DOI] [PubMed] [Google Scholar]
- Kaur S., Prajapati S.K., Kalyanaraman K., Mohmmed A., Joshi H., Chauhan V.S. Plasmodium vivax dihydrofolate reductase point mutations from the Indian subcontinent. Acta Trop. 2006;97(2):174–180. doi: 10.1016/j.actatropica.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Khim N., Andrianaranjaka V., Popovici J., Kim S., Ratsimbasoa A., Benedet C., Barnadas C., Durand R., Thellier M., Legrand E., Musset L., Menegon M., Severini C., Nour B.Y.M., Tichit M., Bouchier C., Mercereau-Puijalon O., Ménard D. Effects of mefloquine use on plasmodium vivax multidrug resistance. Emerg. Infect. Dis. 2014;20(10):1637–1644. doi: 10.3201/eid2010.140411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A., Popovici J., Menard D., Serre D. Plasmodium vivax transcriptomes reveal stage-specific chloroquine response and differential regulation of male and female gametocytes. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-08312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsaeree P., Khongsuk P., Leartsakulpanich U., Chitnumsub P., Tarnchompoo B., Walkinshaw M.D., Yuthavong Y. Crystal structure of dihydrofolate reductase from Plasmodium vivax: pyrimethamine displacement linked with mutation-induced resistance. Proc. Natl. Acad. Sci. U.S.A. 2005;102(37):13046–13051. doi: 10.1073/pnas.0501747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsinczky M., Fischer K., Chen N., Baker J., Rieckmann K., Cheng Q. Sulfadoxine resistance in plasmodium vivax is associated with a specific amino acid in dihydropteroate synthase at the putative sulfadoxine-binding site. Antimicrob. Agents Chemother. 2004;48(6):2214–2222. doi: 10.1128/AAC.48.6.2214-2222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhen K.L., Chatterjee A.K., Rottmann M., Gagaring K., Borboa R., Buenviaje J., Chen Z., Francek C., Wu T., Nagle A., Barnes S.W., Plouffe D., Lee M.C.S., Fidock D.A., Graumans W., van de Vegte-Bolmer M., van Gemert G.J., Wirjanata G., Sebayang B. KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment, and prevention of disease transmission. Antimicrob. Agents Chemother. 2014;58(9):5060–5067. doi: 10.1128/AAC.02727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeia-Andrade S., Menezes M.J., de Sousa T.N., Silvino A.C.R., de Carvalho J.F., Salla L.C., Nery O.A., de Melo G.N.P., Corder R.M., Rodrigues P.T., Ferreira M.U. Monitoring the efficacy of chloroquine-primaquine therapy for uncomplicated plasmodium vivax malaria in the main transmission hot spot of Brazil. Antimicrob. Agents Chemother. 2019;63(5) doi: 10.1128/AAC.01965-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimanis M.L., Jaidee A., Sriprawat K., Kaewpongsri S., Suwanarusk R., Barends M., Phyo A.P., Russell B., Renia L., Nosten F. Plasmodium vivax susceptibility to ferroquine. Antimicrob. Agents Chemother. 2010;54(5):2228–2230. doi: 10.1128/AAC.01572-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek-Uthai U., Suwanarusk R., Ruengweerayut R., Skinner-Adams T.S., Nosten F., Gardiner D.L., Boonma P., Piera K.A., Andrews K.T., Machunter B., McCarthy J.S., Anstey N.M., Price R.N., Russell B. Stronger activity of human immunodeficiency virus type 1 protease inhibitors against clinical isolates of Plasmodium vivax than against those of P. falciparum. Antimicrobial Agents and Chemotherapy. 2008;52(7):2435–2441. doi: 10.1128/AAC.00169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang J., Li Q., Hu Y., Ruan Y., Tao Z., Xia H., Qiao J., Meng L., Zeng W., Li C., He X., Zhao L., Siddiqui F.A., Miao J., Yang Z., Fang Q., Cui L. Ex vivo susceptibilities of Plasmodium vivax isolates from the China-Myanmar border to antimalarial drugs and association with polymorphisms in Pvmdr1 and Pvcrt-o genes. PLoS Neglected Trop. Dis. 2020;14(6) doi: 10.1371/journal.pntd.0008255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Hansen E., DeSimone T.M., Moreno Y., Junker K., Bei A., Brugnara C., Buckee C.O., Duraisingh M.T. Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nat. Commun. 2013;4:1638. doi: 10.1038/ncomms2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.T., Hathaway N.J., Saunders D.L., Lon C., Balasubramanian S., Kharabora O., Gosi P., Sriwichai S., Kartchner L., Chuor C.M., Satharath P., Lanteri C., Bailey J.A., Juliano J.J. Using amplicon deep sequencing to detect genetic signatures of plasmodium vivax relapse. J. Infect. Dis. 2015;212(6):999–1008. doi: 10.1093/infdis/jiv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos-Cuentas A., Casapia M., Chuquiyauri R., Hinojosa J.