Abstract
Objectives
We applied a novel Outbreak Costing Tool (OCT), developed by the US Centers for Disease Control and Prevention (CDC), to estimate the costs of investigating and responding to an anthrax outbreak in Tanzania. We also evaluated the OCT's overall utility in its application to a multisectoral outbreak response.
Methods
We collected data on direct costs associated with a human and animal anthrax outbreak in Songwe Region (December 2018 to January 2019) using structured questionnaires from key-informants. We performed a cost analysis by entering direct costs data into the OCT, grouped into seven cost categories: labor, office, travel and transport, communication, laboratory support, medical countermeasures, and consultancies.
Results
The total cost for investigating and responding to this outbreak was estimated at 102,232 United States dollars (USD), with travel and transport identified as the highest cost category (62,536 USD) and communication and consultancies as the lowest, with no expenditure, for the combined human and animal health sectors.
Conclusions
Multisectoral investigation and response may become complex due to coordination challenges, thus allowing escalation of public health impacts. A standardized framework for collecting and analysing cost data is vital to understanding the nature of outbreaks, in anticipatory planning, in outbreak investigation and in reducing time to intervention. Pre-emptive use of the OCT will also reduce overall and specific (response period) intervention costs for the disease. Additional aggregation of the costs by government ministries, departments and tiers will improve the use of the tool to enhance sectoral budget planning for disease outbreaks in a multisectoral response.
Keywords: Anthrax, Cost analysis, Infectious disease outbreak, One Health, Outbreak Costing Tool, Tanzania
Highlights
-
•
Direct cost estimation for a human-animal anthrax outbreak in Tanzania during 2018–2019, using a novel Outbreak Costing Tool;
-
•
Application of a standardized Outbreak Costing Tool facilitates One Health and encourages multisectoral collaboration;
-
•
Multisectoral outbreak costing data informs planning against disease outbreaks, such as Rift Valley fever, zoonotic influenza and rabies.
1. Introduction
Anthrax (Bacillus anthracis) is a zoonotic bacterial infection that primarily affects herbivores, although all mammals are vulnerable to the disease [1]. B. anthracis spores can survive in soil for years, if not exposed to ultraviolet rays [2]. Livestock species, including cattle, sheep, and goats, as well as wild ruminants, are highly susceptible to infection through ingestion of soil contaminated with B. anthracis spores [2]. Human infection is usually via contact with infected animals or animal products [3] and may manifest as cutaneous, inhalational or gastrointestinal infection [4]. The worldwide incidence of anthrax is generally decreasing, however, the disease is endemic throughout Africa [2].
In Tanzania, this frequently reported bacterial zoonosis [5,6], has been prioritized alongside rabies, viral haemorrhagic fevers (including Rift Valley fever), zoonotic avian influenza, human African trypanosomiasis and brucellosis [7,8]. Through the Global Health Security Agenda (GHSA), Tanzania is addressing anthrax prevention and control and has developed the National Strategy for Prevention and Control of Anthrax in Humans and Animals, 2018–2023. This strategy was supported by the Food and Agriculture Organization of the United Nations (FAO) and received funding from the United States Agency for International Development (USAID) [9].
The FAO, the World Health Organization (WHO), and the World Organization for Animal Health (OIE) have performed a series of evaluative assessments in Tanzania [[10], [11], [12]]. These assessments, in conjunction with the global One Health guidance [13], highlight both the need for the implementation of standardized outbreak investigation tools and sustainable funding for outbreak response that incorporates a One Health approach. The One Health approach is defined by the Tripartite (WHO, FAO, and OIE) as an approach used to address a health threat at the human-animal-environment interface, across all relevant sectors and disciplines, with the ultimate goal of achieving optimal health outcomes for both people and animals [13]. Cost analyses for infectious disease outbreaks, especially those that involve multisectoral investigations, are not routinely implemented. Additionally, studies that have estimated the costs of investigating and responding to outbreaks have often focused on high-income countries [14,15], with only a few examples from low- and middle-income countries [16]. Reasons for this disparity include lack of detailed cost data availability and the absence of a standardized approach for collecting such data [14]. Costing tools that facilitate a One Health approach are limited. However, if costs of investigating and responding to outbreaks could be collected using a standardized and multisectoral approach, planning and informing sustainable funding for multisectoral outbreak investigation and response activities could be greatly improved. Specifically, availability of cost data could: increase budget transparency, allow adaptation and incorporation of the most cost-effective response activities; reduce response times to outbreak alerts by improving access to funding; facilitate development of an outbreak costing database that could be used as a baseline estimate in the case of future human and animal disease outbreaks; and improve national capacity to respond to, and limit the scale of, infectious disease outbreaks.
In 2018, the US Centers for Disease Control and Prevention (CDC), in collaboration with RTI International (formerly Research Triangle Institute), developed an Outbreak Costing Tool (OCT) that can be used to conduct cost analysis for a range of disease outbreak scenarios [17]. The OCT was developed to strengthen global health security by enhancing countries' ability to prevent, detect, and respond to public health emergencies and infectious disease threats. The tool is applicable to both human and animal specific disease outbreaks and outbreaks that require a multisectoral response (e.g., zoonotic disease outbreaks), allowing for the incorporation of costs from multisector stakeholders. In the case of outbreaks that require a multisector response, the implementation of this tool can help facilitate the costing of collaborative outbreak investigations and response activities through improving understanding, communication and reporting. The OCT was first piloted in this study, where its application could assist the Tanzanian human, animal and environmental health ministerial sectors in planning future activities and addressing One Health challenges with respect to estimating the costs of outbreak investigation and response and could potentially assist other countries in the same way. It should be noted that other health specific costing tools have been utilized for health programming and evaluation of disease outbreaks, foodborne illnesses and zoonoses including CostIt [18]; SurvCost 1.0 [19]; WHO-CHOICE [[20], [21], [22], [23], [24], [25], [26]].
