Abstract
Objective: To review the safety and efficacy of apixaban for the treatment of nonvalvular atrial fibrillation or venous thromboembolism in patients receiving peritoneal dialysis (PD). Data Sources: A PubMed and MEDLINE search was conducted through December 2020 using the following keywords and Medical Subject Headings (MeSH) terms alone or in various combinations: apixaban, peritoneal dialysis, continuous ambulatory peritoneal dialysis, end-stage renal disease, and hemodialysis. Study Selection and Data Extraction: English-language studies evaluating clinical outcomes pertaining to the use of apixaban in end stage renal disease (ESRD), which included patients receiving peritoneal dialysis were eligible for inclusion. Data Synthesis: Four studies were identified that met inclusion for this review, all retrospective in nature. These studies compared the safety and efficacy of apixaban with standard therapy in ESRD included patients on dialysis, with a very limited number of subjects receiving PD. In these studies, apixaban was shown to be potentially safer and more effective than warfarin. Outcomes did not differentiate between patients receiving PD or not. Conclusions: Use of apixaban in patients receiving PD may be safe and effective based on data from limited patients. Pharmacokinetics and pharmacodynamics of apixaban in the PD setting is an important question that clinicians should consider with use of this medication in the ESRD population. More studies focusing on the PD population are needed to better assess the use of apixaban in this understudied population.
Keywords: apixaban, DOACs, peritoneal dialysis, end-stage renal disease, hemodialysis
Introduction
According to the National Institute of Health, more than 661 000 Americans in the United States have chronic kidney disease and 468 000 are on dialysis.1 Approximately 11% of dialysis is performed via peritoneal dialysis (PD), an alternative method to intermittent hemodialysis (IHD) in the treatment of renal failure.2 Basic principles of dialysis include 2 separate compartments—blood and dialysate—separated by a semipermeable membrane that allows the solute and fluid to be transferred. In PD, the dialysate compartment is the peritoneal cavity, which is separated by the peritoneal membrane, a semipermeable membrane.3 The peritoneal cavity is utilized as the dwell space for the fluids and solutes to be exchanged before the dialysate fluid is removed.3 A major difference between IHD and PD include the duration of time. Peritoneal dialysis utilizes a prolonged dwell time that allows for the removal of solutes and fluids to occur over many hours, while IHD typically occurs over 3 to 5 hours thrice a week.3,4 In patients with end-stage renal disease (ESRD) undergoing dialysis, IHD and PD add additional considerations to disease state management, including medication management, specifically renal dose adjustments.
Along with ESRD, patients undergoing dialysis might have concomitant disease states such nonvalvular atrial fibrillation (NVAF) and/or venous thromboembolism (VTE). Patients with NVAF require anticoagulation therapy to prevent cardioembolic stroke depending on their CHA2DS2VASc score.5 Stroke prevention for NVAF and treatment of VTE occur with an anticoagulant, which may include warfarin or a direct oral anticoagulant (DOAC).5,6 DOACs describe a class of medications composed of anti-Xa inhibitors including apixaban, rivaroxaban, and edoxaban, and the direct thrombin inhibitor, dabigatran. Traditionally, warfarin has been the medication of choice for stroke prevention in NVAF. In the setting of ESRD, anticoagulation for NVAF is controversial as patients are at high risk of both stroke and systemic