Abstract
Background
Dairy products like ice cream, yogurt and buttermilk are consumed widely due to their rich taste but these products lack anthocyanins, which are polyphenol and exhibit great antioxidant activity in both in vivo and in vitro studies. Therefore, adding a natural source of these antioxidants to the commonly consumed dairy product will be beneficial to the masses. Among all the sources, black carrots are the extraordinary and cheapest source of anthocyanins, which are commonly consumed as a natural fermented drink. In this study, an attempt has been made to examine the feasibility of black carrot concentrate as an ingredient into dairy products.
Methodology
Ice cream, yogurt and buttermilk were prepared by incorporating black carrot concentrate at 2.5, 5.0, 7.5 and 10% level and were subjected to sensory analysis. The most acceptable products with 7.5% black carrot concentrate were analyzed for minerals, polyphenols and antioxidant activity. Effects of storage on physicochemical, microbial and sensory attributes of black carrot concentrate incorporated dairy products were further analyzed.
Results
Sensory analysis revealed that black carrot concentrate could be used up to 7.5% as an ingredient into dairy product with high acceptability. Significant improvement in mineral content (Mg and Fe), polyphenols and antioxidant activity were reported in black carrot concentrate added dairy products. Developed dairy products exhibited an excellent amount of 24.52–113.27 mg/100g anthocyanins. Flavonoids increased by 14.52–34.62 times and Folin-Ciocalteu reducing capacity increased by 26.39–35.87 times in experimental dairy products. The storage study revealed that ice cream could be stored for more than 60 days, yogurt up to 5 days and buttermilk up to 10 days with excellent stability attributes.
Conclusion
Incorporation of black carrot concentrate at the level of 7.5% resulted in high acceptability and exceptional nutraceutical property of dairy products. Incorporation of black carrot concentrate into dairy products would enhance the nutraceutical properties and mineral content of food, which could be highly significant in preventing hidden hunger and oxidative stress-induced disorders in developing countries.
Keywords: Black carrot concentrate, Functional food, Anthocyanins, Ice-cream, Yogurt, Buttermilk
Black carrot concentrate; Functional food; Anthocyanins; Ice-cream; Yogurt; Buttermilk.
1. Introduction
Antioxidants are widely used in the food industry as synthetic food additives to enhance the stability of fats by preventing their oxidative deterioration. Lately, there has been a growing interest in natural antioxidants and their impact on health and disease prevention. India is witnessing an accelerated rise in the prevalence of oxidative stress-induced diseases like hypertension, diabetes, CVD and cancer (Uttara et al., 2009). Free radicals are generated due to various physiological conditions which have a hostile effect on the intestinal epithelium cells. Free radical damage to biological molecules such as lipids, proteins and nucleic acids is considered to be linked to a variety of degenerative diseases that are the leading causes of disability and death in India. Their contribution to the burden of disease is expected to increase over the next 25 years (Patel et al., 2011).
A great number of fruits and vegetables, spices and medicinal plants contain bioactive components demonstrating free radical scavenging activity. Bioactive compounds especially polyphenols are said to have a major role in the reduction of oxidative stress. Polyphenols are regarded as secondary metabolites, which does not demonstrate any specific metabolic properties in plant cells (Xiao et al., 2015), but are essential for the nutritional and sensory characteristics of plant foods. Epidemiological and nutrition studies have shown that polyphenols have a significant role in the prevention of oxidative stress-induced diseases such as diabetes, cancer and heart diseases (Pandey and Grover 2020; Costa et al., 2017; Pandey and Rizvi 2009). Polyphenols have been exhibited to show a wide range of physiological benefits as free radical scavenging activity, anti-inflammatory, anti-tumor and anti-diabetic characteristics (Xiao et al., 2014; Xiao et al., 2014a). Lately, there has been a growing focus on novel antioxidants originated from natural sources. Fruits and vegetables are gaining wide attention pertaining to their richness in different categories of polyphenols (Nabavi et al., 2013).
Black carrot is a common Indian root vegetable, which can be easily cultivated during the winter season. This crop is a tremendous source of anthocyanin pigments and is extremely high in polyphenols. It has depicted exceptionally great antioxidant potential in both in-vitro and in-vivo studies (Narayan and Vankataraman 2000; Karakaya et al., 2001; Kaur and Kapoor 2002; Glei et al., 2003; Ravindra and Narayan 2003; Uyan et al., 2004). Punjab Agricultural University (PAU), Ludhiana has developed India's second black carrot variety ‘Punjab Black Beauty’ for the benefits of farmers, which also promises to address the needs of a huge malnourished section of the Indian population. Black carrots are considered to be the cheapest source of anthocyanin as this crop is available at a very low price (8–15 Rs/kg) in India. Black carrots are grossly underutilized and do not receive much consumer acceptance as a vegetable despite having the obvious advantages as a source of natural food colourant, natural antioxidant and rich mineral content. Despite having numerous health benefits, not enough research work is being done to promote the utilization of black carrots in the food sector.
Dairy products are an integral part of the human diet throughout the globe. It posses an abundant amount of nutrients such as proteins, calcium and vitamins. India is one of the leading producer and consumer of dairy products in the world. Fruit fortified dairy products are already in the market and are highly accepted by consumers. Dairy products can be effectively used as food carriers for natural colourant derived from black carrot polyphenols and anthocyanins. Keeping in mind the wide availability of dairy products in the Indian population, this study was undertaken to develop polyphenols enriched dairy products such as ice cream, yogurt and buttermilk using black carrot concentrate. The addition of black carrot concentrate as a natural food additive will not only enhance the polyphenol content in the diet but will also help to improve the utilization of black carrots.
2. Materials and methods
Punjab Agricultural University, Ludhiana has developed India's second black carrot variety ‘Punjab Black Beauty’ as a solution for preventing hidden hunger and oxidative stress-induced disorders in developing countries like India. Black carrot concentrate of ‘Punjab black beauty’ variety was obtained from Pun-juice Punjab Agro factory, Hoshiarpur, Punjab. The other ingredients used for product development were obtained from the local market of Ludhiana, Punjab.
2.1. Preparation of dairy products
Three dairy products namely ice cream, yogurt and buttermilk were developed for this study by using black carrot concentrate as an ingredient. The ice cream was developed by the method of Hashim and Shamsi (2016). Ingredient used were fresh whole milk (250 ml), whipped cream (200 g), sugar, (70 g), skim milk powder (30 g) and cornflour (15 g). The ice cream samples were prepared in triplicate. Milk was boiled in a non-stick pan. Corn flour was mixed in two tablespoons of water and then added to the boiling milk. It was stirred continuously for two to three minutes. Then sugar and milk powder was incorporated and mixed properly to avoid the development of lumps. The mixture was then transferred from a muslin cloth into the container and froze in a deep freezer for 2 h. The cream was added in order to achieve its fat content to 5%. The prepared mixture was divided into four equal batches. One batch was treated as control and the experimental batches were prepared by substituting cream added mixture with pasteurized black carrot concentrate at different levels (0, 5.0, 7.5 and 10%). The ice cream was collected and then filled in small cups and stored at -20 °C till analyzed.