Our study's primary aim was to use the OCT to retrospectively generate a cost estimate for investigation and response activities associated with an anthrax outbreak. The secondary aim was to evaluate the utility of the OCT in its application to a multisectoral infectious disease outbreak investigation and response.
2. Methods
2.1. Outbreak location
Between December 2018 and January 2019, an outbreak of human and livestock anthrax occurred in the Momba District, Songwe Region, situated in Tanzania's southern highlands. A multisectoral response was mounted from the national to regional level through to the affected district. The 2017 census estimated a human population in excess of 198,269 for Momba District [27], with this rural community comprising largely agro-pastoralist populations [28]. The predominant small holder livestock species in this area are poultry, followed by cattle, goats, pigs, and sheep [29].
2.2. Outbreak description and response
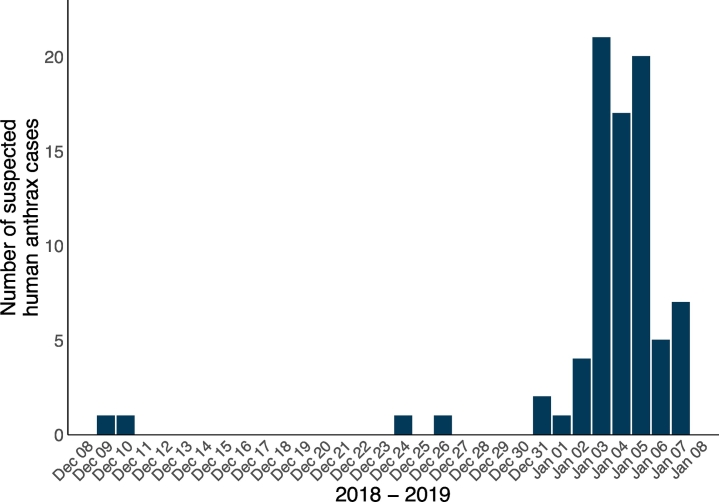
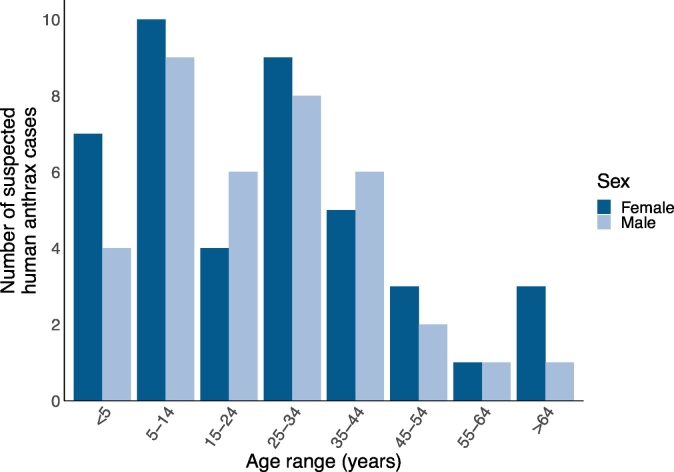
On January 3, 2019, the Ward Councillor for Nzoka Ward in Momba District, Songwe Region, notified the district and regional authorities of a suspected anthrax outbreak in Nzoka Village. The national health authority, the Ministry of Health, Community Development, Gender, Elderly and Children (MoHCDGEC) dispatched a team for investigation on January 10, 2019. Upon outbreak investigation, it was found that 81 suspected human cases were reported between December 9, 2018, and January 7, 2019 (Fig. 1). Suspected human cases had symptoms including rash, blisters, ulcers and inflamed oedematous skin. Forty-four (54.3%) suspected human cases were female (Fig. 2). The median age of suspected human cases was 25 years (range: 1–75 years) for 79 (97.5%) of suspected human cases for whom age data was available (Fig. 2). Four (4.9%) fatalities of 81 suspected human cases were reported, two of the four (50.0%) fatalities were female and the median age was 49 years (range: 13–70 years). These four clinically suspected human case fatalities were laboratory confirmed as anthrax by Gram staining for bacilli demonstration and real-time polymerase chain reactions at the Tanzanian Veterinary Laboratory Agency on January 15, 2019. Subsequent samples were dispatched in batches for testing. Diagnostic testing for the outbreak was conducted in accordance with the government of Tanzania and WHO-FAO-OIE case definitions for confirmed human anthrax [3,7].
Fig. 1.
Timeline of the number of reported suspected human anthrax cases (n = 81) during the outbreak period in Songwe Region, Tanzania, from December 2018 to January 2019.
Fig. 2.
Age range (years) and sex distribution for reported suspected human anthrax cases (n = 79 with data available) during the outbreak period in Songwe Region, Tanzania, from December 2018 to January 2019.
