Abstract
The Wnt signaling pathway regulates physiological processes such as cell proliferation and differentiation, cell fate decisions, and stem cell maintenance and, thus, plays essential roles in embryonic development, but also in adult tissue homeostasis and repair. The Wnt signaling pathway has been associated with heart development and repair and has been shown to be crucially involved in proliferation and differentiation of progenitor cells into cardiomyocytes. The investigation of the role of the Wnt signaling pathway and the regulation of its expression/activity in atrial fibrillation has only just begun. The present minireview (I) provides original data regarding the expression of Wnt signaling components in atrial tissue of patients with atrial fibrillation or sinus rhythm and (II) summarizes the current state of knowledge of the regulation of Wnt signaling components’ expression/activity and the contribution of the various levels of the Wnt signal transduction pathway to the processes of the development, maintenance, and progression of atrial fibrillation.
Keywords: Atrial fibrillation, atrial remodeling, atrial fibrillation risk factors, Wnt signaling pathway
Impact statement
Atrial fibrillation (AF) is characterized by rapidly occurring atrial remodeling. MAP kinase Erk1/2 and BMP/TGF-β1 signaling pathways mediate AF-dependent remodeling. Wnt signaling pathway has been associated with cardiovascular disease, data concerning its contribution to AF-development and progression, especially to atrial remodeling, are scarce. We give a comprehensive description of basal expression of Wnt-signaling pathway components in human atria and demonstrate that there are substantially dysregulated during AF. Major risk factors for AF including diabetes and hypertension strongly influence activity/expression of the Wnt signaling pathway and available data are reviewed. In conclusion, dysregulation of the Wnt signaling pathway caused by AF risk factors and by AF itself contributes significantly to atrial remodeling and to stabilization of AF. Improved understanding of the precise role of Wnt signaling in AF-mediated remodeling processes could inform the development of novel, targeted interventions for preventing atrial remodeling and AF progression.
Introduction
The Wnt signaling pathway, which is evolutionary highly conserved, regulates a multitude of processes involving proliferation, differentiation, migration, maintenance of progenitor cell states, cell fate decisions, apoptosis and, thus, is crucially implicated in embryonic development, adult tissue homeostasis, regeneration and repair as well as malignant growth and metastasis.1–5 Wnt signaling has been linked to heart development 6 and has been shown to affect cardiomyocyte differentiation including that of stem/progenitor cells into cardiomyocytes.7 Various cardiac and vascular diseases are characterized by the dysregulation of the Wnt signaling pathway (excellently reviewed in Foulquier et al.8) With regard to arrhythmia, available data are sparse and mostly relate to ventricular disorders and cells.
Pathophysiology of atrial fibrillation
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with a prevalence of 2% in the general population younger than 65 years.9–12 In addition to severe clinical symptoms like palpitations, dizziness, dyspnea etc., AF is the single most important factor for ischemic stroke in the population over 75 years of age.13 In about 90% of cases, AF occurs in the presence of other cardiac diseases which include hypertensive heart disease, congestive heart failure, or valve diseases.14 AF rarely develops (10%) in the absence of cardiac abnormalities (“lone” AF).9,12,15 Established risk factors for AF include hypertension, hyperaldosteronemia, diabetes, obesity, heart failure, valve disease, hyperthyroidism, and age. Three forms of AF can be differentiated: 1. Paroxysmal AF involves self-terminating AF episodes with durations that vary from seconds to days. 2. Persistent AF that lasts indefinitely until terminated by medical interventions. 3. Permanent AF that cannot be terminated by pharmacological or electrical cardioversion.
AF therapy aims at three main goals: the prevention of thromboembolic complications, reduction of AF-related symptoms, and termination of the arrhythmia when appropriate.
In the past few decades, our understanding of the pathophysiology of AF has improved significantly. AF is associated with substantial changes within the atrial tissue—atrial remodeling—that act to further increase the AF susceptibility and, thus, contribute to the AF maintenance and progression.15 One of the most important pathophysiological mechanism called “electrical remodeling” has shown that AF begets AF.16 Electrical remodeling could be observed very rapidly after the onset of AF episodes (within hours) and is characterized by an AF-induced progressive shortening of the atrial action potential (AP) and effective refractory period (ERP), thereby decreasing the “wavelength” of re-entry circuits, which favors the persistence of the arrhythmia. Mechanistically, alterations in the expression, localization, and/or activity of e.g. ion channels and GAP junction proteins are largely responsible for this electrical remodeling.17–20 The AF-induced remodeling processes further comprise: structural remodeling, hallmarks of which are atrial fibrosis 21–25 and atrial adipositas,26–29 and contractile as well as endocardial and mitochondrial remodeling.30–34 These complex changes occurring in fibrillating atria produce clinically relevant manifestations as they increase AF susceptibility and progression and stimulate AF-associated diseases and, thus, according to a recent consensus paper, are defined as “atrial cardiomyopathy.35
Signaling pathways that contribute to AF-induced atrial remodeling identified so far include MAP kinase, ERK1/2, BMP/TGFβ, SGK/CTGF, as well as the generation of an adipocyte/adipositas-related expression profile induced by, e.g., PPARγ.26–28,36,37
The further characterization of the activity and impact of different signal transduction pathways on the development of such structural and molecular abnormalities may offer new therapeutic approaches to AF.
This review will focus on the expression and activity of the Wnt signaling pathway in fibrillating atrial tissue and its contribution to AF-induced atrial remodeling.