Yogurt was developed with the method described by De (2006). Yogurt culture NCDC 144 (L. delbrukii subspbulgaricus and S. thermophiles, 1:1) was procured from NDRI, Karnal. Milk was preheated to 35–40 °C, filtered to remove extraneous matter. Skim milk powder was added to milk to adjust the solids-not-fat content to approximately 12–15 %. This mixture was preheated to 60 °C and homogenized. The contents and black carrot concentrate was pasteurized to 90 °C for 30 min, after that the temperature was brought down to 43–44 °C. The mixture was then inoculated with 2% bulk starter and stirred briefly to ensure proper mixing. The mix was filled into packages taking care that the temperature does not fall below 41 °C during the filling operation. It was made sure that the time interval between inoculation and filling do not exceed 45 min. The packages were incubated without further agitation at 42 °C for about 4–5 h, till a titrable acidity of 0.75 % was reached. Then it was placed under refrigeration to cool to 5–7 °C. The black carrot concentrate enriched yogurt samples were processed similarly as control however, pasteurized black carrot concentrate at the selected percentage (0%, 5.0%, 7.5 and 10%) was added prior to the addition of culture to these samples.
For the preparation of buttermilk, double toned milk was purchased from Verka Dairy, Ludhiana, Punjab, India and stored at 4 °C. A bacterial culture NCDC 144 (L. delbrukii subspbulgaricus and S. thermophiles, 1:1) was procured from NDRI, Karnal and stored at -18 °C until used. The milk was constituted of 1.5% fat and 9.0% SNF. The milk was heated to 42 °C followed by inoculation with culture. The culture was thoroughly mixed into the milk. The inoculated milk was then shifted to the pre-sterilized beakers with lids of 1-litre capacity. The yogurt samples were incubated at 42 °C in an incubator for 7 h for proper fermentation. After the desired curd setting, the curd was subjected to agitation for 90 s at 10,000 rpm using a blending machine (CelloBlend-N-Mix300, India). Finally, pasteurized cold water was added and thoroughly homogenized. Black carrot concentrate incorporated buttermilk sample treatments were processed in the same fashion as control except the step involving the addition of pasteurized black carrot concentrate at a selected percentage (0%, 5.0%, 7.5 and 10%) prior to the addition of culture (Figure 1).
Figure 1.
Representation of dairy products developed by incorporating black carrot concentrate; Ice cream prepared by incorporating black carrot concentrate (A); Yogurt prepared by incorporating black carrot concentrate (B); Buttermilk prepared by incorporating black carrot concentrate (C). Control- Standard product; 5%- product developed by incorporating 5 % black carrot concentrate; 7.5%- product developed by incorporating 7.5 % black carrot concentrate; 10%- product developed by incorporating 10% black carrot concentrate.
2.2. Sensory analysis
A panel of 10 trained members was formed who were between the age group 22–55 years, and medically fit to perform sensory analysis. Informed consent was obtained from all the panel members before evaluation. The panel members were selected from the Department of Food and Nutrition, Punjab Agricultural University, Ludhiana. The formulated treatments were evaluated fresh as well as during storage for sensory attributes using 9 point hedonic scale (Larmond, 1970) (Annexure I, II and III).
2.3. Determination of physicochemical properties
Formulated products were assessed for total soluble solids (TSS), titratable acidity and pH. TSS content was determined by using a hand refractometer (Erma, Japan) with scale ranging from 0 to 32o Brix. The observations were expressed as oBrix at 20 °C. The titratable acidity was determined according to the method of (Ranganna 1997). The pH was evaluated with the help of a digital pH meter.
2.4. Determination of minerals
Developed products were assessed for mineral content viz. magnesium, zinc and iron (Piper 1950).
2.5. Determination of anthocyanins, flavonoids content and Folin-Ciocalteu reducing capacity
Total anthocyanin content of the samples was estimated by the method of Rabino et al. (1977). A fresh sample of 1 g was taken and crushed finely. 10 ml of 1% methanolic HCl (w/v) was added and it was kept overnight at 4 °C. The next day, sample extracts were filtered and absorbance was taken at 530 and 657 nm. The total anthocyanin content of the sample extract was calculated using the formula A530 – 0.33 A657. This formula is used to correct for the contribution of chlorophyll and its degradation products in acid solution to the absorbance of extracts at 530 nm (Mancinelli et al. 1975). The total anthocyanin content was expressed as cyanidin 3-glucoside (Cyd 3-glu) mg/100g of the fresh sample weight. The estimation of total flavonoids was done by the assay described by Zhishen et al. (1999) and results were expressed as catechin equivalents (CE)/100g of fresh sample.
Folin-Ciocalteu reducing capacity was analysed using Folin-Ciocalteu assay by the method of Swain and Hillis (1959). The Folin-Ciocalteu (FC) assay is an electron transfer based assay that represents the reducing capacity of a sample. It is usually expressed as total phenols of the extract in the literature (Keskin-Šašić et al., 2012; ; Marques et al., 2012; Vizzotto et al., 2006). Repeatable studies have reported that Folin-Ciocalteu assay is not exclusive to phenols as it can react with ascorbic acid, sodium bisulphite, reducing sugars, metals and reducing amino acids. As a result, this could deviate from the actual value of total phenolic content (Chen et al., 2015). Considering the reaction of Folin-Ciocalteu reagent with non-phenolic substances present in the sample that can reduce the Folin-Ciocalteu reagent, this assay has been denominated as ‘Folin-Ciocalteu reducing capacity’. This reaction takes place in the aqueous medium thus it determines the reducing capacity of water-soluble constituents in the sample (Granato et al., 2016).
In brief, 1 g of sample taken and it was refluxed with 80% methanol in a round bottom flask for 2 h and The extract was filtered and the residue was refluxed further for an additional 1 h. The extract was collected and total volume was made to 100 ml with 80% methanol. A sample extract of 0.5 ml was taken in a test tube and 0.5 ml of distilled water was added to it. Further, 5 ml of FC reagent was added and this solution was kept at rest for 5 min. 1 ml of saturated solution of sodium carbonate was added and the solution was stirred properly and kept at rest in a dark place for 60 min. The absorbance of the sample was measured at 725 nm using spectronic- 20 spectrophotometer. A standard curve was plotted against Gallic acid as standard. Folin-Ciocalteu reducing capacity was calculated as mg gallic acid equivalent (mg GAE/100g) of fresh sample.
