Abstract
Objective: To review the evidence and recommendations for the use of adjunctive corticosteroid therapy in community-acquired pneumonia (CAP). Data Sources: A literature search was conducted using PubMed (1993 to November 2020) using the search terms corticosteroids AND community-acquired pneumonia. Study Selection and Data Extraction: Pertinent randomized controlled trials, systematic reviews, and meta-analyses assessing the efficacy and safety of adjunctive corticosteroids in patients with pneumonia were evaluated for inclusion. Data Synthesis: Studies suggest that corticosteroids reduce time to clinical stability and length of hospital stay, but data regarding other important clinical outcomes, such as mortality, are limited. The greatest margin of benefit appears to be in patients with severe CAP. Evidence consistently demonstrates hyperglycemia as the most common adverse effect of corticosteroid therapy in CAP. Safety concerns regarding the potential impact of corticosteroids on the rate of CAP-related rehospitalizations require further investigation. Relevance to Patient Care and Clinical Practice: This review summarizes literature evaluating the efficacy and safety of adjunctive corticosteroids in patients with CAP. It also includes a discussion on current guideline recommendations, patient selection, corticosteroid regimens, adverse effect considerations, limitations, and future directions in this area of research. Conclusions: Studies reviewed suggest that corticosteroids are relatively beneficial and safe in patients with CAP, with the greatest benefit in severe CAP. Currently, the routine use of corticosteroids is not recommended by clinical practice guidelines with the exception of CAP and refractory septic shock. Further research is needed to better define the ideal role of corticosteroids in CAP.
Keywords: corticosteroids, community-acquired pneumonia, prednisone, methylprednisolone, severe, nonsevere, pneumonia
Introduction
Despite medical advancements, community-acquired pneumonia (CAP) continues to be a highly prevalent disease associated with significant morbidity and mortality. In the United States, an estimated 1.5 million adults are hospitalized annually as a result of CAP and an alarming 1 of every 3 are predicted to die within 1 year.1 According to data reported by the World Health Organization, lower respiratory infections account for the leading infectious cause of mortality worldwide.2 With its significant prevalence and associated consequences, optimizing the management of patients with CAP remains of critical importance.
The pathogenesis of CAP begins with the invasion of bacteria into the lower respiratory tract via inhalation, aspiration of oropharyngeal contents, or hematogenous spread from an extrapulmonary site of infection. Once within the lower airways, microorganisms infiltrate alveoli and replicate while alveolar macrophages attempt phagocytosis. If unable to completely clear these organisms, the immune response is escalated through the release of inflammatory cytokines. This local inflammatory response may lead to damage of the lung parenchyma. As a result, patients with CAP typically present with dyspnea, cough, sputum production, pleuritic chest pain, crackles during lung expansion, and infiltrates or consolidations on chest radiograph.3,4
In some patients, the local inflammatory response is insufficient in controlling infection. Cytokines are released into the bloodstream to recruit inflammatory cells into the lungs, resulting in systemic inflammation. This accounts for the systemic signs and symptoms of pneumonia, such as fever, tachycardia, and leukocytosis. If the systemic response becomes dysregulated and uncontrolled, serious complications can occur as a result of the direct tissue injury and organ damage. Pneumonia can progress to bacteremia, sepsis, acute respiratory distress syndrome, multiorgan failure, and death.3
The Pneumonia Severity Index (PSI) is a clinical prediction model for CAP that classifies patients by estimated disease severity and mortality risk.5 This scoring criteria offers a more comprehensive and objective assessment for risk stratification and is often utilized in research. Points are assigned based on certain patient demographics, comorbidities, physical examination, and laboratory and radiographic findings. Patients are then classified into low (classes I-III), moderate (class IV), or high-risk (class V) groups based on total point score. Patients within PSI risk classes I, II, and III have a lower estimated mortality risk of <1%. Mortality risk is higher for patients within PSI risk classes IV and V at 9.3% and 27%, respectively. Trials evaluating adjunctive corticosteroid therapy in CAP often include the PSI as a measure of CAP severity. In clinical practice, the PSI can be used as a decision-aid to determine whether patients require hospitalization. The PSI has also been used for prediction of clinically important outcomes, such as length of hospital stay and admission to the intensive care unit (ICU).5
Corticosteroids are hormones secreted by the adrenal cortex that have widespread impact on metabolism, immune response, and stress. Corticosteroids are composed of 2 major classes: mineralocorticoids and glucocorticoids. Mineralocorticoids (ie, aldosterone) predominately affect sodium and water retention. Glucocorticoids (ie, cortisol) have profound metabolic effects on carbohydrates, proteins, and lipids. Glucocorticoids also have anti-inflammatory actions through their inhibition of inflammatory mediator production and release.6,7 Table 1 illustrates a comparison of the properties of commonly used synthetic glucocorticoids, including respective anti-inflammatory (glucocorticoid) and salt-retaining (mineralocorticoid) potencies.8
Table 1.