Wnt signaling pathway
The Wnt signaling pathway is mediated by Wnt ligands which represent a family of 19 secreted, palmitoleic acid-modified, short-reaching glycoproteins. They act on nearby cells by binding to receptor complexes that consist of 1 of the 10 members of the frizzled receptors (FZD; core receptor) and the co-receptor, low density lipoprotein receptor-related protein 5 or 6 (LRP5/6) 4 to initiate the canonical or non-canonical (e.g.) Wnt pathways. In the canonical Wnt/β-catenin pathway, receptor activation leads to an inhibition of glycogen synthase 3β (GSK-3β) mediated phosphorylation of β-catenin and, thus, prevents its degradation in the proteasome. Resulting cytosolic accumulation of β-catenin favors its translocation into the nucleus where induction of Wnt target gene expression is achieved by recruiting and activating transcription factors such as TCF7L2 and TCF4/LEF. While members of the Wnt 1 family (Wnt1, 2, 3a, 8, 8 b, and 10 b) bring about the stabilization of catenin, representatives of the Wnt5a (Wnt 4, 5a, 5 b, 6, 7, and 11) family cannot. The non-canonical Wnt pathways are β-catenin independent and include calcium-dependent (Wnt/Ca2+), small GTPase-dependent, and the planar cell polarity (Wnt/PCP) pathways.3 Non-canonical Wnt signaling can be initiated by frizzled receptors or by frizzled receptors in combination with associated receptor tyrosine kinases such as receptor tyrosine kinase-like orphan receptor 2 (Ror2) or receptor-like tyrosine kinase (RYK).38 It has been proposed that it is the combination of receptor, co-receptor, and ligand that determines which specific Wnt pathway is actually activated 38,39 and what the resulting cellular effects are. The strict separation of canonical and non-canonical signal pathways is becoming increasingly blurred and a convergent model of Wnt signaling has been proposed in which Wnt/Ca2+ and Wnt/β-catenin pathways act in a coordinated interdependent manner.40
Another layer of complexity is added to the Wnt signaling pathway by the existence of various families of soluble or membrane bound Wnt-inhibitors (excellently reviewed in Cruciat and Niehrs41) of which SFRPs and DKKs are the best characterized ones with respect to cardiac tissue.
Wnt signaling in atrial fibrillation
Structural remodeling
Atrial fibrosis represents a hallmark of structural remodeling during AF. Atrial fibrosis is regularly observed in patients with AF or corresponding animal or in vitro models (rapid pacing (RAP)). Wnt signaling has been shown to contribute to fibrotic processes in the heart. Recently, Wnt3A but not Wnt5A was shown to activate β-catenin-dependent Wnt signaling and profibrotic target gene expression in cardiac fibroblasts.42 Mechanistically, AF-dependent atrial fibrosis has been linked to elevated atrial expression levels of Snail1 that were found to be associated with increased expression of canonical Wnt 1, Wnt3A, and Wnt8A and the non-canonical family members Wnt5A and Wnt11.43 Adenoviral overexpression of miR-27b-3p in rats reduced the duration and incidence of induced AF episodes and, notably, this was accompanied by reduced atrial fibrosis and fibrotic gene expression and diminished phospho-β-catenin and Wnt3A levels.44 A similar decrease in profibrotic gene expression and fibrosis could be observed in primary rat fibroblasts upon over-expression of DACT2, which in HL-1 cells also reduced the phosphorylation of β-catenin.45 These data support the view that AF or RAP is associated with a preferential activation of canonical, β-catenin-dependent WNT signaling.
Electrical remodeling
Increased levels of Cx43, lower Cx40/Cx43 ratios, and the lateralization of Cx43 expression represent well-established hallmarks of the AF-dependent electrical remodeling.15,46 Rapid-pacing of neonatal cardiomyocytes was shown to promote the rapid translocation of β-catenin into the nucleus where this transcriptional co-activator contributed to increased expression levels of connexin-43 (Cx43).47
Genetic factors
The homeobox transcription factor Nkx2.5 is implicated in heart development and growth and was shown to be associated with a genetic variation that underlies AF.48 In HL-1 cardiomyocytes, silencing of Nkx2.5 resulted in decreased expression of Wnt11 49 which is in line with the crucial function of Wnt signaling in development.
Right (RA) and left atria (LA) produce quantitatively or qualitatively divergent responses to AF or AF-related stimuli.50 Accordingly, genetic variants of PITX2, a transcription factor involved in determination of “leftness” have been associated with AF. In PITX2 deficient mice, expression of AF-associated genes, including Wnt8a, is severely impaired and, vice versa, over-expression of Wnt8a in vitro modulates microRNA expression profiles to the same extent as PITX2 loss of function does 90.
RNA sequencing has been applied in a recent study to identify AF-related genes differentially expressed between paired human left and right atrial appendages.51 Pathway enrichment analysis on the 157 down- and 90 up-regulated genes revealed that Wnt5A-dependent internalization of Fzd2, Fzd5, and the ROR2 reactome was among the only five categories shared between left and right atrial appendages. The analysis further revealed a greater involvement of left atrial genes in the Wnt signaling pathway.51
Experimental analyses
Re-examination of transcriptome data that were obtained from atrial tissue of pigs subjected to acute rapid-pacing in vivo 37 revealed a significant down-regulation of the non-canonical Wnt5A (−3.1-fold), ROR1 (−2.8-fold), and SFRP3 (−2.7-fold), as well as a 1.7-fold increase in Wnt9B mRNA levels after 7 h of pacing. Interestingly, the anti-arrhythmic multi-channel blocker, dronedarone, increased the mRNA levels of Fzd1 (1.7-fold), SFRP1 (1.6-fold), DKK3 (1.4-fold), and decreased that of SFRP3 (−0.78-fold) in the border zone in pigs subjected to anterior ischemia/reperfusion myocardial infarction.36
Against this background, it is not surprising that dysregulation of the Wnt signaling pathway is associated with the development, maintenance, and progression of AF. A comprehensive analysis, however, of the basal expression of Wnt signaling pathway components and the impact of atrial fibrillation or rapid-pacing on this expression are lacking so far.
Here, a comparative description of the expression of components of the Wnt-signaling pathways, including ligands (Wnts), receptors (frizzled), co-receptors (LRP5/6, Ror2, Ryk), signal transducers (GSK-3β), transcription factors/co-activators of transcription (TCF7-L2; β-catenin), target genes (CCND1; CCND2), and Wnt inhibitors (DKKs, SFRPs) by RT-qPCR in right atrial tissue samples from patients with AF compared to that of SR is provided.
