Abstract
Biocontrol of root-knot nematode has attracted increasing attention over the past two decades. The inconsistent field performance of biocontrol agents, which is caused by soil fungistasis, often restricts their commercial application. There is still a lack of research on the genes involved in biocontrol fungi response to soil fungistasis, which is important for optimizing practical applications of biocontrol fungi. In this study, the lactoylglutathione lyase-encoding AOL_s00004g335 in the nematophagous fungi Arthrobotrys oligospora was knocked out, and three mutant strains were obtained. The hyphal growth of mutants on the three media was almost the same as that of the wild-type strain, but mutants had slightly higher resistance to NaCl, SDS, and H2O2. Methylglyoxal (MG) significantly increased the resistance of A. oligospora to ammonia, but decreased the resistance to benzaldehyde. Furthermore, the resistance of the mutants to soil fungistasis was largely weakened and MG could not increase the resistance of A. oligospora to soil fungistasis. Our results revealed that MG has different effects on the fungistatic roles of ammonia and benzaldehyde and that lactoylglutathione lyase is very important for A. oligospora to resist soil fungistasis.
Keywords: lactoylglutathione lyase, methylglyoxal, soil fungistasis, ammonia, benzaldehyde
Introduction
Plant-parasitic nematodes cause much more annual damage compared to pests, and they lead to more than 100 billion dollars of global agricultural loss every year (Coyne et al., 2018). The root-knot nematode (RKN), Meloidogyne spp., accounts for about 50% of the losses caused by all plant-parasitic nematodes (Abad et al., 2008; Singh et al., 2015). The RKN also causes plant wounds, through which microbial pathogens infect the plant, and this frequently leads to the underestimation of the damage caused by nematodes (Basso et al., 2020). In the past decades, the main control method against Meloidogyne spp. has been chemical nematicides. However, many chemical nematicides (e.g., methyl bromide) have been forbidden in recent years because of their strong toxicity (Mao et al., 2017). Avermectin, fosthiazate, and fluopyram are the main chemical nematicides used at present. Except for pesticide residues in food, the prolonged use of nematicides also results in drug resistance in nematodes (Wolstenholme et al., 2004; Ghosh et al., 2012), so new alternative methods for controlling nematode diseases are necessary. Nematode biocontrol agents can meet these requirements, and has been attracting more and more attention from researchers (Li et al., 2015; Zhang et al., 2020).
Great effort has been made by scientists to develop nonchemical and eco-friendly RKN management strategies. Many microorganisms, such as Pochonia chlamydosporia (Atkins et al., 2003), Purpureocillium lilacinum (Mo et al., 2019), Muscodor albus (Riga, 2008), Trichoderma harzianum (Sharon et al., 2001), and Arthrobotrys oligospora (Singh et al., 2013) are effective in controlling RKN. Although some of these microbes have been developed as nematicides, inconsistent field performance often restricts the commercial development of biocontrol agents against RKN, and biocontrol microbes are often not considered as acceptable alternatives for pesticides (Chen et al., 2020). This inconsistency can be caused by a large number of biotic and abiotic factors in soil, including interaction with non-target organisms, damage by other pathogens and pests, degree of rhizosphere colonization, and physical and chemical composition of the rhizosphere (Zhang et al., 2020). The outcomes of some of these factors are named as soil fungistasis.
Dobbs and Hinson first proposed the concept of soil fungistasis in 1953 to describe the widespread occurrence of the inhibition of fungal spore germination or growth of fungal hyphae in the soils (Dobbs and Hinson, 1953). Regarding the mechanism of fungistasis generation, decades of research have indicated that the combined role of nutritional deficiency and inhibitory factors induces it (Garbeva et al., 2011). Several studies have shown that nutritional competition is related to soil fungistasis (Lockwood, 1964; Mondal and Hyakumachi, 1998; Legrand et al., 2019). In contrast, many inhibitory factors have been identified, including aluminum (Ko and Hora, 1972), ammonia (Ko, 1974), and ethylene (Balis, 1976). Since 2000, many volatile fungistatic compounds originating from soil microorganisms have been reported, including methylamine, trimethylamine, acetamide, and benzaldehyde (Xu et al., 2004; Zou et al., 2007). Production of ammonia by Streptomyces species was shown as a low-cost and long-distance antibiotic strategy (Avalos et al., 2019). Furthermore, there are several indications that soil microbial community structure, activity and diversity are determinants of soil fungistasis (Termorshuizen et al., 2006; Janvier et al., 2007; Rotenberg et al., 2007). Moreover, interactions within the soil microbial community may play a significant role in soil fungistasis (Wietse et al., 2007; Garbeva, 2010). After almost seven decades of research, it is clear that soil microbial activities lie at the heart of fungistasis, and these activities result in fungal nutrient deficiency or accumulation of fungistatic compounds (Garbeva et al., 2011; Li et al., 2020).
Although the causes of soil fungistasis are almost clear after decades of research, the molecular mechanisms underlying how soil fungistasis repress the germination and growth of fungi, and how fungi respond to soil fungistasis have not been elucidated. These issues are important to optimize practical applications of biocontrol fungi against RKN. Quantitative proteomics revealed proteomic changes in the conidia of the nematode-trapping fungus Arthrobotrys oligospora in response to two fungistatic factors, ammonia (Liu et al., 2018) and benzaldehyde (Liu et al., 2019). The functions of these differentially regulated proteins in response to ammonia or benzaldehyde are yet to be fully determined.
Among these proteins, lactoylglutathione lyase (EC:4.4.1.5), encoded by AOL_s00004g335, was upregulated more than two folds under fungistatic stress induced by ammonia or benzaldehyde. Lactoylglutathione lyase belongs to the glyoxalase system, which is a detoxification system of methylglyoxal (MG). MG is a by-product of glycolysis and a highly reactive substance with strong oxidant and glycosylation properties (Leoncini et al., 1989; Nomura et al., 2010; Bankapalli et al., 2015). The accumulated MG can react with proteins, DNA and other biomolecules, leading to irreversible structural damage and loss of function (Inoue and Kimura, 1995; Du et al., 2006; Shaheen et al., 2014). Therefore, the elimination of MG by the glyoxalase system is essential (Toth et al., 2014). It has been reported that MG concentration in various plant species increases 2 to 6 folds in response to salinity, drought, and cold. The accumulation of MG results in the inhibition of seed germination, and the lactoylglutathione lyase plays an important role in maintaining MG levels in plants under normal and abiotic stress conditions (Yadav et al., 2005). In this study, we aimed to reveal the function of MG and lactoylglutathione lyase in soil fungistasis.