2.6. Determination of total antioxidant activity
The chemical diversity of biological antioxidants makes it challenging to separate and measure individual oxidants from the food matrix. Therefore measurement of total antioxidant activity directly from food extract offers a viable alternative. In this study, two in vitro antioxidant assays namely ferric reducing antioxidant power (FRAP), 2,2-azinobis (3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) were performed by the methods described by Benzie and Strain (1999) and Miller and Rice-Evans (1997) respectively. In both assays, Trolox was used as a standard and the results were represented as μmol Trolox equivalent (TE) per gram fresh weight of the sample.
2.7. Storage stability
Experimental samples with 7.5% black carrot concentrate were analyzed to study the effect of storage on product stability and quality attributes. Ice cream samples were stored in 50 ml airtight plastic cups at -20 °C for 60 days. The samples were analyzed for total solids (AOAC 2010), pH, acidity, total plate count (TPC), yeast and mould count (YMC) and sensory attributes at an interval of 15 days. Yogurt and buttermilk samples were stored for 15 days at 4 °C in airtight plastic containers and glass bottles respectively and were evaluated for pH, acidity and TPC, YMC and sensory characteristics at a regular interval of 5 days. Total Plate Count (TPC) and yeast and mould count (YMC) were evaluated as per the method described by David and Frankhausar (2015).
2.8. Statistical analysis
The results were expressed as mean ± standard deviation. Data were analyzed by Kruskal Wallis H-test for sensory analysis and student's t-test to analyze the difference between control and acceptable formulated experimental sample with 7.5% black carrot concentrate incorporation. Effect of storage on quality attributes of dairy products was analyzed by ANOVA (Analysis of variance), comparing average interval using the SPSS software, version 18.0 (Stats oft Inc. USA).
3. Results and discussion
3.1. Analysis of black carrot concentrate
Physicochemical, polyphenols, reducing capacity and total antioxidant activity of black carrot concentrate are presented in Table 1. The flavonoids, anthocyanins, Folin-Ciocalteu reducing capacity and total antioxidant activity of black carrot concentrate were exceptionally high as compared to the fresh black carrot. Flavonoids content in concentrate increased by 28 times with an excellent concentration of 1682.66 mg/100g of anthocyanin. Analysis of Folin-Ciocalteu reducing capacity and total antioxidant capacity confirmed exceptionally high reducing capacity and antioxidant activity of black carrot concentrate.
Table 1.
Physicochemical, Polyphenolic content and antioxidant activity of black carrot concentrate.
| TSS (0Brix) | Acidity (mg/100 g) | Total Flavonoids (mg CE/100 g) | Anthocyanins (mg/100 g) | Folin-Ciocalteu reducing capacity (mg GAE/100 g) | Total antioxidant capacity |
||
|---|---|---|---|---|---|---|---|
| ABTS assay (μmolTE/g) | FRAP assay (μmolTE/g) | ||||||
| Fresh Black carrot Roots | 8.00 ± 0.10 | 0.25 ± 0.01 | 82.73 ± 1.62 | 233.32 ± 11.14 | 283.53 ± 5.50 | 21.79 ± 1.03 | 12.28 ± 0.41 |
| Black carrot concentrate | 40.02 ± 2.70 | 1.29 ± 0.12 | 2314.22 ± 73.16 | 1682.66 ± 35.69 | 8571.90 ± 25.64 | 31.33 ± 1.26 | 23.32 ± 0.87 |
Data are mean ± SD (n = 3).
FRAP (ferric reducing antioxidant power).
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).
3.2. Sensory evaluation
The hedonic test was used to assess the degree of liking of dairy products developed by using different levels of black carrot concentrate. The incorporation of black carrot concentrate imparted reddish-purple colour to the ice cream which was liked very much by the panellists. Ice cream with 7.5% black carrot concentrate showed the highest liking scores for its appearance and colour, which reduced significantly at 10% level. The texture, flavour, taste and overall acceptability of ice cream with black carrot concentrate at substitution levels of 0–7.5% depicted the highest liking score (Table 2).
Table 2.
Sensory analysis of black carrot concentrate incorporated ice cream.
| Ice cream | Appearance | Colour | Texture | Flavour | Taste | Overall acceptability | |
|---|---|---|---|---|---|---|---|
| Control | 8.50 | 8.30 | 8.20 | 8.10 | 8.00 | 8.10 | |
| Experimental | 5.0% | 8.30 | 8.10 | 8.25 | 8.00 | 8.05 | 8.20 |
| 7.5% | 8.50 | 8.45 | 8.30 | 8.01 | 8.20 | 8.25 | |
| 10.0% | 8.35 | 8.00 | 8.10 | 7.80 | 7.70 | 7.50 | |
| χ2 value (Kruskal–Wallis test) | 12.29∗ | 10.10∗ | 1.23 NS | 9.52∗ | 11.73∗ | 9.71∗ | |
∗∗ Significant at 1% level of significance (p < 0.01) ∗Significant at 5% level of significance (p < 0.05), NS - Non-significant, Control – Product developed with standard recipe, Experimental-product developed by incorporating black carrot concentrate at different levels into standard recipe.
Sensory evaluation of yogurt samples showed that appearance and colour were acceptable up to 7.5 % black carrot concentrate incorporation, as the level was increased to 10% the liking scores decreased significantly (Table 3). The liking scores for other parameters viz., consistency, flavour, taste and overall acceptability established the same observations. The flavour and taste of black carrot concentrate became more prominent and peculiar at the 10% level which further affected all the sensory parameters. Therefore, yogurt sample with 7.5% black carrot concentrate was found as the best acceptable sample for further analysis.
Table 3.
Sensory analysis of black carrot concentrate incorporated yogurt.
| Yogurt | Appearance | Colour | Consistency | Flavour | Taste | Overall acceptability | |
|---|---|---|---|---|---|---|---|
| Control | 8.50 | 8.50 | 8.50 | 8.20 | 8.15 | 8.20 | |
| Experimental | 5.0% | 8.10 | 8.20 | 8.10 | 7.85 | 8.05 | 8.00 |
| 7.5% | 8.00 | 7.80 | 8.00 | 7.80 | 7.85 | 7.80 | |
| 10.0% | 6.50 | 6.60 | 6.50 | 6.40 | 6.65 | 6.50 | |
| χ2 value (Kruskal–Wallis test) | 18.06∗ | 20.47∗∗ | 14.02∗∗ | 12.85∗∗ | 12.54∗∗ | 15.42∗∗ | |
∗∗ Significant at 1% level of significance (p < 0.01) ∗Significant at 5% level of significance (p < 0.05), NS - Non-significant, Control – Product developed with standard recipe, Experimental-product developed by incorporating black carrot concentrate at different levels into standard recipe.