-C., Kerr N., Rosario M., Toovey S., Arch R.H., Phillips M.A., Rozenberg F.D., Bath J., Ng C.L., Cowell A.N., Winzeler E.A., Fidock D.A., Baker M., Möhrle J.J., Hooft van Huijsduijnen R., Gobeau N. Antimalarial activity of single-dose DSM265, a novel plasmodium dihydroorotate dehydrogenase inhibitor, in patients with uncomplicated Plasmodium falciparum or Plasmodium vivax malaria infection: a proof-of-concept, open-label, phase 2a study. The Lancet. Infectious Diseases. 2018;18(8):874–883. doi: 10.1016/S1473-3099(18)30309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos-Cuentas A., Lacerda M.V.G., Hien T.T., Vélez I.D., Namaik-larp C., Chu C.S., Villegas M.F., Val F., Monteiro W.M., Brito M.A.M., Costa M.R.F., Chuquiyauri R., Casapía M., Nguyen C.H., Aruachan S., Papwijitsil R., Nosten F.H., Bancone G., Angus B. Tafenoquine versus primaquine to prevent relapse of plasmodium vivax malaria. N. Engl. J. Med. 2019;380(3):229–241. doi: 10.1056/NEJMoa1802537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Ally M., Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Blood. 2020;136(11):1225–1240. doi: 10.1182/blood.2019000944. [DOI] [PubMed] [Google Scholar]
- Maguire J.D., Krisin null, Marwoto H., Richie T.L., Fryauff D.J., Baird J.K. Mefloquine is highly efficacious against chloroquine-resistant Plasmodium vivax malaria and Plasmodium falciparum malaria in Papua, Indonesia. Clin. Infect. Dis. 2006;42(8):1067–1072. doi: 10.1086/501357. An Official Publication of the Infectious Diseases Society of America. [DOI] [PubMed] [Google Scholar]
- Marfurt J., Chalfein F., Prayoga P., Wabiser F., Kenangalem E., Piera K.A., Fairlie D.P., Tjitra E., Anstey N.M., Andrews K.T., Price R.N. Ex vivo activity of histone deacetylase inhibitors against multidrug-resistant clinical isolates of Plasmodium falciparum and P. vivax. Antimicrobial Agents and Chemotherapy. 2011;55(3):961–966. doi: 10.1128/AAC.01220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt J., de Monbrison F., Brega S., Barbollat L., Müller I., Sie A., Goroti M., Reeder J.C., Beck H.-P., Picot S., Genton B. Molecular markers of in vivo Plasmodium vivax resistance to amodiaquine plus sulfadoxine-pyrimethamine: mutations in pvdhfr and pvmdr1. J. Infect. Dis. 2008;198(3):409–417. doi: 10.1086/589882. [DOI] [PubMed] [Google Scholar]
- McIntosh H.M., Olliaro P. Artemisinin derivatives for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2000;2:CD000256. doi: 10.1002/14651858.CD000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara C.W., Lee M.C.S., Lim C.S., Lim S.H., Roland J., Nagle A., Simon O., Yeung B.K.S., Chatterjee A.K., McCormack S.L., Manary M.J., Zeeman A.-M., Dechering K.J., Kumar T.R.S., Henrich P.P., Gagaring K., Ibanez M., Kato N., Kuhen K.L. Targeting Plasmodium PI(4)K to eliminate malaria. Nature. 2013;504(7479):248–253. doi: 10.1038/nature12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikov A., Galinsky K., Rogov P., Fennell T., Van Tyne D., Russ C., Daniels R., Barnes K.G., Bochicchio J., Ndiaye D., Sene P.D., Wirth D.F., Nusbaum C., Volkman S.K., Birren B.W., Gnirke A., Neafsey D.E. Hybrid selection for sequencing pathogen genomes from clinical samples. Genome Biol. 2011;12(8):R73. doi: 10.1186/gb-2011-12-8-r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo G.C., Monteiro W.M., Siqueira A.M., Silva S.R., Magalhães B.M.L., Alencar A.C.C., Kuehn A., del Portillo H.A., Fernandez-Becerra C., Lacerda M.V.G. Expression levels of pvcrt-o and pvmdr-1 are associated with chloroquine resistance and severe Plasmodium vivax malaria in patients of the Brazilian Amazon. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0105922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard D., Khim N., Beghain J., Adegnika A.A., Shafiul-Alam M., Amodu O., Rahim-Awab G., Barnadas C., Berry A., Boum Y., Bustos M.D., Cao J., Chen J.-H., Collet L., Cui L., Thakur G.-D., Dieye A., Djallé D., Dorkenoo M.A. A worldwide map of plasmodium falciparum K13-propeller polymorphisms. N. Engl. J. Med. 2016;374(25):2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkah N.K., Schafer C., Kappe S.H.I. Humanized mouse models for the study of human malaria parasite biology, pathogenesis, and immunity. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto O., Amato R., Ashley E.A., MacInnis B., Almagro-Garcia J., Amaratunga C., Lim P., Mead D., Oyola S.O., Dhorda M., Imwong M., Woodrow C., Manske M., Stalker J., Drury E., Campino S., Amenga-Etego L., Thanh T.-N.N., Tran H.T. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat. Genet. 2015;47(3):226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohring F., Hart M.N., Rawlinson T.A., Henrici R., Charleston J.A., Diez Benavente E., Patel A., Hall J., Almond N., Campino S., Clark T.G., Sutherland C.J., Baker D.A., Draper S.J., Moon R.W. Rapid and iterative genome editing in the malaria parasite Plasmodium knowlesi provides new tools for P. vivax research. ELife. 2019;8 doi: 10.7554/eLife.45829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S., Liong K.-Y., Lim E.-H., Huang X., Zhu L., Preiser P.R., Bozdech Z. Structural polymorphism in the promoter of pfmrp2 confers Plasmodium falciparum tolerance to quinoline drugs. Mol. Microbiol. 2014;91(5):918–934. doi: 10.1111/mmi.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons B., Croon J.J., van der Star W., van der Kaay H.J. Erythrocytic schizogony and invasion of Plasmodium vivax in vitro. Int. J. Parasitol. 1988;18(3):307–311. doi: 10.1016/0020-7519(88)90138-5. [DOI] [PubMed] [Google Scholar]