Materials and Methods
Strains and Vectors
The nematode-trapping fungus Arthrobotrys oligospora ATCC24927 was purchased from the American Type Culture Collection and maintained on corn meal agar (CMA) plate at 4°C (Yang et al., 2013). Its genome, containing a 40.07-Mb assembled sequence, was reported by our lab in 2011 (Yang et al., 2011). Plasmid pCSN44 was stored in Escherichia coli strain DH5а, and used to amplify the hygromycin B resistance gene hph, which is a selection marker for a gene knockout (Jiang et al., 2017; Zhang et al., 2019). Plasmid pRS426 was used as a backbone plasmid for constructing a gene knockout plasmid, and Saccharomyces cerevisiae FY834, a uracil auxotrophic strain, was used as a host strain for recombinational cloning procedures (Xie et al., 2019; Xie et al., 2020).
AOL_s00004g335 Knockout Vector Construction
The disruption vector of AOL_s00004g335 was constructed using a modified yeast cloning procedure (Li et al., 2019; Wang et al., 2019). The 2183 bp upstream homologous fragment and the 1956-bp downstream homologous fragment of AOL_s00004g335 were amplified via PCR using primer pairs 335-5f/5r, and 335-3f/3r, respectively ( Supplementary Materials, Table S1 ). The hygromycin cassette was obtained via PCR using plasmid pCSN44 as a template, and the primer pair hphF/hphR was also used. The amplified fragments had 21-bp tails homologous to the hygromycin cassette (hph). The two homologous fragments of AOL_s00004g335 were co-transformed into yeast strain FY834 along with the hph cassette and gapped yeast shuttle vector (pRS426). The endogenous homologous recombination system of yeast created a circular plasmid, and the final disruption vector (pRS426-g335-hph) was recovered via transformation into E. coli. The recombinant plasmid extracted from E. coli DH5a was confirmed as the correct plasmid using PCR and DNA sequencing.
Protoplast-Based Gene Knockout
The A. oligospora ATCC24927 was cultured in 250 ml Erlenmeyer flask containing 150 ml liquid TG (1 % tryptone, and 1 % glucose) medium for 36 h at 28°C, and then the hyphae were collected and used to prepare protoplasts according to a previously described method (Tunlid et al., 1999; Leng et al., 2008; Park et al., 2011). The knockout cassette fragment was amplified with primers 335-5f and 335-3r using plasmid pRS426-g335-hph as a template, and then transformed into A. oligospora following a protoplast-based protocol (Tunlid et al., 1999; Leng et al., 2008). Next, the transformants were selected and confirmed via PCR according to a previously reported method (Liu et al., 2018; Liu et al., 2019), using primers yz-5f and yz-3r ( Supplementary Table S1 ).
Hyphae Growth and Conidia Yield of the Wild-Type and Mutant Strains Under Different Conditions
The hyphae growth of wild-type and mutant strains was measured on 9 cm plates containing PDA, TG, and TYGA solid media at 28°C according to a previously reported method (Li et al., 2019). The radius of colonies was measured every day for 5 days.
To compare the stress response capabilities, the hyphae growth of these strains was also tested on 9 cm plates using TG medium which contained the following: 0.01% and 0.02% SDS; 0.1, 0.2, and 0.3 M NaCl; 5, 10, and 15 mM H2O2; 1, 2, and 4μl MG solution (~40% in water, Sigma-Aldrich, USA) per 5 mL TG medium. The hyphal growth was evaluated by measuring the radius of the colonies for 5-7 days.
The sporulation capacity of these strains was analyzed according to the following method. Hypha plugs (0.5 cm in diameter) from the wild-type and mutant strains were inoculated at the center of Corn Meat Agar medium in 250 mL Erlenmeyer flasks and cultured at 28°C for 14 days. Then, 30 ml of sterile water and properly sterilized glass beads were added into the Erlenmeyer flask and shaken slightly. After that, 30 ml water-containing conidia was filtered to remove the hyphae debris using six layers of lens paper, and 10 μl of conidia suspension was used to count the number of conidia using a hemocytometer.
Three replicates of all the experiments described above were performed.
Comparison of Conidial Germination Rates Between the Wild-Type and Mutant Strains Under Normal or Methylglyoxal Suppression Conditions
The conidia of the wild-type and mutant strains were collected according to the method mentioned above. The conidia suspension was then cultured in 100 mL Erlenmeyer flasks containing deionized water, placed in constant temperature shaker, and shook at 160 rpm at 28°C. Spore germination rates were observed microscopically after 0, 4, 8, and 12 h according to described method (Liu et al., 2018).
To test the conidial germination rate under methylglyoxal (MG) suppression, 100 μl conidia suspension of wild-type or mutant strains were spread on 2% water agar (WA) plates supplemented with MG. Precisely 5 ml of WA medium, containing 2, 3, 4, 5, and 6 μl of MG solution (~40%, Sigma-Aldrich®), was prepared. The plates were sealed with two layers of parafilm (Solarbio, USA), and cultured at 28°C for 24 h. Then, conidial germination rates were detected.
Three replicates of all the experiments described above were performed.
Mutant Strain Resistance to Fungistatic Stress of Ammonia or Benzaldehyde
Fungistasis was often quantified based on spore germination via microscopic observation (Lockwood, 1977), and the ability of mutant strains to resist the fungistatic stress of ammonia or benzaldehyde was quantified by detecting the conidial germination rates. Precisely 5 ml of WA medium was added to one side of a two-compartment Petri dish (9cm in diameter), and 100 μl of conidial suspension was spread on the WA medium. Exactly 6, 6.5, 7, 8, and 9 μl of ammonia water (25-28%, Guanghua Sci-Tech Co., Ltd., China) or 0.4, 0.6, 0.8, and 1.0 μl of benzaldehyde, respectively, was dropped on the sterilized cotton on the other side of the Petri dish. After sealing with parafilm, the plates were cultured in incubator (Tiancheng experimental instrument, Shanghai, China) at 20°C for 24 h, and then conidial germination rates were detected. Three replicates were performed.
Evaluating the Effect of MG on the Fungistatic Role of Ammonia or Benzaldehyde
To evaluate the effect of MG on the fungistatic role of ammonia or benzaldehyde, 5 mL of WA medium, containing different volumes of MG solution, was added to one side of a two-compartment Petri dish. Three replicates were set. Other procedures were the same as the method mentioned above. The conidial germination rate of wild-type and mutant strains was detected after 24 h.
Wild-type and Mutant Strain Resistance to Soil Fungistasis
According to the reported method (Liu et al., 2020), soil and deionized water were added to a beaker at a mass ratio of 2.5:1, 1:1, and 1:2.5, and were mixed well to produce a high fungistatic soil suspension, a medium fungistatic soil suspension, and a low fungistatic soil suspension, respectively. The fresh conidia of wild-type and mutant strains were transferred into a dialysis bag (300 kDa; Spectrum, USA), and the dialysis bag was then placed into three kinds of soil suspension at 28°C, with the agitation of the soil suspension using a magnetic stirrer, as described previously (Liu et al., 2020). After 24 h, the dialysis bags were removed from the soil suspension, and the conidia germination rate was tested microscopically.
