Abstract
As one of the largest volume flavor ingredients, vanillin remains an attractive target for development of a cost-effective and sustainable process to manufacture. Presented here is newly available data on the production of vanillin via fermentation in an engineered strain of Saccharomyces cerevisiae grown on sucrose ex-sugarcane. The use of the C4 plant source of carbohydrate resulted in a δ13C mean stable isotope ratio of -14.43 ‰ (SD = 0.24) relative to the V-PDB standard and a δ2H mean stable isotope ratio of -122.8 ‰ (SD = 2.9) relative to the SMOW standard by IRMS. The abundance of 14C in the fermentation derived vanillin averaged 14.01 dpm/gC (SD = 0.09) by AMS measurement. These data are compared to historical data collected on vanillin derived from a number of sources to provide a more holistic view on vanillin bulk isotope data based on its method of manufacture.
Keywords: Vanilla, Vanillin, Isotope ratio, δ13C, δ2H
Vanilla, Vanillin, Isotope ratio, δ13C, δ2H.
1. Introduction
Consumer demand for natural flavorings has resulted in intensive research to capitalize on alternative raw materials and processes to generate natural ingredients for flavor creation following local and regional regulatory guidelines (Gallage and Moller, 2015). Vanillin, the main driver of vanilla flavorings, is the most sought after natural ingredient. Processes developed to manufacture natural vanillin can be 20 times the cost of artificial vanillin. The bioconversion of ferulic acid derived from rice bran via the endogenous pathway of Streptomyces setonii was adopted early as a natural process to manufacture vanillin by fermentation (Figure 1) (Muheim et al., 2006). Eugenol and curcumin have also been employed as natural raw materials to manufacture vanillin by bioconversion (Overhage et al., 1999; Bharti et al., 2011). Low per batch throughput and fluctuations in raw material availability and costs have created uncertainty in natural vanillin supply and price. This can, unfortunately, lead to adulteration of vanilla extract and vanilla flavors to offset costs which have been a recognized problem for 100 years or more (Gnadinger, 1925). Adulteration can be accomplished through silent replacement or supplementing more expensive natural vanillin or vanilla extracts with the significantly cheaper artificial version. Analytical methods including Isotope Ratio Mass Spectrometry (IRMS) and Sight-specific Natural Isotope Fractionation Nuclear Magnetic Resonance (SNIF-NMR) have been developed to identify the botanical origin of a natural vanillin sample versus vanillin produced by artificial or otherwise regionally unaccepted methods as a line of defense against adulteration practices (Hoffman and Salb, 1979; Culp and Noakes, 1992; Cochennec, 2013). Recently, data was compiled on vanillin created from multiple bioconversion paths and compared to its origin from vanilla bean, lignin, guaiacol or even glucose (Geißler et al., 2017; Wilde et al., 2019). It is anticipated that the biosynthesis of vanillin with sucrose ex-sugarcane will result in a cluster from other known sources of vanillin reported to date. The 14C and bulk δ13C and δ2H data have been acquired and reported for the first time for the biosynthesis of vanillin from sucrose ex-sugarcane in an engineered yeast strain. The availability of this new data will aid in the determination of the origin of vanillin used in a flavoring claimed as natural in a commercial food or beverage composition (Krueger and Krueger, 1983; Schipilliti et al., 2016; Perini et al., 2019).
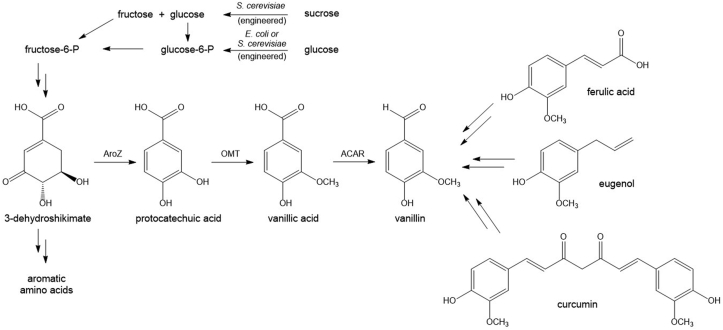
Figure 1.
Various routes to producing vanillin via fermentation; 3-DHS: 3-dehydroshikimic acid, AroZ: protocatechuic acid synthase, OMT: O-methyltransferase, ACAR: aryl carboxylic acid reductase.