The liking scores of all the experimental samples of buttermilk for sensory parameters viz., appearance, colour, consistency, flavour and taste were at par with control and showed high acceptability (Table 4). However, the overall acceptability of 10% black carrot concentrate was found to be the lowest. Therefore, buttermilk sample with 7.5% black carrot concentrate was selected and stored at refrigerated temperature (4 °C) in airtight glass bottles for further analysis.
Table 4.
Sensory analysis of black carrot concentrate incorporated buttermilk.
| Buttermilk | Appearance | Colour | Consistency | Flavour | Taste | Overall acceptability | |
|---|---|---|---|---|---|---|---|
| Control | 8.00 | 8.20 | 8.30 | 8.05 | 8.50 | 8.10 | |
| Experimental | 5.0 % | 8.20 | 8.40 | 8.25 | 8.10 | 8.30 | 8.20 |
| 7.5% | 8.25 | 8.35 | 8.35 | 8.20 | 8.50 | 8.40 | |
| 10.0% | 8.00 | 8.05 | 8.15 | 7.80 | 8.10 | 7.80 | |
| χ2 value (Kruskal–Wallis test) | 1.11 NS | 0.63NS | 1.60NS | 5.60NS | 1.31NS | 8.58∗ | |
∗∗ Significant at 1% level of significance (p < 0.01) ∗Significant at 5% level of significance (p < 0.05), NS - Non-significant, Control – Product developed with standard recipe, Experimental-product developed by incorporating black carrot concentrate at different levels into standard recipe.
The sensory evaluation results pointed out that incorporation of black carrot concentrate in dairy products up to 7.5% gives the best overall acceptability. However, dairy products containing 10% black carrot concentrate were rated comparatively lower, which might be due to an excessive amount of polyphenolic compounds, which can negatively affect the sensory attributes of food (Drewnowski and Gomez- Carneros, 2000). The change in product colour was quite evident as represented in Figure 1. The initial colour of the products incorporated with 5% black carrot concentrate was reddish-purple, which became darker as the concentration increased. Experimental dairy products with 7.5% black carrot concentrate were analysed further along with control.
3.3. Physicochemical properties
Physicochemical properties of ice cream, yogurt and buttermilk with 7.5% black carrot concentrate are presented in Table 5. A significant increase in TSS (oBrix) was observed in all the experimental dairy products having 7.5% black carrot concentrate. This might be due to the higher TSS of black carrot concentrate (40 oBrix). The addition of black carrot concentrate (7.5%) caused a significant increase in the acidity of the ice cream sample. This increase in acidity might be attributed to the acidity of black carrot concentrate. The results were in accordance with Kaur (2014) who reported that incorporation of ginger juice significantly (p < 0.01) increased the titratable acidity and decreased pH in ice cream. Hwang et al. (2009) also reported similar results in grape wine lees incorporated ice cream. With respect to yogurt and buttermilk samples, incorporation of black carrot concentrate (7.5%) showed significantly higher pH value and lower acidity than control. Higher pH and acidity could be due to the higher lactic acid content of the control. Mudgil and Barak (2016) who developed functional buttermilk by soluble fibre fortification also observed similar results.
Table 5.
Physicochemical characteristics of black carrot concentrate incorporated dairy products.
| Products | TSS (0brix) | Acidity (% Lactic Acid) | pH | |
|---|---|---|---|---|
| Ice cream | Control | 25.38 ± 0.34 | 0.16 ± 0.00 | 6.60 ± 0.10 |
| Experimental (7.5%) | 26.86 ± 0.51 | 0.23 ± 0.01 | 6.41 ± 0.09 | |
| t-value | 4.18∗ | 7.56∗∗ | 2.43NS | |
| Yogurt | Control | 8.11 ± 0.30 | 0.61 ± 0.03 | 4.13 ± 0.02 |
| Experimental (7.5%) | 10.30 ± 0.70 | 0.51 ± 0.05 | 4.52 ± 0.19 | |
| t-value | 4.98∗∗ | 2.97∗ | 3.54∗ | |
| Buttermilk | Control | 1.60 ± 0.07 | 0.62 ± 0.01 | 4.83 ± 0.01 |
| Experimental (7.5%) | 2.50 ± 0.10 | 0.58 ± 0.01 | 4.96 ± 0.01 | |
| t-value | 12.77∗∗ | 4.90∗∗ | 14.70∗∗ | |
∗∗ Significant at 1% level of significance (p < 0.01), ∗Significant at 5% level of significance (p < 0.05), NS - Non significant, Control – Product developed with standard recipe, Experimental- Product developed by incorporating 7.5% black carrot concentrate into the standard recipe.
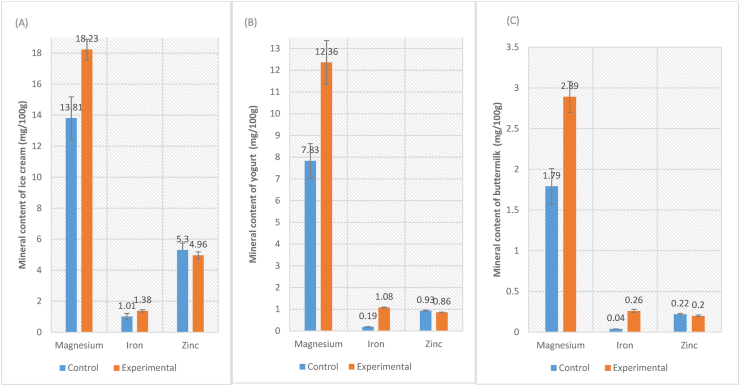
3.4. Mineral content
The incorporation of black carrot concentrate into dairy products resulted in a significant increase in magnesium and iron content, however, zinc content was not affected (Figure 2). Mg content was found to be increased by 32% in ice cream, 57.9% in yogurt and 61.5% in buttermilk, whereas iron content increased from 1.4 times in ice cream to 6.5 times in buttermilk. The higher values of these estimated minerals in experimental buttermilk might be attributed to the higher content of respective minerals in black carrot (Nicolle et al., 2004). These results suggested that black carrot concentrate could also be considered a good source of iron, which might help in combating anaemia, one of the biggest malnutrition problems of India. However, further study is required to assess their bioavailability. The promotion of such indigenous vegetables in diet might help alleviate food insecurity and malnutrition in developing countries (Kamga et al., 2013).
Figure 2.
Mineral content of black carrot concentrate incorporated dairy products. Ice cream prepared by incorporating black carrot concentrate (A); Yogurt prepared by incorporating black carrot concentrate (B); Buttermilk prepared by incorporating black carrot concentrate (C). Control- Standard product; Experimental- Product developed by incorporating 7.5% black carrot concentrate into the standard recipe. Values are expressed on fresh weight basis. Bars represent standard deviation of means (n = 3).