Sixteen cattle deaths were reported during the human outbreak by the Ministry of Livestock and Fisheries (MoLF) [30]. Two cattle carcasses earlier consumed by the human cases were identified as the probable source of human infection. Samples were not collected from carcasses due to a number of reasons, including lack of stereotypical anthrax symptoms, slaughtering and handling of meat from potentially infected carcasses without inspection by a designated livestock officer [6], and a delay in human diagnosis. Following the confirmation of human anthrax, no further livestock carcasses nor wildlife carcasses were reported. Therefore, animal confirmatory testing was not performed during the outbreak period.
Outbreak response livestock vaccination was conducted until January 16, 2019, covering 64,348 animals across the Momba District. Other prevention and control measures employed included a provision of health education to communities in the affected village on how to prevent transmission of the disease, raising health seeking behaviour, safe disposal of carcasses and strengthening surveillance activities both in human and livestock sectors. There was no local government emergency budget available for this outbreak, and all direct costs were primarily incurred by the Tanzanian national government (i.e., MoLF and the MoHCDGEC) and a number of development partners, including the FAO, US CDC, WHO, and USAID.
2.3. Questionnaire data collection for outbreak costs
Data on costs associated with the outbreak were collected from May to June 2019, from all sectors involved, including human, animal and environmental health government representatives. Twenty human, animal and environmental health sector government officials were approached in-person (three human health, eight animal health, and two One Health specialists), by phone (two human health and one animal health specialist) and email (two human health, one animal health and one environmental health specialist) for participation in outbreak costing data collection. Those individuals with knowledge of costs associated with the outbreak were selected as initial key-informants. Following informed consent, respondents completed a structured questionnaire pertaining to one of seven OCT independent cost categories: labor, office materials and equipment, travel and transport, communication, laboratory support, medical countermeasures, and consultancies. Each cost category questionnaire was designed to answer every field within each OCT cost category. When a respondent did not have knowledge on specific aspects of a cost category, either the respondent conferred with a colleague for further information or the respondent made a suggestion of a knowledgeable colleague that could complete the remaining cost category fields, and this individual was approached for participation. Questionnaire responses were cross-verified by additional government officials where possible to generate more robust costing estimates and reduce questionnaire bias [31]. This cross-verification was conducted by additional key-informant questionnaire administration at the regional, zonal or national government levels.
2.4. Outbreak Costing Tool (OCT) description
Costing analysis for the investigation and response costs associated with the anthrax outbreak was performed using the OCT. The OCT offered a standardized, Excel-based approach to recording and summarizing outbreak costing data. While the costing tool allowed entry of information from multiple sectors, it did not allow the breakdown of costs incurred by each sector, thus all costs incurred were represented as totals across all sectors involved. Questionnaire data were entered into each spreadsheet of the OCT. Within the OCT, seven cost-related categories were split into one labor and six non-labor activities, with non-labor cost categories including: office materials and equipment, travel and transport, communication, laboratory support, medical countermeasures, and consultancies. Individual cost items listed within the OCT for each non-labor cost category are given in Table 1.
Table 1.
Outbreak Costing Tool individual cost items for each non-labor cost category.
| Office materials and equipment | Travel and transport | Communication | Laboratory support | Medical countermeasures | Consultancies |
|---|---|---|---|---|---|
| Stationeries | Fuel costs | Airtime for national radio broadcasts | Personal protective equipment | Drugs for prevention: Vaccines | Database development |
| Printing/copies | Rented or hired vehicles | Airtime for national television broadcasts | Syringes | Antibiotic prophylaxis | Database management |
| Rented building space | Parking | Advertisements in national newspapers | Pipettes | Quarantine | Data collection |
| Rented equipment | Purchased vehicles | Airtime for local radio broadcasts | Reagents | Closing of food premises | Data analyses |
| Rented furniture | Maintenance and repair costs | Airtime for local television broadcasts | Shipment of materials | Animal culls | Field epidemiology |
| Internet | Lodging | Advertisements in local newspapers | Specimen collection | Disposal or decontamination of contaminated items | Biology/entomology |
| Cellular data | Per diem expenses (e.g., food) | Wall poster advertisements | Specimen transport | Water chlorination | Training |
| Speciality software | Airfare for deployed personnel | T-shirts to raise outbreak awareness | Specimen processing | Impregnated bed nets | Risk communication and media trainings |
| Mobile phones | Taxi and bus fares | Identification of pathogens | Development of case management guidelines for safety hazards (e.g., zoonotic, food safety) | ||
| Solar panels to charge phones and computers | Data management | ||||
| Global Positioning System devices | Data analysis and results | ||||
| Mobile hotspots | Waste management |
Per cost category, the OCT incorporated individual items associated with that category, the quantity and total cost per item, and the percentage cost for each item in relation to three pre-defined outbreak periods. These outbreak periods included: the initial response period (i.e., preparation, outbreak verification, outbreak diagnosis, case verification, case diagnosis, case definition construction, case recording, epidemiology description, hypothesis development, hypothesis evaluation and finalization, and reconciling evidence); the outbreak response period (i.e., implementing infection control and prevention measures); and the follow-up and reporting period (i.e., initiating or maintaining surveillance and dissemination of findings). A results spreadsheet summarized the data, facilitating interpretation of outputs. All cost estimates were calculated in local currency, Tanzanian shillings (TSH), and converted to equivalent United States dollars (USD), using the average exchange rate for June 2019 (1 TSH = 0.000434 USD [32]).