In addition, to test the ability of exogenous MG in inducing A. oligospora resistance to soil fungistasis, 1 ml MG was added to 3 ml of conidia suspension, and incubated at 28°C for 5, 10, 15, 20, 25, and 30 min. Then, MG was removed by centrifugation, and the conidia were collected and resuspended in 3 ml sterilized water. Finally, the conidia suspension was transferred into a dialysis bag, placed into a soil suspension, and used to detect the conidia germination rate as described above.
Three replicates of all the experiments were performed in this section.
Statistical Analyses
All the statistical analyses were performed using GraphPad Prism 8.0.1 (GraphPad Software Inc., San Diego, CA, USA). Multiple t test of this software was used to analyze the germination rates among different samples.
Results
Verification of Gene Knockout Mutants
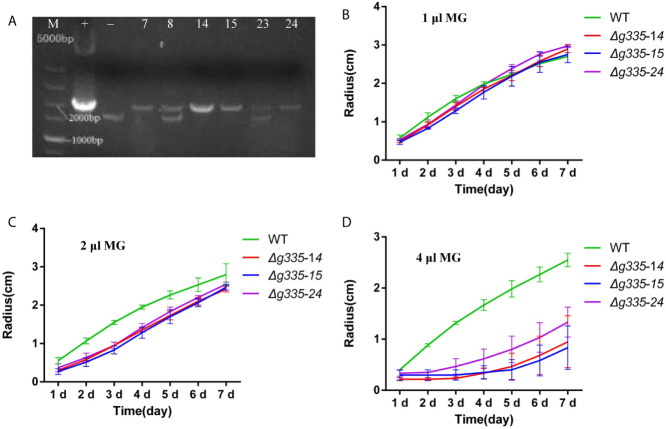
To study the function of lactoylglutathione lyase and MG in response to fungistatic stress, the AOL_s00004g335, encoding lactoylglutathione lyase, was knocked out. The genomic DNA of hygromycin-resistant transformants was isolated and used as templates for PCR verification of knockout mutants, using the genomic DNA of the wild-type strain as a negative control and the plasmid pRS426-g335-hph as a positive control. The transformant, which had a 2018-bp amplified fragment similar to the positive control, was positive, and the transformants with 1638-bp and 2018-bp amplified fragments were negative. Three positive transformants (g335-14, g335-15, and g335-24) were obtained ( Figure 1A ). Further verification of the knockout mutants was performed by measuring their hyphal growth on the TG medium containing different amounts of MG. On TG medium containing 1 μl MG solution per 5 ml medium, the hyphal growth of three transformants (Δg335-14, Δg335-15, and Δg335-24) were almost the same as that of the wild-type strain ( Figure 1B ). However, when MG concentration in the TG medium increased, the hyphal growth of the three transformants was severely impaired ( Figures 1C, D ). This result suggested that the resistance of mutants to MG was reduced and indirectly verified that the strains Δg335-14, Δg335-15, and Δg335-24 were positive transformants.
Figure 1.
Verification of AOL_s00004g335 knock-out mutants. (A) PCR verification of knock-out mutants. +, positive control. -, negative control. M, DNA marker. No. 14, 15, and 24 are positive transformants. (B–D) Verification of the knock-out mutants by measuring the hyphal growth on the TG medium containing different amounts of MG. 1, 2, 4 μl of MG solutions per 5 ml medium were used in (B–D), respectively. WT, wild-type strain. Δg335-14, Δg335-15, and Δg335-24: AOL_s00004g335 knock-out mutants.
The Effect of AOL_s00004g335 Deletion on Hyphal Growth and Stress Resistance
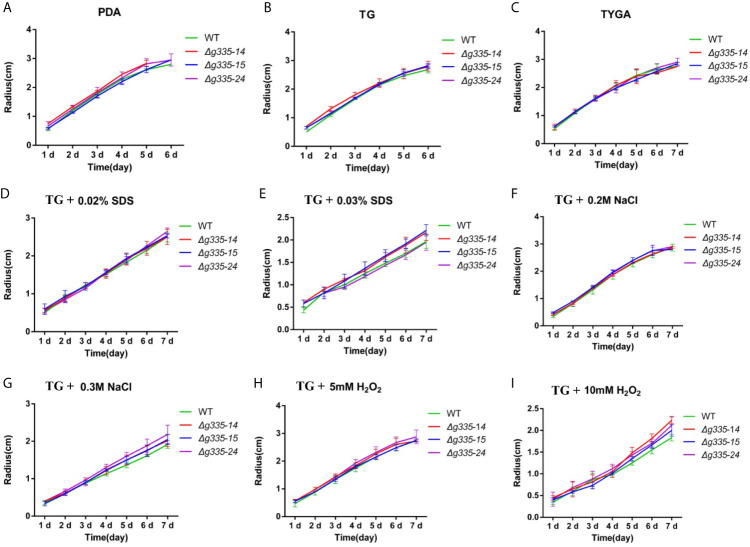
To examine whether the disruption of lactoylglutathione lyase influenced the growth of A. oligospora, the hyphal growth of wild-type and mutant strains on PDA, TG, and TYGA media was determined ( Figures 2A–C ). Compared with the wild-type strain, the three mutant strains had almost the same growth rate, indicating that AOL_s00004g335 deletion had no obvious influence on hyphal growth. In addition, there was no notable difference in the sporulation capacity between these mutants and the wild-type strain (data not shown).
Figure 2.
Measurement of the hyphal growth of mutants under normal or stressful condition. (A–C) The hyphal growth rates of mutant and wild-type strain on PDA, TG, and TYGA media. (D, E) The hyphal growth rates of mutant and wild-type strain on TG medium containing 0.02% (D), and 0.03% (E) sodium dodecyl sulfate (SDS). (F, G) The hyphal growth rates of mutant and wild-type strain on TG medium containing 0.2M (F), and 0.3M (G) sodium chloride (NaCl). (H, I) The hyphal growth rates of mutant and wild-type strain on TG medium containing 5mM (H), and 10 mM (Fig. I) hydrogen peroxide (H2O2). WT, wild-type strain. Δg335-14, Δg335-15, and Δg335-24: AOL_s00004g335 knock-out mutants.