- Moon R.W., Hall J., Rangkuti F., Ho Y.S., Almond N., Mitchell G.H., Pain A., Holder A.A., Blackman M.J. Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 2013;110(2):531–536. doi: 10.1073/pnas.1216457110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S. Role and regulation of glutathione metabolism in plasmodium falciparum. Molecules. 2015;20(6):10511–10534. doi: 10.3390/molecules200610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Nkhoma S.C., Serre D., Zimmerman P.A., Gorena K., Daniel B.J., Nosten F., Anderson T.J.C., Cheeseman I.H. Single-cell genomics for dissection of complex malaria infections. Genome Res. 2014;24(6):1028–1038. doi: 10.1101/gr.168286.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Will No Longer . National Institutes of Health (NIH); 2015. Support Biomedical Research on Chimpanzees.https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-will-no-longer-support-biomedical-research-chimpanzees November 18. [Google Scholar]
- Nkhoma E.T., Poole C., Vannappagari V., Hall S.A., Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cell Mol. Dis. 2009;42(3):267–278. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Nkhoma S., Molyneux M., Ward S. Molecular surveillance for drug-resistant Plasmodiumfalciparum malaria in Malawi. Acta Trop. 2007;102(2):138–142. doi: 10.1016/j.actatropica.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Nogueira F., Lopes D., Alves A.C., Rosario V. E. do. Plasmodium falciparum multidrug resistance protein (MRP) gene expression under chloroquine and mefloquine challenge. J. Cell Anim. Biol. 2008;2(1):10–20. doi: 10.5897/JCAB.9000118. [DOI] [Google Scholar]
- Noisang C., Meyer W., Sawangjaroen N., Ellis J., Lee R. Molecular detection of antimalarial drug resistance in plasmodium vivax from returned travellers to NSW, Australia during 2008–2018. Pathogens. 2020;9(2):101. doi: 10.3390/pathogens9020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunt M.H., Han J.-H., Wang B., Aye K.M., Aye K.H., Lee S.-K., Htut Y., Kyaw M.P., Han K.T., Han E.-T. Clinical and molecular surveillance of drug resistant vivax malaria in Myanmar (2009-2016) Malar. J. 2017;16(1):117. doi: 10.1186/s12936-017-1770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson K.N., Ketchum M.A., Rimer J.D., Vekilov P.G. Mechanisms of hematin crystallization and inhibition by the antimalarial drug chloroquine. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112(16):4946–4951. doi: 10.1073/pnas.1501023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord R., Alexander N., Dunyo S., Hallett R., Jawara M., Targett G., Drakeley C.J., Sutherland C.J. Seasonal carriage of pfcrt and pfmdr1 alleles in Gambian Plasmodium falciparum imply reduced fitness of chloroquine-resistant parasites. J. Infect. Dis. 2007;196(11):1613–1619. doi: 10.1086/522154. [DOI] [PubMed] [Google Scholar]
- Orjuela-Sánchez P., de Santana Filho F.S., Machado-Lima A., Chehuan Y.F., Costa M.R.F., Alecrim M., das G.C., del Portillo H.A. Analysis of single-nucleotide polymorphisms in the crt-o and mdr1 genes of Plasmodium vivax among chloroquine-resistant isolates from the Brazilian Amazon region. Antimicrob. Agents Chemother. 2009;53(8):3561–3564. doi: 10.1128/AAC.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parobek C.M., Lin J.T., Saunders D.L., Barnett E.J., Lon C., Lanteri C.A., Balasubramanian S., Brazeau N., DeConti D.K., Garba D.L., Meshnick S.R., Spring M.D., Chuor C.M., Bailey J.A., Juliano J.J. Selective sweep suggests transcriptional regulation may underlie Plasmodium vivax resilience to malaria control measures in Cambodia. Proc. Natl. Acad. Sci. U.S.A. 2016;113(50):E8096–E8105. doi: 10.1073/pnas.1608828113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava Z., Handayuni I., Wirjanata G., To S., Trianty L., Noviyanti R., Poespoprodjo J.R., Auburn S., Price R.N., Marfurt J. Expression of plasmodium vivax crt-o is related to parasite stage but not ex vivo chloroquine susceptibility. Antimicrob. Agents Chemother. 