2. Materials and methods
2.1. Vanillin samples
The biosynthesis of vanillin from sucrose via the intermediacy of 3-dehydroshikimate (Figure 1) can be accomplished by recombinant expression of the appropriate pathway genes by stable integration onto the genome of Saccharomyces cerevisiae parent strain CEN.PK2 when expressing the appropriate phosphopantetheinyl transferase resulting in accumulation of vanillin in the fermentation broth (Hansen et al., 2009; Hansen et al., 2014).
2.2. Chemicals
Sucrose derived from sugarcane was supplied by C&H (ASR Group, West Palm Beach, FL, USA) which was used as the primary carbon source for the production of vanillin by fermentation in the engineered yeast strain. Inorganic salts and trace metals were Reagent Plus grade (Sigma-Aldrich, St. Louis, MO, USA). Vitamins, L-lysine HCl, maltose, myo-inositol and succinate were Bioreagent grade (Sigma-Aldrich, St. Louis, MO, USA). Ethyl acetate and hexanes were reagent grade (Acros Organics, Fair Lawn, NJ, USA). Water and acetonitrile were HPLC grade (Acros Organics, Fair Lawn, NJ, USA). Silica gel was ultrapure grade, 60-200μ (Acros Organics, Fair Lawn, NJ, USA).
2.3. Fermentation conditions and media
A single colony of the engineered vanillin producing yeast strain grown on agar plates was used to inoculate a 500 mL baffled seed flask containing 60 mL of YM1 media containing 4% sucrose (C&H), 5 g/L-lysine HCl, 6 g/L succininc acid, salts (8 g/L KH2PO4, 15 g/L (NH4)2SO4, 6.15 g/L MgSO4∗7H2O), trace metals (0.0575 g/L ZnSO4∗7H2O, 0.0032 g/L CuSO4, 0.0032 g/L MnCl2∗4H2O, 0.0047 g/L CoCl2∗6H2O, 0.0048 g/L Na2MoO4∗2H2O, 0.028 g/L FeSO4∗7H2O, 0.029 g/L CaCl2∗2H2O, 0.117 g/L EDTA) and vitamins (0.0006 g/L Biotin, 0.0024 g/L p-Aminobenzoic acid, 0.012 g/L nicotinic acid, 0.03 g/L myo-inositol, 0.012 g/L pyridozine HCl, 0.012 g/L thiamine HCl, 0.012 g/L calcium pantothenate), and grown in a shaker at 28 °C, 200 RPM for 21 h. The seed flask culture was used to inoculate a 0.5-L Biostat Q Plus Fermenter (Sartorius Stedim Biotech, Göttingen, Germany) containing 240 mL of YM1 media described above. The nutrient feed to the fermentor was a 100 g/L pure sucrose feedstock. The initial pulse was 2 g TRS/L at a rate of 5 g/L/h (TRS refers to total reducing sugars that can be measured by hydrolysis of sucrose to glucose plus fructose). The fermentor feed rate was then adjusted using an algorithm based on the culture demand for carbon, as indicated by rises in dissolved oxygen. The fermentation was run aerobically at a temperature of 30 °C and pH 5.0 (controlled by ammonium hydroxide additions) until the dissolved oxygen reached 0%. The agitation was then controlled in order to maintain an oxygen utilization rate of 15 mmol O2/L/h for 5 days. Culture was removed as needed to prevent overflow. Salts, trace metals and vitamins were also added daily. Foaming was controlled with the addition of 0.1 mL L-61 Antifoam to the fermentation media at the beginning and subsequently added as needed. At the conclusion of the run, a sample of filtered broth was checked by UHPLC analysis and found to accumulate the following metabolites: 300 mg/L isovanillyl alcohol, 275 mg/L protocatechuic acid, 100 mg/L protocatechuic alcohol, 150 mg/L protocatechuic aldehyde, 200 mg/L vanillic acid, 125 mg/L vanillin and 775 mg/L vanillyl alcohol. To isolate the vanillin, the remainder of the broth was separated from the cell mass by centrifugation. The clarified broth was extracted with ethyl acetate (2 × 100 mL per 400 mL clarified broth). Combined ethyl acetate extracts were dried over 10 g MgSO4, gravity filtered through fluted Whatman filter paper and concentrated in vacuo by rotary evaporation to an oily solid. Vanillin was isolated by silica gel column chromatography using a gradient of 40–60% ethyl acetate in hexanes. Pooled fractions containing pure vanillin were dried in vacuo by rotary evaporation to afford 45 mg vanillin as a cream/yellow solid and 99+% purity.