2.5. Descriptive statistics
Total suspected human anthrax cases were evaluated over the outbreak period. Confirmed human cases were visualized by age and sex distributions. For each of the seven cost categories and three outbreak periods summarized using the OCT, total costs and cost proportions, presented as total percentage costs, were visualized using R software version 3.6.1 [33] and the ggplot2 R package [34].
3. Results
In total, 18 of 20 (90.0%) government officials from district to national level and cutting across multiple sectors, who are directly involved in the outbreak investigation and response activities were able to provide information to populate the questionnaire cost data. The remaining two individuals were not directly involved in the outbreak response but contributed technical information. An estimated 5045 labor hours were spent on outbreak investigation and response activities by at least 29 district, region, zonal and national government employees across human, animal and environmental health sectors.
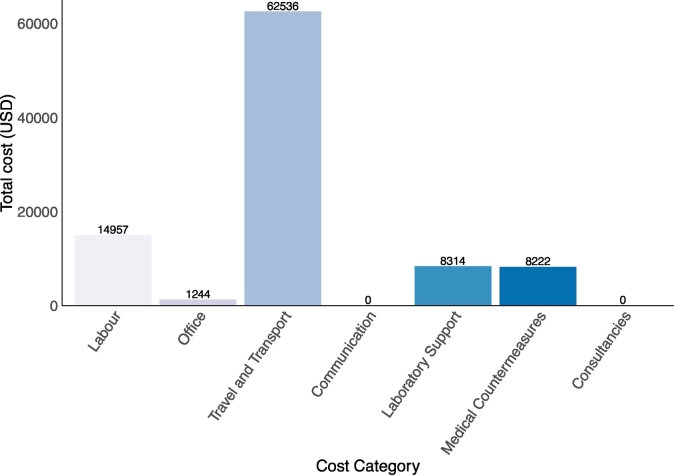
The total cost for the entire multisectoral outbreak response, including projected hours for ongoing activities, was approximately 102,232 USD (235,016,666 TSH) across all sectors. The total estimate for labor costs was 14,957 USD (34,384,666 TSH), the remaining non-labor cost categories combined were estimated at 87,276 USD (200,634,483 TSH). The category with the highest cost was travel and transport and the lowest cost categories were communication and consultancies, reporting zero costs. The lack of reported cost for the communication cost category was due to the provision of free of charge health education services by local media houses and the engagement of traditional and religious leaders in educating the affected communities. The travel and transport cost category represented approximately 61.2% of the total outbreak investigation and response costs. Medical countermeasures accounted for 14.9%, labor costs 14.3%, laboratory support 8.1% and office materials and equipment 1.2% of the total costs (Fig. 3).
Fig. 3.
Total cost per individual cost category in United States dollars (USD) for an anthrax outbreak in Songwe Region, Tanzania, from December 2018 to January 2019.
Note: The equivalent cost categories of total expenditure in Tanzanian shillings (TSH) are as follows: Labor; 34,384,666 TSH (14,957 USD), Office; 2,860,000 TSH (1244 USD), Travel and Transport; 143,760,000 TSH (62,536 USD), Communication; 0 TSH (0 USD), Laboratory Support; 19,112,000 TSH (8314 USD), Medical Countermeasures; 18,900,000 TSH (8222 USD), and Consultancies; 0 TSH (0 USD).
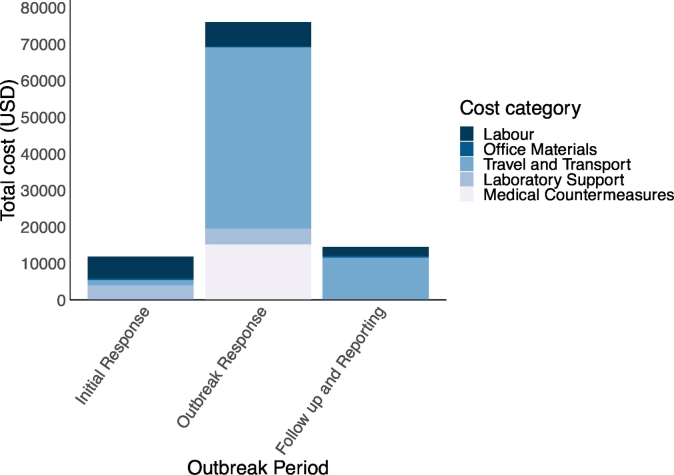
Multisectoral outbreak investigation and response activity costs for the three outbreak response periods (initial response, outbreak response, and follow-up and reporting periods) are shown in Fig. 4. The outbreak response period was the costliest of the three outbreak periods, representing 74.3% of the overall costs (75,944 USD; 175,583,746 TSH), then follow up and reporting at 14.2% (14,498 USD; 33,329,177 TSH). The initial response was estimated at 11.5% (11,790 USD; 27,103,742 TSH). Considering cost category distribution per response period, travel and transport incurred the majority of costs during the outbreak response period.
Fig. 4.
Total individual category costs in United States dollars (USD) across three defined outbreak investigation and response periods for an anthrax outbreak in Songwe Region, Tanzania, from December 2018 to January 2019.