thromboembolism and bleeding.7 Information regarding both safety and efficacy in this population is vital. Warfarin has been shown to significantly lower stroke risk in patients on IHD in some studies, but DOACs may be preferred for numerous reasons, including ease of dosing, lack of routine monitoring, and fewer drug-drug and drug-food interactions for patients with NVAF.7-9 Specifically, the 2019 American College of Cardiology/American Heart Association/Heart Rhythm Society Focused Update of the 2014 Guideline for the Management of Patient with Atrial Fibrillation recommend either warfarin or apixaban in patients requiring anticoagulation who have ESRD and/or are on dialysis.5 Conversely, for VTE, the American Society of Hematology 2020 Guidelines for Management of VTE cite that renal insufficiency must be considered when selecting an anticoagulant and that ongoing studies evaluating apixaban use in ESRD will further establish its use for VTE.10 At present, apixaban is the only DOAC indicated for use in patients with a creatinine clearance (CrCl) of <30 mL/min.11
Dosing of apixaban is dependent on indication: for prevention of stroke in patients with NVAF apixaban is dosed at 5 mg twice daily, unless they have 2 of the 3 following criteria: age >80 years, weight <60 kg, and/or SCr >1.5 mg/dL, wherein then the dose is 2.5 mg twice daily.11 For initial treatment of acute VTE, apixaban is dosed at 10 mg twice daily for 7 days, followed by 5 mg twice daily. Dosing recommendations for patients with NVAF undergoing hemodialysis align similarly, with recommended doses of 5 mg twice daily unless the patient is above 80 years of age or weighs less than 60 kg. For treatment of patients with VTE on hemodialysis, there are no dose adjustment recommendations provided by the manufacturer.11
Currently, limited studies exist regarding the pharmacodynamics of use of apixaban in dialysis. Wang and colleagues12 revealed that IHD has very little impact on apixaban clearance, as only 27% of apixaban is eliminated by renal mechanisms.12 Stanton and colleagues13 showed apixaban to have comparable safety and efficacy to warfarin in preventing stroke and other cardiac complications in patients with severe renal impairment or ESRD on IHD.13 While apixaban may be safe and efficacious in patients receiving IHD, the question remains regarding its use in patients receiving PD. This remains an important clinical question, as patients on dialysis are often excluded from studies that these guidelines for therapy are based on, and PD makes up about 11% of all dialysis types, yet has a varied mechanism and impact on medications.2,14 The purpose of this study is to review the safety and efficacy of apixaban for treatment of NVAF or VTE in the setting of PD.
Data Sources
An English language PubMed and MEDLINE search was conducted through December 2020 using the following keywords and Medical Subject Headings (MeSH) terms alone or in various combinations: apixaban, peritoneal dialysis, continuous ambulatory peritoneal dialysis, end stage renal disease, and hemodialysis. All published human studies evaluating clinical outcomes pertaining to the use of apixaban in ESRD which included patients receiving PD were eligible for inclusion. Articles from the literature search were evaluated for study inclusion by at least 2 reviewers to eliminate the possibility of selection bias. References from published articles were reviewed for additional citations for study inclusion.
Results
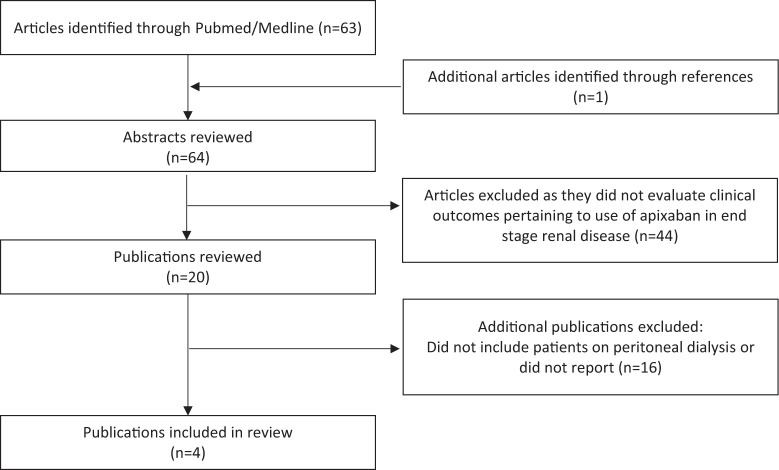
Four studies evaluating apixaban in PD met the inclusion criteria for this review. Figure 1 highlights the inclusion and exclusion of literature.
Figure 1.
Flowchart of publication inclusion.
