Abstract
Vitamin A is a fat-soluble vitamin involved in essential functions including growth, immunity, reproduction, and vision. The vitamin A Dietary Reference Intakes (DRIs) for North Americans suggested that a minimally acceptable total liver vitamin A reserve (TLR) is 0.07 µmol/g, which is not explicitly expressed as a vitamin A deficiency cutoff. The Biomarkers of Nutrition for Development panel set the TLR cutoff for vitamin A deficiency at 0.1 µmol/g based on changes in biological response of several physiological parameters at or above this cutoff. The criteria used to formulate the DRIs include clinical ophthalmic signs of vitamin A deficiency, circulating plasma retinol concentrations, excretion of vitamin A metabolites in the bile, and long-term storage of vitamin A as protection against vitamin A deficiency during times of low dietary intake. This review examines the biological responses that occur as TLRs are depleted. In consideration of all of the DRI criteria, the review concludes that induced biliary excretion and long-term vitamin A storage do not occur until TLRs are >0.10 µmol/g. If long-term storage is to continue to be part of the DRI criteria, vitamin A deficiency should be set at a minimum cutoff of 0.10 µmol/g and should be set higher during times of enhanced requirements where TLRs can be rapidly depleted, such as during lactation or in areas with high infection burden. In population-based surveys, cutoffs are important when using biomarkers of micronutrient status to define the prevalence of deficiency and sufficiency to inform public health interventions. Considering the increasing use of quantitative biomarkers of vitamin A status that indirectly assess TLRs, i.e. the modified-relative-dose response and retinol-isotope dilution tests, setting a TLR as a vitamin A deficiency cutoff is important for users of these techniques to estimate vitamin A deficiency prevalence. Future researchers and policymakers may suggest that DRIs should be set with regard to optimal health and not merely to prevent a micronutrient deficiency.
Keywords: Biliary excretion, dietary reference intakes, vitamin A status
Impact statement
Public health practice uses prevalence above and below biomarker cutoffs to evaluate the presence of a condition at the group or population level. The vitamin A (VA) Dietary Reference Intakes (DRIs) for North Americans suggested that a total liver VA concentration of 0.07 µmol/g is minimally acceptable and is often interpreted as a VA deficiency (VAD) cutoff. An expert panel working on biomarkers for VA assessment recommended that the cutoff for VAD should be 0.10 µmol/g liver based on biological responses above this cutoff using similar criteria as in DRI formulation. This paper reviews evidence for defining the cutoff for VAD at 0.10 µmol/g. This is increasingly important because of widespread use of quantitative biomarkers of VA status, such as modified-relative-dose response and retinol-isotope dilution tests. Future research and policy evaluation may recommend an even higher concentration for special circumstances, such as in women of reproductive age or association with optimal health.
Introduction
Vitamin A (VA) is an essential fat-soluble micronutrient that is involved in many physiological functions in humans including cellular differentiation and epithelial integrity, growth and development, immune function, reproduction, and vision.1,2 VA is primarily stored in the liver for immediate use as retinol and long-term storage as retinyl esters.2 In 2001, the Dietary Reference Intakes (DRIs) for North Americans suggested that a liver VA concentration of at least 0.07 µmol/g (equivalent to 20 µg/g liver weight) in adults is a minimally acceptable liver reserve,1 but never defined it as a vitamin A deficiency (VAD) cutoff. Some researchers and policymakers have adopted this value as the liver cutoff for VAD. For the purposes of this review, the liver VA concentration will be referred to as total liver VA reserves (TLRs) and includes the sum of preformed VA as retinol and all retinyl esters. In 2016, the Biomarkers of Nutrition for Development (BOND) expert VA panel reviewed the biological evidence for a TLR cutoff to be used to define VAD and recommended that 0.1 µmol/g liver be used in studies evaluating VA status.2 This article summarizes and expands on those findings and the implications for setting a TLR as VAD.
The estimated average requirement (EAR) for any nutrient is a numerical value that is meant to meet the requirements of half of the healthy people in any defined group.1 The DRIs state that the following criteria were used to estimate average VA requirements using the minimally acceptable TLR:1
The minimal acceptable liver reserve is estimated to be 20 µg/g and is based on the concentration at which (1) no clinical signs of a deficiency are observed, (2) adequate plasma retinol concentrations are maintained (Loerch et al., 1979),3 (3) induced biliary excretion of vitamin A is observed (Hicks et al., 1984),4 and (4) there is a protection against a vitamin A deficiency for approximately 4 months while the person consumes a vitamin A-deficient diet.
In the DRI criteria, there are only two primary research studies cited and both were performed in rats.3,4 No human research studies are cited in support of these criteria to establish EARs, which are further discussed below. Another paper cited in the DRIs with reference to the 0.07 µmol/g TLR, is a commentary on adopting retinol-isotope dilution (RID) techniques to measure total body VA stores and estimate TLRs in humans.5 The RID test involves administering a dose of retinyl ester labeled with either deuterium or 13C, taking appropriately timed blood samples, and analyzing the serum with mass spectrometry.2 The commentary in the DRIs cites multiple studies that measured TLRs directly in human liver but no biological evidence is reviewed to support a cutoff for VAD.5 The author does not propose 0.07 µmol/g liver as a VAD cutoff, but instead suggests that in humans “saturation” occurs between 0.07 and 0.105 µmol/g.5 This saturation refers to the TLR that is necessary to reach a plateau in plasma retinol-binding protein (RBP) concentrations.5 RBP, also referred to as RBP4, is the carrier protein of retinol in plasma that is under homeostatic control over a wide range of TLRs.2
Review of the dietary reference intake criteria
This review will investigate the four DRI criteria used to establish EARs for VA and suggest that a VAD cutoff should be chosen that maintains VA balance such that degradation and excretion match intake, changes for different life cycles with increased needs, and perhaps reflects optimal health through storage of VA and carotenoids. The focus of this review is the VAD cutoff and does not review the evidence for setting hypervitaminosis A or toxicity, which is still under debate.2,6 Briefly, toxic effects, including hypertrophy and elevated serum retinyl esters, have occurred in humans at a TLR of ∼3 µmol/g liver7 and this cutoff has been proposed as a reflection of toxicity.6 In 1990, Olson set the cutoff for VA toxicity at 1.05 µmol/g liver,8 but more human data are needed to distinguish between hypervitaminotic TLRs (excessive VA without clinical signs) and toxicity with measurable clinical and biological outcomes.