The growth rates of wild-type and mutant strains were also compared on the TG medium with different stress factors, including NaCl, SDS, and H2O2. The mutant strains exhibited the same growth rate as wild-type on the TG medium containing a low concentrations of NaCl (0.2 M), SDS (0.02%), and H2O2 (5 mM) ( Figures 2D, F, H ), but the growth rates of the mutant strains were slightly faster than those of the wild-type on the TG medium containing high concentrations of NaCl (0.3 M), SDS (0.03%), and H2O2 (10 mM) ( Figures 2E, G, I ). These results indicated that the mutant strains had relatively higher stress resistance to some extent.
AOL_s00004g335 Deletion Increases Ammonia Resistance of Mutant Strains Significantly
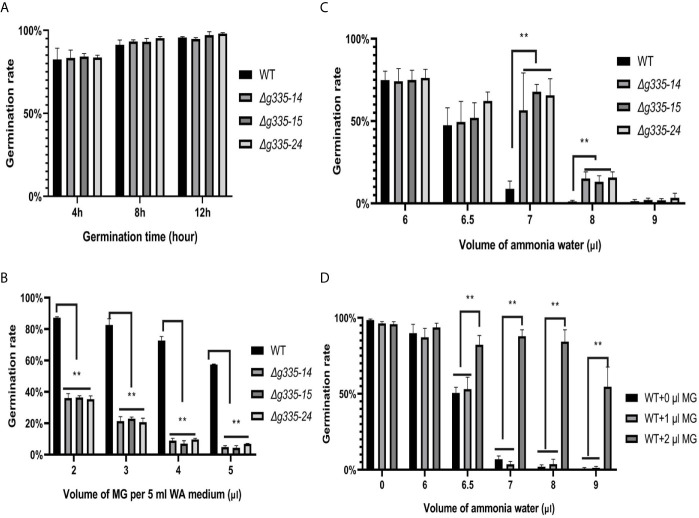
Compared with the wild-type strain, the conidial germination rates of A. oligospora on WA medium after 4, 8, and 12 h were not influenced by the deletion of AOL_s00004g335 ( Figure 3A ). However, the germination rates of mutant strains at 24 h on the WA medium containing MG decreased severely (less than 40%) even when only 2 μl 40% MG solution was added. Under the same condition, the germination rate of wild-type strain was more than 80% ( Figure 3B ). These results suggested that the lactoylglutathione lyase encoded by the AOL_s00004g335 was very important for A. oligospora to detoxify MG.
Figure 3.
Measurement of ammonia resistance of mutant strains on WA medium. (A) Determination of conidial germination rates of mutant and wild-type strains at 4, 8, and 12 hours. (B) Determination of conidial germination rates (24 h) of mutant and wild-type strains on WA medium containing different volume of MG. (C) Determination of the fungistatic effect of ammonia on the conidial germination rates (24h) of mutant and wild-type strain. (D) Exogenous MG relieved the fungistatic inhibition of ammonia on the conidial germination of wild-type strain. WT, wild-type strain. Δg335-14, Δg335-15, and Δg335-24: AOL_s00004g335 knock-out mutants. **P < 0.01.
The germination rates of mutant and wild-type strains were also determined and compared under the fungistatic stress of ammonia. As shown in Figure 3C , the ammonia resistance of the mutant strains increased significantly (p<0.01). The germination rate of the wild-type strain was less than 10% under the fungistatic stress of 7 μl ammonia water, which was far less than those of the mutant strains (56-67%). Even under the fungistatic stress of 8 μl ammonia water, there was still ~15% of conidia of mutant strains that could germinate. The deletion of AOL_s00004g335 might result in the accumulation of MG, and we wondered whether the accumulated MG improved ammonia resistance of the mutant strains. Therefore, different amounts of 40% MG solution were added into the WA medium, and germination rates of the wild-type strain were determined under the fungistatic stress of ammonia ( Figure 3D ). Surely, 2 μl MG solution significantly improved the germination rates of A. oligospora under the fungistatic stress of 6.5, 7, 8, and 9 μl ammonia water.
AOL_s00004g335 Deletion Weakens the Benzaldehyde Resistance of Mutant Strains
The above results showed that deletion of AOL_s00004g335 increased the resistance of mutant strains against SDS, NaCl, H2O2, and the fungistatic factor ammonia. In order to evaluate the resistance of mutant strains against another fungistatic factor, we tested the benzaldehyde resistance of mutant strains by observing the conidial germination rate. As shown in Figure 4A , conidial germination rates of AOL_s00004g335 knockout mutant strains were significantly (p <0.01) lower than those of the wild-type strain under the fungistatic stress of 0.2, 0.4, and 0.6 μl benzaldehyde. Besides, 1 or 2 μl of MG had almost no inhibitory effect on the conidial germination of the wild-type strain when benzaldehyde was absent, but it increased the inhibition effect of benzaldehyde ( Figure 4B ). Approximately 60% and 40% of wild-type conidia germinated in the presence of 0.2 and 0.4μl benzaldehyde, respectively; furthermore, the addition of 1 or 2 μl of MG resulted in significantly (p <0.01) lower conidial germination rates.
Figure 4.
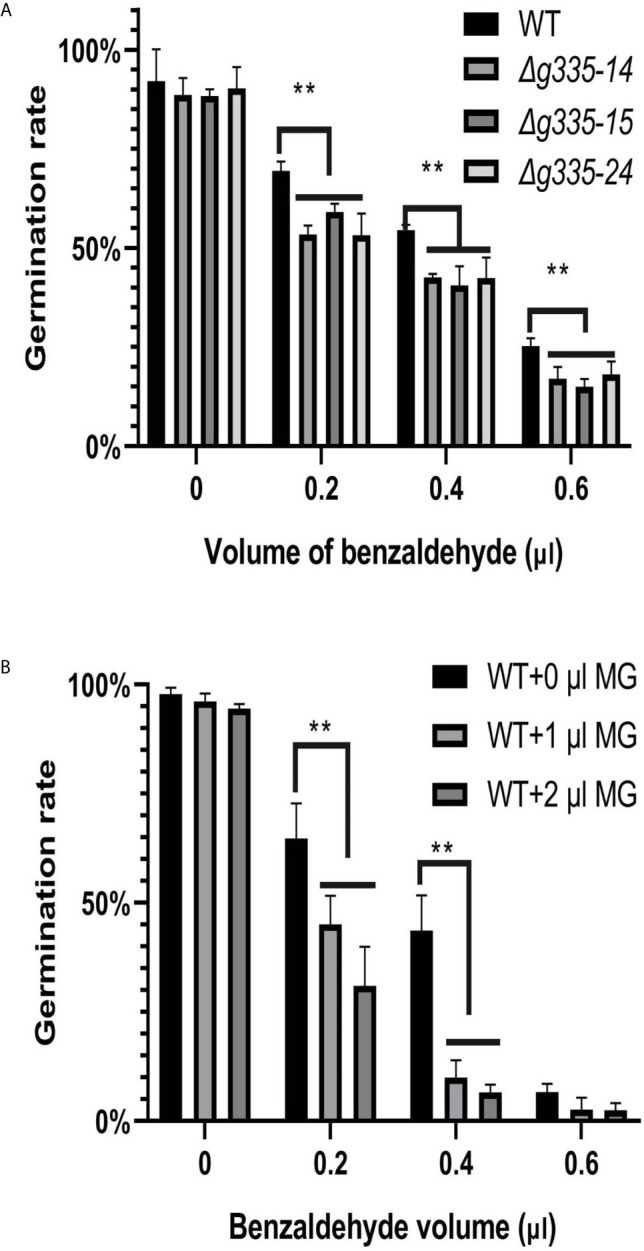
Measurement of benzaldehyde resistance of mutant strains on WA medium. (A) Determination of conidial germination rates (24 h) of mutant and wild-type strains on WA medium containing different volume of benzaldehyde. (B) Exogenous MG increased the fungistatic inhibition of benzaldehyde on conidial germination of wild-type strain. WT: wild-type strain. Δg335-14, Δg335-15, and Δg335-24: AOL_s00004g335 knock-out mutants. **P < 0.01.