2.4. UPLC assay conditions
To quantify the amount of vanillin produced, the samples were analyzed on an Agilent Vanquish™ Flex Binary UHPLC System (Santa Clara, CA, USA) with a diode array detector. The binary mobile was made up of (A) 1.4% sulfuric acid v/v in water and (B) 100% acetonitrile at a flow rate of 1 mL/min with the following gradient: [gradient time, (min) mobile phase A, (%)]; [(0.00, 88), (0.5,88), (1.25, 85), (2.25, 83), (3.0, 82), (3.5, 88), (4.0, 88)].
2.5. Sample analysis
Isotope analysis was carried out by the Center for Applied Isotope Studies at the University of Georgia based on vanillin samples isolated from five replicate fermentations (Culp and Noakes, 1990; Culp and Noakes, 1992; Culp, 2017; Center for Applied Isotope Studies, University of Georgia, 2020). Samples were run in triplicate and reported with one Standard Deviation from the mean at 95% confidence under ISO/IEC1702S:2017 validation parameters.
14C measurements were carried out on a 500 kV Pelleton Tandem accelerator mass spectrometer (National Electrostatics Corporation, Middleton, WI, USA) versus an Oxalic acid I standard reference material (SRM4990C, National Institute for Standards and Technology, Gaithersburg, MD, USA) and 14C devoid graphite (International Atomic Energy Agency, Vienna, Austria) as a negative control. Data was analyzed with the aid of the ABC software package (National Electrostatic Corporation). The 14C activities are reported as disintegrations per min per gram of carbon (dpm/gC) which can be used to differentiate between a fossil fuel derived chemical (dpm/gC approaching 0) versus a chemical derived from a plant source where the present day 14C activity is approximately 13.6 dpm/gC (Culp, 2017).
IRMS measurements were carried out on a Thermo-Finnigan MAT253 dual-inlet isotope ratio mass spectrometer (Thermo-Finnigan, San Jose, CA, USA) coupled with the Isodat 3.0 software package for data analysis. The 13C/12C measured in the CO2 from sample combustion was compared relative to CO2 gas obtained from international Vienna-Pee Dee Belemnite (V-PDB) reference standard and the secondary standard USGS71 (Reston Stable Isotope Laboratory, Reston, VA, USA) with the certified value of -10.5 ± 0.03‰. The 2H/1H measured in the H2 from sample combustion was compared relative to H2 gas obtained from Standard Mean Ocean Water (SMOW) reference standard and the USGS71 secondary standard with the certified value of -4.9 ± 1‰. The values were denoted in δ in relation to V-PDB for δ13C and in relation to SMOW for δ2H, according to the following equation (Coplen, 2011):
| δ iE = (RSA - Rstd)/Rstd, | (1) |
where i is the mass number of the heavier isotope of the element E (C or H), RSA is the respective isotope ratio (13C/12C or 2H/1H) of the sample and Rstd is the relevant internationally recognized reference material. The δ values were multiplied by 1000 and expressed in units “per mil” (‰).
3. Results
Vanillin remains an interesting target for the development of a sustainable route to a natural ingredient while keeping pace with annual demand at a lower cost. One such pathway reported by Li and Frost (1998) converts cheap carbohydrate feedstocks into vanillin by siphoning 3-dehydroshikimate from aromatic amino acid biosynthesis to vanillic acid in an engineered strain of E. coli followed by reduction with the aryl carboxylic acid reductase (ACAR) enzyme isolated from N. crassa in three steps. More recently, the pathway has been fully engineered in yeast and E. coli to take advantage of the pathway efficiency from primary metabolism while relying on cheap, readily available carbohydrate feedstocks (Hansen et al., 2009; Hansen et al., 2014; Kunjapur et al., 2014).