Criterion 1
The first DRI criterion for a minimally acceptable TLR is “no clinical signs of a deficiency are observed.”1 In the DRIs, no TLR is suggested as to when clinical signs of VAD are present. In the literature, the TLR at which overt ocular VAD clinical signs occur is dangerously low, and immune function is already impaired.2 Xerophthalmia (i.e. sequelae associated with the eye) from VAD in most individuals likely does not occur until TLR are <0.02 µmol/g.8 A serious infection or illness occurring during this low TLR range would lead to mortality in children if not treated. One case report of a four-year-old child with corneal xerosis had a higher TLR of 0.035 µmol/g and a serum retinol concentration of 0.31 µmol/L.9 The World Health Organization (WHO) has set the prevalence of serum retinol concentrations ≤0.70 µmol/L to define different degrees of public health significance for VAD.10 For example, a severe public health problem is defined by 20% or more of evaluated children with serum retinol ≤0.70 µmol/L and a mild degree defined as 2–9%.10,11 Furthermore, xerophthalmia is complicated by other co-deficiencies of macro- and micronutrients in malnourished humans.
Common childhood infections such as diarrhea, respiratory disease, and measles may lead to TLR depletion, which can precipitate clinical eye signs of VAD.9,12 WHO recommends high-dose VA supplements in areas of greatest VAD risk to children under the age of five years.13 However, a 2016 systematic review could not find compelling evidence that VA supplementation prevented blindness in children with measles.14 Nonetheless, three meta-analyses have determined that high-dose VA supplementation can prevent 23–30% of mortality in children under the age of five years.15–17 High-dose VA supplementation only transiently increases TLRs in children with low dietary intake,18,19 indicating that supplementation alone may not allow enough long-term storage to prevent depletion during illness.
In summary, Criterion 1, which is based on overt ocular signs of VAD, is met with a low TLR that does not support optimal health in humans. Based on the availability of quantitative VA biomarkers that determine VAD before xerophthalmia is present, setting a TLR for defining VAD before ocular signs manifest is of utmost importance.
Criterion 2
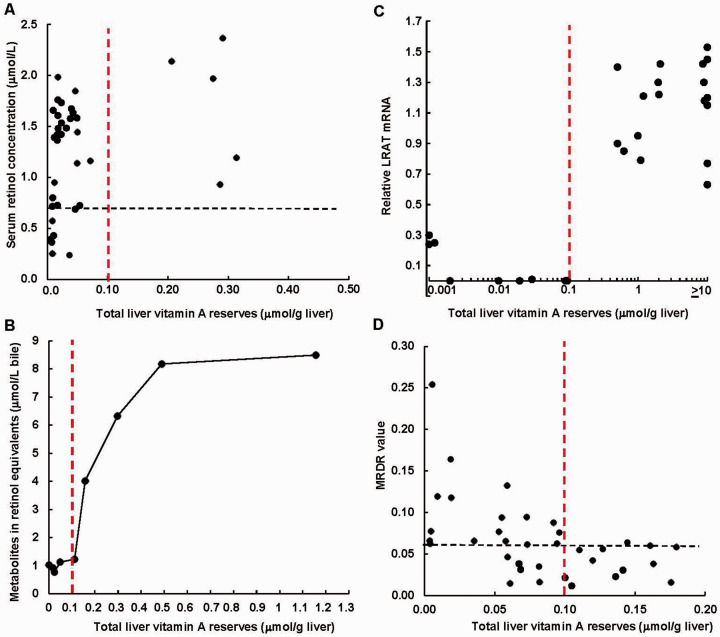
The second criterion to support a minimal TLR is “adequate plasma retinol concentrations are maintained.”1 The sole paper supporting this criterion is a rat study.3 Rats are an established mammalian model for VA deficiency studies.20 However, in the Loerch et al. study, there were no rats in the 0.07 to 0.2 µmol/g TLR range (Figure 1(a)) and plasma retinol concentrations were maintained >0.7 µmol/L in almost all rats that were not treated with retinoic acid, which lowered plasma concentrations,3 a confounding factor in that study for translation to humans. Thus, basing a minimally acceptable TLR on this rat paper is limited, and more recent human studies are discussed below. Circulating plasma retinol is bound to RBP. Serum is the preferred sample matrix and retinol is denatured from RBP during analysis. Serum retinol concentration is a common population biomarker of VAD but it is not sensitive to change over a broad range of TLRs due to homeostasis between plasma and hepatic stores.2
Figure 1.
The relationship between total liver vitamin A reserves (TLRs) in µmol/g liver and four biological responses. (a) The relationship of plasma retinol concentrations to TLRs in individual rats under various vitamin A regimens (data estimated from Loerch et al.).3 The black dashed line is the World Health Organization’s suggested serum retinol concentration cutoff for vitamin A deficiency at ≤0.70 µmol/L,10,11 indicating 22% sensitivity against a TLR of 0.10 µmol/g liver. (b) Excretion of retinol metabolites expressed as retinol equivalents in bile after rats received a tracer dose of tritiated retinyl acetate (data from Hicks et al.4; points are means of 5 to 12 rats). (c) Relative expression of LRAT mRNA compared with young rat control liver (data estimated from Zolfaghari and Ross)21; points are a pool of two rat livers, values ≥10 µmol/g liver were set to 10 µmol/g. (d) The modified-relative-dose response (MRDR) values in individual piglets after a variety of dietary interventions (data from Tanumihardjo).22 The black dashed line is the MRDR value cutoff for vitamin A deficiency (3, 4-didehyroretinol to retinol ratio ≥0.060). The red dotted line highlights 0.10 µmol/g liver in each panel. [Note: The TLR scales have been converted to µmol/g liver from the original figures for direct comparisons]. (A color version of this figure is available in the online journal.)