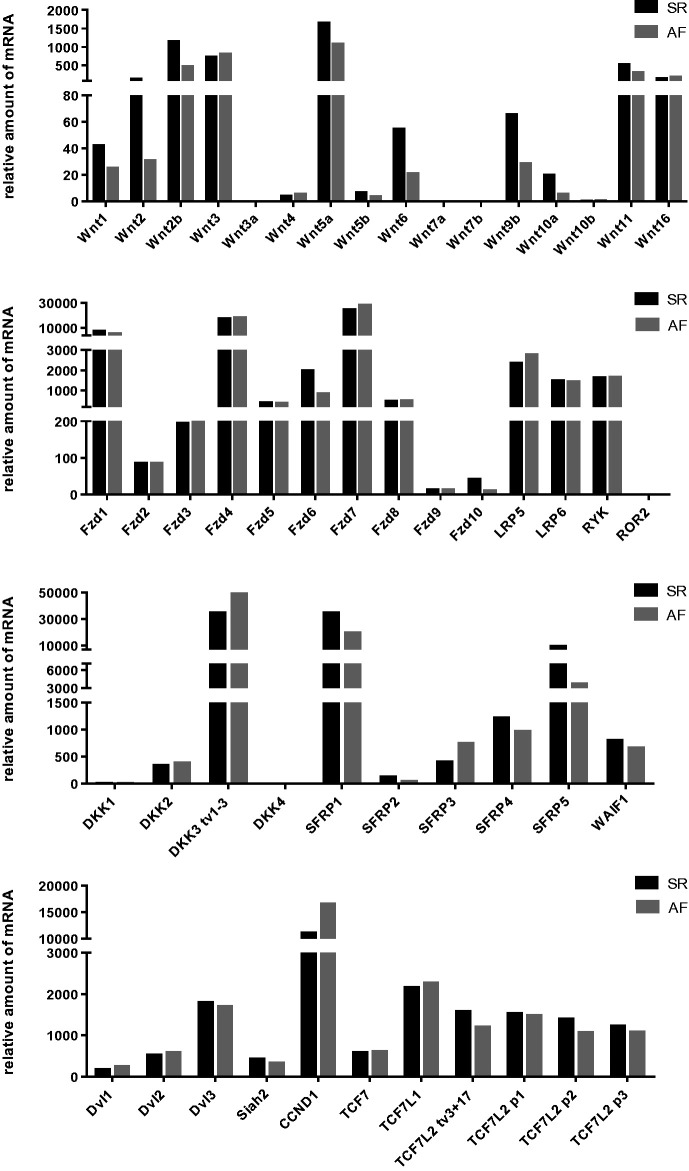
As shown in Figure 1, the expression of a broad panel of Wnt components could be detected in pooled samples of human atrial tissue of patients with AF (n = 9; three paroxysmal AF, four persistent AF, two permanent AF) or SR (n = 11). Patients and methods have been described in detail in a previous study,26 except that patients #21, 42, 73, 77 (AF) and #29 and 72 (SR) were not included here. Detailed information on primers is given in Supplementary Table S1. Highest mRNA amounts could be detected for the Wnt family members Wnt2, Wnt2B, Wnt3, Wnt5A, Wnt11, and Wnt16, the receptors Fzd1, Fzd4, Fzd7, the Wnt inhibitors SFRP1, DKK3, and WAIF1, the intracellular signal transducer Dvl3, the transcription factor TCF7L1, and the Wnt target gene, cyclin D1 (Figure 1). On the contrary, Wnt3A, Wnt7A, Wnt7B, ROR2, and DKK4 were the only components of the Wnt pathway that were not expressed in any sample (Cq value ≥38). Generally, low expression levels were observed for Wnt4, Wnt5B, Wnt10A, Wnt10B, Fzd2, Fzd3, Fzd5, Fzd8, Fzd9, Fzd10, DKK1, DKK2, SFRP2, and Dvl2. All other components of the Wnt pathway were found to be expressed at moderate levels (Figure 1).
Figure 1.
Relative expression levels of Wnt signaling pathway components in atrial tissue of patients with atrial fibrillation (AF) or with sinus rhythm (SR). mRNA amounts have been determined by RT-qPCR of pooled tissue samples (AF: n = 9; SR n = 11).
Atrial tissue samples from patients with AF showed substantial alterations in the mRNA expression profile of Wnt signaling components. As could be concluded from the results obtained with pooled samples, AF is characterized by decreased mRNA expression levels (>1.5-fold change) of the majority of Wnt family members as well as of the receptor Fzd6 and increased expression of Wnt inhibitors (Table 1). In contrast, the Wnt target gene, cyclin D1, showed higher expression levels in AF samples as it could be also observed for Dvl1, DKK3, and SFRP3.
Table 1.
Wnt signaling pathway components differentially expressed between pooled atrial samples from patients with AF relative to SR.
| Wnt signaling pathway components | Fold change |
|---|---|
| Ligands | |
| Wnt1 | −1.75 |
| Wnt2 | −4.17 |
| Wnt2b | −2.63 |
| Wnt4 | 1.73 |
| Wnt5a | −1.61 |
| Wnt5b | −1.96 |
| Wnt6 | −2.00 |
| Wnt9b | −2.44 |
| Receptors | |
| Fzd6 | −1.85 |
| Intracelluler signal transducers | |
| Dvl1 | 1.89 |
| Dvl2 | 1.42 |
| Inhibitors | |
| DKK3 | 1.58 |
| SFRP1 | −1.59 |
| SFRP2 | −2.94 |
| SFRP3 | 2.05 |
| SFRP5 | −2.17 |
| Transcription factors | |
| Cyclin D1 (CCND1) | 1.92 |
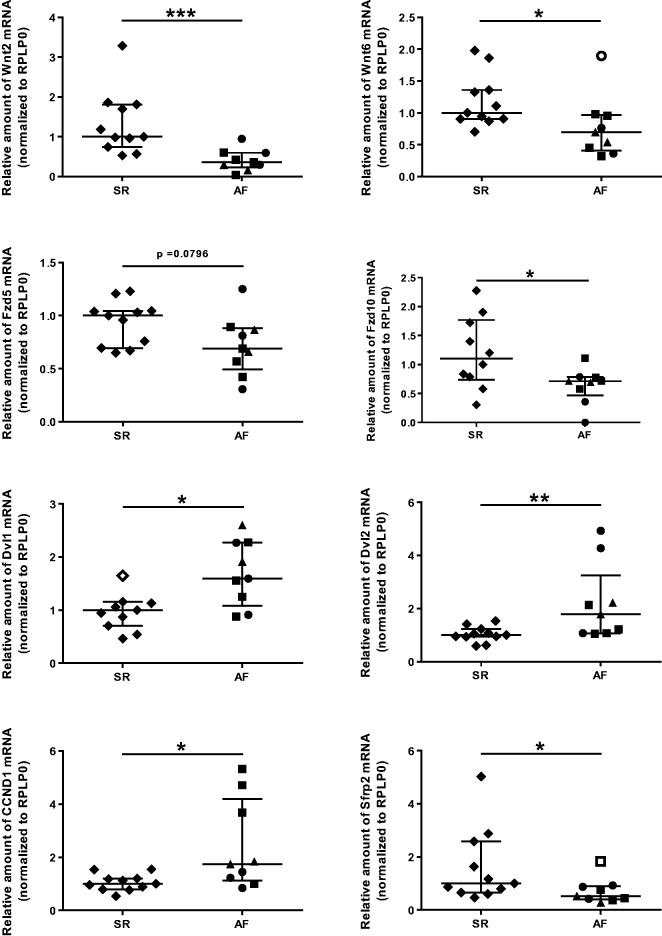
Data obtained by pooled sample analysis could be confirmed by analyzing individual samples for selected targets. As shown in Figure 2, the observed AF-dependent changes of mRNA levels in individual atrial tissue samples are statistically significant for Wnt2, Wnt6, Fzd10, Dvl1, Dvl2, cyclin D1, and SFRP2. Furthermore, there was a tendency towards down-regulation of Fzd5 in AF (Figure 2).