AOL_s00004g335 Is Important for A. oligospora Resistance to Soil Fungistasis
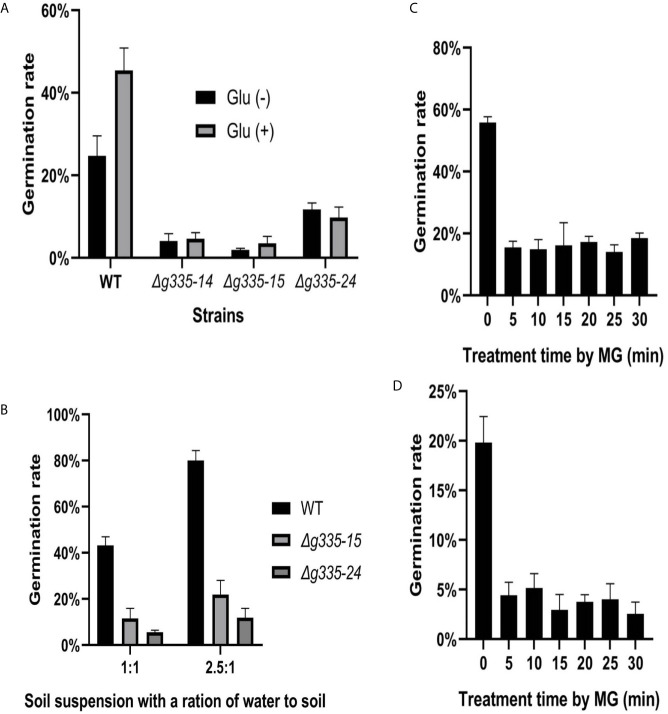
The deletion of AOL_s00004g335 resulted in different responses of A. oligospora to fungistatic factors ammonia and benzaldehyde. The function of this gene in response to soil fungistasis was further studied. Compared to that of the wild-type strain, the conidial germination rate of the three mutant strains (Δg335-14, Δg335-15, and Δg335-24) decreased significantly in the high fungistatic soil suspension ( Figure 5A ), and the relief effect of soil fungistasis by glucose observed in the wild-type strain disappeared in the mutant strains. In addition, the conidial germination of mutant strains was worse than that of the wild-type strain in soil suspensions with less fungistatic intensity ( Figure 5B ), especially in low fungistatic soil suspension. This soil suspension had a little fungistatic effect on wild-type conidia, which had a germination rate of ~80%, but the mutant strains had a germination rate of less than 20%. These results suggested that lactoylglutathione lyase encoded by AOL_s00004g335 is important for A. oligospora resistance to soil fungistasis.
Figure 5.
The function determination of AOL_s00004g335 in resisting soil fungistasis. (A) The conidial germination rates (24 h) of mutant and wild type strains were determined in high fungistatic soil suspension (glu-), and the relief effect of conidial germination rates by glucose was compared in high fungistatic soil suspension (glu+). (B) Determine the conidial germination rates (24h) of mutant and wild type strains in medium fungistatic soil suspension (1:1), or low fungistatic soil suspension (2.5:1). (C, D) The conidia of wild type strain were treated by MG for different time, then placed in high fungistatic soil suspension (C), or medium fungistatic soil suspension (D) for 24h, and determine the conidial germination rate. WT, wild-type strain. Δg335-14, Δg335-15, and Δg335-24: AOL_s00004g335 knock-out mutants. Glu(-), conidia suspension without glucose. Glu(+), conidia suspension with glucose.
We wondered whether exogenous MG can induce the resistance of A. oligospora to soil fungistasis. Hence, the conidia of A. oligospora were treated with approximately 2.17 μM MG for 5 to 30 min, and the conidial germination rates were detected in high, and medium fungistatic soil suspensions ( Figures 5C, D ). The results showed that none of the treatments improved the resistance of A. oligospora to soil fungistasis; conversely, all treatments significantly reduced conidial germination rates (p <0.01).
Discussion
Here, we aimed to reveal the function of MG and lactoylglutathione lyase in soil fungistasis and found that that MG had contrary effects on the fungistatic roles of ammonia and benzaldehyde. MG relieved the fungistatic role of ammonia, but increased the fungistatic role of benzaldehyde. Moreover, lactoylglutathione lyase is necessary for A. oligospora to resist soil fungistasis.
Resistance to soil fungistasis is very important for RKN biocontrol fungi to exert their control effect; however, there is little known about this. Based on proteomics data, it was found that lactoylglutathione lyase was upregulated more than two folds under fungistatic stress induced by ammonia or benzaldehyde. Lactoylglutathione lyase belongs to the glyoxalase system and detoxifies MG. Our results showed that lactoylglutathione lyase was the main detoxification enzyme of MG, and its deletion had no negative effect on the growth of A. oligospora on PDA, TG, and TYGA; furthermore, the mutant strains had even higher resistance to NaCl, SDS, H2O2 ( Figure 2 ), and ammonia ( Figure 3C ). This might be due to the signaling function of MG that might accumulate in mutant strains after the deletion of lactoylglutathione lyase. It was reported that MG concentration varies between 30–75 μM in various plant species and it increases 2 to 6 folds in response to salinity, drought, and cold stress conditions (Yadav et al., 2005), and it was considered as a signal molecule, which can improve the resistance of plants to abiotic stress by activating the glyoxalase system and oxidation-antioxidation system (Bless et al., 2017; Li et al., 2017a; Li et al., 2017b; Majláth et al., 2020). Moreover, our results were consistent with the results of these studies.