A retrospective cohort study of Medicare beneficiaries completed by Siontis and colleagues included in the US Renal Data System (a national system that collects, analyzes, and distributes information about kidney disease) characterized the use of apixaban in patients with NVAF or atrial flutter and ESRD on dialysis. This study included the largest subpopulation of patients receiving PD.14 This study also aimed at determining apixaban’s clinical efficacy outcomes when compared with warfarin. A total of 25 523 patients with ESRD on dialysis were included in the study and a subset of 1377 (5.4%) patients were receiving PD. Although the interventions included the DOACs rivaroxaban, dabigatran, apixaban, and edoxaban, only apixaban was used in the comparative analysis with warfarin due to the lack of utilization of the other agents. Overall, 23 172 patients were on warfarin whereas 2351 were on apixaban (either 2.5 mg or 5 mg twice daily). Although there were no differences in safety outcomes such as bleeding between both doses, the 5 mg apixaban dose was associated with lower risks of stroke/systemic embolism (hazard ratio [HR] = 0.61, 95% confidence interval [CI] = 0.37-0.98, P = .04) when compared with warfarin, unlike the 2.5-mg dose, which was not statistically significant (HR = 1.11, 95% CI = 0.82-1.50; P = .49). When compared with warfarin, the apixaban 5 mg twice daily dose and the apixaban 2.5 mg twice daily dose resulted in significantly lower risk of major bleeding (HR = 0.72, 95% CI = 0.69-1.12, P = 0.29; HR = 0.71, 95% CI = 0.56-0.91, P = .007). Subgroup analyses for patients on PD was not reported by the investigators.14
A retrospective, single-center matched-cohort study was conducted by Stanton and colleagues13 to evaluate the safety and efficacy of apixaban in patients with severe renal impairment. Patients were matched by age (≥18 years) and admission date to the study institution (between January 30, 2014, and December 31, 2015). In addition to patients on dialysis (IHD and PD), this study also included patients with severe renal impairment (creatinine clearance <25 mL/min and SCr >2.5 mg/dL). All patients received at least 1 dose of apixaban or warfarin during the study period. One hundred and forty-six patients were included in the study with an equal amount of patients in each arm (73 in the warfarin arm and 73 in the apixaban arm); however, only 42 patients on dialysis made up the patient population, 4 of whom were receiving PD. Outcomes of this study revealed that there was a higher rate of major bleeding in the warfarin arm than apixaban (17.8% vs 9.6%). Composite bleeding rates were also higher with warfarin than apixaban (27.4% vs 21.9%); however, these results were not found to be significant (P = .149 and P = .442). Similar results were seen in major bleeding or composite bleeding risk when comparing patients on IHD or PD to those not receiving dialysis, and these results were not statistically significant. Stroke risk was shown to be similar between the warfarin and apixaban group, with only 8 patients experiencing a stroke. This outcome was not broken down into patient receiving dialysis or not. Recurrent VTE did not occur in either of the treatment groups.13
A retrospective, single-center study of 375 patients with ESRD receiving IHD or PD evaluated clinical outcomes of patients taking either apixaban or warfarin.15 Of the 375 patients with ESRD screened for inclusion for any indication requiring anticoagulation, 74 were included in the apixaban group and 50 in the warfarin group. There was only one patient receiving PD included (in the warfarin arm). Outcomes revealed that warfarin had a higher risk of bleeding events compared with apixaban (42% vs 18.9%; P = .01). Of these 35 bleeding events, major bleeding occurred in 15 of these cases, and was more prevalent in the warfarin group (22%) than apixaban group (5.4%). Recurrent VTE was also shown to have a more frequent occurrence in the warfarin group than the apixaban group (28.6% vs 4.4%, respectively; P = .99).15
Another retrospective, single-center study conducted at a Veterans Affairs Hospital compared safety and efficacy outcomes between apixaban and warfarin in patients with chronic kidney disease (CKD) stage 4, CKD stage 5, and on dialysis.16 A total of 111 patients were included, 54 in the apixaban group with 1 patient receiving PD, and 57 in the warfarin group with no patients receiving PD. No differences were found between groups in major bleeding or incidence of stroke or thromboembolism; however, there were increased rates of minor bleeding (26% vs 6%, P = .004) and composite bleeding (46% vs 20%; P = .004) in the warfarin group. There were also no significant differences in major bleeding when patients were sorted by CKD stage. Individual outcomes for the patient receiving PD was not reported.16
Discussion
There are a limited amount of studies that have incorporated or reported on apixaban-related outcomes in patients receiving PD. Two large retrospective studies reviewed compared apixaban with warfarin use in patients on dialysis.13,14 The percentage of patients receiving PD in each study was very low, with 5.4% of patients receiving PD being the highest percentage of the population. Outcomes of these studies showed that apixaban was potentially safer and more effective than warfarin.13,14,16 Although the increased bleeding risk with warfarin use was deemed insignificant in some studies, they did not differentiate outcomes in IHD versus PD and grouped patients together as “dialysis” patients.13-16
Dosing recommendations for apixaban vary based on age, weight, and renal function.11 Both of the studies including patients receiving PD utilized the 2 possible dosing recommendations of 2.5 mg or 5 mg twice daily.13,14 Studies did not differentiate if patients receiving IHD or PD received a different dose based on any criteria other than the 3 characteristics previously mentioned, indicating that dosing in dialysis patients may not differ significantly from patients without renal impairment.13,14 This lack of data available for patients receiving PD leaves many questions regarding the pharmacokinetic and pharmacodynamic properties of apixaban.