Figure 2.
Quantitative analysis of expression levels of Wnt signaling pathway components in atrial tissue of patients with atrial fibrillation (AF) or with sinus rhythm (SR). mRNA amounts have been determined by RT-qPCR of individual atrial tissue samples (AF n = 9; SR n = 11). Data are given as scatter plots with interquartile range (IQR) ± 1.5 × IQR with outliers as indicated (Tukey method) (* P < 0.05; ** P < 0.01; ***P < 0.001). ♦ SR, ● paroxysmal AF, ▪ persistent AF, ▴ permanent AF, ⋄○□ outliers.
The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway,52 and therefore induction of cyclin D1 mRNA expression in AF seems to be fully consistent with an activation of the pathway under these conditions. In support of this view, we found the amounts of Dvl1 also increased in response to AF as shown previously.53 When considering, however, the transcriptional down-regulation of Wnt and frizzled family members, it is tempting to speculate that these changes are secondary to the increase and activation of β-catenin and aims to cut off any potentially harmful signal input via Wnt pathways. Mechanistically, an alternative activation of β-catenin in the setting of AF could be due to increased Akt/GSK-3β/β-catenin signaling. This pathway has been demonstrated just recently to indeed induce atrial fibrosis.54
AF risk factors and Wnt signaling
As mentioned above, advanced age, arterial hypertension, valvular disease, diabetes mellitus, and hyperthyroidism represent major risk factors for AF. All these clinical conditions enhance AF susceptibility in an additive manner; the prevalence of persistent AF increases steadily depending on the number of such conditions present. Proper treatment of concomitant disease and risk factors is not always associated with an adequately lowered risk for AF development. In diabetic patients, for example, remodeling processes go on and cardiovascular mortality worsens with duration of diabetes.55 Furthermore, the presence of AF risk factors is often diagnosed by chance or with a considerable delay and patient’s compliance to therapy represents another important issue.
To what extent these AF risk factors are capable of modulating Wnt signal transduction and, thereby, may contribute to increased susceptibility, maintenance, and progression of AF is presented below.
Arterial hypertension
About 60–80% of AF patients suffer from hypertension.56 Hypertension is an independent predictor of AF,57 and it contributes to AF progression. Hypertension is associated with increased plasma and tissue levels of angiotensin II (AngII). The same is true for AF,58,59 where increased expression/activity of ACE has been identified as an underlying mechanism.60 A few hours of rapid atrial pacing are sufficient to elevate plasma levels of AngII suggesting a very early involvement in the pathology of AF.58,59,61,62 The elevated levels of AngII mediate tissue remodeling and exert direct pro-arrhythmogenic effects in human atrial tissue.
The effects of angiotensin are mediated by several intracellular signal transduction pathways which include the mitogen-activated protein kinase, Erk1/2, and the Wnt signaling pathway.60,63 AngII increased the amounts of active β-catenin and expression of Wnt target genes, MYC and CCND1, in neonatal cardiomyocytes.64 In neonatal rat ventricular cardiomyocytes, AngII elevated mRNA levels of Wnt1, Wnt3A, Fzd2, Dvl1, Dvl2, and WISP1, a finding that could be confirmed for selected targets, Dvl2 and WISP1, at the protein level.53 Plasma levels of total β-catenin and Wnt3 are higher in hypertensive patients compared to normotensive controls.65 β-catenin just recently has been identified as a master regulator controlling gene expression of essential components of the renin-angiotensin system including angiotensinogen, renin, ACE, AT1R, and AT2R.66,67 The application of ICG-001, a specific inhibitor of β-catenin-dependent gene transcription, can prevent the AngII-mediated increase in blood pressure.66 Vice versa, chronic infusion of AngII was found to induce the expression of eight Wnt family members in the kidney.66 AngII has been further shown to stimulate the expression of SFRP5 in an AT1R/Rho/ROCK1/JNK-mediated manner.68
Volume-overload was shown to decrease β-catenin expression in an AT1R-dependent mechanism in a rabbit model.69 Phospho-myosin phosphatase target subunit (p-MYPT1), a marker of Rho/ROCK kinase activation, has been implicated in high-salt dependent hypertension.70 It could be shown that Wnt5A is indispensable for the AngII-mediated induction of p-MYPT1 human vascular smooth muscle cells.70
Diabetes mellitus
Patients with impaired glucose tolerance or diabetes mellitus (DM) have an increased risk of developing AF, and about 15% of AF patients suffer from DM.71–73 An excellent recent study demonstrated that acute hyperglycemia leads to a prolongation of conduction velocity and atrial effective refractory period (AERP) in healthy pigs.74 This prolongation of the AERPs has rather protective effects with regard to AF inducibility.74 The authors conclude that longer-lasting hyperglycemia promotes AF by structural atrial remodeling, which is in full accordance with clinical observations and animal studies.75,76
The putative contribution of altered Wnt signaling pathway activity/expression to the AF-induced atrial structural remodeling in diabetic patients has not been specifically addressed so far. Results obtained in other fields clearly show that hyperglycemia leads to dysregulation of the Wnt signaling pathway on several levels, and thus it is reasonable to assume similar mechanisms apply to AF-dependent atrial remodeling processes. In neonatal rat cardiac fibroblasts, hyperglycemia resulted in increased levels of Wnt3, cytoplasmic, and nuclear β-catenin, whereas that of Axin-2 and phospho-β-catenin appeared to be decreased.77 It is the inhibition of the Wnt pathway that seems to be responsible for the malformation of the heart caused by gestational diabetes.78
High glucose levels have been shown to amplify Wnt signaling in various types of cancer (excellently reviewed in Garcia-Jimenez et al.79) providing an example of how metabolism can effectively rearrange vital cellular signaling pathways.