Vanillin for this study was produced by fermentation from sucrose by siphoning the metabolite 3-dehydroshikimate from aromatic amino acid biosynthesis in three steps as first disclosed by Li and Frost (1998). Sucrose from sugarcane is abundantly available and represents some of the lowest cost carbon source for microbial growth and natural flavor ingredient manufacture. Sucrose enters into glycolysis through the common intermediate fructose-6-phosphate (Figure 1). As shown in Table 1, fermentation derived vanillin from yeast grown on sucrose ex-sugarcane resulted in a mean 14C activity of 14.01 dpm/gC (SD = 0.09). These measurements are in line with the atmospheric steady state of 13.6 dpm/gC for CO2 uptake during photosynthesis. Live plants reach a 14C equilibrium with the atmosphere which is maintained through continued uptake of CO2 during photosynthesis and radioactive decay. On the other hand, fossil fuels have mean 14C activities approaching 0 due to complete radioactive decay. The 14C measurements indicate that the carbon in the vanillin can be traced back to a recently harvested plant source (Culp, 2017). Although this data alone cannot authenticate the vanillin samples as natural, it does at least provide a line of evidence that the vanillin was derived from a recently harvested plant source and not derived from fossil fuel intermediates.
Table 1.
Isotopic analysis of vanillin from sucrose ex-sugarcane.
| Vanillin Source | 14C [dpm/gC] | SD∗ [dpm/gC] | δ13C [‰] | SD∗ [‰] | δ2H [‰] | SD∗ [‰] |
|---|---|---|---|---|---|---|
| sucrose ex-sugarcane | 14.03 | 0.14 | -14.09 | 0.13 | -125 | 1 |
| sucrose ex-sugarcane | 13.95 | 0.08 | -14.50 | 0.06 | -124 | 1 |
| sucrose ex-sugarcane | 14.08 | 0.08 | -14.40 | 0.04 | -124 | 1 |
| sucrose ex-sugarcane | 14.12 | 0.08 | -14.83 | 0.05 | -124 | 1 |
| sucrose ex-sugarcane | 13.87 | 0.07 | -14.33 | 0.11 | -117 | 2 |
| Average | 14.01 | 0.09 | -14.43 | 0.24 | -122.8 | 2.9 |
Standard Deviation at 95% confidence.
The carbon isotope ratio for vanillin derived from sucrose ex-sugarcane via fermentation was measured to have a mean stable isotope ratio of -14.43‰ (SD = 0.24) relative to V-PDB (Table 1). Sugarcane exhibits Hatch-Slack CO2 fixation during photosynthesis. During the course of CO2 fixation, a C4 intermediate is formed on the pathway to making carbohydrates to store as energy. The pathway exhibits less overall enrichment of 12C versus the heavier 13C isotope which results in a lower mean stable isotope ratio relative to the V-PDB standard (Culp and Noakes, 1990). Vanillin obtained by fermentation of sucrose ex-sugarcane falls within the accepted ‰ range for a C4 plant. On the other hand, Calvin CO2 fixation during photosynthesis exhibits more enrichment of 12C versus the heavier 13C isotope due to the kinetic isotope effect. This type of CO2 fixation forms a C3 intermediate on the path to storing energy in the form of carbohydrates. The metabolites formed from the cascade of bioconversions that take place from Calvin CO2 fixation exhibits a larger measured mean stable isotope ratio relative to the V-PDB standard (Culp and Noakes, 1990). Vanillin obtained by biotransformation of ferulic acid ex-rice has been traditionally accepted as a natural flavor ingredient with a carbon mean stable isotope ratio of -36.23‰ (SD = 0.46) relative to V-PDB (Table 2). Ferulic acid from rice results in a vanillin exhibiting a bulk δ13C consistent with a C3 plant (Culp and Noakes, 1990). As disclosed by Geißler and co-workers, the mean stable isotope ratio shifts to -19.45‰ (SD = 0.62) when ferulic acid is derived from corn which is in line with expectations for C4 plants (Table 2) (Geißler et al., 2017). More recently, Wilde and co-workers published results on the conversion of glucose derived presumably from a C4 plant source using the same aromatic amino acid intermediate siphoning strategy (Wilde et al., 2019). In their work, the bulk δ13C for vanillin made from glucose, speculated to be obtained from corn, was measured to have a mean stable isotope ratio of -12.5‰ relative to V-PDB which is similar to the sucrose results derived from the C4 pathway in sugarcane (Table 2).
Table 2.