Comparison of Glucocorticoid Properties.8
| Glucocorticoid | GC potency | MC potency | Equivalent dose | Half-life (hours) |
|---|---|---|---|---|
| Hydrocortisone | 1 | 1 | 20 mg | 8-12 |
| Prednisone | 4 | 0.8 | 5 mg | 18-36 |
| Methylprednisolone | 5 | 0.5 | 4 mg | 18-36 |
| Dexamethasone | 25 | 0 | 0.75 mg | >36 |
Abbreviations: GC, glucocorticoid; MC, mineralocorticoid.
Glucocorticoids have activity against peripheral leukocytes and other markers that play a role in the inflammatory response to infection. Inflammation occurs with the release of cytokines and subsequent infiltration of leukocytes into the affected tissue, such as the lung parenchyma in pneumonia. Glucocorticoids block cytokine expression and synthesis, inhibit leukocyte migration to sites of inflammation, and promote other anti-inflammatory effects such as phagocytic removal of cellular debris. Glucocorticoids also have immunosuppressive effects through apoptosis of immune cells. These actions may provide benefit in opposing the detrimental effects of immune system hyperactivity and dysregulation in sepsis.9
Corticosteroids are associated with a range of potential adverse effects due to their widespread actions.7 Adverse effects from short-term corticosteroid use include hyperglycemia, fluid retention, hypertension, and neuropsychiatric effects. Long-term and/or high-dose corticosteroids can increase risk of bacterial and fungal infections, impaired wound healing, weight gain, diabetes, osteoporosis, depressive disorders, child growth suppression, and hirsutism. Abrupt withdrawal of corticosteroid therapy may cause exacerbation of the underlying disease and acute adrenal insufficiency due to hypothalamus-pituitary-adrenal axis suppression.8,9
The adjunctive use of corticosteroids is currently recommended as part of the standard management of several conditions, including chronic obstructive pulmonary disease, meningitis, tuberculosis, and septic shock. The hypothesized benefit of adjunctive corticosteroids in the management of CAP has been a subject of continued research. One proposed mechanism is attributed to corticosteroid-induced downregulation of cytokine production and inhibition of immune cell migration to the lungs, which may reduce pulmonary inflammation and prevent respiratory failure in severe CAP. Another proposed mechanism is related to normalization of the hypothalamus-pituitary-adrenal axis in patients with an inadequate adrenal response to infection due to critical illness-related corticosteroid insufficiency, which is the mechanism for corticosteroids in septic shock. Corticosteroids can also prevent Jarisch-Herxheimer-like reactions on antibiotic initiation in patients with a high bacterial burden. Although these are a few pathophysiologic hypotheses, the true effect of steroids in CAP is not fully known. In addition, individual patient response to steroids may vary due to the range of CAP presentations in terms of severity and etiology of infection.9,10 A number of randomized controlled trials (RCTs), systematic reviews, and meta-analyses have sought to characterize the potential benefits and harms of adjunctive corticosteroid therapy in the management of CAP.
Data Sources
A search was conducted using PubMed to identify literature published in the English language from January 1993 to November 2020 that evaluated the use of adjunctive corticosteroids in the treatment of patients with CAP. Key search terms included corticosteroids AND community-acquired pneumonia.
Study Selection and Data Extraction
Pertinent RCTs, systematic reviews, and meta-analyses were considered for inclusion. Out of the available literature, one RCT and 2 systematic reviews and meta-analyses were selected based on their size, quality, and scope. Clinical practice guidelines on the management of CAP were also reviewed and included for discussion in this article.
Results
Randomized Controlled Trial: Blum et al11
In 2015, Blum and colleagues published a RCT comparing a 7-day course of prednisone 50 mg daily versus placebo in 802 patients hospitalized with CAP. The primary outcome was time to clinical stability, defined as stable vital signs for at least 24 hours as well as mental status return to baseline and ability for oral intake. Secondary outcomes included time to hospital discharge, pneumonia-associated complications, and the incidence of all-cause mortality, recurrence of pneumonia, or readmission to the hospital within 30 days. Prespecified subgroup analyses of patient age, C-reactive protein (CRP) level, severity of CAP based on PSI score, bacteremia, and chronic obstructive pulmonary disease diagnosis were also evaluated to determine whether a specific subset of patients derived greater benefit from adjunctive corticosteroid therapy.