Surprisingly, exogenous MG significantly increased the resistance of A. oligospora to fungistasis by ammonia. No report suggests that MG can react with ammonia directly, but MG may well be transformed into other compounds in vivo, mainly lactate (Ghosh et al., 2016; Bari et al., 2019), which can react with ammonia. In addition, the induced resistance of A. oligospora to ammonia by exogenous MG might exist because 1 μl of MG hardly increased the resistance of A. oligospora to ammonia ( Figure 3D ). However, the resistance supplied by MG was not always effective; for example, MG increased the fungistatic role of benzaldehyde, and there was a synergistic effect between benzaldehyde and MG ( Figure 4 ).
The fungistatic role of benzaldehyde can partially attribute to the cellular toxicity of MG. In our recent research on A. oligospora ATCC24927, we found that lactoylglutathione lyase was upregulated more than two folds under the fungistatic stress induced by ammonia or benzaldehyde (Liu et al., 2018; Liu et al., 2019). Although the obvious accumulation of MG in conidia was not detected using high-performance liquid chromatography under these two fungistatic stresses (Data not shown), the upregulation of lactoylglutathione lyase still suggested the accumulation of MG (Zuin et al., 2005). On the one hand, 2 μl of MG (about 2.6 μM) inhibited the conidial germination of mutant strains significantly ( Figure 3B ), it means the concentration of MG in conidia is far lower than that in the plant. And on the other hand, MG is easy to associate with proteins and DNA (Inoue and Kimura, 1995; Shaheen et al., 2014; Toth et al., 2014); therefore, most of the produced MG in conidia may not be dissociated and detected.
Although the proteomics data shown that lactoylglutathione lyase was upregulated more than two folds under fungistatic stress induced by ammonia or benzaldehyde, it is difficult to evaluate enzyme activity of lactoylglutathione lyase in conidia. There are four MG degradation pathways in eucaryotic cell (Mostofa et al., 2018), lactate is the degradation product in three of the pathways, including the lactoylglutathione lyase pathway. And lactate can also be produced by glycolytic pathway. what’s more, MG is a high reactive molecule which can react with proteins and DNA. So, it difficult to evaluate enzyme activity by measuring the consumption of MG or production of lactate. Besides, after the deletion of AOL_s00004g335, compensatory degradation of MG by other pathways would increase. Under fungistatic stress induced by ammonia or benzaldehyde, upregulation of lactoylglutathione lyase could help conidia limit MG to low concentration and avoid the toxicity of high concentration MG.
This study also suggested that MG might be involved in the fungistatic role of soil, and the detoxification of MG is important for A. oligospora to resist soil fungistasis. Compared to benzaldehyde, the lactoylglutathione lyase played more important role in soil fungistasis. Although MG can also activate the stress response in fungi (Zuin et al., 2005; Takatsume et al., 2006), compared to a single fungistatic factor, soil is a very complex fungistatic environment containing many fungistatic factors, the stress response induced by exogenous MG (2.17μM) was not enough for A. oligospora to resist soil fungistasis; in contrast, it enhanced the fungistatic role of soil ( Figures 5C, D ).
This study provides an insight into the participation of MG in the fungistatic role of several factors, including soil, and enriched our understanding of stress response to soil fungistasis in the nematode-trapping fungi.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author ContributionS
Authors XL, N-MH, and L-XT contributed equally to this work. XL and N-MH carried out the main experiments. L-XT mainly drafted the manuscript. M-HM and TL designed the study, analyzed the data, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was financed by the National Natural Science Foundation Program of China (31960022 and 31870091), the Department of Science and Technology of Yunnan Province (202001BB050057 and 2019ZG00901), and the Ten-thousands Talents Program in Yunnan Province (YNWR-CYJS-2019042 and YNWR-QNBJ-2018153). The authors declare that this research was also financed by China Tobacco Yunnan Industrial Co. Ltd. (2019530000241018). The funder had no involvement with the study.
Conflict of Interest
Author Z-YL was employed by Puer Corporation of Yunnan Tobacco Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Editage for linguistic assistance (www.editage.cn) during the preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.640823/full#supplementary-material
References
- Abad P., Gouzy J., Aury J. M., Castagnone-Sereno P., Danchin E. G., Deleury E., et al. (2008). Genome Sequence of the Metazoan Plant-Parasitic Nematode Meloidogyne Incognita . Nat. Biotechnol. 26, 909–915. 10.1038/nbt.1482 [DOI] [PubMed] [Google Scholar]
- Atkins S. D., Hidalgo-Diaz L., Kalisz H., Mauchline T. H., Hirsch P. R., Kerry B. R. (2003). Development of a New Management Strategy for the Control of Root-Knot Nematodes (Meloidogyne Spp) in Organic Vegetable Production. Pest Manage. Sci. 59, 183–189. 10.1002/ps.603 [DOI] [PubMed] [Google Scholar]
- Avalos M., Garbeva P., Raaijmakers J. M., Wezel G. (2019). Production of Ammonia as a Low-Cost and Long-Distance Antibiotic Strategy by Streptomyces Species. ISME J. 14, 1–15. 10.1038/s41396-019-0537-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balis C. (1976). Ethylene-Induced Volatile Inhibitors Causing Soil Fungistasis. Nature 259, 112–114. 10.1038/259112a0 [DOI] [Google Scholar]