Furthermore, hyperglycemia has been established as a main cause of endothelial dysfunction and also in this context it is associated with enhanced Wnt signaling activity/expression.80
Hyperthyroidism
Thyroid dysfunction, and hyperthyroidism in particular, increases AF vulnerability and incidence as well as cardiac dysfunction.81,82 Even subclinical hyperthyroidism increases the risk for developing AF by about 3-fold.83 Adequate therapy of thyroid disease often terminates AF and due to improved clinical management of thyroid disease, thyroid dysfunction is relatively rare in current AF populations.15,84 AF occurs in 15% of patients with hyperthyroidism compared to 4% of people in the general population.84
Studies in a rat thyroidectomy model have shown that hyperthyroid rats exhibited cardiac hypertrophy, increased cardiac function, and electrophysiological changes in heart rates and AERP.81,83
Genome-wide association studies (GWAS) have identified risk variants linked to AF. SNPs in the vicinity of the paired-like homeodomain transcription factor 2, PITX2, show highest linkage significance.85 PITX2 is also involved in maintaining “leftness” in atria of mice and humans.86 Prolonged hypertension and, as it has been shown more recently, also hyperthyroidism down-regulate PITX2 expression which in turn increases Wnt signaling (induction of Wnt8) and dysregulates miRNAs and ion channels as it was previously observed in PITX2 insufficiency.87 Impaired redox signaling very likely contributes to the increased AF incidence in the setting of hyperthyroidism and impaired PITX2/Wnt/miRNA signaling.88
Conclusions
Literature data and experimental results shown here demonstrate that Wnt signaling pathway components are abundantly expressed in atrial tissue. Both the existence of AF risk factors and the presence of AF itself provoke a substantial dysregulation of Wnt signaling pathway activity/expression. Although these changes are varied and complex, AF/RAP and the AF risk factors, diabetes, hypertension, AngII, and hyperthyroidism have in common the capability of predominantly stimulating stabilization and nuclear translocation of β-catenin. In full agreement with the proposed convergence model, AF/RAP is characterized by a drastic dysregulation of also non-canonical Wnt signaling. Data suggest that β-catenin contributes to most of the adverse effects that AF exerts on the myocardium and future work should verify this by means of pharmacological inhibition or models of genetic deficiency. Furthermore, for further work, the urgent questions arise (I) to what extent this effect come about through genuine Wnt signaling or alternative activation of catenin, and (II) whether the observed substantial dysregulation of Wnt pathway expression is secondary to and downstream of β-catenin. Current data suggest a rather protective effect resulting from Wnt/β-catenin down-regulation/inhibition but, again, pharmacological and genetic tool should be employed to assess consequences for AF inducibility and AF-dependent atrial remodeling.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370221994086 for WNT signaling in atrial fibrillation by Carmen Wolke, Elmer Antileo and Uwe Lendeckel in Experimental Biology and Medicine
ACKNOWLEDGMENTS
We thank Manja Möller, Ines Schultz for their excellent technical assistance.
AUTHORS’ CONTRIBUTIONS: All authors contributed to the design, writing, review, and editing of the manuscript. CW and UL performed experiments, collected, and analyzed data.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Uwe Lendeckel https://orcid.org/0000-0002-0684-9959
SUPPLEMENTAL MATERIAL: Supplemental material for this article is available online.