Although higher serum retinol concentration cutoffs for VAD have been considered (i.e. 1.05 µmol/L),23 WHO has not adopted a higher cutoff. In US adult cadavers, a serum retinol concentration cutoff at both 0.7 and 1 µmol/L had 83% sensitivity to determine VAD (TLRs ≤0.10 µmol/g liver).7 However, increasing the serum retinol concentration deficiency cutoff to <1 µmol/L decreased specificity from 76 to 57%.7 During severe VAD, we surmise from available evidence that as long as the animals or humans remain relatively healthy and do not contract an infection, an adequate serum retinol concentration can be maintained through increased recycling. It is well-known that the carrier RBP is an acute phase reactant and decreases during infection and inflammation.24,25 In Zambian children with high TLRs, including a large majority experiencing hypervitaminosis A due to overlapping preformed VA interventions,26 serum retinol concentrations were depressed below 0.7 µmol/L in 17% of the cohort,27 which would be considered a moderate public health problem by WHO criteria despite the absence of VAD.11 By shifting the cutoff to include the influence of inflammation, measured by C-reactive protein and α1-acid-glycoprotein, the prevalence of low serum retinol concentrations decreased to 2.3%.27
As with overt clinical eye signs, serum retinol concentration is not a good measure of TLRs until TLRs are extremely low. The first reports that serum retinol concentration did not reflect TLRs were in the 1940s and 1950s. In 1942 in rats, Lewis et al. reported a TLR of 0.036 µmol/g, while serum retinol was maintained >1.05 µmol/L.28 In 1954, High et al. reported a very low TLR of <0.015 µmol/g and a serum retinol concentration of >1.0 µmol/L.29 A similar phenomenon was observed in a more recent rat study where serum retinol concentrations were maintained at 1.37 ± 0.21 µmol/L but TLRs were 0.0051 ± 0.0029 µmol/g,30 which is 20-times lower than the cutoff proposed for deficiency.2 Another rodent model that has been established for provitamin A carotenoid research is the Mongolian gerbil, which metabolizes provitamin A carotenoids similarly to humans.20 In recent gerbil studies (n = 130), serum retinol concentrations were maintained ≥0.70 µmol/L until a critically low TLR of 0.01 µmol/g. Even at that critical TLR, 38% of the gerbils (3 out of 8) were maintaining a “healthy” serum retinol concentration.31 In these studies, serum retinol had 12% sensitivity to predict VAD at a measured TLR of 0.10 µmol/g (unpublished observations). This is very similar to Thai schoolchildren who were maintaining healthy serum retinol concentrations of 1.19 ± 0.22 µmol/L but 65% had TLRs <0.1 µmol/g predicted by RID.27
Criterion 2 is based on plasma retinol concentration maintenance; however, due to homeostasis, plasma retinol concentrations are not reflective of TLRs until the liver is almost exhausted of VA,2 as noted above in multiple research studies in animals and humans. Furthermore, the influence of infection and inflammation on plasma retinol depression24,25 complicates its use as a VAD biomarker because illness is common in populations afflicted with VAD. The TLR that negatively impacts plasma retinol concentrations is similar to that for overt clinical signs, which are part of Criterion 1.
Criterion 3
The third criterion is the point at which “induced biliary excretion of VA is observed”.1,4 A rat study is cited in the DRIs in support of biliary excretion of VA as being important in VA metabolism.4 Bile, which is secreted from hepatocytes, is an essential component in lipid emulsification and absorption.32 Bile collects in the gallbladder and is released into the small intestine for digestion and micelle formation with fat-soluble vitamins for absorption.33 Many drugs, toxins, and other compounds, including VA, are eliminated from the body through biliary excretion.32 In order to retain VA, biliary excretion of VA does not substantially occur during VAD. Furthermore, cholic, chenodeoxycholic, and tauroursodeoxycholic bile acids in the liver were lower in VA-deficient gerbils with a TLR of 0.064 ± 0.05 µmol/g compared with VA-adequate gerbils at 0.45 ± 0.11 µmol/g.34 It is interesting to note that the rat study cited in the DRIs did not observe an increase in biliary excretion of VA until TLRs were between 0.11 and 0.16 µmol/g (Figure 1(b)),4 supporting the cutoff of 0.10 µmol/g suggested by the BOND expert panel as being biologically relevant.2 Other researchers did not find an increase in VA metabolite excretion until TLRs were >0.2 µmol/g in older rats.35 VA metabolite excretion may be affected by age as reported in younger and older rats,4,35 suggesting that children have a lower TLR than adults to define VAD, but there are some subtleties in the experimental design of the older rat study35 that need to be considered. Because of the allocated differences in dietary VA intake among the rat groups, a continuum in TLRs was not achieved among nine groups. Two sharp increases in urinary and fecal VA excretion were demonstrated. The first between 0.055 ± 0.004 and 0.23 ± 0.021 µmol/g and the next between 0.23 ± 0.021 and 1.01 ± 0.15 µmol/g eight days after dosing with tritiated retinyl acetate.35 However, the first groups received different amounts of radiolabeled VA, 0.07 versus 0.35 µmol retinol equivalents.35 The difference in excretion between TLRs of 0.055 ± 0.004 and 0.23 ± 0.021 µmol/g may partially be explained by a 5-times increase in tracer dose between the groups.
The excretion of VA-metabolites in bile is considered a protective mechanism to reduce excessive storage of VA.1,4 However, in both rat studies that used tritiated retinyl acetate as a tracer and liquid scintillation counting for analysis,4,35 a plateau in metabolite excretion occurred but at different TLRs. In the younger rats, the plateau was >0.5 µmol/g;4 in the older rats, it did not occur until TLRs were >1 µmol/g.35 This range is consistent with young piglets that increased VA catabolism when TLRs reached 0.7 µmol/g.36 Preschool children in South Africa appeared to catabolize VA after the governmental-mandated, WHO-supported high-dose retinyl palmitate supplements when TLRs were >1 µmol/g.37 These children continued to accrue hepatic VA from repeated supplements over their life time, in part, due to adequate dietary intake.37
Bile acid and VA homeostatic mechanisms are more intricately associated than merely excretion.33 The farnesoid X receptor (FXR), an orphan nuclear receptor that requires activation by bile acids, is involved in hepatic VA storage.38 In FXR-null mice, hepatic retinol and retinyl esters were depleted and reintroduction of two FXR isoforms restored hepatic TLRs.38 The lack of excretion of bile acids until TLRs are >0.1 µmol/g suggests that significant storage for long-term utilization does not occur until TLRs are above this threshold.