3.5. Anthocyanins, flavonoids content and Folin-Ciocalteu reducing capacity
The major bioactive compound in black carrot is polyphenols and anthocyanins, which is well known for its colouring properties and strong antioxidant activity (Algarra et al., 2014; Akhtar et al., 2017; Pandey and Grover 2020). The total anthocyanin content of black carrot concentrate was 1682.66 mg/100g and as expected the anthocyanins were detected only in dairy products containing black carrot concentrate (Table 6). The anthocyanin content of experimental dairy products ranged from 24.52 to 113.27 mg/100g. In recent years, the use of polyphenolic compounds such as anthocyanins has been extensively studied for their inhibitive effect in various chronic and degenerative diseases (Vauzour et al., 2010). The pharmacological effect of anthocyanins compounds has been associated with their molecular structure. The suppressive effect of anthocyanins on oxidative stress-induced diseases and inhibition of inflammatory pathways have been established in several studies (Juránek and Bezek 2005; Zweier et al., 1987). The strength of the antioxidant potential of anthocyanins depends on the total number of free hydroxyl group around the pyrone ring and the high number of hydroxyl groups dissipated throughout its molecular structure (Heymes et al., 2003). Therefore, the incorporation of anthocyanin-rich black carrot concentrate in dairy products can result in additional health benefits. The flavonoids content of developed dairy products were also analyzed and results are shown in Table 6. The control samples represented a negligible amount of flavonoids, whereas experimental samples represented excellent content. This infers that dairy products possessed some amounts of polyphenols that are possibly derived from the milk. O'Connell and Fox (2001) reported ‘phenolic compounds can be found in milk from animal feed, amino acid catabolism, and/or from the environment.’ Polyphenols may also increase during the heating of milk due to Maillard's reaction. The flavonoids content of black carrot concentrate was reported to be 2314.22 mg CE/100g (Table 1). A comparison of the estimated content of polyphenols in dairy products and expected values suggested that some degradation might have occurred during processing. However, despite the loss of polyphenols after processing, all experimental dairy products containing black carrot concentrate showed significantly higher concentrations of phenols when compared with the control. The Folin-Ciocalteu reducing capacity of products showed a similar pattern as total flavonoid content. The reducing capacity increased significantly by 26–35 times upon incorporation of black carrot concentrate at 7.5% level. The black carrot polyphenols are reported to possess better stability in comparison to other fruit polyphenols (Day et al., 2009). This feature offers black carrot as one of the most recommended natural sources of bioactive compounds such as anthocyanins.
Table 6.
Polyphenols content and antioxidant activity of black carrot concentrate incorporated dairy products.
| Products | Anthocyanin (mg/100g) | Total Flavonoids (mg CE/100g) | Folin-Ciocalteu reducing capacity (mg GAE/100g) | Total antioxidant capacity |
||
|---|---|---|---|---|---|---|
| ABTS (μmolTE/g) | FRAP (μmol TE/g) | |||||
| Ice cream | Control | ND | 4.02 ± 0.96 | 14.32 ± 1.32 | 3.01 ± 0.03 | 1.01 ± 0.02 |
| Experimental | 98.09 ± 11.31 | 139.21 ± 12.33 | 513.63 ± 57.15 | 24.64 ± 0.49 | 13.48 ± 0.34 | |
| t-value | 15.02∗∗ | 18.93∗∗ | 15.13∗∗ | 76.61∗∗ | 63.98∗∗ | |
| Yogurt | Control | ND | 10.36 ± 0.93 | 19.36 ± 1.03 | 3.53 ± 0.02 | 1.57 ± 0.02 |
| Experimental | 113.27 ± 10.41 | 165.91 ± 18.39 | 544.30 ± 61.99 | 25.31 ± 0.33 | 13.94 ± 0.33 | |
| t-value | 18.85∗∗ | 14.63∗∗ | 14.67∗∗ | 115.76∗∗ | 65.81∗∗ | |
| Buttermilk | Control | ND | 2.23 ± 0.44 | 4.44 ± 0.86 | 2.08 ± 0.07 | 0.83 ± 0.04 |
| Experimental | 24.52 ± 2.41 | 32.39 ± 2.56 | 117.19 ± 11.26 | 15.11 ± 0.1 | 7.03 ± 0.14 | |
| t-value | 17.62∗∗ | 20.11∗∗ | 17.29∗∗ | 184.16∗∗ | 74.34∗∗ | |
Data are mean ± SD (n = 3).
∗∗ Significant at 1% level of significance (p < 0.01), ∗Significant at 5% level of significance (p < 0.05), NS - Non significant, Control – Product developed with standard recipe, Experimental- Product developed by incorporating 7.5% black carrot concentrate into the standard recipe.
ND: Not detectible.
FRAP (Ferric reducing antioxidant power).
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).
3.6. Total antioxidant capacity
Total antioxidant capacity is a unique parameter that measures the strength of a sample to quench free radicals. The antioxidant activity of dairy products was greatly improved by the incorporation of black carrot concentrate. The total antioxidant capacity of all the experimental dairy products was significantly higher in comparison to control. The increase in antioxidant capacity was observed to be 7–8 times in ABTS assay 8–13 times in FRAP assay with the addition of black carrot concentrate at 7.5% into dairy products. High concentrations of polyphenols, flavonoids and anthocyanins in black carrot are the main contributors to their high antioxidant capacity. In addition, black carrot anthocyanins are more acylated which confers higher antioxidant activity than other monomeric anthocyanins (Kammerer et al., 2004). The results were in accordance with Cam et al. (2013) who reported an increase in total antioxidant capacity with the addition of pomegranate peel phenolics and pomegranate seed oil in ice cream. Improvement in the antioxidant activity of the experimental sample might be attributed to the incorporation of polyphenols including anthocyanins, which had been proved to have high antioxidant properties (Grassmann et al., 2007). These results are in agreement with Prior et al. (1998) who demonstrated a linear relationship between antioxidant activity and total phenolic content. Both in vitro assays viz. ABTS and FRAP used for the analysis of the total antioxidant capacity of developed dairy products were found to be equally simple, economical and consistent. Presently, ABTS assay is the preferred method for analyzing the antioxidant capacity of anthocyanins containing samples, as the radical produced in this method has a maximum absorption at a wavelength of 734 nm, therefore it reduces the chances of interference of anthocyanins which absorb between the wavelength of 460–550 nm (Kuskoski et al., 2006). Prior et al. (2005) and Van den Berg et al. (1999) concluded that the antioxidant capacity assay can not truly reflect the “total antioxidant capacity” of phenolic products but it can be used to provide a ranking order of antioxidants.