- Bankapalli K., Saladi S., Awadia S. S., Goswami A. V., Samaddar M., D’Silva P. (2015). Robust Glyoxalase Activity of Hsp31, a ThiJ/DJ-1/PfpI Family Member Protein, is Critical for Oxidative Stress Resistance in Saccharomyces Cerevisiae . J. Biol. Chem. 290, 26491–26507. 10.1074/jbc.M115.673624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari L. D., Atlante A., Armeni T., Kalapos M. P. (2019). Synthesis and Metabolism of Methylglyoxal, S-D-lactoylglutathione and D-lactate in Cancer and Alzheimer’s Disease. Exploring the Crossroad of Eternal Youth and Premature Aging. Ageing Res. Rev. 53, 100915. 10.1016/j.arr.2019.100915 [DOI] [PubMed] [Google Scholar]
- Basso M. F., Lourenço-Tessutti I. T., Mendes R., Pinto C., Bournaud C., Gillet F. X., et al. (2020). MiDaf16-like and MiSkn1-like Gene Families are Reliable Targets to Develop Biotechnological Tools for the Control and Management of Meloidogyne Incognita . Sci. Rep. 10, 6991. 10.1038/s41598-020-63968-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bless Y., Ndlovu L., Gokul A., Keyster M. (2017). Exogenous Methylglyoxal Alleviates Zirconium Toxicity in Brassica Rapa L. Seedling Shoots. S. Afr. J. Bot. 109, 327. 10.1016/j.sajb.2017.01.030 [DOI] [Google Scholar]
- Chen J., Li Q. X., Song B. (2020). Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 68, 12175–12188. 10.1021/acs.jafc.0c02871 [DOI] [PubMed] [Google Scholar]
- Coyne D. L., Laura C., Dalzell J. J., Claudius-Cole A. O., Solveig H., Nessie L., et al. (2018). Plant-parasitic Nematodes and Food Security in Sub-Saharan Africa. Annu. Rev. Phytopathol. 56, 381–403. 10.1146/annurev-phyto-080417-045833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs C. G., Hinson W. H. (1953). A Widespread Fungistasis in Soils. Nature 172, 197–199. 10.1038/172197a0 [DOI] [PubMed] [Google Scholar]
- Du J., Zeng J., Ou X., Ren X., Cai S. (2006). Methylglyoxal Downregulates Raf-1 Protein Through a Ubiquitination-Mediated Mechanism. Int. J. Biochem. Cell Biol. 38, 1084–1091. 10.1016/j.biocel.2005.10.019 [DOI] [PubMed] [Google Scholar]
- Garbeva P. (2010). Inter-Specific Interactions Between Carbon-Limited Soil Bacteria Affect Behavior and Gene Expression. Microb. Ecol. 58, 36–46. 10.1007/s00248-009-9502-3 [DOI] [PubMed] [Google Scholar]
- Garbeva P., Hol W. H. G., Termorshuizen A. J., Kowalchuk G. A., De Boer W. (2011). Fungistasis and General Soil Biostasis - A New Synthesis. Soil Biol. Biochem. 43, 469–477. 10.1016/j.soilbio.2010.11.020 [DOI] [Google Scholar]
- Ghosh R., Andersen E. C., Shapiro J. A., Gerke J. P., Kruglyak L. (2012). Natural Variation in a Chloride Channel Subunit Confers Avermectin Resistance in C. Elegans . Science 335, 574–578. 10.1126/science.1214318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Kushwaha H. R., Hasan M. R., Pareek A., Sopory S. K., Singla-Pareek S. L. (2016). Presence of Unique Glyoxalase III Proteins in Plants Indicates the Existence of Shorter Route for Methylglyoxal Detoxification. Sci. Rep. 6, 18358. 10.1038/srep18358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Kimura A. (1995). Methylglyoxal and Regulation of its Metabolism in Microorganisms. Adv. Microb. Physiol. 37, 177–227. 10.1016/S0065-2911(08)60146-0 [DOI] [PubMed] [Google Scholar]
- Janvier C., Villeneuve F., Alabouvette C., Edel-Hermann V., Mateille T., Steinberg C. (2007). Soil Health Through Soil Disease Suppression: Which Strategy From Descriptors to Indicators? Soil Biol. Biochem. 39, 1–23. 10.1016/j.soilbio.2006.07.001 [DOI] [Google Scholar]
- Jiang D., Zhou J., Bai G., Xing X., Tang L., Yang X., et al. (2017). Random Mutagenesis Analysis and Identification of a Novel C(2)H(2)-type Transcription Factor From the Nematode-Trapping Fungus Arthrobotrys Oligospora . Sci. Rep. 7, 5640. 10.1038/s41598-017-06075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W. H. (1974). Isolation and Identification of a Volatile Fungistatic Substance From Alkaline Soil. Phytopathology 64, 1398. 10.1094/Phyto-64-1398 [DOI] [Google Scholar]
- Ko W. H., Hora K. F. (1972). Identification of an Al Ion as a Soil Fungitoxin. Soil Sci. 113, 42–45. 10.1097/00010694-197201000-00008 [DOI] [Google Scholar]
- Legrand F., Chen W., Cobo-Diaz J. F., Picot A., Floch G. L. (2019). Co-Occurrence Analysis Reveal That Biotic and Abiotic Factors Influence Soil Fungistasis Against Fusarium Graminearum . FEMS Microbiol. Ecol. 95, fiz056. 10.1093/femsec/fiz056 [DOI] [PubMed] [Google Scholar]
- Leng W. C., Liu T., Li R., Yang J., Wei C. D., Zhang W. L., et al. (2008). Proteomic Profile of Dormant Trichophyton Rubrum Conidia. BMC Genomics 9, 303. 10.1186/1471-2164-9-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini G., Maresca M., Buzzi E. (1989). Inhibition of the Glycolytic Pathway by Methylglyoxal in Human Platelets. Cell Biochem. Funct. 7, 65–70. 10.1002/cbf.290070111 [DOI] [PubMed] [Google Scholar]
- Li Z. G., Duan X. Q., Min X., Zhou Z. H. (2017. a). Methylglyoxal as a Novel Signal Molecule Induces the Salt Tolerance of Wheat by Regulating the Glyoxalase System, the Antioxidant System, and Osmolytes. Protoplasma 254, 1995–2006. 10.1007/s00709-017-1094-z [DOI] [PubMed] [Google Scholar]
- Li Z. G., Duan X. Q., Xia Y. M., Wang Y., Zhou Z. H., Min X. (2017. b). Methylglyoxal Alleviates Cadmium Toxicity in Wheat (Triticum Aestivum L). Plant Cell Rep. 36, 367–370. 10.1007/s00299-016-2070-3 [DOI] [PubMed] [Google Scholar]
- Li X., Garbeva P., Liu X., Klein Gunnewiek P. J. A., Clocchiatti A., Hundscheid M. P. J., et al. (2020). Volatile-Mediated Antagonism of Soil Bacterial Communities Against Fungi. Environ. Microbiol. 22, 1025–1035. 10.1111/1462-2920.14808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Huang Y., Chen X. X., Long X., Yang Y. H., Zhu M. L., et al. (2020). Comparative Transcriptomics Reveals Features and Possible Mechanisms of Glucose-Mediated Soil Fungistasis Relief in Arthrobotrys Oligospora . Front. Microbiol. 10, 3143. 10.3389/fmicb.2019.03143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Tian D. W., Zou L. J., Liu F. Y., Can Q. Y., Yang J. K., et al. (2018). Quantitative Proteomics Revealed Partial Fungistatic Mechanism of Ammonia Against Conidial Germination of Nematode-Trapping Fungus Arthrobotrys Oligospora ATCC24927. Int. J. Biochem. Cell Biol. 98, 104–112. 10.1016/j.biocel.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Liu T., Zou L. J., Tian D. W., Can Q. Y., Zhu M. L., Mo M. H., et al. (2019). Proteomic Changes in Arthrobotrys Oligospora Conidia in Response to Benzaldehyde-Induced Fungistatic Stress. J. Proteomics 192, 358–365. 10.1016/j.jprot.2018.09.016 [DOI] [PubMed] [Google Scholar]