The population most affected by VAD is preschool children. In areas where VAD is most severe, enteropathy caused by environmental enteric dysfunction is common.39 In Malawi, children with environmental enteric dysfunction had lower serum bile acids compared with children who did not.40 The authors suggest that this may be related to impaired return to systemic circulation and not liver disease, which may cause reduced synthesis. However, these same children would consequently excrete more bile acids, which would not be a consequence of VA status. They would need to synthesize more bile acids in the liver to compensate for this reduction.40 Thus, the relationship between VA status and bile acid secretion is complex. Nonetheless, evaluation of serum bile acid profiles in conjunction with quantitative RID tests may be useful to better diagnose hypervitaminosis A and should be explored. WHO recommends that at least two biological markers be used to assess VAD.10,11 Perhaps two biomarkers should be used for hypervitaminosis A evaluation considering the current trend in overlapping preformed VA interventions in some countries.26
Criterion 3 links bile acid secretion to VA homeostasis.1 The evidence cited in the DRIs regarding VA metabolite secretion in bile4 supports a higher TLR to define VAD than 0.07 µmol/g, suggesting that it is even >0.10 µmol/g. More research is needed to determine the relationship of serum bile acid profiles with quantitative measures of VA status.
Criterion 4
Criterion 4 suggests that at the minimally acceptable TLR “there is a protection against VAD for approximately 4 months while the person consumes a VA-deficient diet”.1 To store retinol in the liver to achieve this four months of protection, lecithin:retinol acyl transferase (LRAT) catalyzes the transfer of fatty acids to retinol to form retinyl esters. In rats, messenger RNA of the enzyme LRAT was not upregulated until TLRs were between a concentration >0.1 and <0.4 µmol/g liver (Figure 1(c)).21 In a knockout mouse model, LRAT was essential for retinyl ester storage in the eye and liver.41 Therefore, very little to no long-term storage can occur until this liver reserve, i.e. >0.1 µmol/g, is satisfied. If LRAT is not available in the liver, retinyl esters will not be significantly synthesized and stored for long-term use. Nevertheless, some redundancy exists in the synthesis of retinyl esters as demonstrated in isolated stellate cells. In cultured stellate cells from LRAT knock-out mice, acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) appears to catalyze the formation of retinyl esters, but the lipid droplet size is smaller.42
The distribution of retinol and retinyl esters shifts between hepatocytes and stellate cells with increasing degrees of VAD. When TLRs are adequate and excessive, most VA is stored as retinyl esters in the stellate cells.2 In a limited study, cells isolated from rats (n = 3/group) with severe VAD (0.004 ± 0.001 µmol/g liver) demonstrated that most of the VA (83.5%) was in the non-stellate parenchymal cells and 60% of TLRs was present as “free” retinol in the stellate cells and not as the storage ester form43 to draw upon during times of low dietary VA intake.1 With increasing TLRs, the amount in the parenchymal cells decreased to 37% and 18% at TLRs of 0.051 ± 0.011 and 0.101 ± 0.031 µmol/g, respectively.43 Higher TLRs were not investigated.
Below a TLR of 0.1 µmol/g, RBP begins to accumulate in the liver awaiting its ligand. In VA-deficient rats, unbound apo-RBP accumulation was three times higher in the liver than VA control rats at a TLR of approximately 0.1 µmol/g.44 This indicates that RBP synthesis is not associated with VA status and is maintained during VAD. The modified-relative-dose response (MRDR) test monopolizes on this phenomenon by administering a challenge dose of 3,4-didehydroretinyl acetate and taking a single blood sample 4 to 7 h later.2 The ratio of 3,4-didehydroretinol to retinol in the serum, referred to as the MRDR value, is an indication of VA status and distinguishes between VAD and adequate status.2 In piglets undergoing the MRDR test for VAD,22 the test became positive (an MRDR value ≥0.060 indicating VAD) in 63% of the piglets between 0.07 and 0.10 µmol/g liver (Figure 1(d)). A complete review of the dose–response tests in animals and humans against TLRs as the gold standard revealed 80% sensitivity for the MRDR test using a TLR of 0.10 µmol/g to define VAD.45
In contrast to Criteria 1 and 2, Criterion 4, which states long-term VA storage is essential, supports a higher TLR cutoff than 0.07 µmol/g for VAD based on evidence of inducement of LRAT expression21 and MRDR positivity.22,45 This is in comparison with Criterion 3, which suggests that the TLR cutoff needs to be >0.10 µmol/g to ensure protection from VAD for at least four months.1
Dietary reference intake formula considerations
In the mathematical formula for the EARs described in the DRIs, the concepts included are the percent of VA stores lost each day, the minimally acceptable liver value of 0.07 µmol/g discussed above, the liver weight to body weight ratio, body weight reference values, the ratio of body to liver stores, and efficiency of storage.1 A uniform liver weight as body weight ratio is used: “The liver weight:body weight ratio is 1:33 (0.03 or 3%) and is an average of ratios for infants and adults”.1 Liver size as a proportion of body weight decreases as humans age. A variety of formulae exist in transplantation literature to estimate liver weight. In order for TLRs to be estimated using RID, recommended % body weight as liver weight are 4–4.2, 3, and 2.4% for infants, children, and adults, respectively.46 While we do not recommend that the DRIs be tailored to the individual at this time, we have confirmed the liver weight to body weight ratios used with RID in two cohorts using formulae based on surface area. In 70 Burkinabe children aged 7 to 12 years, the calculated mean value for liver weight was 2.97 ± 0.21% of body weight47 using the pediatric Herden et al. formula.48 Using a simplified formula by Yoshizumi et al.,49 calculations in Zambian women resulted in a range of values from 1.7 to 2.6%.50 The range in women was greater than in the children, but encompasses the 2.4% recommended for the RID test when applied to adults but not the 3% used in the DRI calculations.1,46
Summary of dietary reference intake criteria
The minimally acceptable TLR of 0.07 µmol/g was used to formulate DRIs but is not referred to as a VAD cutoff. From data behind the criteria, severe VAD as noted by Criteria 1 and 2 occurs at a TLR of approximately 0.02 µmol/g, but this does not allow for enhanced biliary excretion and long-term storage for protection against VAD as noted by Criteria 3 and 4, respectively. A TLR of at least 0.10 µmol/g is needed to induce biliary excretion and upregulate LRAT expression for long-term VA storage as retinyl esters. As recommended by the BOND expert panel, the VAD cutoff should be a TLR of at least 0.10 µmol/g.2 TLRs less than this neither induce biliary excretion nor storage in humans or provide for special conditions, such as lactation, which is explored below.