A total of 785 patients were included in the intention-to-treat analysis with 392 in the prednisone group and 393 assigned to placebo. The median vital signs at baseline within both groups met primary outcome criteria for clinical stability, which provides some context when assessing the overall level of critical illness within this study. Based on PSI classification, about half of patients had lower versus higher severity pneumonia (PSI classes I-III vs IV-V, respectively). Twenty percent of patients had a diagnosis of diabetes mellitus at baseline.
The primary outcome of time to clinical stability was shorter in the prednisone group compared with the placebo group (3 days vs 4.4 days; hazard ratio = 1.33 [95% confidence interval (CI) = 1.15-1.5, P < .0001]). Patients in the prednisone group also had a shorter median time to effective hospital discharge (6 days vs 7 days; hazard ratio = 1.19 [95% CI = 1.04-1.38, P = .012]). No significant differences were observed in the overall incidence of pneumonia-associated complications and rates of recurrent pneumonia, hospital readmission, and all-cause mortality. The incidence of inpatient hyperglycemia requiring treatment with insulin was significantly higher in the prednisone group (19% vs 11%; odds ratio [OR] = 1.96 [95% CI = 1.31-2.93]). Other corticosteroid-related adverse effects were infrequent and similar between groups. There was no evidence of effect modification in any of the prespecified subgroups.
The authors concluded that treatment with prednisone for 7 days in patients hospitalized with CAP shortens time to clinical stability without an increase in complications. Based on study definitions, a 1.4-day reduction was seen in time to clinical stability as well as a 1-day reduction in time to hospital discharge. Although the true significance of clinical stability is uncertain, time to hospital discharge is a clinically important and appropriate outcome for evaluation of efficacy in a trial of this size. A potential limitation of the primary outcome definition is that it does not account for other factors that may affect the determination of clinical stability, such as physical examination findings or persistence of significant pneumonia symptoms. Despite being one of the largest RCTs on this topic at the time of publication, this study is limited by its sample size for other important endpoints, such as mortality. In addition, the subgroup analyses were not adequately powered to detect a difference. With promising results for potential benefit of corticosteroids in patients with CAP, this trial likely influenced subsequent research on this topic.
IDSA Systematic Review and Meta-Analysis: Briel et al12
A systematic review and meta-analysis by Briel and colleagues in 2018 utilized individual patient data (IPD) from 6 RCTs to evaluate adjunctive corticosteroid therapy in adults hospitalized with CAP.11,13-17 Previous RCTs investigating this topic were limited in their evaluation of subgroup differences due to inadequate statistical power. With the inclusion of multiple RCTs, one objective of this meta-analysis was to explore these subgroup differences and identify whether a specific subset of patients may derive greater benefit from corticosteroids.
Of the 6 RCTs included in this IPD meta-analysis, 3 specifically studied adults with severe CAP.15-17 However, these trials were smaller in size, representing only 218 (15%) of the 1509 patients in the meta-analysis. Two RCTs included all patients hospitalized with CAP regardless of severity and one included adults with CAP not requiring intensive care.11,13,14 The RCTs specifically studying patients with severe CAP each used an intravenous (IV) corticosteroid (ie, hydrocortisone, methylprednisolone).15-17 The other 3 RCTs used prednisone 50 mg orally daily, prednisolone 40 mg orally or IV daily, and dexamethasone 5 mg IV daily.11,13,14 The average duration of corticosteroid therapy between all included RCTs was 6.5 days.
In total, 748 patients received corticosteroid therapy and 758 were assigned to placebo. Approximately 10% of patients were directly admitted to the ICU and 75% had 2 or more systemic inflammatory response syndrome (SIRS) criteria. Based on PSI classification, about half of the patients were considered to have severe CAP. About 17% of patients had a diagnosis of diabetes mellitus at baseline.