In an open-label, parallel group, single-dose study, the pharmacokinetic and pharmacodynamic of apixaban was studied in patients with ESRD.12 The study included 8 healthy patients and 8 patients with ESRD on IHD. In this study, healthy patients received 1 dose of apixaban 5 mg and the patients with ESRD received 2 doses of apixaban 5 mg separated by 7 days, which was given 2 hours before IHD and immediately after IHD. The half-life was 13 hours versus 20 hours in patients with ESRD and healthy patients, respectively. When comparing Cmax (maximum concentration) and AUCinf (area under the curve, measuring exposure to drug) with healthy subjects, it was found to be 10% lower and 36% higher. About 6.7% of apixaban dose was recovered in the dialysate. Pharmacodynamic studies showed only a small change in international normalized ratio, prothrombin time, and activated partial thromboplastin time between the 2 groups. The study concluded that since CLr in ESRD subjects was negligible, apixaban was eliminated by other routes as well. About 27% of total clearance was due to renal clearance. Due to the pharmacokinetics, pharmacodynamics, and safety findings of this study, the authors concluded that apixaban can be used without dose modification in patients with ESRD maintained on IHD. This study aids in understanding the usage and possible difficulties in patients with ESRD maintained on dialysis.12
Another study by Mavrakanas and colleagues17 found in comparison that the 5 mg twice daily dose would accumulate, leading to supratherapeutic levels after multiple doses of apixaban.17 Unlike the study completed by Wang and colleagues,12 Mayrakanas and colleagues17 evaluated the use of apixaban in 7 patients receiving 2.5 mg twice daily for 8 days rather than only 2 doses. Drug concentrations were measured on days 1 and 8 (non-dialysis days) to compare area under the curve. On day 9 after the first dose of the day, apixaban levels were monitored hourly during dialysis, which resulted in the apixaban levels reducing by 4%. After a 5-day washout period, 5 of the 7 patients received 5 mg twice daily for 8 days. The area under the curve concentration and trough levels were above the 90th percentile for patients on the 5 mg dose with preserved renal function. The authors concluded that patients receiving IHD should receive the 2.5 mg twice daily dose of apixaban as this achieved drug concentrations similar to the median level of patients receiving 5 mg twice daily dose with preserved renal function.17
Nevertheless, the current recommendation for renal dose adjustments for patients with NVAF on hemodialysis are 5 mg twice daily unless the patient is 80 years and older, or has a weight less than 60 kg.11 This is supported by the retrospective cohort study from Sionis, which showed that while 2.5 mg twice daily has a significant lower risk in major bleeding events compared with warfarin, only the 5 mg dose has a significant lower risks of stroke/systemic embolism when compared with warfarin.14
There have been several studies evaluating the use of apixaban in IHD, but the use in PD still remains a question. Both IHD and PD work in a relatively similar manner to filter blood. As studies have concluded that apixaban can be safely used in IHD and renal excretion represents a smaller portion of the total excretion, then it may be reasonable to conclude that apixaban use in PD would be safe and effective as well.