Current dietary reference intakes are too high
Human evidence suggests that the current DRIs for North Americans, which are largely based on mathematical calculations, are too high, even though they were originally formulated to maintain a minimally acceptable TLR of 0.07 µmol/g.1 Considering that governmental fortification mandates often use the EARs to recommend fortificant levels, it is prudent to reevaluate these values in light of recent biological evidence in humans. TLRs were more than four times higher than 0.07 µmol/g in US women consuming the current EAR of 500 µg retinol activity equivalents (RAE)/d.51 In two highly controlled feeding studies, 257 µg RAE maintained TLRs of 1.13 ± 0.41 µmol/g in Zambian children, and 330 µg RAE maintained 0.46 ± 0.32 µmol/g in US women.52 In agreement with the findings in children, a mathematical analysis of RID data from children in Bangladesh, the Philippines, Guatemala, and Mexico predicted that if the DRI is consumed, current DRIs were sufficient to attain TLRs >0.07 µmol/g by one week of age.53
Moreover, the TLR cutoff to define VAD should be tailored to age, sex, and life cycle categories. Special life cycle events may warrant a higher VAD cutoff, which is especially true for lactation. While VA stores during pregnancy are important for fetal development, the production of healthy breast milk needs a larger boost from either diet or liver stores. Lactating women can quickly deplete their VA stores if dietary intake is not increased during this period. In a recent study in Zambian women, the lactating women’s total body VA stores were 25% lower than non-lactating women; the estimated overall TLR prevalence ≤0.10 µmol/g was 48% using the RID test.50 Based on studies in lactating swine54 and a sensitivity evaluation of the MRDR test against TLRs,45 a TLR of 0.20 µmol/g responded positively (MRDR value ≥0.060), which indicated accumulated RBP during this life stage at this higher TLR. Thus, a TLR of 0.20 µmol/g may be more appropriate than 0.10 µmol/g to define VAD in women of reproductive age and may be needed to ensure adequate hepatic stores to support VA breast milk content. A TLR of 0.10 µmol/g was estimated to only provide 75 days of protection from total VA exhaustion in these Zambian women,50 which is not enough to support exclusive breastfeeding for 6 months as recommended by WHO.55 Recently, a TLR value of 0.14 µmol/g was evaluated in lactating Thai women in conjunction with RID and a fortified rice intervention; this TLR would only allow 100 days of protection from severe VAD during breastfeeding.56
The effect of maternal VA status on offspring is not entirely known.57 In piglets, retention of high dose VA supplements was lower when the mothers had a greater degree of VAD.58 Poorer retention in the liver was observed with moderate VA doses (25,000 and 50,000 IU) in piglets from mothers on a VAD depletion regimen for a longer period (11–17% retention) than in those whose mothers had better VA status (35–38% retention). Although both piglet control groups had VAD, the offspring from the mothers that were more VA deficient were not able to accrue appreciable storage and all group TLR means were <0.1 µmol/g after treatment.58 High doses administered to piglets, such as 100,000 and 200,000 IU, result in low retention values,36,58 in part due to excretion of unabsorbed dose in the feces.36
Factors future dietary reference intake evaluations should consider
The current DRI values are 20 years old59 and as noted above, the relationship to TLRs in the formulation did not have much data behind the assignment of a TLR value as VAD or even minimally acceptable. Future DRI panels will have more data to consider at reevaluation. For example, more is known about the contribution of provitamin A carotenoids to VA efficacy and how this differs among individuals.59 Perhaps future DRIs should consider polymorphisms in key carotenoid metabolic enzymes. While it is considered beneficial to have carotenoids in circulation due to their epidemiological association with disease reduction,60 accumulation of provitamin A carotenoids is dependent on VA status. In Mongolian gerbils, appreciable tissue accumulation of provitamin A carotenoids does not occur until an approximate TLR of 0.4 µmol/g.19,31,61 Below this threshold, most absorbed provitamin A carotenoids are converted to VA with efficient bioefficacy factors.19 Between 0.4 and 0.7 µmol/g, which might be considered an optimal TLR range, provitamin A carotenoids are converted as needed with some storage and above 0.7 µmol/g, the bioefficacy factor increases (i.e. low efficiency of cleavage and more carotenoid storage).19 Thus, the optimal TLR range of 0.4 to 0.7 µmol/g renders balance with preformed VA and provitamin A dietary intakes. High concentrations of serum provitamin A carotenoids have been associated with high TLRs and elevated serum retinyl esters in Zambian and Malawian children evaluated with the RID and MRDR tests, respectively.62,63
Future DRIs should also consider what VAD cutoff prevents abnormal hepatic pathology in humans and set it above this value to ensure protection. In Mongolian gerbils, moderate cirrhosis and fibrosis were observed in animals with TLRs ≤0.07 µmol/g liver,61 suggesting that this minimal TLR does not promote optimal liver health. This is in agreement with severe cirrhosis found in an adult cadaver with VAD.7 While the liver has a remarkable capacity for regeneration,64 it is unknown what degree of injury or scarring from fibrosis is reversible.65 Fibrosis is mainly caused from activation of stellate cells to become fibrogenic during injury.64,66 Crosstalk is essential between different liver cells in the regenerative process.65 Hypothetically, balance among VA concentrations in hepatocytes and stellate cells is needed to promote a healthy liver. Titration of TLRs along the continuum and TLR association with hepatic injury in humans is needed to understand optimal TLRs for health.
Conclusions and future directions
Future research should determine if circulating carotenoids in plasma and tissue storage support optimal health including inflammation reduction and immune response enhancement.60 If so, a marginal status may be an interval that extends to 0.4 µmol/g (below which the provitamin A carotenoids are efficiently cleaved), which is biologically meaningful, but more human data are needed. At the current time, it is unknown what TLR supports optimal immune function, but VA supplementation enhanced T-regulatory cells in low birth weight Bangladeshi infants.67 Furthermore, maternal VA status affects fetal immunity structure;57 however, it is unknown if this is associated with maternal TLR storage or immediate VA supplementation effects.