2015;60(1):361–367. doi: 10.1128/AAC.02207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.D., Amato R., Auburn S., Miotto O., Almagro-Garcia J., Amaratunga C., Suon S., Mao S., Noviyanti R., Trimarsanto H., Marfurt J., Anstey N.M., William T., Boni M.F., Dolecek C., Hien T.T., White N.J., Michon P., Siba P. Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat. Genet. 2016;48(8):959–964. doi: 10.1038/ng.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I., Gabryszewski S.J., Johnston G.L., Dhingra S.K., Ecker A., Lewis R.E., de Almeida M.J., Straimer J., Henrich P.H., Palatulan E., Johnson D.J., Coburn-Flynn O., Sanchez C., Lehane A.M., Lanzer M., Fidock D.A. Balancing drug resistance and growth rates via compensatory mutations in the Plasmodium falciparum chloroquine resistance transporter. Mol. Microbiol. 2015;97(2):381–395. doi: 10.1111/mmi.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.A., Lotharius J., Marsh K., White J., Dayan A., White K.L., Njoroge J.W., El Mazouni F., Lao Y., Kokkonda S., Tomchick D.R., Deng X., Laird T., Bhatia S.N., March S., Ng C.L., Fidock D.A., Wittlin S., Lafuente-Monasterio M. A long-duration dihydroorotate dehydrogenase inhibitor (DSM265) for prevention and treatment of malaria. Sci. Transl. Med. 2015;7(296):296ra111. doi: 10.1126/scitranslmed.aaa6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.A., White K.L., Kokkonda S., Deng X., White J., Mazouni F.E., Marsh K., Tomchick D.R., Manjalanagara K., Rudra K.R., Wirjanata G., Noviyanti R., Price R.N., Marfurt J., Shackleford D.M., Chiu F.C.K., Campbell M., Jimenez-Diaz M.B., Bazaga S.F. A triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor (DSM421) with improved drug-like properties for treatment and prevention of malaria. ACS Infect. Dis. 2016;2(12):945–957. doi: 10.1021/acsinfecdis.6b00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo A.P., Lwin K.M., Price R.N., Ashley E.A., Russell B., Sriprawat K., Lindegardh N., Singhasivanon P., White N.J., Nosten F. Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial. Clin. Infect. Dis. 2011;53(10):977–984. doi: 10.1093/cid/cir631. An Official Publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preechapornkul P., Imwong M., Chotivanich K., Pongtavornpinyo W., Dondorp A.M., Day N.P.J., White N.J., Pukrittayakamee S. Plasmodium falciparum pfmdr1 amplification, mefloquine resistance, and parasite fitness. Antimicrob. Agents Chemother. 2009;53(4):1509–1515. doi: 10.1128/AAC.00241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.N., Cassar C., Brockman A., Duraisingh M., van Vugt M., White N.J., Nosten F., Krishna S. The pfmdr1 gene is associated with a multidrug-resistant phenotype in plasmodium falciparum from the western border of Thailand. Antimicrob. Agents Chemother. 1999;43(12):2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.N., Marfurt J., Chalfein F., Kenangalem E., Piera K.A., Tjitra E., Anstey N.M., Russell B. In vitro activity of pyronaridine against multidrug-resistant Plasmodium falciparum and Plasmodium vivax. Antimicrob. Agents Chemother. 2010;54(12):5146–5150. doi: 10.1128/AAC.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price Ric N., von Seidlein L., Valecha N., Nosten F., Baird J.K., White N.J. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. The Lancet. Infectious Diseases. 2014;14(10):982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrittayakamee S., Vanijanonta S., Chantra A., Clemens R., White N.J. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J. Infect. Dis. 1994;169(4):932–935. doi: 10.1093/infdis/169.4.932. [DOI] [PubMed] [Google Scholar]
- Pukrittayakamee S., Viravan C., Charoenlarp P., Yeamput C., Wilson R.J., White N.J. Antimalarial effects of rifampin in Plasmodium vivax malaria. Antimicrob. Agents Chemother. 1994;38(3):511–514. doi: 10.1128/aac.38.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulcini S., Staines H.M., Lee A.H., Shafik S.H., Bouyer G., Moore C.M., Daley D.A., Hoke M.J., Altenhofen L.M., Painter H.J., Mu J., Ferguson D.J.P., Llinás M., Martin R.E., Fidock D.A., Cooper R.A., Krishna S. Mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, enlarge the parasite's food vacuole and alter drug sensitivities. Sci. Rep. 2015;5(1):14552. doi: 10.1038/srep14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj D.K., Mu J., Jiang H., Kabat J., Singh S., Sullivan M., Fay M.P., McCutchan T.F., Su X.-Z. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J. Biol. Chem. 2009;284(12):7687–7696. doi: 10.1074/jbc.M806944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel G.W., Clark M.A., Kanjee U., Goldberg J.M., MacInnis B., Menezes M.J., Ferreira M.U., Duraisingh M.T. Plasmodium vivax transcriptional profiling of low input cryopreserved isolates through the intraerythrocytic development cycle. PLoS Neglected Trop. Dis. 2020;14(3) doi: 10.1371/journal.pntd.0008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel G.W., Clark M.A., Kanjee U., Lim C., Shaw-Saliba K., Menezes M.J., Mascarenhas A., Chery L., Gomes E., Rathod P.K., Ferreira M.U., Duraisingh M.T. Enhanced ex vivo plasmodium vivax intraerythrocytic enrichment and maturation for rapid and sensitive parasite growth assays. Antimicrob. Agents Chemother. 2018;62(4) doi: 10.1128/AAC.02519-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P.N., Uplekar S., Kayal S., Mallick P.K., Bandyopadhyay N., Kale S., Singh O.P., Mohanty A., Mohanty S., Wassmer S.C., Carlton J.M. A method for amplicon deep sequencing of drug resistance genes in plasmodium falciparum clinical isolates from India. J. Clin. Microbiol. 2016;54(6):1500–1511. doi: 10.1128/JCM.00235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff A., Siswantoro H., Kenangalem E., Maristela R., Wuwung R.M., Laihad F., Ebsworth E.P., Anstey N.M., Tjitra E., Price R.N. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369(9563):757–765. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M.B., Saliba K.J., Caruana S.R., Kirk K., Cowman A.F. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403(6772):906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- Reiling S.J., Rohrbach P. Monitoring PfMDR1 transport in plasmodium falciparum. Malar. J. 2015;14(1):270. doi: 10.1186/s12936-015-0791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaut C., Berry A., Chevalley S., Reybier K., Morlais I., Parzy D., Nepveu F., Benoit-Vical F., Valentin A. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 2008;7:45. doi: 10.1186/1475-2875-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach P., Sanchez C.P., Hayton K., Friedrich O., Patel J., Sidhu A.B.S., Ferdig M.T., Fidock D.A., Lanzer M. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum. EMBO J. 2006;25(13):3000–3011. doi: 10.1038/sj.emboj.7601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann M., McNamara C., Yeung B.K.S., Lee M.C.S., Zou B., Russell B., Seitz P., Plouffe D.M., Dharia N.V., Tan J., Cohen S.B., Spencer K.R., González-Páez G.E., Lakshminarayana S.B., Goh A., Suwanarusk R., Jegla T., Schmitt E.K., Beck H.-P. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329(5996):1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B., Chalfein F., Prasetyorini B., Kenangalem E., Piera K., Suwanarusk R., Brockman A., Prayoga P., Sugiarto P., Cheng Q., Tjitra E., Anstey N.M., Price R.N. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 2008;52(3):1040–1045. doi: 10.1128/AAC.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B.M., Udomsangpetch R., Rieckmann K.H., Kotecka B.M., Coleman R.E., Sattabongkot J. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 2003;47(1):170–173. doi: 10.1128/aac.47.1.170-173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell Bruce, Suwanarusk R., Borlon C., Costa F.T.M., Chu C.S., Rijken M.J., Sriprawat K., Warter L., Koh E.G.L., Malleret B., Colin Y., Bertrand O., Adams J.H., D'Alessandro U., Snounou G., Nosten F., Rénia L. A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood. 2011;118(13):e74–81. doi: 10.1182/blood-2011-04-348748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sà J.M., Cannon M.V., Caleon R.L., Wellems T.E., Serre D. Single-cell transcription analysis of Plasmodium vivax blood-stage parasites identifies stage- and species-specific profiles of expression. PLoS Biol. 2020;18(5) doi: 10.1371/journal.pbio.3000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá J.M., Kaslow S.R., Moraes Barros R.R., Brazeau N.F., Parobek C.M., Tao D., Salzman R.E., Gibson T.J., Velmurugan S., Krause M.A., Melendez-Muniz V., Kite W.A., Han P.K., Eastman R.T., Kim A., Kessler E.G., Abebe Y., James E.R., Chakravarty S. Plasmodium vivax chloroquine resistance links to pvcrt transcription in a genetic cross. Nat. Commun. 2019;10(1):1–10. doi: 10.1038/s41467-019-12256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá, Nomura T., Neves J. d'Arc, Baird J.K., Wellems T.E., del Portillo H.A. Plasmodium vivax: allele variants of the mdr1 gene do not associate with chloroquine resistance among isolates from Brazil, Papua, and monkey-adapted strains. Exp. Parasitol. 2005;109(4):256–259. doi: 10.1016/j.exppara.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Sanchez C.P., Rotmann A., Stein W.D., Lanzer M. Polymorphisms within PfMDR1 alter the substrate specificity for anti-malarial drugs in Plasmodium falciparum. Mol. Microbiol. 2008;70(4):786–798. doi: 10.1111/j.1365-2958.2008.06413.x. [DOI] [PubMed] [Google Scholar]