The primary outcome of all-cause mortality at 30 days showed no difference in the corticosteroid group compared with placebo (5% vs 5.9%; OR = 0.75 [95% CI = 0.46-1.21, P = .24]). However, time to clinical stability (3 vs 4 days; OR = −1.03 [95% CI = −1.62 to −0.43, P = .001]) and length of hospital stay (7 vs 8 days; OR = −1.15 [95% CI= −1.75 to −0.55, P < .001]) were both approximately 1 day shorter in the group receiving corticosteroids. Rates of both early and late treatment failure were similar between groups. Raising a potential safety concern, an increase in CAP-related rehospitalization within 30 days of discharge was observed in the corticosteroid group (5% vs 2.7%; OR = 1.85 [95% CI = 1.03-3.32, P = .04]; NNH [number needed to harm] = 45). In addition, there was a higher incidence of inpatient hyperglycemia requiring insulin with the use of corticosteroids (22% vs 12%; OR = 2.15 [95% CI = 1.60-2.90]). In this IPD meta-analysis, the prespecified subgroup analyses for the primary outcome did not demonstrate statistically significant differences. However, the subgroups that assessed CAP severity based on PSI classification, initial ICU admission, and SIRS criteria each demonstrated a trend toward potential mortality benefit in patients with more severe illness.
The authors concluded that adjunctive treatment with corticosteroids in patients hospitalized with CAP reduces time to clinical stability and length of stay without a significant effect on mortality. Adverse outcomes include a potential increase in risk for CAP-related rehospitalization and hyperglycemia. The design of this study as an IPD meta-analysis confers a number of strengths. Access to individual patient data allows for the standardization of definitions and analysis across studies. Most of the included trials met high methodological quality standards, thereby increasing internal validity and minimizing the potential for bias. The large study population consisting of more than 1500 patients improves statistical power in studying important outcomes. However, 3 eligible RCTs that specifically evaluated patients with severe CAP were excluded from this meta-analysis due to inability to obtain individual patient data. Concern has been expressed that the exclusion of these RCTs limited the power of mortality assessment in this subpopulation of patients with severe CAP.18 Another potential limitation of this meta-analysis is confounding related to the difference in management of patients across trials (ie, corticosteroid regimens, antimicrobial therapy).
Cochrane Systematic Review and Meta-Analysis: Stern et al19
In December 2017, an updated Cochrane review and meta-analysis assessing the efficacy and safety of corticosteroids in the treatment of pneumonia was published by Stern and colleagues.19 With the inclusion of 17 RCTs conducted worldwide, this meta-analysis examined a much larger sample population of 2264 total patients. Of the 13 RCTs conducted in adults with CAP, 7 trials only included adults with severe CAP.15-17,20-23 However, a total of 9 trials provided data for 995 adults with severe CAP in this meta-analysis overall.
The corticosteroid interventions differed between the RCTs in terms of the agent, dose, and duration of therapy. However, most trials used steroid doses equivalent to 40 to 50 mg of prednisone per day for a total of 5 to 7 days. Table 2 outlines the corticosteroid interventions for each trial included in this meta-analysis.11,13-17,20-26
Table 2.
Cochrane Systematic Review and Meta-Analysis.19
| Trial | Number of patients | Population | Corticosteroid regimen | Duration of therapy |
|---|---|---|---|---|
| Mikami et al24 | 31 | Inpatient with CAP (non-ICU) | Prednisolone 40 mg IV daily | 3 days |
| Meijvis et al13 | 304 | Inpatient with CAP (non-ICU) | Dexamethasone 5 mg IV daily | 4 days |
| McHardy et al25 | 126 | Inpatient with CAP (any severity) | Prednisolone 20 mg PO daily | 7 days |
| Hatakeyama et al26 | 30 | Inpatient with CAP (any severity) | Methylprednisolone 20 mg daily | 3 days |
| Snijders et al14 | 213 | Inpatient with CAP (any severity) | Prednisolone 40 mg PO or IV daily | 7 days |
| Blum et al11 | 785 | Inpatient with CAP (any severity) | Prednisone 50 mg PO daily | 7 days |
| Marik et al20 | 30 | Inpatient with severe CAP | Hydrocortisone 10 mg/kg IV × 1 dose | 1 dose |
| Confalonieri et al15 | 46 | Inpatient with severe CAP | Hydrocortisone 200 mg IV bolus, then 10 mg/h | 7 days |
| El-Ghamrawy et al21 | 34 | Inpatient with severe CAP | Hydrocortisone 200 mg IV bolus, then 10 mg/h | 7 days |
| Sabry et al22 | 80 | Inpatient with severe CAP | Hydrocortisone 200 mg IV bolus, then 12.5 mg/h | 7 days |
| Fernández-Serrano et al16 | 56 | Inpatient with severe CAP | Methylprednisolone 200 mg IV bolus, then tapering infusion (3.3-0.8 mg/h) | 9 days |
| Nafae et al23 | 80 | Inpatient with severe CAP | Hydrocortisone 200 mg IV bolus, then 10 mg/h | 7 days |
| Torres et al17 | 120 | Inpatient with severe CAP | Methylprednisolone 0.5 mg/kg IV BID | 5 days |
Abbreviations: CAP, community-acquired pneumonia; ICU, intensive care unit; IV, intravenous; PO, by mouth; BID, twice daily.