The criteria used to define a minimally acceptable TLR in the formulation of the 2001 VA DRIs for North Americans are largely based on two rat studies, one of which supported 0.1 µmol/g as being biologically meaningful,4 and the other could not differentiate between 0.07 and 0.2 µmol/g because of lack of data in that region.3 Furthermore, the DRI formulation does not define a specific TLR as VAD. More recent data in a variety of animals and humans suggest that defining VAD at 0.07 µmol/g liver does not support a healthy liver or provide storage to draw upon during times of low dietary intake. This review supports the value determined by the BOND expert panel in 2016, i.e. 0.1 µmol/g liver,2 as a cutoff to define VAD but further suggests that a higher value should be considered during different life stages, such as lactation, where storage becomes critical to prevent VAD in vulnerable women. Moreover, in children with high infection burden, urinary losses and impaired absorption occur;60 therefore, higher TLRs are needed to prevent VAD. In addition to the DRI criteria, biomarkers of VA and carotenoid status have different working TLR ranges of utility (Table 1). Current applications of RID where a numerical value for TLR is estimated, warrant a closer evaluation of what defines VAD. Public health surveillance systems that may apply more robust measures of TLRs, such as MRDR and RID tests, are accustomed to determining prevalence above and below a VAD cutoff.
Table 1.
Review of the total liver vitamin A (VA) reserve as it relates to the dietary reference intake (DRI) criteria used to formulate estimated average requirements and to biomarkers of VA and carotenoid status.
| DRI criteria | Total liver VA reserve µmol/g | Model | References |
|---|---|---|---|
| (1) No clinical signsXerophthalmic sequelae | <0.02 | Predicted for children | Olson8* |
| (2) Adequate plasma retinolDepressed serum retinolconcentrations | >0.005<0.02<0.01<0.1 | RatsPredicted for childrenGerbilsAdult humans | Lewis et al.,28 High et al.,29 Riabroy et al.30Olson8*Kaeppler et al.31Olsen, Suri et al.7 |
| (3) Induced biliary excretionEnhanced biliary secretion or excretion | >0.1 | Rats | Hicks et al.4Varma and Beaton35 |
|
(4) Protection for 4 monthsmRNA upregulation of LRAT |
>0.1 |
Rats |
Zolfaghari and Ross21 |
|
Biomarkers of VA status |
|
|
|
| Positive dose response tests | <0.10 | Piglets, calves, rats, humans | Sheftel and Tanumihardjo45* |
| Elevated provitaminA carotenoids | >0.7 >1 | Gerbils (liver) Humans (serum) | Tanumihardjo19*Sowa et al.61Mondloch et al.62 |
| Elevated serum retinyl esters | ∼3 | Humans | Olsen, Suri et al.7 |
| Retinol isotope dilution | Continuum | RatsNon-human primatesHumans | Tanumihardjo68 Escaron et al.69Tanumihardjo et al.2*Tanumihardjo19* |
| Hepatic biopsy | Continuum | Humans | Olsen, Suri et al.7 |
These references are either a chapter in a book or reviews of multiple studies.
ACKNOWLEDGMENTS
The author would like to thank Devika Suri, Jesse Sheftel, Veronica Lopez-Teros, Jevin Lorte, and Wai Hlaing Bwar for commenting on the manuscript during preparation and revision, and Jesse Sheftel for assistance with Figure 1.
Footnotes
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review is part of a seed grant from the Global Health Institute at the University of Wisconsin-Madison.
ORCID iD: Sherry A Tanumihardjo https://orcid.org/0000-0002-7022-1760
References
- 1.Institute of Medicine Food and Nutrition Board. Vitamin A. In: ■ Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press, 2001, pp. 82–161. [PubMed] [Google Scholar]
- 2.Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of nutrition for development (BOND) – vitamin A review. J Nutr 2016; 146:1816S–48S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loerch JD, Underwood BA, Lewis KC. Response of plasma levels of vitamin A to a dose of vitamin A as an indicator of hepatic vitamin A reserves in rats. J Nutr 1979; 109:778–86 [DOI] [PubMed] [Google Scholar]
- 4.Hicks VA, Gunning DB, Olson JA. Metabolism, plasma transport and biliary excretion of radioactive vitamin A and its metabolites as a function of liver reserves of vitamin A in the rat. J Nutr 1984; 114:1327–33 [DOI] [PubMed] [Google Scholar]
- 5.Olson JA. New approaches to methods for the assessment of nutritional status of the individual. Am J Clin Nutr 1982; 35:1166–8 [DOI] [PubMed] [Google Scholar]
- 6.Tanumihardjo SA. The dawn of a new era of vitamin A assessment. J Nutr 2020; 150:185–7 [DOI] [PubMed] [Google Scholar]
- 7.Olsen K, Suri DJ, Davis C, Sheftel J, Nishimoto K, Yamaoka Y, Toya Y, Welham NV, Tanumihardjo SA. Serum retinyl esters are positively correlated with analyzed total liver vitamin A reserves collected from US adults at time of death. Am J Clin Nutr 2018; 108:997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson J. Vitamin A. Present knowledge in nutrition. 6th ed. Washington (DC): International Life Sciences Institute, 1990, p. 101 [Google Scholar]
- 9.World Health Organization. Control of vitamin A deficiency and xerophthalmia. Report of a Joint WHO/UNICEF/USAID/Helen Keller International/IVACG meeting. World Health Organization technical report series, 1982, 672
- 10.WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva, Switzerland: World Health Organization, 2011 [Google Scholar]
- 11.WHO. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluation intervention programmes. Geneva, World Health Organization, 1996. [Google Scholar]
- 12.Hussey GD, Klein M. Measles-induced vitamin A deficiency. Ann N Y Acad Sci 1992; 669:188–94 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Guideline: vitamin A supplementation in infants and children 6-59 months of age. Geneva, Switzerland: World Health Organization, 2011 [Google Scholar]
- 14.Bello S, Meremikwu MM, Ejemot-Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database Syst Rev 2016; 4:CD007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, Harvey B. Effectiveness of vitamin A supplementation in control of young child morbidity and mortality in developing countries – nutrition policy discussion paper No. 13. Geneva (Switzerland): UN, 1993