Note: The equivalent cost categories of total expenditure in Tanzanian shillings (TSH) for the Initial Response Period are as follows: Labor; 13,531,242 TSH (5886 USD), Office; 1,144,000 TSH (498 USD), Travel and Transport; 3,285,000 TSH (1429 USD), Laboratory Support; 9,143,500 TSH (3977 USD), Medical Countermeasures; 0 TSH (0 USD). For the Outbreak Response Period: Labor; 15,203,246 TSH (6613 USD), Office; 572,000 TSH (249 USD), Travel and Transport; 113,940,000 TSH (49,564 USD), Laboratory Support; 9,968,500 TSH (4336 USD), and Medical Countermeasures; 34,900,000 TSH (15,182 USD). For the Follow up and Reporting Period: Labor; 5,650,177 TSH (2458 USD), Office; 1,144,000 TSH (498 USD), Travel and Transport; 26,535,000 TSH (11,523 USD), Laboratory Support; 0 TSH (0 USD), and Medical Countermeasures; 0 TSH (0 USD). The cost categories: communication and consultancies, are not shown as there was no associated expenditure.
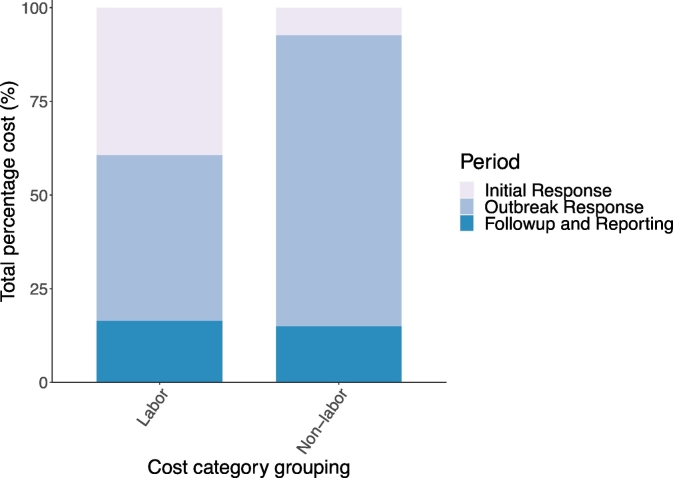
Breaking down costs broadly into labor and non-labor across all sectors, the greater percentage of labor costs were nearly evenly split between the initial response (39.4%) and the outbreak response (44.2%) periods, with follow-up and reporting making up the remainder (16.4%) of labor costs. Whereas the majority of the non-labor costs were estimated to have been incurred during the outbreak response period (77.7%). Non-labor costs for the initial response period and follow-up and reporting period, were 7.3% and 15.0%, respectively (Fig. 5). Additionally, the use of OCT allowed for the review of the index case and reclassification of earlier misdiagnosis.
Fig. 5.
Percentage labor and non-labor costs across three defined outbreak investigation and response periods for an anthrax outbreak in Songwe Region, Tanzania, from December 2018 to January 2019.
Note: Non-labor category grouping includes the cost categories: office supplies and equipment, travel and transport, laboratory support and medical countermeasures. The cost categories: communication and consultancies, are not shown as there was no associated expenditure.
4. Discussion
Our aim was to estimate the costs of multisectoral investigation and response activities associated with an anthrax outbreak that occurred in Songwe Region, Tanzania, between December 2018 and January 2019. We used a novel OCT to collect and analyse cost data associated with investigating and responding to the outbreak. In addition, we aimed to evaluate the overall utility of the OCT in its application to an infectious disease outbreak that required a multisectoral investigation and response. To our knowledge, this is the first attempt at using a standardized outbreak costing tool to estimate the costs of multisectoral investigation and response activities for an anthrax outbreak in sub-Saharan Africa.
The OCT was able to estimate comprehensive costs (102,232 USD) associated with investigating and responding to an anthrax outbreak in Tanzania, divided between labor (15.7%) and non-labor (84.3%) costs. These estimated costs cover the entirety of the outbreak, from the initial response (12.4%), through to outbreak response (72.4%), and follow-up and reporting (15.2%) periods. The transport and travel cost category had the largest reported costs, which included vehicle, lodging and general travel-related expenses, such as vehicle hire, fuel costs, and per diems for outbreak rapid response team members. The second largest cost category was medical countermeasures, which comprised drugs (both medical and veterinary), and control measures, such as quarantine and the closing of food premises. Outbreak items with the greatest cost included hired vehicles and vaccines. All cost categories had a percentage of total cost split across the three main response periods: initial response period, outbreak response period, and follow-up and reporting period. The majority of costs were incurred during the outbreak response period. In terms of outbreak budget planning, this suggests that activities involving the implementation of disease outbreak control and prevention measures are the driving cost components during the course of an outbreak event.
Understanding the distribution of estimated costs associated with different aspects of outbreak investigation and response activities can assist in effective budgeting and planning for future outbreaks. First, the ability to detect and reduce the scale of an outbreak is tremendously beneficial, as only a portion of the initial outbreak response costs will be required, and potential subsequent costs can be avoided. In addition, during an outbreak, an effective notification system following the index case and a rapid response may reduce the overall costs incurred in managing an outbreak. Although, it is evident that cost will be incurred during outbreak investigation and response activities, innovative approaches must be used by technical officers, planners and policy makers to reduce the cost burden of such activities where feasible. For instance, 1) the utilization of more regional and district-level officers to reduce long distance travel to outbreak sites, 2) the availability of outbreak investigation and response materials and consumables to facilitate rapid response, and 3) peace-time coordinated simulation exercises to pre-empt cost saving measures before public health events occur, may assist in this regard. This tool can also serve as an aid in resource-poor settings where budget constraints are more severe and thus can assist in priority setting and resource allocation.