The primary outcome of all-cause mortality within 30 days demonstrated a significant benefit of adjunctive corticosteroids in all adults with CAP (relative risk [RR] = 0.66 [95% CI = 0.47-0.92]). However, this difference appeared to be attributed to adults with severe CAP (RR = 0.58 [95% CI = 0.40-0.84]) as there was no significant mortality difference observed in adults with nonsevere CAP (RR = 0.95 [95% CI = 0.45-2.00]).
Corticosteroid therapy significantly reduced the incidence of early clinical failure in all adults regardless of CAP severity classification (RR = 0.40 [95% CI 0.23-0.70]). However, the effect was larger in the subgroup of patients with severe CAP (RR = 0.32 [95% CI = 0.15-0.70]) compared with adults with nonsevere CAP (RR = 0.68 [95% CI = 0.56-0.83]). Both time to clinical cure and duration of hospital stay were reduced in the corticosteroid arm compared with placebo (−1.8 days [95% CI = −2.5 to −1.2] and −2.9 days [95% CI −4.3 to −0.9], respectively).
Although more adverse events occurred in the corticosteroid group, no statistically significant difference was found between groups overall (RR = 1.21 [95% CI = 0.99-1.47]). For the individual adverse outcomes of gastrointestinal bleeding, neuropsychiatric events, and adverse cardiac events, no significant differences were identified, but there was a significantly higher rate of hyperglycemia in the corticosteroid arm compared with placebo (RR = 1.72 [95% CI = 1.38-2.14]).
The authors concluded that the results of this meta-analysis support the use of corticosteroids in adults with severe CAP due to reduced mortality, clinical failure, complication rates, length of hospitalization, and time to clinical cure. They indicated that adults with nonsevere CAP may derive benefit as well, but without survival advantage.
A major strength of this meta-analysis is the large study population that provided adequate power to assess mortality. The large sample size also improved the assessment of subgroup analyses based on CAP severity. However, there are also several limitations of this meta-analysis. Allocation concealment was unclear in some of the trials, which may increase risk of selection bias. In addition, there were differences in outcome definitions of early clinical failure and time to clinical cure between trials. This inconsistency can lead to indirectness and lower the quality of evidence. Heterogeneity, as indicated by the I2 index, was used to quantify the percentage of variation across studies due to heterogeneity rather than chance. Some secondary outcome analyses demonstrated significant heterogeneity, lowering the quality of evidence. However, the primary outcome of mortality is an objective measure and demonstrated minimal heterogeneity, offering reassurance in this result. Overall, this Cochrane meta-analysis contributed to evidence supporting the use of adjunctive corticosteroid therapy specifically in adults with severe CAP.
Discussion
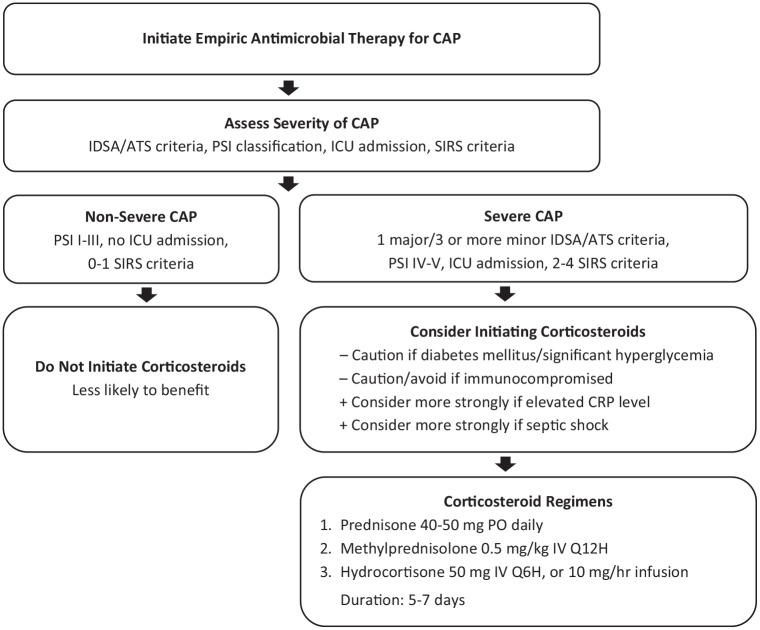
A number of clinical trials and meta-analyses performed over the past couple of decades have sought to identify the impact of corticosteroids in the treatment of CAP. Some trials have shown significant benefit, such as reducing hospital length of stay, while others have demonstrated a potential for harm. The corticosteroid interventions (eg, agent, dose, duration) as well as the patient populations (eg, CAP severity, study sample sizes) have varied significantly making comparison difficult. With inconsistent results, the utilization of corticosteroids as adjunctive therapy in CAP has remained controversial. Figure 1 provides an algorithm that may be considered for the use of adjunctive corticosteroids in the management of CAP after further investigation based on the evidence and discussion presented in this review article.