Isotopic analysis of vanillin from various sources.
| Vanillin Source | δ13CBulk [‰] | δ2HBulk [‰] | Reference |
|---|---|---|---|
| curcumin | -27.8 | -155 | Geißler et al. (2017) |
| curcumin | -28.8 | -135 | Geißler et al. (2017) |
| curcumin | -29.7 | -134 | Geißler et al. (2017) |
| curcumin | -28.6 | -134 | Geißler et al. (2017) |
| curcumin | -30.4 | -128 | Geißler et al. (2017) |
| curcumin | -27.8 | -155 | Geißler et al. (2017) |
| curcumin∗ | -29.06 (SD = 0.79) | -137.2 (SD = 8.1) | |
| eugenol | -32.2 | -62 | Geißler et al. (2017) |
| eugenol | -32.4 | -88 | Geißler et al. (2017) |
| eugenol | -32.1 | -84 | Geißler et al. (2017) |
| eugenol | -30.4 | -70 | Geißler et al. (2017) |
| eugenol | -32.4 | -114 | Geißler et al. (2017) |
| eugenol∗ | -31.90 (SD = 0.67) | -83.6 (SD = 15.7) | |
| ferulic acid ex-rice | -36.9 | -167 | Geißler et al. (2017) |
| ferulic acid ex-rice | -36.3 | -170 | Geißler et al. (2017) |
| ferulic acid ex-rice | -35.9 | -162 | Geißler et al. (2017) |
| ferulic acid ex-rice | -37.3 | -174 | Geißler et al. (2017) |
| ferulic acid ex-rice | -36.1 | -159 | Geißler et al. (2017) |
| ferulic acid ex-rice | -35.4 | -158 | Geißler et al. (2017) |
| ferulic acid ex-rice | -35.7 | -162 | Geißler et al. (2017) |
| ferulic acid ex-rice∗ | -36.23 (SD = 0.46) | -164.4 (SD = 4.1) | |
| ferulic acid ex-corn | -19.0 | -97 | Geißler et al. (2017) |
| ferulic acid ex-corn | -19.9 | -99 | Geißler et al. (2017) |
| ferulic acid ex-corn∗ | -19.45 (SD = 0.62) | -98.0 (SD = 1.4) | |
| guaiacol | -25.9 | 104 | Geißler et al. (2017) |
| guaiacol | -28.3 | 66 | Geißler et al. (2017) |
| guaiacol | -28.4 | 49 | Geißler et al. (2017) |
| guaiacol | -28.6 | 63 | Geißler et al. (2017) |
| guaiacol | -29.5 | -3 | Geißler et al. (2017) |
| guaiacol | -30.7 | 53 | Geißler et al. (2017) |
| guaiacol | -29.4 | 61 | Geißler et al. (2017) |
| guaiacol | -27.1 | -25 | Geißler et al. (2017) |
| guaiacol | -27.1 | 117 | Geißler et al. (2017) |
| guaiacol | -29.6 | 21 | Geißler et al. (2017) |
| guaiacol | -29.6 | 107 | Geißler et al. (2017) |
| guaiacol∗ | -28.56 (SD = 0.79) | 55.7 (SD = 25.3) | |
| lignin | -28.1 | -182 | Geißler et al. (2017) |
| lignin | -27.9 | -182 | Geißler et al. (2017) |
| lignin | -27.4 | -175 | Geißler et al. (2017) |
| lignin∗ | -27.80 (SD = 0.33) | -179.7 (SD = 3.7) | |
| vanilla bean | -20.5 | -59 | Geißler et al. (2017) |
| vanilla bean | -20.4 | -73 | Geißler et al. (2017) |
| vanilla bean | -20.5 | -60 | Geißler et al. (2017) |
| vanilla bean | -20.2 | -73 | Geißler et al. (2017) |
| vanilla bean | -21.8 | -83 | Geißler et al. (2017) |
| vanilla bean∗ | -20.68 (SD = 0.50) | -69.6 (SD = 7.9) | |
| glucose ex-C4 | -12.5 | -94.8 | Wilde et al. (2019) |
Mean value for each vanillin source with Standard Deviation at 95% confidence.