The use of the OCT in our project had many strengths. The costing tool we used provided a standardized framework for recording and analysing data that allowed us to generate rapid cost estimates. The OCT is also adaptable to a range of infectious disease outbreak scenarios, and the incorporation of multisectoral costs is possible. This aspect is particularly useful in the case of zoonotic disease outbreaks, highlighting the tool's utility in facilitating the evaluation of outbreak investigation and response activities that require a One Health approach. Additionally, the OCT was able to incorporate costs incurred by local to national government departments, meaning that a single cost analysis could be performed for direct costs incurred in an outbreak. The OCT was able to incorporate additional cost categories and items where necessary, making the tool flexible to different outbreak scenarios and investigation and response activities.
Since the costing tool allowed entry of information from multiple sectors and did not allow the breakdown of costs incurred by each sector, it may present a limitation where sectoral contributions to a central budget for field activities have to be implemented among key sectors. Another limitation of our investigation was the use of small number of participants to obtain the cost data, as this may have influenced the cost estimates generated. Respondent sample size and response times could be improved through the prospective collection of these data in person, wherever feasible, by a designated government representative. It should be noted that the tool was applied approximately 5–6 months after the outbreak event; hence, the study may be subjected to a degree of recall bias. We made an effort to reduce this effect by cross-validating the information obtained from the key informants with additional informants within the same institutions. However, it is likely that some degree of inconsistency exists in qualitative information obtained from respondents, especially where official record keeping is limited. Therefore, cost data generated here should be considered as an approximate baseline for these anthrax outbreak investigation and response activities.
We have several recommendations for future use of the OCT. The OCT could be implemented multiple times for different government departments, generating separate cost analyses if required. The OCT could be further expanded so as to allow for quick analysis of government sector-specific costs incurred. This type of designation could highlight where sectors might need to invest more resources or coordinate more effectively across sectors for future response efforts. The OCT could also include the estimation of indirect costs associated with infectious disease outbreaks, such as those costs incurred by family members of individuals directly affected by the outbreak. The OCT was utilized retrospectively in our study, but could possibly be more effective being implemented prospectively, for example during an outbreak simulation exercise. Prospective application would help to reduce recall bias by outbreak investigation and response participants. Routine, prospective implementation of the OCT could be performed by regional-level, government monitoring and evaluation representatives. Costing analysis data could then be assessed during national or subnational government after action reviews to ensure effective communication of cost analysis results.
Previously, cost analyses for infectious disease outbreaks have been viewed as a challenge due to the general lack of cost data available [15]. The availability of simple, fast and adaptable tools, such as the OCT, could prove key in effectively collecting robust cost data for infectious disease outbreaks. Overall, the availability of multisector costing data recorded and analysed in the OCT can be shared with appropriate stakeholders to be used as a baseline to estimate costs to guide preparation for future anthrax or other infectious disease outbreak events, such as Rift Valley fever, zoonotic influenza and rabies. This would assist in building capacity to respond to outbreaks via the justification for, and most effective allocation of, outbreak investigation and response funds. The evaluation of the application of this OCT can also assist in its effective application to other infectious disease outbreaks, therefore helping to address the need for standardized methodology in cost data collection and reporting. Particularly in the case of infectious disease outbreaks that require a multisectoral response, the implementation of a standardized costing tool can help inform and improve One Health related preparedness and response activities.
Of added benefit, the use of the One Health approach and OCT allowed for the review and reclassification of the index case (earlier misdiagnosis as extra pulmonary tuberculosis, based on the clinical signs and symptoms). The application of this tool also assisted national authorities in re-prioritizing response-related resources for this outbreak response and possibly in subsequent anthrax outbreaks in Tanzania based on lessons learnt [30]. Furthermore by using a One Health approach, it opened a network of collaboration between the public health and veterinary laboratories in the effective diagnosis of human cases of anthrax and allowed the Ministry of Livestock and Fisheries to allocate resources for the strategic vaccination of approximately 50,000 livestock in the affected region [30].
Questionnaires to target specific cost head for the Outbreak Costing Tool.
Supplementary material 2.
Funding
This work was supported by the FAO ECTAD Global Health Security Agenda-Zoonotic Disease and Animal Health in Africa (GHSA-ZDAH) project funded by USAID, Washington, D.C., USA [project number: OSRO/GLO/507/USA]. RFB received funding from the UK Biotechnology and Biological Sciences Research Council, the UK Department for International Development, the UK Economic & Social Research Council, the UK Medical Research Council, the UK Natural Environment Research Council and the UK Defence Science and Technology Laboratory, under the Zoonoses and Emerging Livestock Systems (ZELS) programme [grant number BB/N503563/1]. The OCT development, in co-collaboration with the US CDC and RTI International, was supported in part by contract number # 200-2017-F-96261, funded to RTI International by the US CDC.
Disclaimer
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the USAID, US CDC, and FAO.