Although studies reviewed were specific to patients with ESRD, there were very few patients receiving PD included, limiting the applicability of such studies to the specific PD patient population. In the study by Siontis and colleagues14 mentioned above, out of the 25 553 participants, only 1377 were receiving PD.14 Studies by Reed and colleagues,15 Herndon and colleagues,16 and Stanton and colleagues13 only included 1, 1, and 4 patients receiving PD, respectively. Globally, among trials, demographics are likely representative of a PD population as a whole but it is important to note that the majority of patients were above 55 years of age. A multicenter approach for future studies on this topic would provide a larger study population including PD population. In addition to the reduced combined sample size, all 4 studies were retrospective studies examining the exposures to risk and the outcomes. Although this type of study design might be easier to conduct, results can be subject to bias and confounding if the study is not well controlled.
Apixaban is a frequently used alternative to warfarin in patients with ESRD receiving dialysis due to pharmacokinetic data and increasing experience with its use in this patient population. Many providers have become comfortable with the use of apixaban in renal dysfunction, including with its dose adjustments and use of apixaban may continue to grow with time. While ESRD patients, regardless of whether they are receiving IHD or PD, would have a similar lack of renal function, the differences in apixaban clearance in between type of dialysis used could vary and is not well known. Additionally, patients receiving PD may be subject to other effects pertaining to safety outcomes of bleeding rates. It remains important to identify how the clinical response to apixaban may vary in patients receiving PD. Overall, questions still remain regarding safety and efficacy, given limited patients receiving PD included in studies and other trials not reporting on dialysis modalities.
Conclusion
The majority of studies comparing the safety and efficacy of apixaban to standard therapy in ESRD included patients on IHD and very few participants receiving PD. Apixaban appears to be safe and effective within these studies and pharmacokinetic studies in IHD may potentially be extrapolated with caution to patients receiving PD. Despite this, further studies specific for patients receiving PD are needed to evaluate the pharmacokinetics, pharmacodynamics, and safety and efficacy as well as recommended dose of apixaban in PD.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Meredith L. Howard  https://orcid.org/0000-0003-4514-3948
https://orcid.org/0000-0003-4514-3948
References
- 1. National Institute of Diabetes and Digestive and Kidney Diseases. Peritoneal dialysis. Accessed December 29, 2020. https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/peritoneal-dialysis
- 2. Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533-544. doi: 10.1681/ASN.2011060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pratsinis A, Devuyst O, Leroux JC. Peritoneal dialysis beyond kidney failure? J Control Release. 2018;282:3-12. doi: 10.1016/j.jconrel.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 4. Jameson MD, Wiegmann TB. Principles, uses and complications of hemodialysis. Med Clin North Am. 1990;74:945-960. doi: 10.1016/s0025-7125(16)30528-4 [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann S, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125-e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel report. Chest. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 7. Bonde AN, Lip GYH, Kamper A, et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol. 2014;64:2471-2482. doi: 10.1016/j.jacc.2014.09.051 [DOI] [PubMed] [Google Scholar]
- 8. Ronco F, Mazzone P, Hosseinian L, Genovesi S. Recent advances in stroke prevention in patients with atrial fibrillation and end-stage renal disease. Cardiorenal Med. 2017;7:207-217. doi: 10.1159/000470856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adeboyeje G, Sywestrzak G, Barron JJ, et al. Major bleeding risk during anticoagulation with warfarin, dabigatran, apixaban, or rivaroxaban in patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23:968-978. doi: 10.18553/jmcp.2017.23.9.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693-4738. doi: 10.1182/bloodadvances.2020001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Apixaban [package insert]. Wolters Kluwer Health, Inc; Riverwoods, IL. [Google Scholar]
- 12. Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharm. 2016;56:628-636. doi: 10.1002/jcph.628 [DOI] [PubMed] [Google Scholar]
- 13. Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37:412-419. doi: 10.1002/phar.1905 [DOI] [PubMed] [Google Scholar]
- 14. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519-1529. doi: 10.1161/CIRCULATIONAHA.118.035418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. 2018;2:291-298. doi: 10.1002/rth2.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herndon K, Guidry TJ, Wassell K, et al. Characterizing the safety profile of apixaban versus warfarin in moderate to severe chronic kidney disease at a Veterans Affairs Hospital. Ann Pharmacother. 2020;54:554-560. doi: 10.1177/1060028019897053 [DOI] [PubMed] [Google Scholar]
- 17. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241-2248. doi: 10.1681/ASN.2016090980 [DOI] [PMC free article] [PubMed] [Google Scholar]