3.7. Storage stability
Results in Table 7 shows the effect of storage on different quality attributes of experimental dairy products incorporated with 7.5% black carrot concentrate. A non-significant change in total solids, a significant increase in acidity and a decrease in pH was observed in ice cream during storage. The increase in acidity during storage might be due to the formation of lactic acid by lactic acid bacteria (Murtaza et al., 2004). Bajwa et al. (2003) also reported a decrease of 2.71% in pH value during 40 days of storage in ice cream containing strawberry pulp. During the storage period of 60 days, the total plate count of ice cream decreased significantly from 3.33 to 1.82 log10 cfu/g. The reduction in the total plate count (TPC) was due to the destruction of bacteria at low temperatures. The TPC values for the ice cream samples were within the acceptable levels as per ISI specifications i.e. 250 log10 cfu/ml maximum (De 2006). This might be attributed to the formation of ice crystals which disrupts the cell membrane of microbes resulting in a decrease in microbial load (Davidson et al., 2000). Black carrot concentrate also had a high antioxidant capacity, which prevents oxidation of fat thus increases the shelf life of the product. Goraya (2013) also reported a decline in TPC of ice cream with amla products during the storage period of 60 days. Yeasts and moulds were not detected throughout the 2 months of the storage study, however, a gradual decrease in overall acceptability scores of all the ice cream samples (8.25–7.89) was reported. However, scores were found to be in a highly acceptable range throughout the period.
Table 7.
Effect of storage on physicochemical, microbial and sensory attributes of black carrot concentrate incorporated dairy products.
| Storage period (days) | Total solids (%) | pH | Acidity (%Lactic Acid) | TPC (log10 cfu/g) | YMC (log10 cfu/g) | Overall acceptability |
|---|---|---|---|---|---|---|
|
Ice cream | ||||||
| 0 | 31.77 ± 0.93a | 6.41 ± 0.001a | 0.230 ± 0.002d | 3.33 ± 0.03a | ND | 8.25 ± 0.05a |
| 15 | 31.98 ± 0.53a | 6.40 ± 0.001a | 0.233 ± 0.001d | 3.15 ± 0.02b | ND | 8.20 ± 0.02a |
| 30 | 32.13 ± 0.43a | 6.39 ± 0.001b | 0.238 ± 0.002c | 3.03 ± 0.01c | ND | 8.01 ± 0.01b |
| 45 | 32.24 ± 0.35a | 6.38 ± 0.005bc | 0.245 ± 0.001b | 2.01 ± 0.01d | ND | 7.95 ± 0.05bc |
| 60 | 32.56 ± 0.3a | 6.37 ± 0.002bc | 0.250 ± 0.001a | 1.82 ± 0.01e | ND | 7.89 ± 0.03c |
| pH | Acidity (%) | TPC (log10 cfu/g) | YMC (log10 cfu/g) | Overall acceptability | |
|---|---|---|---|---|---|
|
Yogurt | |||||
| 0 | 4.52 ± 0.06a | 0.51 ± 0.01a | 8.90 ± 0.01a | ND | 7.80 ± 0.05a |
| 5 | 4.10 ± 0.02b | 0.53 ± 0.01a | 7.75 ± 0.02b | ND | 7.65 ± 0.06b |
| 10 | 3.85 ± 0.06c | 0.57 ± 0.01b | 6.60 ± 0.04c | ND | 6.80 ± 0.10c |
| 15 |
3.73 ± 0.05d |
0.61 ± 0.00b |
3.83 ± 0.02d |
ND |
6.30 ± 0.05d |
|
Buttermilk | |||||
| 0 | 4.96 ± 0.02d | 0.30 ± 0.01b | 4.16 ± 0.01d | ND | 8.40 ± 0.05a |
| 5 | 4.38 ± 0.01c | 0.33 ± 0.01b | 4.25 ± 0.02c | ND | 8.20 ± 0.01b |
| 10 | 4.01 ± 0.01b | 0.39 ± 0.01a | 4.35 ± 0.01b | ND | 7.30 ± 0.02c |
| 15 | 3.84 ± 0.01a | 0.42 ± 0.01a | 4.39 ± 0.01a | ND | 6.80 ± 0.05d |
Value are mean ± SD, Value in columns followed by different superscript differ significantly at 5 % level.
ND-Not Detectable.
Ice cream, Yogurt and Buttermilk sample contain 7.5% black carrot concentrate.
TPC: Total Plate count.
YMC: Yeast and mould count.
For fermented dairy products, pH and acidity are key quality defining parameters as they determine their organoleptic properties, shelf-life quality and microbial safety. The pH values of the yogurt sample declined significantly from 4.52 to 3.73 after the 15th day of storage. The acidity values gradually increased from 0.51 to 0.61% during the storage in black carrot concentrate added yogurt after 15 days of storage. Beal et al. (2001) observed an increased production of lactic acid and galactose in the first 2 weeks due to increased microbial metabolic activity with lactose utilization by lactic acid bacteria. There was a significant reduction in TPC in yogurt sample due to a lower pH and higher acidic processing conditions during storage. A decline in the number of lactobacilli bacteria was also reported in fruit yogurt during storage by Canganella et al. (1998) and Vahedi et al. (2008). The probiotic bacteria must reach the intestine in an active state and in adequate quantities i.e. 6-7 log10 cfu/g of product in order to exert health benefits. The black carrot concentrate enriched yogurts maintained recommended probiotic quantity up to ten days of storage period at 4 °C. Yeast and mould counts were not identified over the storage period of yogurt sample. The overall acceptability scores of the yogurt samples declined significantly after 5 days of storage. Thus, best acceptability up to 5 days could be considered for yogurt. The decline in overall acceptability might be attributed to the increased acidity of yogurt during storage.
The acidity of buttermilk sample increased significantly till the 15th day of storage. The results were in line with Patel et al. (2017) who observed a rise in acidity of buttermilk samples during storage at refrigerated condition. pH values of buttermilk showed a gradual decline during the storage at 4 °C. Rao (2003) also reported a decrease in pH of buttermilk samples from an initial value of 4.14 to 4.11 on the 12th day of storage. During storage of buttermilk, a significant rise in the TPC value was reported up to the 15th day from 4.16 to 4.36 log10 cfu/g. A steady rise in TPC was also observed by Patel et al. (2017) during storage of buttermilk supplemented with Moringa. Yeast and mould counts were not identified in the experimental buttermilk sample throughout the storage period. A decrease in scores for overall acceptability of the buttermilk samples was observed throughout the storage period, which dropped significantly after 10 days of storage (7.30). The decline in overall acceptability was attributed to the increased acidity of buttermilk during storage. Thus, it can be concluded that 7.5 % black carrot concentrate enriched buttermilk can be well accepted up to 10 days of storage at 4 °C temperature.