- Schneider K.A., Escalante A.A. Fitness components and natural selection: why are there different patterns on the emergence of drug resistance in Plasmodium falciparum and Plasmodium vivax? Malar. J. 2013;12:15. doi: 10.1186/1475-2875-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe M.L., Ranjitkar S., Rajakaruna R.S., Amerasinghe P.H., Morales F., Pearce R., Ord R., Leslie T., Rowland M., Gadalla N.B., Konradsen F., Bygbjerg I.C., Roper C., Alifrangis M. Multiple origins of mutations in the mdr1 gene—a putative marker of chloroquine resistance in P. Vivax. PLoS Neglected Trop. Dis. 2015;9(11) doi: 10.1371/journal.pntd.0004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn N., Rarau P., Manong D., Salib M., Siba P., Reeder J.C., Rogerson S.J., Genton B., Mueller I. Effectiveness of artemether/lumefantrine for the treatment of uncomplicated Plasmodium vivax and P. falciparum malaria in young children in Papua New Guinea. Clin. Infect. Dis. 2013;56(10):1413–1420. doi: 10.1093/cid/cit068. An Official Publication of the Infectious Diseases Society of America. [DOI] [PubMed] [Google Scholar]
- Sharrock W.W., Suwanarusk R., Lek-Uthai U., Edstein M.D., Kosaisavee V., Travers T., Jaidee A., Sriprawat K., Price R.N., Nosten F., Russell B. Plasmodium vivax trophozoites insensitive to chloroquine. Malar. J. 2008;7:94. doi: 10.1186/1475-2875-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A.B.S., Valderramos S.G., Fidock D.A. Pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 2005;57(4):913–926. doi: 10.1111/j.1365-2958.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- Silva S.R., Almeida A.C.G., da Silva G.A.V., Ramasawmy R., Lopes S.C.P., Siqueira A.M., Costa G.L., Sousa T.N., Vieira J.L.F., Lacerda M.V.G., Monteiro W.M., de Melo G.C. Chloroquine resistance is associated to multi-copy pvcrt-o gene in Plasmodium vivax malaria in the Brazilian Amazon. Malar. J. 2018;17(1):267. doi: 10.1186/s12936-018-2411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S.R., Almeida A.C.G., da Silva G.A.V., Ramasawmy R., Lopes S.C.P., Siqueira A.M., Costa G.L., Sousa T.N., Vieira J.L.F., Lacerda M.V.G., Monteiro W.M., de Melo G.C. Chloroquine resistance is associated to multi-copy pvcrt-o gene in Plasmodium vivax malaria in the Brazilian Amazon. Malar. J. 2018;17(1):267. doi: 10.1186/s12936-018-2411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotin A., Mahami-Oskouei M., Ahmadpour E., Parsaei M., Rostami A., Emami S., Gholipour S., Farmani M. Global assessment of genetic paradigms of Pvmdr1 mutations in chloroquine-resistant Plasmodium vivax isolates. Trans. R. Soc. Trop. Med. Hyg. 2020;114(5):339–345. doi: 10.1093/trstmh/traa002. [DOI] [PubMed] [Google Scholar]
- Stepniewska K., Ashley E., Lee S.J., Anstey N., Barnes K.I., Binh T.Q., D'Alessandro U., Day N.P.J., de Vries P.J., Dorsey G., Guthmann J.-P., Mayxay M., Newton P.N., Olliaro P., Osorio L., Price R.N., Rowland M., Smithuis F., Taylor W.R.J. In vivo parasitological measures of artemisinin susceptibility. J. Infect. Dis. 2010;201(4):570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Kirkman L.A., Fujioka H., Wellems T.E. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell. 1997;91(5):593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- Sumawinata I.W., Bernadeta null, Leksana B., Sutamihardja A., Purnomo null, Subianto B., Sekartuti null, Fryauff D.J., Baird J.K. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am. J. Trop. Med. Hyg. 2003;68(4):416–420. [PubMed] [Google Scholar]
- Summers R.L., Dave A., Dolstra T.J., Bellanca S., Marchetti R.V., Nash M.N., Richards S.N., Goh V., Schenk R.L., Stein W.D., Kirk K., Sanchez C.P., Lanzer M., Martin R.E. Diverse mutational pathways converge on saturable chloroquine transport via the malaria parasite's chloroquine resistance transporter. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111(17):E1759–E1767. doi: 10.1073/pnas.1322965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutanto I., Endawati D., Ling L.H., Laihad F., Setiabudy R., Baird J.K. Evaluation of chloroquine therapy for vivax and falciparum malaria in southern Sumatra, western Indonesia. Malar. J. 2010;9:52. doi: 10.1186/1475-2875-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanarusk, Chavchich M., Russell B., Jaidee A., Chalfein F., Barends M., Prasetyorini B., Kenangalem E., Piera K.A., Lek-Uthai U., Anstey N.M., Tjitra E., Nosten F., Cheng Q., Price R.N. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J. Infect. Dis. 2008;198(10):1558–1564. doi: 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanarusk, Russell B., Chavchich M., Chalfein F., Kenangalem E., Kosaisavee V., Prasetyorini B., Piera K.A., Barends M., Brockman A., Lek-Uthai U., Anstey N.M., Tjitra E., Nosten F., Cheng Q., Price R.N. Chloroquine resistant plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PloS One. 2007;2(10):e1089. doi: 10.1371/journal.pone.0001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala-Harrison S., Jacob C.G., Arze C., Cummings M.P., Silva J.C., Dondorp A.M., Fukuda M.M., Hien T.T., Mayxay M., Noedl H., Nosten F., Kyaw M.P., Nhien N.T.T., Imwong M., Bethell D., Se Y., Lon C., Tyner S.D., Saunders D.L. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J. Infect. Dis. 2015;211(5):670–679. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talundzic E., Ndiaye Y.D., Deme A.B., Olsen C., Patel D.S., Biliya S., Daniels R., Vannberg F.O., Volkman S.K., Udhayakumar V., Ndiaye D. Molecular epidemiology of plasmodium falciparum kelch13 mutations in Senegal determined by using targeted amplicon deep sequencing. Antimicrob. Agents Chemother. 2017;61(3) doi: 10.1128/AAC.02116-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W.R.J., Thriemer K., von Seidlein L., Yuentrakul P., Assawariyathipat T., Assefa A., Auburn S., Chand K., Chau N.H., Cheah P.Y., Dong L.T., Dhorda M., Degaga T.S., Devine A., Ekawati L.L., Fahmi F., Hailu A., Hasanzai M.A., Hien T.T. Short-course primaquine for the radical cure of Plasmodium vivax malaria: a multicentre, randomised, placebo-controlled non-inferiority trial. Lancet (London, England) 2019;394(10202):929–938. doi: 10.1016/S0140-6736(19)31285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjitra E., Baker J., Suprianto S., Cheng Q., Anstey N.M. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob. Agents Chemother. 2002;46(12):3947–3953. doi: 10.1128/aac.46.12.3947-3953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townell N., Looke D., McDougall D., McCarthy J.S. Relapse of imported Plasmodium vivax malaria is related to primaquine dose: a retrospective study. Malar. J. 2012;11:214. doi: 10.1186/1475-2875-11-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun K.M., Imwong M., Lwin K.M., Win A.A., Hlaing T.M., Hlaing T., Lin K., Kyaw M.P., Plewes K., Faiz M.A., Dhorda M., Cheah P.Y., Pukrittayakamee S., Ashley E.A., Anderson T.J.C., Nair S., McDew-White M., Flegg J.A., Grist E.P.M. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. The Lancet. Infectious Diseases. 2015;15(4):415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pluijm R.W., Imwong M., Chau N.H., Hoa N.T., Thuy-Nhien N.T., Thanh N.V., Jittamala P., Hanboonkunupakarn B., Chutasmit K., Saelow C., Runjarern R., Kaewmok W., Tripura R., Peto T.J., Yok S., Suon S., Sreng S., Mao S., Oun S. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. The Lancet. Infectious Diseases. 2019;19(9):952–961. doi: 10.1016/S1473-3099(19)30391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del C M Vargas-Rodríguez R., da Silva Bastos M., Menezes M.J., Orjuela-Sánchez P., Ferreira M.U. Single-nucleotide polymorphism and copy number variation of the multidrug resistance-1 locus of plasmodium vivax: local and global patterns. Am. J. Trop. Med. Hyg. 2012;87(5):813–821. doi: 10.4269/ajtmh.2012.12-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.M., Pinapati R.S., Cheeseman I.H., Camargo N., Fishbaugher M., Checkley L.A., Nair S., Hutyra C.A., Nosten F.H., Anderson T.J.C., Ferdig M.T., Kappe S.H.I. Plasmodium falciparum genetic crosses in a humanized mouse model. Nat. Methods. 2015;12(7):631–633. doi: 10.1038/nmeth.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga M. Isabel, Dhingra S.K., Henrich P.P., Straimer J., Gnädig N., Uhlemann A.-C., Martin R.E., Lehane A.M., Fidock D.A. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat. Commun. 2016;7:11553. doi: 10.1038/ncomms11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga Maria Isabel, Osório N.S., Ferreira P.E., Franzén O., Dahlstrom S., Lum J.K., Nosten F., Gil J.P. Complex polymorphisms in the plasmodium falciparum multidrug resistance protein 2 gene and its contribution to antimalarial response. Antimicrob. Agents Chemother. 2014;58(12):7390–7397. doi: 10.1128/AAC.03337-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrely K.M., Kumar S., Li X., Vaughan A.M. Humanized mice and the rebirth of malaria genetic crosses. Trends Parasitol. 2020;36(10):850–863. doi: 10.1016/j.pt.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzier L.H., Coyle R., Singh S., Sanderson T., Rayner J.C. Plasmodium knowlesi as a model system for characterising Plasmodium vivax drug resistance candidate genes. PLoS Neglected Trop. Dis. 2019;13(6) doi: 10.1371/journal.pntd.0007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg-van der Wel A.M., Zeeman A.