References
- 1.Farzaneh M, Rahimi F, Alishahi M, Khoshnam SE. Paracrine mechanisms involved in mesenchymal stem cell differentiation into cardiomyocytes. Curr Stem Cell Res Ther 2019; 14:9–13 [DOI] [PubMed] [Google Scholar]
- 2.Blankesteijn WM. Interventions in WNT signaling to induce cardiomyocyte proliferation: crosstalk with other pathways. Mol Pharmacol 2020; 97:90–101 [DOI] [PubMed] [Google Scholar]
- 3.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov 2014; 13:513–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017; 169:985–99 [DOI] [PubMed] [Google Scholar]
- 5.Wiese KE, Nusse R, van Amerongen R. Wnt signalling: conquering complexity. Develop 2018; 145:dev165902. [DOI] [PubMed] [Google Scholar]
- 6.Schinner S. Wnt-signalling and the metabolic syndrome. Horm Metab Res 2009; 41:159–63 [DOI] [PubMed] [Google Scholar]
- 7.Kadari A, Mekala S, Wagner N, Malan D, Koth J, Doll K, Stappert L, Eckert D, Peitz M, Matthes J, Sasse P, Herzig S, Brustle O, Ergun S, Edenhofer F. Robust generation of cardiomyocytes from human iPS cells requires precise modulation of BMP and WNT signaling. Stem Cell Rev Rep 2015; 11:560–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT signaling in cardiac and vascular disease. Pharmacol Rev 2018; 70:68–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982; 306:1018–22 [DOI] [PubMed] [Google Scholar]
- 10.Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415:219–26 [DOI] [PubMed] [Google Scholar]
- 11.Shapiro W, Klein G. Alterations in cardiac function immediately following electrical conversion of atrial fibrillation to normal sinus rhythm. Circulation 1968; 38:1074–84 [DOI] [PubMed] [Google Scholar]
- 12.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham study. JAMA 1985; 254:3449–53 [PubMed] [Google Scholar]
- 13.Hart RG, Halperin JL. Atrial fibrillation and stroke: concepts and controversies. Stroke 2001; 32:803–8 [DOI] [PubMed] [Google Scholar]
- 14.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the cardiovascular health study). Am J Cardiol 1994; 74:236–41 [DOI] [PubMed] [Google Scholar]
- 15.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011; 91:265–325 [DOI] [PubMed] [Google Scholar]
- 16.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92:1954–68 [DOI] [PubMed] [Google Scholar]
- 17.Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol 1998; 9:1378–93 [DOI] [PubMed] [Google Scholar]
- 18.Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH, Chen YS, Huang SK, Tseng YZ, Lien WP. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol 1999; 33:1231–7 [DOI] [PubMed] [Google Scholar]
- 19.Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circulation Res 1999; 84:776–84 [DOI] [PubMed] [Google Scholar]
- 20.van der Velden HM, van Kempen MJ, Wijffels MC, van Zijverden M, Groenewegen WA, Allessie MA, Jongsma HJ. Altered pattern of connexin40 distribution in persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol 1998; 9:596–607 [DOI] [PubMed] [Google Scholar]
- 21.Bukowska A, Lendeckel U, Krohn A, Keilhoff G, ten Have S, Neumann KH, Goette A. Atrial fibrillation down-regulates renal neutral endopeptidase expression and induces profibrotic pathways in the kidney. Europace 2008; 10:1212–7 [DOI] [PubMed] [Google Scholar]
- 22.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997; 96:1180–4 [DOI] [PubMed] [Google Scholar]
- 23.Lendeckel U, Arndt M, Wrenger S, Nepple K, Huth C, Ansorge S, Klein HU, Goette A. Expression and activity of ectopeptidases in fibrillating human atria. J Mol Cell Cardiol 2001; 33:1273–81 [DOI] [PubMed] [Google Scholar]
- 24.Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation 2002; 105:2672–8 [DOI] [PubMed] [Google Scholar]
- 25.Thijssen VL, Ausma J, Liu GS, Allessie MA, van Eys GJ, Borgers M. Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol 2000; 9:17–28 [DOI] [PubMed] [Google Scholar]
- 26.Chilukoti RK, Giese A, Malenke W, Homuth G, Bukowska A, Goette A, Felix SB, Kanaan J, Wollert HG, Evert K, Verheule S, Jais P, Hatem SN, Lendeckel U, Wolke C. Atrial fibrillation and rapid acute pacing regulate adipocyte/adipositas-related gene expression in the atria. Int J Cardiol 2015; 187:604–13 [DOI] [PubMed] [Google Scholar]
- 27.Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, Claus P, LePrince P, Nicoletti A, Jalife J, Wolke C, Lendeckel U, Jais P, Willems R, Hatem SN. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J 2017; 38:53–61 [DOI] [PubMed] [Google Scholar]
- 28.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K, Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 2015; 36:795–805a [DOI] [PubMed] [Google Scholar]
- 29.Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res 2014; 102:205–13 [DOI] [PubMed] [Google Scholar]
- 30.Bukowska A, Schild L, Keilhoff G, Hirte D, Neumann M, Gardemann A, Neumann KH, Röhl F-W, Huth C, Goette A, Lendeckel U. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp Biol Med 2008; 233:558–74 [DOI] [PubMed] [Google Scholar]
- 31.Girerd N, Scridon A, Bessiere F, Chauveau S, Geloen A, Boussel L, Morel E, Chevalier P. Periatrial epicardial fat is associated with markers of endothelial dysfunction in patients with atrial fibrillation. PLoS One 2013; 8:e77167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation 1996; 94:2968–74 [DOI] [PubMed] [Google Scholar]
- 33.Jeganathan J, Saraf R, Mahmood F, Pal A, Bhasin MK, Huang T, Mittel A, Knio Z, Simons R, Khabbaz K, Senthilnathan V, Liu D, Sellke F, Matyal R. Mitochondrial dysfunction in atrial tissue of patients developing postoperative atrial fibrillation. Ann Thor Surg 2017; 104:1547–55 [DOI] [PubMed] [Google Scholar]