4. Conclusion
In this study, black carrot concentrate enriched dairy products were developed and analysed for sensory attributes, physicochemical characteristics, polyphenols and antioxidant capacity and storage stability. The results showed that black carrot concentrate at the level of 7.5% could be used in the development of acceptable dairy products. The incorporation of black carrot concentrate markedly increased the total anthocyanin, total phenols, total flavonoids, antioxidant activity, magnesium and iron content of dairy products without significantly affecting their sensory attributes. We may also infer that the ‘Punjab Black Beauty’ variety of Indian black carrot can be meritoriously incorporated into dairy products to exhibit higher bioactive compounds, antioxidant activity and mineral content.
Declarations
Author contribution statement
Pragya Pandey: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kiran Grover: Conceived and designed the experiments.
Tarsem Singh Dhillon, Amarjeet Kaur: Contributed reagents, materials, analysis tools or data.
Mohammed Javed: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work was supported by INSPIRE fellowship from DST, India. The author would like to thank Dr Mudit Chandra (Assistant Scientist, Department of Veterinary Microbiology, College of Veterinary Science, GDVASU, Ludhiana Punjab, India) for supporting to carry out microbiological work of this paper in their lab.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2021.e06880.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- Akhtar S., Rauf A., Imran M., Qamar M., Riaz M., Mubarak M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: a review. Trends Food Sci. Technol. 2017;66:36–47. [Google Scholar]
- Algarra M., Fernandes A., Mateus N., Freitas V., Joaquim C.G., Silva E.D., Casado J. Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J. Food Composition Anal. 2014;33:71–76. [Google Scholar]
- AOAC International Official methods of analysis. Assoc. Off. Anal. Chem. 2010 (eighteenth ed.) Washington DC. [Google Scholar]
- Bajwa U.A., Huma N., Ehsan B., Jabbar K., Khurrama A. Effect of different concentration of strawberry pulp on the properties of ice cream. Int. J. Agric. Biol. 2003;15:635–637. [Google Scholar]
- Béal C., Fonseca F., Corrieu G. Resistance to freezing and frozen storage of Streptococcus thermophiles is related to membrane fatty acid composition. J. Dairy Sci. 2001;84(11):2347–2356. doi: 10.3168/jds.S0022-0302(01)74683-8. [DOI] [PubMed] [Google Scholar]
- Benzie I.F., Strain J.J. [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Cam M., Erdogan F., Aslan D., Dinc M. Enrichment of functional properties of ice cream with pomegranate by-products. J. Food Sci. 2013;10:1543–1550. doi: 10.1111/1750-3841.12258. [DOI] [PubMed] [Google Scholar]
- Canganella F., Ovidi M., Paganini S., Vettraino A.M., Bevilacqua L., Trovatelli L.D. Survival of undesirable micro-organisms in fruit yogurts during storage at different temperatures. Food Microbiol. 1998;15(1):71–77. [Google Scholar]
- Chen L.Y., Cheng C.W., Liang J.Y. Effect of esterification condensation on the Folin–Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015;170:10–15. doi: 10.1016/j.foodchem.2014.08.038. [DOI] [PubMed] [Google Scholar]
- Costa C., Tsatsakis A., Mamoulakis C., Teodoro M., Briguglio G., Caruso E., Tsoukalas D., Margina D., Dardiotis E., Kouretas D., Fenga C. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017;110:286–299. doi: 10.1016/j.fct.2017.10.023. [DOI] [PubMed] [Google Scholar]
- David B., Frankhausar Pour plate technique for bacterial enumeration. 2015. http://biology.clc.uc.edu/frankhauser/Labs/Microbiology/Meat_Milk/Pour_Plate.html (Cited from.
- Davidson R.H., Duncan S.E., Hackney C.R., Eigel W.N., Boling J.W. Probiotic culture survival and implications in fermented frozen yogurt characteristics. J. Dairy Sci. 2000;83(4):666–673. doi: 10.3168/jds.S0022-0302(00)74927-7. [DOI] [PubMed] [Google Scholar]
- Day L., Seymour R.B., Pitts K.F. Incorporation of functional ingredients into foods. Trends Food Sci. Technol. 2009;20:388–395. [Google Scholar]
- De S. first ed. Oxford University Press; New Delhi: 2006. Outlines of Dairy Technology: Ice Cream; pp. 182–183. [Google Scholar]
- Drewnowski A., Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 2000;72:1424–1435. doi: 10.1093/ajcn/72.6.1424. [DOI] [PubMed] [Google Scholar]
- Glei M., Matuschek M., Steiner C., Bohm V., Persin C., Pool-Zobel B.L. Initial in vitro toxicity testing of functional foods rich in catechins and anthocyanins in human cells. Toxicol. Vitro. 2003;17:723–729. doi: 10.1016/s0887-2333(03)00099-7. [DOI] [PubMed] [Google Scholar]
- Goraya R.K. Punjab Agricultural University; Ludhiana, India: 2013. Functionality and Quality Assessment of Amla Incorporated Ice Cream. M.Sc. thesis. [Google Scholar]
- Granato Daniel, Santos Jânio Sousa, Maciel Laércio Galvão, Nunes Domingos Sávio. Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. Trends Anal. Chem. 2016 [Google Scholar]
- Grassmann J., Schnitzler W.H., Habegger R. Evaluation of different coloured carrot cultivars on antioxidative capacity based on their carotenoid and phenolic contents. Int. J. Food Sci. Nutr. 2007;58:603–611. doi: 10.1080/09637480701359149. [DOI] [PubMed] [Google Scholar]
- Hashim I., Shamsi K.S.A. Physiochemical and sensory properties of ice-cream sweetened with date syrup. MOJ Food Proc. Technol. 2016;2(3):1–4. [Google Scholar]
- Heymes C., Bendall J.K., Ratajczak P., Cave A.C., Samuel J.-L., Hasenfuss G. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- Hwang J.Y., Shyu Y.S., Hsu C.K. Grape wine lees improves the rheological and adds antioxidant properties to ice cream. LWT-Food Sci. Technol. 2009;42(1):312–318. [Google Scholar]
- Juránek I., Bezek S. Controversy of free radical hypothesis: reactive oxygen species–cause or consequence of tissue injury? Gen. Physiol. Biophys. 2005;24:263–278. [PubMed] [Google Scholar]
- Kamga R.T., Kouamé C., Atangana A.R., Chagomoka T., Ndango R. Nutritional evaluation of five African indigenous vegetables. J. Hortic. Res. 2013;21(1):99–106. [Google Scholar]
- Kammerer D., Carle R., Schieber A. Characterization of phenolic acids in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004;18:1331–1340. doi: 10.1002/rcm.1496. [DOI] [PubMed] [Google Scholar]
- Karakaya S., El S.N., Tas A.A. Antioxidant activity of some food containing phenolic compounds. Int. J. Food Sci. Nutr. 2001;52:501–508. [PubMed] [Google Scholar]