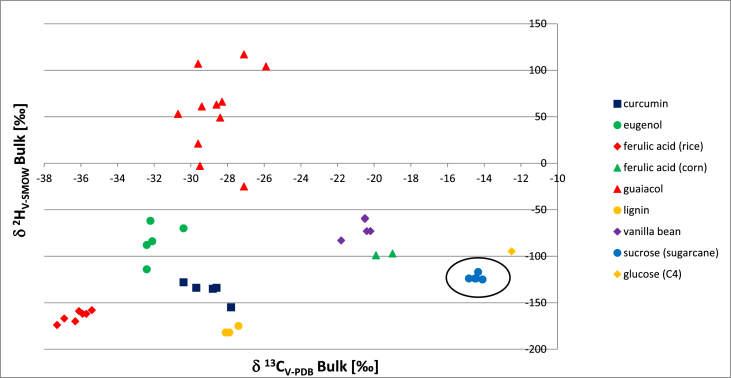
The bulk δ13C relative to V-PDB for curcumin, eugenol, lignin and guaiacol are less distinct and requires coupling with the bulk δ2H values to differentiate the samples (Culp and Noakes, 1992; Geißler et al., 2017). The bulk δ2H for vanillin derived from sucrose ex-sugarcane had a mean stable isotope ratio of -122.8‰ (SD = 2.9) relative to SMOW. Although vanillin derived from sucrose via fermentation results in a carbon isotope ratio away from previous reports, coupling with the hydrogen isotope ratio results in a cluster from other known pathways to access vanillin (Figure 2). A bulk of the artificial vanillin is derived from lignin or fossil fuel sourced guaiacol. However, a recently developed process for naturally sourced guaiacol may change its status as a flavor ingredient in some regions (Verdier and Madelaine, 2019). The bioconversion of naturally sourced curcumin to vanillin fits the criteria for a natural flavor ingredient while vanillin derived from eugenol is dependent on the process employed to generate the ingredient (Gallage and Moller, 2015). Isolation of vanillin from the extracts of Vanilla planifolia or Vanilla tahitensis results in a bulk δ13C mean stable isotope ratio of -20.68‰ (SD = 0.50) (Table 2). This can be attributed to the Vanilla orchid exhibiting a combination of Calvin and Hatch-Slack CO2 fixation during photosynthesis which is unique to CAM plants (Culp and Noakes, 1990).
Figure 2.
2D-plot of δ2H and δ13C bulk values for vanillin derived from multiple raw material expressed as the ‰ relative to SMOW (hydrogen) and V-PDB (carbon). Values combined from Tables 1 and 2.
4. Discussion and conclusion
The flavor ingredient vanillin can be derived from a number of raw materials by recruitment of synthetic, biosynthetic or biotransformation methodologies. Samples of vanillin obtained by fermentation with sucrose ex-sugarcane as the carbon source had a 14C mean activity of 14.01 dpm/gC (SD = 0.09) which is within expectations of present day CO2 fixation. Bulk δ13C and δ2H values for multiple vanillin samples have been previously published with the exception of biosynthesis from sucrose via the intermediacy of 3-dehydroshikimate (Culp and Noakes, 1992; Geißler et al., 2017; Wilde et al., 2019). Vanillin derived from glucose, presumed to be sourced from corn, was reported to have a bulk δ13C of -12.5‰ and δ2H of -94.8‰ (Wilde et al., 2019). This work has demonstrated that biosynthesis of vanillin from sucrose ex-sugarcane, a plant classified as C4 for CO2 fixation, to have a bulk δ13C mean stable isotope of -14.43‰ (SD = 0.24) relative to V-PDB and bulk δ2H mean stable isotope ratio of -122.8‰ (SD = 2.9) relative to SMOW. The 2-dimensional plot of both sets of data points results in a cluster that is near the results reported from glucose presumed to be derived from a C4 plant and discernible from other sources of vanillin reported to date. This data provides a more holistic view on vanillin qualities available for flavor creation and enables its identification when deployed in food and beverages.
Declarations
Author contribution statement
Chad A. Hansen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare the following conflict of interests: Chad A. Hansen; [is an employee of Givaudan Taste & Wellbeing].
Additional information
No additional information is available for this paper.