Author contributions
RFB, NMM, JEBH, GW, FOF conceptualized the project. RFB, JB, EM, NMM, HEN, ESS performed data collection. NMM, JEBH, CB, YJM, GW, FOF supervised the project. MAW, SS, CHC contributed to the development of the OCT. MAW provided training on implementation of the OCT. RFB, NMM, FOF performed data analyses and visualization. JEBH, HEN, ESS, CB, YJM, GW, FOF conducted project administration. All authors contributed to writing, reviewing and revising the manuscript.
Ethical clearance
Written consent was obtained from participants, or oral consent for telephone participants. All key-informants were informed of their rights as a participant and their right to discontinue participation at any time. The Directorate of Veterinary Services, Ministry of Livestock and Fisheries, United Republic of Tanzania provided permission to carry out the study.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge: US CDC for access to the OCT and implementation training provided for FAO and other partners in Tanzania; Sandi Brown for coordinating the OCT training in Tanzania; RTI International for co-development of the OCT in collaboration with US CDC; USAID, Tanzania, specifically Raz Stevenson, Elizabeth Williams and Lisa Kramer for facilitating rapid approvals for GHSA fund utilization for this intervention; Veterinary, medical and environmental health employees from Momba district and Songwe and Mbeya regions for jointly responding to this outbreak and also providing relevant information and insights to authors for preparation of this manuscript; Tanzania Veterinary Laboratory Agency, and Sokoine University of Agriculture, Tanzania contributors for diagnosis and effective feedbacks on confirmatory diagnoses; Ministry of Livestock and Fisheries, Ministry of Health, Community Development, Gender, Elderly and Children, and Prime Minister's Office – One Health Coordinating Desk, Tanzania representatives for their support, coordination and facilitating linkages with field officers. Individuals interested in using the OCT should contact Mahlet A. Woldetsadik, PhD, (mwoldetsadik@cdc.gov), Division of Global Health Protection, Center for Global Health, US CDC, Atlanta, GA, USA.
References
- 1.Mock M., Fouet A. Anthrax. Annu. Rev. Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 2.Fasanella A., Galante D., Garofolo G., Jones M.H. Anthrax undervalued zoonosis. Vet. Microbiol. 2010;140:318–331. doi: 10.1016/j.vetmic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Fourth ed. WHO Press; Geneva, Switzerland: 2008. Anthrax in Humans and Animals. [Google Scholar]
- 4.Cieslak T.J., Eitzen E.M. Clinical and epidemiologic principles of anthrax. Emerg. Infect. Dis. 1999;5:552–555. doi: 10.3201/eid0504.990418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lembo T., Hampson K., Auty H., Beesley C.A., Bessell P., Packer C., Halliday J., Fyumagwa R., Hoare R., Ernest E., Mentzel C., Mlengeya T., Stamey K., Wilkins P.P., Cleaveland S. Serologic surveillance of anthrax in the Serengeti ecosystem, Tanzania, 1996-2009. Emerg. Infect. Dis. 2011;17:387–394. doi: 10.3201/eid1703.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mwakapeje E.R., Høgset S., Fyumagwa R., Nonga H.E., Mdegela R.H., Skjerve E. Anthrax outbreaks in the humans - livestock and wildlife interface areas of Northern Tanzania: a retrospective record review 2006-2016. BMC Public Health. 2018;18:106. doi: 10.1186/s12889-017-5007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Government of Tanzania . 2018. Guidelines for Surveillance of Prioritized Zoonotic Diseases for Human and Animal Health in the United Republic of Tanzania. [Google Scholar]
- 8.Centers for Disease Control and Prevention . 2017. One Health Zoonotic Disease Prioritization for Multisectoral Engagement in Tanzania. [DOI] [Google Scholar]
- 9.Government of Tanzania National Strategy for Prevention and Control of Anthrax in Humans and Animals 2018–2023. 2018. https://www.mifugouvuvi.go.tz/uploads/publications/sw1602245078-NATIONAL%20STRATEGY%20ON%20PREVENTATION%20AND%20CONTROL%20OF%20ANTHRAX%20IN%20HUMANS%20AND%20ANIMALS.pdf
- 10.World Organisation for Animal Health . 2016. PVS Evaluation Follow-Up Mission Report Tanzania. [Google Scholar]
- 11.World Health Organization . 2017. Joint External Evaluation of IHR Core Capacities of the United Republic of Tanzania. [Google Scholar]
- 12.Food and Agriculture Organization of the United Nations . 2017. Surveillance Evaluation Tool (SET) piloting mission in Tanzania 12th - 21st June 2017 - Evaluation report. [Google Scholar]
- 13.World Health Organization, Food and Agriculture Organization of the United Nations, World Organisation for Animal Health Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. 2019. http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/EN_TripartiteZoonosesGuide_web.pdf (accessed 25 February 2021)
- 14.Brodszky V., Beretzky Z., Baji P., Rencz F., Péntek M., Rotar A., Tachkov K., Mayer S., Simon J., Niewada M., Hren R., Gulácsi L. Cost-of-illness studies in nine Central and Eastern European countries. Eur. J. Health Econ. 2019;20:155–172. doi: 10.1007/s10198-019-01066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luyten J., Beutels P. Costing infectious disease outbreaks for economic evaluation. Pharmacoeconomics. 2009;27:379–389. doi: 10.2165/00019053-200927050-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wallace A.S., Masresha B.G., Grant G., Goodson J.L., Birhane H., Abraham M., Endailalu T.B., Letamo Y., Petu A., Vijayaraghavan M. Evaluation of economic costs of a measles outbreak and outbreak response activities in Keffa Zone, Ethiopia. Vaccine. 2014;32:4505–4514. doi: 10.1016/j.vaccine.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 17.US Centers for Disease Control and Prevention, Centre for Global Health, Division of Global Health and Protection, Office of the Director . 2018. Outbreak Costing Tool. [Google Scholar]
- 18.Adam T., Aikins M., Evans D. CostIt Software (Costing Intervention Template) 2007. https://www.who.int/choice/toolkit/cost_it/en/ Available at: accessed 24 April 2021.