- Kaur D. 2014. Assessment of Quality and Stability of Ginger Incorporated Ice Cream (Doctoral Dissertation, Punjab Agricultural University, Ludhiana) [Google Scholar]
- Kaur C., Kapoor H.C. Antioxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002;37:153–161. [Google Scholar]
- Keskin-Šašić I., Tahirović I., Topčagić A., Klepo L., Salihović M.B., Ibragić S., Toromanović J., Ajanović A., Velispahić E. Total phenolic content and antioxidant capacity of fruit juices. Bull. Chemist. Technol. Bosnia Herzegovina. 2012;39:25–28. [Google Scholar]
- Kuskoski E.M., Asuero A.G., Troncoso A.M., Fett R. Antioxidant activity of pulps of tropical fruits. Application of the ABTS method. Alimentaria. 2006;376:67–70. [Google Scholar]
- Larmond E. Food Research Institute, Central Experimental Farm, Canada Department of Agriculture; Ottawa, Canada: 1970. Methods for sensory evaluation of food. Publication 1284. [Google Scholar]
- Mancinelli A.L., Yang C.P.H., Lindquist P., Anderson O.R., Rabino I. Photocontrol of anthocyanin synthesis: III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol. 1975;55(2):251–257. doi: 10.1104/pp.55.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M.R., Paz D.D., Batista L.P.R., Barbosa C.D.O., Araújo M.A.M., Moreira-Araújo R.S.D.R. An in vitro analysis of the total phenolic content, antioxidant power, physical, physicochemical, and chemical composition of Terminalia Catappa Linn fruits. Food Sci. Technol. 2012;32(1):209–213. [Google Scholar]
- Miller N.J., Rice-Evans C. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic. Res. 1997;26:195–199. doi: 10.3109/10715769709097799. [DOI] [PubMed] [Google Scholar]
- Mudgil D., Barak S. Development of functional buttermilk by soluble fibre fortification. Agro Food Ind. Hi-Tech. 2016;27(2):44–47. [Google Scholar]
- Murtaza M.A., Huma N.U.Z.H.A.T., Mueen-Ud-Din G., Shabbir M.A., Mahmood S.H.A.H.I.D. Effect of fat replacement by fig addition on ice cream quality. Int. J. Agric. Biol. 2004;6(1):68–70. [Google Scholar]
- Nabavi S.F., Nabavi S.M., Setzer W.N., Nabavi S., Nabavi S.A., Ebrahimzadeh M.A. Antioxidant and anti-hemolytic activity of lipid-soluble bioactive substances in avocado fruits. Fruits. 2013;68:185–193. [Google Scholar]
- Narayan M.S., Vankataraman L.V. Characterization of anthocyanins derived from carrot (Daucus carota) cell culture. Food Chem. 2000;70:361–363. [Google Scholar]
- Nicolle C., Simon G., Rock E., Amouroux P., Rémésy C. Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange, and dark-orange carrot cultivars. J. Am. Soc. Hortic. Sci. 2004;129(4):523–529. [Google Scholar]
- O’connell J.E., Fox P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: a review. Int. Dairy J. 2001;11(3):103–120. [Google Scholar]
- Pandey P., Grover K. Characterization of black carrot (Daucus carota L.) polyphenols; role in health promotion and disease prevention: an overview. J. Pharmacogn. Phytochem. 2020;9(5):2784–2792. [Google Scholar]
- Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxi. Med. Cell. Longev. 2009;2 doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Chatterji S., Chisholm D., Ebrahim S., Gopalakrishna G., Mathers C., Mohan V., Prabhakaran D., Ravindran R.D., Reddy K.S. Chronic diseases and injuries in India. The Lancet. 2011;377(9763):413–428. doi: 10.1016/S0140-6736(10)61188-9. [DOI] [PubMed] [Google Scholar]
- Piper C.S. Interscience Pub. Inc.; New York: 1950. Soil and plant analysis; p. 212. [Google Scholar]
- Prior R.L., Cao G., Martin A., Sofic E., McEwen J., O'Brien C., Lischner N., Ehlenfeldt M., Kalt W., Krewer G., Mainland C.M. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998;46(7):2686–2693. [Google Scholar]
- Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Rabino I., Mancinelli A.L., Kuzmanoff K.M. Photocontrol of anthocyanin synthesis: VI. Spectral sensitivity, irradiance dependence, and reciprocity relationships. Plant Physiol. 1977;59:569–573. doi: 10.1104/pp.59.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Edu. 1997:47–48. [Google Scholar]
- Rao A.V. 2003. Selected Technological Parameters for Manufacture of Chhash (M. Sc. Thesis Submitted to Anand Agricultural University, Anand) [Google Scholar]
- Ravindra P.V., Narayan M.S. Antioxidant activity of the anthocyanin from carrot (Daucus carota) callus culture. Int. J. Food Sci. Nutr. 2003;54:349–355. doi: 10.1080/09637480120092134. [DOI] [PubMed] [Google Scholar]
- Swain T., Hillis W.E. The phenolic constituents of Prunusdomestica. I.—the quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10(1):63–68. [Google Scholar]
- Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyan S.E., Baysal T., Yurdagel Ü., El S.N. Effects of drying process on antioxidant activity of purple carrots. Food Nahrung. 2004;48:57–60. doi: 10.1002/food.200300373. [DOI] [PubMed] [Google Scholar]
- Vahedi N., Tehrani M.M., Shahidi F. Optimizing of fruit yoghurt formulation and evaluating its quality during storage. Am.-Eurasian J. Agric. Environ. Sci. 2008;3:922–927. [Google Scholar]
- Van den Berg R., Haenen G.R., van den Berg H., Bast A.A.L.T. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66(4):511–517. [Google Scholar]
- Vauzour D., Rodriguez-Mateos A., Corona G., Oruna-Concha M.J., Spencer J.P.E. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzotto M., Cisneros-Zevallos L., Byrne D.H., Ramming D.W., Okie W.R. 2006. Total phenolic, carotenoid, and anthocyanin content and antioxidant activity of peach and plum genotypes. [Google Scholar]
- Xiao J., Capanoglu E., Jassbi A.R., Miron A. The paradox of natural flavonoid C-glycosides and health benefits: when more occurrence is less research. Biotechnol. Adv. 2014;(14) doi: 10.1016/j.biotechadv.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Xiao J., Chen T., Cao H. Flavonoid glycosylation and biological benefits. Biotechnol. Adv. 2014;(14) doi: 10.1016/j.biotechadv.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Xiao J., Ni X., Kai G., Chen X. Advance in dietary polyphenols as aldose reductases inhibitors: structure–activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2015;55:16–31. doi: 10.1080/10408398.2011.584252. [DOI] [PubMed] [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- Zweier J.L., Flaherty J.T., Weisfeldt M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. U.S.A. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.