Figure 1.
Use of Adjunctive Corticosteroids in Community-Acquired Pneumonia: Management Algorithm: for Future Investigation.
Abbreviations: CAP, community-acquired pneumonia; CRP, C-reactive protein; ICU, intensive care unit; IDSA/ATS, Infectious Diseases Society of America/American Thoracic Society; PSI, Pneumonia Severity Index; SIRS, systemic inflammatory response syndrome.
Guideline Recommendations
The Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) consensus guidelines on the management of CAP published in 2007 do not make any definitive recommendations on the use of corticosteroids.27 However, a new set of clinical practice guidelines for the diagnosis and treatment of CAP were put forth by the IDSA/ATS in August 2019. Within these guidelines, routine use of corticosteroids in adults with nonsevere CAP was not recommended (strong recommendation, high quality of evidence). It also suggested not routinely using corticosteroids in adults with severe CAP (conditional recommendation, moderate quality of evidence) or severe influenza pneumonia (conditional recommendation, low quality of evidence). The guidelines did endorse the Surviving Sepsis Campaign recommendations on the use of corticosteroids in patients with CAP and refractory septic shock. The rationale for these recommendations were based on the lack of data suggesting benefit in nonsevere CAP and limited data in severe CAP as well as the potential risks of corticosteroid therapy. Hyperglycemia was cited as the predominant risk, but concerns for potential increase in rehospitalization rates and uncertainty in the risk of complications 30 to 90 days following corticosteroid therapy were also noted in their rationale.28
Patient Selection
The selection of a patient or population likely to benefit from adjunctive corticosteroid therapy has remained a component of uncertainty in this debate. Available literature suggests that patients with a higher severity of CAP may derive the greatest benefit from corticosteroids.10,29-31 Severe CAP has been variably defined in the literature according to PSI classification (class IV or V), SIRS criteria (2 or more), and ICU admission. The IDSA/ATS guidelines refer to their own validated criteria for defining severe CAP (Table 3).27,28 The rationale for corticosteroid therapy in this population is to suppress the exaggerated, maladaptive inflammatory process often seen in patients with critical illness and respiratory distress due to severe pneumonia.10 Other measures of inflammation, such as CRP, may prove useful in identifying specific patients who may respond favorably to corticosteroids. With the expansion of precision medicine in health care, the use of specific biomarkers may guide the selection of corticosteroid treatment for patients with CAP in the future. Further research is needed to investigate the correlation between specific biomarkers and the inflammatory process in CAP, define potential thresholds for corticosteroid intervention or de-escalation, and evaluate the clinical impact of a biomarker-guided approach to therapy.32,33
Table 3.
| Minor criteria | Major criteria |
|---|---|
| Respiratory rate ≥30 breaths/min PaO2/FIO2 ratio ≤250 Multilobar infiltrates Confusion/disorientation Uremia (BUN ≥20 mg/dL) Leukopeniab (WBC <4000 cells/µL) Thrombocytopenia (platelet count <100 000/µL) Hypothermia (core T < 36 °C) Hypotension requiring aggressive fluid resuscitation |
Septic shock with need for vasopressors Respiratory failure requiring mechanical ventilation |
Abbreviations: IDSA/ATS, Infectious Diseases Society of America/American Thoracic Society; CAP, community-acquired pneumonia; PaO2, partial pressure of arterial oxygen; FIO2, percentage of inspired oxygen; BUN, blood urea nitrogen; WBC, white blood cell count.
Validated definition of severe CAP includes either 1 major criterion or ≥3 minor criteria.
Due to infection alone (ie, not chemotherapy induced).
Corticosteroid Regimen
The optimal pharmacologic strategy in terms of corticosteroid agent, dose, route, frequency, and duration has yet to be clearly defined by the literature. Agents with the most evidence include prednisone and methylprednisolone. Most trials have utilized corticosteroid doses equivalent to 40 to 50 mg of prednisone per day. When methylprednisolone is used, a dosing strategy of 0.5 mg/kg IV every 12 hours is best supported by evidence to date. Corticosteroid treatment has been most frequently studied for total duration of 5 to 7 days without a taper. Table 2 outlines the corticosteroid interventions evaluated by the trials reviewed within this article.