References
- Bharti N., Anand A.L., Gupta R.K. Biotransformation of curcumin to vanillin. Indian J. Chem. 2011;50b:1119–1122. [Google Scholar]
- Center for Applied Isotope Studies, University of Georgia Compound specific isotope testing (14C, C, N, O, & H) 2020. https://cais.uga.edu/service/compound-specific-isotope-testing-14c-c-n-o-h/
- Cochennec C. Natural vanillin obtained by means of bioconversion. Perfum. Flavor. 2013;38:20–25. [Google Scholar]
- Coplen T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011;25:2538–2560. doi: 10.1002/rcm.5129. [DOI] [PubMed] [Google Scholar]
- Culp R. John Wiley & Sons, Ltd.; 2017. Radiocarbon analysis for biofuel quantification in fuel blends. encyclopedia of analytical chemistry: applications, theory and instrumentation; pp. 1–13. [Google Scholar]
- Culp R.A., Noakes J.E. Identification of isotopically manipulated cinnamic aldehyde and benzaldehyde. J. Agric. Food Chem. 1990;38:1249–1255. [Google Scholar]
- Culp R.A., Noakes J.E. Determination of synthetic components in flavors by deuterium/hydrogen isotopic ratios. J. Agric. Food Chem. 1992;40:1892–1897. [Google Scholar]
- Gallage N.J., Moller B.L. Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant. 2015;8:40–57. doi: 10.1016/j.molp.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Geißler K., Greule M., Schafer U., Hans J., Geißler T., Meier L., Keppler F., Krammer G. Vanilla authenticity control by DNA barcoding and isotope data aggregation. Flavour Fragrance J. 2017;32:228–237. [Google Scholar]
- Gnadinger C.B. Detecting the adulteration of vanilla extract. Am. Perfum. Essent. Oil Rev. 1925;20:268–269. [Google Scholar]
- Hansen E.H., Moller B.L., Kock G.R., Bunner C.M., Kristensen C., Jensen O.R., Okkels F.T., Olsen C.E., Motawia M.S., Hansen J. De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker’s yeast (Saccharomyces cerevisiae) Appl. Environ. Microbiol. 2009;75:2765–2774. doi: 10.1128/AEM.02681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Andersen Hvid T., Okkels Thyge F. 2014. A Method of Producing a Low Moleculare Weight Plant Secondary Metabolite in a Yeast Cell. EP1649029 B1. [Google Scholar]
- Hoffman P.G., Salb M. Isolation and stable isotope ratio analysis of vanillin. J. Agric. Food Chem. 1979;27:352–355. [Google Scholar]
- Krueger D.A., Krueger H.W. Carbon isotopes in vanillin and the detection of falsified “natural” vanillin. J. Agric. Food Chem. 1983;31:1265–1268. [Google Scholar]
- Kunjapur A.M., Tarasova Y., Prather K.L.J. Synthesis and accumulation of aromatic aldehydes in an engineered strain of Escherichia coli. J. Am. Chem. Soc. 2014;136:11644–11654. doi: 10.1021/ja506664a. [DOI] [PubMed] [Google Scholar]
- Li K., Frost J.W. Synthesis of vanillin from glucose. J. Am. Chem. Soc. 1998;120:10545–10546. [Google Scholar]
- Muheim A., Muller B., Wetli M. 2006. Process for the Production of Vanillin. EP0885968 B1. [Google Scholar]
- Overhage J., Priefert H., Rabenhorst J., Steinbuchel A. Biotransformation of eugenol to vanillin by a mutant of Pseudomonas sp. HR199 constructed by disruption of the vanillin dehydrogenase (vdh) gene. Appl. Microbiol. Biotechnol. 1999;52:820–828. doi: 10.1007/s002530051598. [DOI] [PubMed] [Google Scholar]
- Perini M., Pianezze S., Strojnik L., Camin F. C and H stable isotope ratio analysis using solid-phase microextraction and gas chromatography-isotope ratio mass spectrometry for vanillin authentication. J. Chromatogr. A. 2019;1595:168–173. doi: 10.1016/j.chroma.2019.02.032. [DOI] [PubMed] [Google Scholar]
- Schipilliti L., Bonaccorsi I.L., Mondello L. Characterization of natural vanilla flavor in foodstuff by HS-SPME and GC-C-IRMS. Flavour Fragrance J. 2016;32:85–91. [Google Scholar]
- Verdier S., Madelaine F. 2019. New Vanillin And/or Ethylvanillin, Process for Their Preparations and Use Thereof. WO2019020773. [Google Scholar]
- Wilde A.S., Frandsen H.L., Fromberg A., Smedsgaard J., Gruele M. Isotopic characterization of vanillin ex-glucose by GC-IRMS – new challenge for natural vanilla flavor authentification? Food Contr. 2019;106:106735. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.