- 34.Schild L, Bukowska A, Gardemann A, Polczyk P, Keilhoff G, Täger M, Dudley SC, Klein HU, Goette A, Lendeckel U. Rapid pacing of embryoid bodies impairs mitochondrial ATP synthesis by a calcium-dependent mechanism – a model of in vitro differentiated cardiomyocytes to study molecular effects of tachycardia. Biochim Biophys Acta 2006; 1762:608–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S, Document R. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016; 18:1455–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bukowska A, Hammwöhner M, Sixdorf A, Schild L, Wiswedel I, Röhl FW, Wolke C, Lendeckel U, Aderkast C, Bochmann S, Chilukoti RK, Mostertz J, Bramlage P, Goette A. Dronedarone prevents microcirculatory abnormalities in the left ventricle during atrial tachypacing in pigs. Br J Pharmacol 2012; 166:964–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chilukoti RK, Mostertz J, Bukowska A, Aderkast C, Felix SB, Busch M, Volker U, Goette A, Wolke C, Homuth G, Lendeckel U. Effects of irbesartan on gene expression revealed by transcriptome analysis of left atrial tissue in a porcine model of acute rapid pacing in vivo. Int J Cardiol 2013; 168:2100–8 [DOI] [PubMed] [Google Scholar]
- 38.Phesse T, Flanagan D, Vincan E. Frizzled7: a promising Achilles' heel for targeting the wnt receptor complex to treat cancer. Cancers 2016; 8:50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 2012; 13:767–79 [DOI] [PubMed] [Google Scholar]
- 40.Thrasivoulou C, Millar M, Ahmed A. Activation of intracellular calcium by multiple wnt ligands and translocation of beta-catenin into the nucleus: a convergent model of wnt/Ca2+ and wnt/beta-catenin pathways. J Biol Chem 2013; 288:35651–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol 2013; 5:a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dzialo E, Rudnik M, Koning RI, Czepiel M, Tkacz K, Baj-Krzyworzeka M, Distler O, Siedlar M, Kania G, Blyszczuk P. WNT3a and WNT5a transported by exosomes activate WNT signaling pathways in human cardiac fibroblasts. Int J Mol Sci 2019; 20:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo F, Yi X, Li M, Fu J, Li S. Snail1 is positively correlated with atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease. Exp Therap Med 2017; 14:4231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lv X, Li J, Hu Y, Wang S, Yang C, Li C, Zhong G. Overexpression of miR-27b-3p targeting Wnt3a regulates the signaling pathway of wnt/beta-catenin and attenuates atrial fibrosis in rats with atrial fibrillation. Oxid Med Cell Longev 2019; 2019:5703764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou J, Huang S, Long Y, Huang J, Yang S, Yao J, Chen G, Yue Y, Liang M, Mei B, Li J, Wu Z. DACT2 regulates structural and electrical atrial remodeling in atrial fibrillation. J Thorac Dis 2020; 12:2039–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Regnier F, De Vivie ER, Dhein S. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol 2001; 38:883–91 [DOI] [PubMed] [Google Scholar]
- 47.Nakashima T, Ohkusa T, Okamoto Y, Yoshida M, Lee JK, Mizukami Y, Yano M. Rapid electrical stimulation causes alterations in cardiac intercellular junction proteins of cardiomyocytes. Am J Physiol Heart Circ Physiol 2014; 306:H1324–33 [DOI] [PubMed] [Google Scholar]
- 48.Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, Surakka I, Mathis MR, Yamazaki M, Crawford RD, Gabrielsen ME, Skogholt AH, Holmen OL, Lin M, Wolford BN, Dey R, Dalen H, Sulem P, Chung JH, Backman JD, Arnar DO, Thorsteinsdottir U, Baras A, O'Dushlaine C, Holst AG, Wen X, Hornsby W, Dewey FE, Boehnke M, Kheterpal S, Mukherjee B, Lee S, Kang HM, Holm H, Kitzman J, Shavit JA, Jalife J, Brummett CM, Teslovich TM, Carey TJ, Gudbjartsson DF, Stefansson K, Abecasis GR, Hveem K, Willer CJ. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet 2018; 50:1234–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Xu S, Li W, Wu L, Wang L, Li Y, Zhou W. Nkx2 5 Insufficiency leads to atrial electrical remodeling through wnt signaling in HL-1 cells. Exp Therap Med 2019; 18:4631–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lendeckel U, Wolke C. Structuring (right) atrial fibrillation: location matters. Europace 2018; 20:906–7 [DOI] [PubMed] [Google Scholar]
- 51.Thomas AM, Cabrera CP, Finlay M, Lall K, Nobles M, Schilling RJ, Wood K, Mein CA, Barnes MR, Munroe PB, Tinker A. Differentially expressed genes for atrial fibrillation identified by RNA sequencing from paired human left and right atrial appendages. Physiol Genomics 2019; 51:323–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 1999; 96:5522–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Guleria RS, Thakur S, Zhang CL, Pan J, Baker KM, Gupta S. Thymosin beta4 prevents angiotensin II-Induced cardiomyocyte growth by regulating wnt/WISP signaling. J Cell Physiol 2016; 231:1737–44 [DOI] [PubMed] [Google Scholar]
- 54.Lin R, Wu S, Zhu D, Qin M, Liu X. Osteopontin induces atrial fibrosis by activating akt/GSK-3beta/beta-catenin pathway and suppressing autophagy. Life Sci 2020; 245:117328. [DOI] [PubMed] [Google Scholar]
- 55.From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol 2009; 103:1463–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, Goette A, Lewalter T, Ravens U, Meinertz T, Breithardt G, Steinbeck G. The registry of the German competence NETwork on atrial fibrillation: patient characteristics and initial management. Europace 2009; 11:423–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdecchia P, Reboldi G, Gattobigio R, Bentivoglio M, Borgioni C, Angeli F, Carluccio E, Sardone MG, Porcellati C. Atrial fibrillation in hypertension: predictors and outcome. Hypertension 2003; 41:218–23 [DOI] [PubMed] [Google Scholar]
- 58.Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res 2003; 60:315–25 [DOI] [PubMed] [Google Scholar]
- 59.Goette A, Bukowska A, Lendeckel U, Erxleben M, Hammwöhner M, Strugala D, Pfeiffenberger J, Röhl FW, Huth C, Ebert MP, Klein HU, Röcken C. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation 2008; 117:732–42 [DOI] [PubMed] [Google Scholar]
- 60.Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol 2000; 35:1669–77 [DOI] [PubMed] [Google Scholar]
- 61.Goette A, Ittenson A, Hoffmanns P, Reek S, Hartung W, Klein H, Ansorge S, Geller JC. Increased expression of P-selectin in patients with chronic atrial fibrillation. Pacing Clin Electrophysiol 2000; 23:1872–5 [DOI] [PubMed] [Google Scholar]
- 62.Tsai CT, Lai LP, Hwang JJ, Chen WP, Chiang FT, Hsu KL, Tseng CD, Tseng YZ, Lin JL. Renin-angiotensin system component expression in the HL-1 atrial cell line and in a pig model of atrial fibrillation. J Hypertension 2008; 26:570–82 [DOI] [PubMed] [Google Scholar]