- 16.Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality: a meta-analysis. JAMA 1993; 269:898–903 [PubMed] [Google Scholar]
- 17.Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev 2010; 12:CD008524. [DOI] [PubMed] [Google Scholar]
- 18.Allen LH, Haskell M. Estimating the potential for vitamin A toxicity in women and young children. J Nutr 2002; 132:2907S–19S [DOI] [PubMed] [Google Scholar]
- 19.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Safety 2008; 7:373–81 [Google Scholar]
- 20.Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW., Jr. Review of animal models in carotenoid research. J Nutr 1999; 129:2271–7 [DOI] [PubMed] [Google Scholar]
- 21.Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase from mouse and rat liver: cDNA cloning and liver-specific regulation by dietary vitamin A and retinoic acid. J Lipid Res 2000; 41:2024–34 [PubMed] [Google Scholar]
- 22.Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011; 94:658S–65S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 2002; 132:2895S–901S [DOI] [PubMed] [Google Scholar]
- 24.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003; 362:2052–8 [DOI] [PubMed] [Google Scholar]
- 25.Larson LM, Namaste SM, Williams AM, Engle-Stone R, Addo OY, Suchdev PS, Wirth JP, Temple V, Serdula M, Northrop-Clewes CA. Adjusting retinol-binding protein concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am J Clin Nutr 2017; 106:390S–401S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanumihardjo SA, Kaliwile C, Boy E, Dhansay MA, van Stuijvenberg ME. Overlapping vitamin A interventions in the United States, Guatemala, Zambia, and South Africa: case studies. Ann N Y Acad Sci 2019; 1446:102–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suri DJ, Tanumihardjo JP, Gannon BM, Pinkaew S, Kaliwile C, Chileshe J, Tanumihardjo SA. Serum retinol concentrations demonstrate high specificity after correcting for inflammation but questionable sensitivity compared with liver stores calculated from isotope dilution in determining vitamin A deficiency in Thai and Zambian children. Am J Clin Nutr 2015; 102:1259–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis JM, Bodanskoy O, Falk KG, McGuire G. Vitamin A requirements in the rat. The relation of vitamin A intake to growth and to concentration of vitamin A in the blood plasma, liver and retina. J Nutr 1942; 23:351–63 [Google Scholar]
- 29.High EG. Studies on the absorption, deposition and depletion of vitamin A in the rat. Arch Biochem Biophys 1954; 49:19–29 [DOI] [PubMed] [Google Scholar]
- 30.Riabroy N, Dever J, Tanumihardjo SA. α-Retinol and 3,4-didehydroretinol support growth in rats when fed at equimolar amounts and α-retinol is not toxic after repeated administration of large doses. Br J Nutr 2014; 111:1373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaeppler M, Smith J, Davis C, Simon P, Tanumihardjo S. Anthocyanin and lycopene content do not affect beta-carotene bioefficacy from multicolored carrots in male Mongolian gerbils. Curr Dev Nutr 2020; 4:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyer JL. Bile formation and secretion. Compr Physiol 2013; 3:1035–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saeed A, Hoekstra M, Hoeke MO, Heegsma J, Faber KN. The interrelationship between bile acid and vitamin A homeostasis. Biochim Biophys Acta Mol Cell Biol Lipids 2017; 1862:496–512 [DOI] [PubMed] [Google Scholar]
- 34.La Frano MR, Brito A, Johnson CM, Wilhelmson B, Gannon B, Fanter RK, Pedersen TL, Tanumihardjo SA, Newman JW. Metabolomics reveals altered hepatic bile acids, gut microbiome metabolites, and cell membrane lipids associated with marginal vitamin A deficiency in a Mongolian gerbil model. Mol Nutr Food Res 2020; 64:e1901319 [DOI] [PubMed] [Google Scholar]
- 35.Varma RN, Beaton GH. Quantitative aspects of the urinary and fecal excretion of radioactive metabolites of vitamin A in the rat. Can J Physiol Pharmacol 1972; 50:1026–37 [DOI] [PubMed] [Google Scholar]
- 36.Gannon BM, Davis CR, Nair N, Grahn M, Tanumihardjo SA. Single high-dose vitamin A supplementation to neonatal piglets results in a transient dose response in extrahepatic organs and sustained increases in liver stores. J Nutr 2017; 147:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Stuijvenberg ME, Dhansay MA, Nel J, Suri D, Grahn M, Davis CR, Tanumihardjo SA. South African preschool children habitually consuming sheep liver and exposed to vitamin A supplementation and fortification have hypervitaminotic A liver stores: a cohort study. Am J Clin Nutr 2019; 110:91–101 [DOI] [PubMed] [Google Scholar]
- 38.Saeed A, Yang J, Heegsma J, Groen AK, van Mil SWC, Paulusma CC, Zhou L, Wang B, Faber KN. Farnesoid X receptor and bile acids regulate vitamin A storage. Sci Rep 2019; 9:19493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crane RJ, Jones KDJ, Berkley JA. Environmental enteric dysfunction: an overview. Food Nutr Bull 2015; 36:S76–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semba RD, Gonzalez-Freire M, Moaddel R, Trehan I, Maleta KM, Khadeer M, Ordiz MI, Ferrucci L, Manary MJ. Environmental enteric dysfunction is associated with altered bile acid metabolism. J Pediatr Gastroenterol Nutr 2017; 64:536–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem 2004; 279:10422–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajat M, Molenaar M, Brouwers JFHM, Vaandrager AB, Houweling M, Helms JB. Hepatic stellate cells retain the capacity to synthesize retinyl esters and to store neutral lipids in small lipid droplets in the absence of LRAT. Biochim Biophys Acta Mol Cell Biol Lipids 2017; 1862:176–87 [DOI] [PubMed] [Google Scholar]
- 43.Batres RO, Olson JA. A marginal vitamin A status alters the distribution of vitamin A among parenchymal and stellate cells in rat liver. J Nutr 1987; 117:874–9 [DOI] [PubMed] [Google Scholar]