Adverse Effect Considerations
Although trials evaluating corticosteroids for use in the CAP population have demonstrated limited safety concerns with short courses of therapy, there are a few adverse effect considerations to mention. One consistently identified adverse effect is hyperglycemia, at times requiring insulin initiation, in corticosteroid treatment groups. This known adverse effect of corticosteroids may be factored into the clinical decision-making process for their use, especially in patients with diabetes or significant hyperglycemia. Immunocompromised patients may serve as a population in which the risk of harm outweighs the potential benefit of adjunctive corticosteroid therapy. These patients were excluded from the majority of trials due to safety concerns of further immunosuppression with corticosteroids. Briel and colleagues found an increase in CAP-related rehospitalizations with the use of corticosteroids in their systematic review and meta-analysis.12 This finding was not replicated in the Cochrane systematic review and meta-analysis, but does introduce a safety concern for the use of adjunctive corticosteroids in CAP and could be an endpoint to confirm or refute with future research.19
Limitations of Research
Individual RCTs evaluating the use of corticosteroids in CAP have been limited in their analysis of important clinical outcomes due to small sample sizes. Some of the major meta-analyses conducted on this subject are limited by heterogeneity to draw definitive conclusions on certain clinical outcomes, such as time to clinical cure and early clinical failure. Variability in CAP trial populations in terms of the severity of illness likely has a significant impact on outcomes with corticosteroid therapy. In addition, the variability in corticosteroid regimens serves as another limiting factor when seeking to apply these results in clinical practice.
Future Direction
Future research is needed to define the optimal corticosteroid regimen for adjunctive use in CAP treatment in terms of agent, dose, route, frequency, and duration of therapy. In addition, further research would be beneficial in determining the optimal patient population to benefit from this therapy, whether using a CAP severity, biomarker-guided, or other approach.
The 2019 ATS/IDSA guidelines highlight the need for large, multicenter, RCTs with well-defined inclusion and exclusion criteria to address the limitations of research to date on this subject. The guidelines also advise that multiple clinically relevant outcomes are measured in order to designate patient subsets that may derive benefit or potential harm from corticosteroid therapy.28
Relevance to Patient Care and Clinical Practice
CAP is a highly prevalent infectious disease that also carries a significant risk for morbidity and mortality. Optimization of therapy is crucial to prevent complications and improve outcomes in patients with CAP. Currently, the standard management of CAP does not include adjunctive corticosteroid therapy. Corticosteroids have shown beneficial effects in the treatment of some disease states but are also known to have significant adverse effects. Historically, there has been limited evidence to recommend their use as a part of the standard management of CAP. Recently published literature and clinical practice guidelines are beginning to offer improved insight and direction for the use of systemic corticosteroids as adjunctive therapy in certain subsets of patients with CAP.
Conclusions
The trial and meta-analyses reviewed in this article present some of the most relevant published data on the use of corticosteroids in CAP. Results from these studies suggest that adjunctive corticosteroid therapy is relatively beneficial and safe in the management of patients with CAP with the greatest margin of benefit demonstrated in patients with severe CAP. Currently, the routine use of corticosteroids in adults with CAP is not recommended by clinical practice guidelines with the exception of those with CAP and refractory septic shock. Further research is needed to better define the ideal patient subset, optimal regimen, and ascertain the full safety concerns with the use of corticosteroids as an adjunctive therapy in the treatment of CAP.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Lindsay K. Harris  https://orcid.org/0000-0002-4704-6247
https://orcid.org/0000-0002-4704-6247
Andrew J. Crannage  https://orcid.org/0000-0002-8898-1493
https://orcid.org/0000-0002-8898-1493
References
- 1. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65:1806-1812. doi: 10.1093/cid/cix647 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Global health estimates: life expectancy and leading causes of death and disability. Accessed December 18, 2020. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates
- 3. Blackford MG, Zasowski EJ. Lower respiratory tract infections. In: DiPiro JT, Yee GC, Posey LM, Haines ST, Nolin TD, Ellingrod V, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. McGraw-Hill; 2020. [Google Scholar]