- 63.Gitau SC, Li X, Zhao D, Guo Z, Liang H, Qian M, Lv L, Li T, Xu B, Wang Z, Zhang Y, Xu C, Lu Y, Du Z, Shan H, Yang B. Acetyl salicylic acid attenuates cardiac hypertrophy through wnt signaling. Front Med 2015; 9:444–56 [DOI] [PubMed] [Google Scholar]
- 64.Yu L, Meng W, Ding J, Cheng M. Klotho inhibits angiotensin II-induced cardiomyocyte hypertrophy through suppression of the AT1R/beta catenin pathway. Biochem Biophys Res Commun 2016; 473:455–61 [DOI] [PubMed] [Google Scholar]
- 65.Yin L, Yao J, Deng G, Wang X, Cai W, Shen J. Identification of candidate lncRNAs and circRNAs regulating WNT3/beta-catenin signaling in essential hypertension. Aging 2020; 12:8261–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao L, Xu B, Zhou L, Tan RJ, Zhou D, Fu H, Li A, Hou FF, Liu Y. Wnt/beta-catenin regulates blood pressure and kidney injury in rats. Biochim Biophys Acta Mol Basis Dis 2019; 1865:1313–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y. Multiple genes of the renin-angiotensin system are novel targets of wnt/beta-catenin signaling. J Am Soc Nephrol 2015; 26:107–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin X, Guo B, Yan J, Yang R, Chang L, Wang Y, Miao C, Liu S, Zhang H, Li Y. Angiotensin II increases secreted frizzled-related protein 5 (sFRP5) expression through AT1 receptor/rho/ROCK1/JNK signaling in cardiomyocytes. Mol Cell Biochem 2015; 408:215–22 [DOI] [PubMed] [Google Scholar]
- 69.Itoh M, Takeishi Y, Nakada S, Miyamoto T, Tsunoda Y, Takahashi H, Kubota I, Tomoike H. Long-term treatment with angiotensin II type 1 receptor antagonist, CV-11974, restores beta-catenin mRNA expression in volume-overloaded rabbit hearts. Heart Vessels 2002; 17:36–41 [DOI] [PubMed] [Google Scholar]
- 70.Kawarazaki W, Mizuno R, Nishimoto M, Ayuzawa N, Hirohama D, Ueda K, Kawakami-Mori F, Oba S, Marumo T, Fujita T. Salt causes aging-associated hypertension via vascular Wnt5a under klotho deficiency. J Clin Invest 2020; 130:4152–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham heart study. JAMA 1994; 271:840–4 [PubMed] [Google Scholar]
- 72.Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins K, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med 2010; 25:853–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latini R, Staszewsky L, Sun JL, Bethel MA, Disertori M, Haffner SM, Holman RR, Chang FF, Giles TD, Maggioni AP, Rutten GE, Standl E, Thomas L, Tognoni L, Califf RM, McMurray JJ. Incidence of atrial fibrillation in a population with impaired glucose tolerance: the contribution of glucose metabolism and other risk factors. A post hoc analysis of the nateglinide and valsartan in impaired glucose tolerance outcomes research trial. Am Heart J 2013; 166:935–40.e1. [DOI] [PubMed] [Google Scholar]
- 74.Manninger M, Zweiker D, Dobrovnik M, van Hunnik A, Rohrer U, Zirngast B, Herbst V, Maechler H, Schotten U, Zirlik A, Scherr D. Acute hyperglycaemia is not associated with the development of atrial fibrillation in healthy pigs. Sci Rep 2020; 10:11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao TF, Suenari K, Chang SL, Lin YJ, Lo LW, Hu YF, Tuan TC, Tai CT, Tsao HM, Li CH, Ueng KC, Wu TJ, Chen SA. Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. Am J Cardiol 2010; 106:1615–20 [DOI] [PubMed] [Google Scholar]
- 76.Kato T, Yamashita T, Sekiguchi A, Sagara K, Takamura M, Takata S, Kaneko S, Aizawa T, Fu LT. What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol 2006; 17:890–4 [DOI] [PubMed] [Google Scholar]
- 77.Hu J, Lu X, Zhang X, Shao X, Wang Y, Chen J, Zhao B, Li S, Xu W, Wie C. Exogenous spermine attenuates myocardial fibrosis in diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress and the canonical wnt signaling pathway. Cell Biol Int 2020; 44:1660–70 [DOI] [PubMed] [Google Scholar]
- 78.Zhao Z. TGFbeta and wnt in cardiac outflow tract defects in offspring of diabetic pregnancies. Birth Defects Res B Dev Reprod Toxicol 2014; 101:364–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garcia-Jimenez C, Garcia-Martinez JM, Chocarro-Calvo A, De la Vieja A. A new link between diabetes and cancer: enhanced WNT/beta-catenin signaling by high glucose. J Mol Endocrinol 2014; 52:R51–66 [DOI] [PubMed] [Google Scholar]
- 80.Durak-Kozica M, Paszek E, Stepien EL. Role of the wnt signalling pathway in the development of endothelial disorders in response to hyperglycaemia. Expert Rev Mol Med 2019; 21:e7. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y, Dedkov EI, Teplitsky D, Weltman NY, Pol CJ, Rajagopalan V, Lee B, Gerdes AM. Both hypothyroidism and hyperthyroidism increase atrial fibrillation inducibility in rats. Circulation 2013; 6:952–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy V, Taha W, Kundumadam S, Khan M. Atrial fibrillation and hyperthyroidism: a literature review. Indian Heart J 2017; 69:545–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bielecka-Dabrowa A, Mikhailidis DP, Rysz J, Banach M. The mechanisms of atrial fibrillation in hyperthyroidism. Thyroid Res 2009; 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, Cobbe S, Breithardt G, Le Heuzey JY, Prins MH, Levy S, Crijns HJGM. Atrial fibrillation management: a prospective survey in ESC member countries: the euro heart survey on atrial fibrillation. Eur Heart J 2005; 26:2422–34 [DOI] [PubMed] [Google Scholar]
- 85.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007; 448:353–7 [DOI] [PubMed] [Google Scholar]
- 86.Kahr PC, Piccini I, Fabritz L, Greber B, Scholer H, Scheld HH, Hoffmeier A, Brown NA, Kirchhof P. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS One 2011; 6:e26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lozano-Velasco E, Hernandez-Torres F, Daimi H, Serra SA, Herraiz A, Hove-Madsen L, Aranega A, Franco D. Pitx2 impairs calcium handling in a dose-dependent manner by modulating wnt signalling. Cardiovasc Res 2016; 109:55–66 [DOI] [PubMed] [Google Scholar]
- 88.Lozano-Velasco E, Wangensteen R, Quesada A, Garcia-Padilla C, Osorio JA, Ruiz-Torres MD, Aranega A, Franco D. Hyperthyroidism, but not hypertension, impairs PITX2 expression leading to wnt-microRNA-ion channel remodeling. PLoS One 2017; 12:e0188473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370221994086 for WNT signaling in atrial fibrillation by Carmen Wolke, Elmer Antileo and Uwe Lendeckel in Experimental Biology and Medicine