- 44.Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol binding protein metabolism by vitamin A status in the rat. J Biol Chem 1972; 247:2542–50 [PubMed] [Google Scholar]
- 45.Sheftel J, Tanumihardjo SA. Systematic review and Meta-analysis of the relative dose response tests to assess vitamin A status. Adv Nutr 2020;31:nmaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gannon BM, Tanumihardjo SA. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr 2015; 145:847–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bationo JF, Zeba AN, Coulibaly ND, Sheftel J, Davis CR, Bassole IHN, Barro N, Ouedraogo JB, Tanumihardjo SA. Liver retinol estimated by 13C-retinol isotope dilution at 7 versus 14 days in Burkinabe schoolchildren. Exp Biol Med 2019; 244:1430–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herden U, Wischhusen F, Heinemann A, Ganschow R, Grabhorn E, Vettorazzi E, Nashan B, Fischer L. A formula to calculate the standard liver volume in children and its application in pediatric liver transplantation. Transpl Int 2013; 26:1217–24 [DOI] [PubMed] [Google Scholar]
- 49.Yoshizumi T, Gondolesi GE, Bodian CA, Jeon H, Schwartz ME, Fishbein TM, Miller CM, Emre S. A simple new formula to assess liver weight. Transplant Proc 2003; 35:1415–20 [DOI] [PubMed] [Google Scholar]
- 50.Kaliwile C, Michelo C, Sheftel J, Davis CR, Grahn M, Bwembya P, Simpungwe E, Mwanza S, Chileshe J, Tanumihardjo SA. Breast milk-derived retinol is a potential surrogate for serum in the 13C-retinol isotope dilution test in Zambian lactating women with vitamin A deficient and adequate status. J Nutr 2021; 151:255–63 [DOI] [PubMed] [Google Scholar]
- 51.Valentine AR, Davis CR, Tanumihardjo SA. Vitamin A isotope dilution predicts liver stores in line with long-term vitamin A intake above the current recommended dietary allowance for young adult women. Am J Clin Nutr 2013; 98:1192–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheftel J, Valentine AR, Hull AK, Fadjarwati T, Gannon BM, Davis CR, Tanumihardjo SA. Findings in 3 clinical trials challenge the accuracy of the Institute of Medicine’s Estimated Average Requirements for vitamin A in children and women. Am J Clin Nutr 2020:nqaa132. DOI: 10.1093/ajcn/nqaa132 [DOI] [PMC free article] [PubMed]
- 53.Ford JL, Lopez-Teros V. Prediction of vitamin A stores in young children provides insights into the adequacy of current dietary reference intakes. Curr Dev Nutr 2020; 4:nzaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Surles RL, Hutson PR, Valentine AR, Mills JP, Tanumihardjo SA. 3, 4-Didehydroretinol kinetics differ during lactation in sows on a retinol depletion regimen and the serum:milk 3, 4-didehydroretinol:retinol ratios are correlated. J Nutr 2011; 141:554–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO and UNICEF. Global strategy for infant and young child feeding. World Health Organization: Geneva, Switzerland, 2003, p.30 [Google Scholar]
- 56.Pinkaew S, Udomkesmalee E, Davis CR, Tanumihardjo SA. Vitamin A-fortified rice increases total body vitamin A stores in lactating Thai women measured by retinol isotope dilution: a double-blind, randomized, controlled trial. Am J Clin Nutr 2021. DOI: 10.1093/ajcn/nqaa418 [DOI] [PubMed]
- 57.Gannon BM, Jones C, Mehta S. Vitamin A requirements in pregnancy and lactation. Curr Dev Nutr 2020; 4:nzaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Surles RL, Mills JP, Valentine AR, Tanumihardjo SA. One-time graded doses of vitamin A to weanling piglets enhance hepatic retinol but do not always prevent a deficient vitamin A status. Am J Clin Nutr 2007; 86:1045–53 [DOI] [PubMed] [Google Scholar]
- 59.Ross AC, Moran NE. Our current dietary reference intakes for vitamin A – now 20 years old. Curr Dev Nutr 2020; 4:nzaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubin LP, Ross AC, Stephensen CB, Bohn T, Tanumihardjo SA. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv Nutr 2017; 8:197–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sowa M, Mourao L, Sheftel J, Kaeppler M, Simmons G, Grahn M, Davis CR, von Lintig J, Simon PW, Pixley KV, Tanumihardjo SA. Overlapping vitamin A interventions with provitamin A carotenoids and preformed vitamin A cause excessive liver retinol stores in male Mongolian gerbils. J Nutr 2020; 150:2912–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mondloch S, Gannon BM, Davis CR, Chileshe J, Kaliwile C, Masi C, Rios-Avila L, Gregory IIJ, Tanumihardjo SA. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores. Am J Clin Nutr 2015; 102:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams A, Tanumihardjo SA, Rhodes EC, Mapango C, Kazembe B, Phiri F, Kang’ombe DD, Sheftel J, Orchardson V, Tripp K, Suchdev PS. Vitamin A deficiency has declined in Malawi, but with evidence of elevated vitamin A in children. Am J Clin Nutr 2021. (in press). DOI: 10.1093/ajcn/nqab004 [DOI] [PMC free article] [PubMed]
- 64.Manco R, Leclercq IA, Clerbaux L-A. Liver regeneration: different sub-populations of parenchymal cells at play choreographed by an injury-specific microenvironment. Int J Mol Sci 2018; 19:4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol 2016; 65:608–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017; 14:397–411 [DOI] [PubMed] [Google Scholar]
- 67.Ahmad SM, Huda MN, Raqib R, Qadri F, Alam MJ, Afsar MNA, Peerson JM, Tanumihardjo SA, Stephensen CB. High-dose neonatal vitamin A supplementation to Bangladeshi infants increases the percentage of CCR9-positive treg cells in infants with lower birthweight in early infancy, and decreases plasma sCD14 concentration and the prevalence of vitamin A deficiency at two years of age. J Nutr 2020; 150:3005–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanumihardjo SA. Vitamin A status assessment in rats using 13C4-retinyl acetate and gas chromatography-combustion isotope ratio mass spectrometry (GCCIRMS). J Nutr 2000; 130:2844–9 [DOI] [PubMed] [Google Scholar]
- 69.Escaron AE, Green MH, Howe JA, Tanumihardjo SA. Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A. J Nutr 2009; 139:2000–6 [DOI] [PMC free article] [PubMed] [Google Scholar]