- 4. Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol. 2013;174:193-202. doi: 10.1111/cei.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243-250. doi: 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 6. Hwang AY, Smith SM, Gums JG. Adrenal gland disorders. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. McGraw-Hill; 2017. [Google Scholar]
- 7. Chrousos GP. Adrenocorticosteroids and adrenocortical antagonists. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2018. [Google Scholar]
- 8. Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63:655-670. doi: 10.4187/respcare.06314 [DOI] [PubMed] [Google Scholar]
- 9. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711-1723. doi: 10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- 10. Garnacho-Montero J, Barrero-García I, Gómez-Prieto MG, Martín-Loeches I. Severe community-acquired pneumonia: current management and future therapeutic alternatives. Expert Rev Anti Infect Ther. 2018;16:667-677. doi: 10.1080/14787210.2018.1512403 [DOI] [PubMed] [Google Scholar]
- 11. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511-1518. doi: 10.1016/S0140-6736(14)62447-8 [DOI] [PubMed] [Google Scholar]
- 12. Briel M, Spoorenberg SMC, Snijders D, et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data metaanalysis. Clin Infect Dis. 2018;66:346-354. doi: 10.1093/cid/cix801 [DOI] [PubMed] [Google Scholar]
- 13. Meijvis SC, Hardeman H, Remmelts HH, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:2023-2030. doi: 10.1016/S0140-6736(11)60607-7 [DOI] [PubMed] [Google Scholar]
- 14. Snijders D, Daniels JM, de Graaff CS, et al. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975-982. doi: 10.1164/rccm.200905-0808OC [DOI] [PubMed] [Google Scholar]
- 15. Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242-248. doi: 10.1164/rccm.200406-808OC [DOI] [PubMed] [Google Scholar]
- 16. Fernández-Serrano S, Dorca J, Garcia-Vidal C, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care. 2011;15:R96. doi: 10.1186/cc10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686. doi: 10.1001/jama.2015.88 [DOI] [PubMed] [Google Scholar]
- 18. Stern A, Leibovici L, Paul M. Corticosteroids reduce mortality in patients with severe community-acquired pneumonia. Clin Infect Dis. 2018;67:1467. doi: 10.1093/cid/ciy336 [DOI] [PubMed] [Google Scholar]
- 19. Stern A, Skalsky K, Avni T, Carrara E, Leibovici L, Paul M. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017;(12):CD007720. doi: 10.1002/14651858.CD007720.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest. 1993;104:389-392. doi: 10.1378/chest.104.2.389 [DOI] [PubMed] [Google Scholar]
- 21. El-Ghamrawy AH, Shokier MH, Esmat AA. Effects of low-dose hydrocortisone in ICU patients with severe community-acquired pneumonia. Egypt J Chest Dis Tuberculosis. 2006;55:91-99. [Google Scholar]
- 22. Sabry NA, Omar EE. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacol Pharm. 2011;2:73-81. doi: 10.4236/pp.2011.22009 [DOI] [Google Scholar]
- 23. Nafae RM, Ragab MI, Amany FM, et al. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egypt J Chest Dis Tuberculosis. 2013;62:439-445. doi: 10.1016/j.ejcdt.2013.03.009 [DOI] [Google Scholar]
- 24. Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007;185:249-255. doi: 10.1007/s00408-007-9020-3 [DOI] [PubMed] [Google Scholar]
- 25. McHardy VU, Schonell ME. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co-operative controlled trial. Br Med J. 1972;4:569-573. doi: 10.1136/bmj.4.5840.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hatakeyama S, Tachibana A, Suzuki K, Okano H. Treatment of aspiration pneumonia with low-dose methylprednisolone and antibiotics [in Japanese]. Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33:51-56. [PubMed] [Google Scholar]
- 27. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72. doi: 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torres A, Chalmers JD, Dela Cruz CS, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 2019;45:159-171. doi: 10.1007/s00134-019-05519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ceccato A, Ferrer M, Barbeta E, Torres A. Adjunctive therapies for community-acquired pneumonia. Clin Chest Med. 2018;39:753-764. doi: 10.1016/j.ccm.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 31. Bi J, Yang J, Wang Y, et al. Efficacy and safety of adjunctive corticosteroids therapy for severe community-acquired pneumonia in adults: an updated systematic review and meta-analysis. PLoS One. 2016;11:e0165942. doi: 10.1371/journal.pone.0165942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shaddock EJ. How and when to use common biomarkers in community-acquired pneumonia. Pneumonia (Nathan). 2016;8:17. doi: 10.1186/s41479-016-0017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gavrilovic S, Andrijevic A, Mujakovic A, Odeyemi Y, Paralija B, Gajic O. Adjunct corticosteroid treatment in patients with pneumonia: a precision medicine approach. Bosn J Basic Med Sci. 2019;19:315-320. doi: 10.17305/bjbms.2019.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]