-M., Nieuwenhuis I.G., van der Werff N.M., Klooster E.J., Klop O., Vermaat L.C., Kocken C.H.M. Dual-luciferase-based fast and sensitive detection of malaria hypnozoites for the discovery of antirelapse compounds. Anal. Chem. 2020;92(9):6667–6675. doi: 10.1021/acs.analchem.0c00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg-van der Wel A., Zeeman A.-M., van Amsterdam S.M., van den Berg A., Klooster E.J., Iwanaga S., Janse C.J., van Gemert G.-J., Sauerwein R., Beenhakker N., Koopman G., Thomas A.W., Kocken C.H.M. Transgenic fluorescent Plasmodium cynomolgi liver stages enable live imaging and purification of Malaria hypnozoite-forms. PloS One. 2013;8(1) doi: 10.1371/journal.pone.0054888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T.E., Walker-Jonah A., Panton L.J. Genetic mapping of the chloroquine-resistance locus on Plasmodium falciparum chromosome 7. Proc. Natl. Acad. Sci. U.S.A. 1991;88(8):3382–3386. doi: 10.1073/pnas.88.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar. J. 2011;10(1):297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2007. Technical Expert Group Meeting on Intermittent Preventive Treatment in Pregnancy (IPTp) July. [Google Scholar]
- WHO . WHO; World Health Organization; 2019. WHO | Intermittent Preventive Treatment in Pregnancy (IPTp)http://www.who.int/malaria/areas/preventive_therapies/pregnancy/en/ [Google Scholar]
- WHO . vol. 1. WHO; 2019. (World Malaria Report 2019). [Google Scholar]
- Wicht K.J., Mok S., Fidock D.A. Molecular mechanisms of drug resistance in plasmodium falciparum malaria. Annu. Rev. Microbiol. 2020;74(1):431–454. doi: 10.1146/annurev-micro-020518-115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilairatana P., Silachamroon U., Krudsood S., Singhasivanon P., Treeprasertsuk S., Bussaratid V., Phumratanaprapin W., Srivilirit S., Looareesuwan S. Efficacy of primaquine regimens for primaquine-resistant Plasmodium vivax malaria in Thailand. Am. J. Trop. Med. Hyg. 1999;61(6):973–977. doi: 10.4269/ajtmh.1999.61.973. [DOI] [PubMed] [Google Scholar]
- Wirjanata G., Handayuni I., Prayoga P., Apriyanti D., Chalfein F., Sebayang B.F., Kho S., Noviyanti R., Kenangalem E., Campo B., Poespoprodjo J.R., Price R.N., Marfurt J. Quantification of Plasmodium ex vivo drug susceptibility by flow cytometry. Malar. J. 2015;14:417. doi: 10.1186/s12936-015-0940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz N., Fall B., Pascual A., Fall M., Baret E., Camara C., Nakoulima A., Diatta B., Fall K.B., Mbaye P.S., Diémé Y., Bercion R., Wade B., Pradines B. Role of Pfmdr1 in in vitro plasmodium falciparum susceptibility to chloroquine, quinine, monodesethylamodiaquine, mefloquine, lumefantrine, and dihydroartemisinin. Antimicrob. Agents Chemother. 2014;58(12):7032–7040. doi: 10.1128/AAC.03494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WWARN Artemether-lumefantrine treatment of uncomplicated Plasmodium falciparum malaria: a systematic review and meta-analysis of day 7 lumefantrine concentrations and therapeutic response using individual patient data. BMC Med. 2015;13:227. doi: 10.1186/s12916-015-0456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WWARN, Dahal P., d'Alessandro U., Dorsey G., Guerin P.J., Nsanzabana C., Price R.N., Sibley C.H., Stepniewska K., Talisuna A.O. Clinical determinants of early parasitological response to ACTs in African patients with uncomplicated falciparum malaria: a literature review and meta-analysis of individual patient data. BMC Med. 2015;13:212. doi: 10.1186/s12916-015-0445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogavel M., Nettleship J.E., Sharma A., Harlos K., Jamwal A., Chaturvedi R., Sharma M., Jain V., Chhibber-Goel J., Sharma A. Structure of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase–dihydropteroate synthase from Plasmodium vivax sheds light on drug resistance. J. Biol. Chem. 2018;293(39):14962–14972. doi: 10.1074/jbc.RA118.004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannes A.M., Teklehaimanot A., Bergqvist Y., Ringwald P. Confirmed vivax resistance to chloroquine and effectiveness of artemether-lumefantrine for the treatment of vivax malaria in Ethiopia. Am. J. Trop. Med. Hyg. 2011;84(1):137–140. doi: 10.4269/ajtmh.2011.09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang L., Soe M.T., Aung P.L., Wei H., Liu Z., Ma T., Huang Y., Menezes L.J., Wang Q., Kyaw M.P., Nyunt M.H., Cui L., Cao Y. Molecular surveillance for drug resistance markers in Plasmodium vivax isolates from symptomatic and asymptomatic infections at the China–Myanmar border. Malar. J. 2020;19(1):281. doi: 10.1186/s12936-020-03354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.