- 19.Zana C.S., Meltzer M.I., Perry H.N. Centers for Disease Control and Prevention, National Center for Prevention, Detection and Control of Infectious Diseases (NCPDCID), Division of Emerging Infections and Surveillance Services (DEISS), U.S. Department of Health and Human Services; 2007. SurvCost 1.0: A Manual to Assist Country and District Public Health Officials in Estimating the Cost of the Implementation of Integrated Disease Surveillance and Response Systems (Beta Test Version)https://www.cdc.gov/globalhealth/healthprotection/idsr/pdf/SurvCost-Manual-Jan-2008.pdf Available at: accessed 24 April 2021. [Google Scholar]
- 20.World Health Organization CHOosing Interventions that are Cost Effective (WHO-CHOICE) 2011. http://www.who.int/choice/en/ Available at: Accessed 24 April 2021. [DOI] [PMC free article] [PubMed]
- 21.Bartsch S.M., Asti L., Nyathi S., Spiker M.L., Lee B.Y. Estimated cost to a restaurant of a foodborne illness outbreak. Public Health Rep. 2018;133(3):274–286. doi: 10.1177/0033354917751129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Candrilli S.D., Kurosky S. RTI Press; Research Triangle Park, NC: 2019. The Response to and Cost of Meningococcal Disease Outbreaks in University Campus Settings: A Case Study in Oregon, United States. RTI Press Publication No. RR-0034-1910. [DOI] [PubMed] [Google Scholar]
- 23.Lee C.T., Katz R., Eaneff S., Mahar M., Ojo O. Action-based costing for national action plans for health security: accelerating progress toward the international health regulations (2005) Health Secur. 2020 doi: 10.1089/hs.2019.0063. S-53-S-63. [DOI] [PubMed] [Google Scholar]
- 24.Spearing N.M., Jensen A., McCall B.J., Neill A.S., McCormack J.G. Direct costs associated with a nosocomial outbreak of Salmonella infection: an ounce of prevention is worth a pound of cure. Am. J. Infect. Control. 2000:54–57. doi: 10.1016/s0196-6553(00)90012-9. [DOI] [PubMed] [Google Scholar]
- 25.Ding Y., Sauerborn R., Xu B., Shaofa N., Yan W., Diwan V.K., Dong H. A cost-effectiveness analysis of three components of a syndromic surveillance system for the early warning of epidemics in rural China. BMC Public Health. 2015;15:1127. doi: 10.1186/s12889-015-2475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan W., Levin A., Hutubessy R.C., Mogasale V. Costing oral cholera vaccine delivery using a generic oral cholera vaccine delivery planning and costing tool (CholTool) Hum. Vaccin Immunother. 2020;16(12):3111–3118. doi: 10.1080/21645515.2020.1747930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Government of Tanzania Tanzania in Figures 2018. 2019. http://www.nbs.go.tz/nbs/takwimu/references/Tanzania_in_Figures_2018.pdf (accessed 25 February 2021)
- 28.Sagamiko F.D., Muma J.B., Karimuribo E.D., Mwanza A.M., Sindato C., Hang’ombe B.M. Sero-prevalence of Bovine Brucellosis and associated risk factors in Mbeya region, Southern highlands of Tanzania. Acta Trop. 2018;178:169–175. doi: 10.1016/j.actatropica.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Government of Tanzania The United Republic of Tanzania 2016/2017 Annual Agricultural Sample Survey Crop and Livestock Report. https://nbs.go.tz/nbs/takwimu/Agriculture/2016_17_AASS_report.pdf, 2017 (accessed 25 February 2021)
- 30.Government of Tanzania . 2019. Investigation of Anthrax Outbreak at Nzoka Ward, Songwe Region. [Google Scholar]
- 31.Broshenka D., Castro A.P. Wood Fuel Survey. 1993. Methods of fact finding, Chapter 4.http://www.fao.org/3/Q1085E/q1085e07.htm accessed 25 February 2021. [Google Scholar]
- 32.XE.com Inc. XE Currency Converter. 2019. www.xe.com (accessed 20 June 2019)
- 33.R Core Team R: A Language and Environment for Statistical Computing. 2019. http://www.r-project.org R Found. Stat. Comput. (accessed 25 February 2021)
- 34.Wickham H., Chang W., Henry L., Pedersen T.L., Takahashi K., Wilke C., Woo K., Yutani H. 2019. ggplot2: Create Elegant data Visualisations Using the Grammar of Graphics. Version 3.2.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questionnaires to target specific cost head for the Outbreak Costing Tool.
Supplementary material 2.