- Li J., Wu R., Wang M., Borneman J., Yang J., Zhang K. Q. (2019). The Ph Sensing Receptor AopalH Plays Important Roles in the Nematophagous Fungus Arthrobotrys Oligospora . Fungal Biol. 123, 547–554. 10.1016/j.funbio.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Li J., Zou C., Xu J., Ji X., Niu X., Yang J., et al. (2015). Molecular Mechanisms of Nematode-Nematophagous Microbe Interactions: Basis for Biological Control of Plant-Parasitic Nematodes. Annu. Rev. Phytopathol. 53, 67–95. 10.1146/annurev-phyto-080614-120336 [DOI] [PubMed] [Google Scholar]
- Lockwood J. L. (1964). Soil Fungistasis. Annu. Rev. Phytopathol. 2, 341–362. 10.1146/annurev.py.02.090164.002013 [DOI] [Google Scholar]
- Lockwood J. L. (1977). Fungistasis in Soils. Biol. Rev. 52, 1–43. 10.1111/j.1469-185X.1977.tb01344.x [DOI] [Google Scholar]
- Majláth I., Éva C., Tajti J., Khalil R., Elsayed N., Darko E., et al. (2020). Exogenous Methylglyoxal Enhances the Reactive Aldehyde Detoxification Capability and Frost-Hardiness of Wheat. Plant Physiol. Biochem. 149, 75–85. 10.1016/j.plaphy.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Mao L., Jiang H., Zhang L., Zhang Y., Sial M. U., Yu H., et al. (2017). Replacing Methyl Bromide With a Combination of 1,3-Dichloropropene and Metam Sodium for Cucumber Production in China. PloS One 12, e0188137. 10.1371/journal.pone.0188137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S. N., Hyakumachi M. (1998). Carbon Loss and Germinability, Viability, and Virulence of Chlamydospores of Fusarium Solanif. Sp. Phaseoli After Exposure to Soil At Different Ph Levels, Temperatures, and Matric Potentials. Phytopathology 88, 148–155. 10.1094/PHYTO.1998.88.2.148 [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Ghosh A., Li Z. G., Siddiqui M. N., Tran L. (2018). Methylglyoxal – a Signaling Molecule in Plant Abiotic Stress Responses. Free Radical Biol. Med. 122, 96–109. 10.1016/j.freeradbiomed.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Mo C., Xie C., Wang G., Liu J., Xiao Y. (2019). Genome-Wide Identification and Characterization of the Cyclophilin Gene Family in the Nematophagous Fungus Purpureocillium Lilacinum . Int. J. Mol. Sci. 20, 2978. 10.3390/ijms20122978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura W., Maeta K., Kita K., Izawa S., Inoue Y. (2010). Methylglyoxal Activates Gcn2 to Phosphorylate eIF2alpha Independently of the TOR Pathway in Saccharomyces Cerevisiae . Appl. Microbiol. Biotechnol. 86, 1887–1894. 10.1007/s00253-009-2411-z [DOI] [PubMed] [Google Scholar]
- Park G., Colot H. V., Collopy P. D., Krystofova S., Crew C., Ringelberg C., et al. (2011). High-Throughput Production of Gene Replacement Mutants in Neurospora Crassa . Methods Mol. Biol. 722, 179–189. 10.1007/978-1-61779-040-9_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga E. (2008). Muscodor Albus, a Potential Biocontrol Agent Against Plant-Parasitic Nematodes of Economically Important Vegetable Crops in Washington State, USA. Biol. Control 45, 380–385. 10.1016/j.biocontrol.2008.01.002 [DOI] [Google Scholar]
- Rotenberg D., Wells A. J., Chapman E. J., Whitfield A. E., Goodman R. M., Cooperband L. R. (2007). Soil Properties Associated With Organic Matter-Mediated Suppression of Bean Root Rot in Field Soil Amended With Fresh and Composted Paper Mill Residuals. Soil Biol. Biochem. 39, 2936–2948. 10.1016/j.soilbio.2007.06.011 [DOI] [Google Scholar]
- Shaheen F., Shmygol A., Rabbani N., Thornalley P. J. (2014). A Fluorogenic Assay for Methylglyoxal. Biochem. Soc Trans. 42, 548–555. 10.1042/BST20140028 [DOI] [PubMed] [Google Scholar]
- Sharon E., Bar-Eyal M., Chet I., Herrera-Estrella A., Kleifeld O., Spiegel Y. (2001). Biological Control of the Root-Knot Nematode Meloidogyne Javanica by Trichoderma Harzianum . Phytopathology 91, 687–693. 10.1094/PHYTO.2001.91.7.687 [DOI] [PubMed] [Google Scholar]
- Singh U. B., Sahu A., Sahu N., Singh R. K., Renu S., Singh D. P., et al. (2013). Arthrobotrys Oligospora-Mediated Biological Control of Diseases of Tomato (Lycopersicon Esculentum Mill.) Caused by Meloidogyne Incognita and Rhizoctonia Solani . J. Appl. Microbiol. 114, 196–208. 10.1111/jam.12009 [DOI] [PubMed] [Google Scholar]
- Singh S., Singh B., Singh A. P. (2015). Nematodes: A Threat to Sustainability of Agriculture. Proc. Environ. Sci. 29, 215–216. 10.1016/j.proenv.2015.07.270 [DOI] [Google Scholar]
- Takatsume Y., Izawa S., Inoue Y. (2006). Methylglyoxal as a Signal Initiator for Activation of the Stress-Activated Protein Kinase Cascade in the Fission Yeast Schizosaccharomyces Pombe . J. Biol. Chem. 281, 9086–9092. 10.1074/jbc.M511037200 [DOI] [PubMed] [Google Scholar]
- Termorshuizen A. J., van Rijn E., van der Gaag D. J., Alabouvette C., Chen Y., Lagerlöf J., et al. (2006). Suppressiveness of 18 Composts Against 7 Pathosystems: Variability in Pathogen Response. Soil Biol. Biochem. 38, 2461–2477. 10.1016/j.soilbio.2006.03.002 [DOI] [Google Scholar]
- Toth A. E., Toth A., Walter F. R., Kiss L., Veszelka S., Ozsvari B., et al. (2014). Compounds Blocking Methylglyoxal-Induced Protein Modification and Brain Endothelial Injury. Arch. Med. Res. 45, 753–764. 10.1016/j.arcmed.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Tunlid A., Aringhman J., Oliver R. P. (1999). Transformation of the Nematode-Trapping Fungus Arthrobotrys Oligospora . FEMS Microbiol. Lett. 173, 111–116. 10.1111/j.1574-6968.1999.tb13491.x [DOI] [PubMed] [Google Scholar]
- Wang J.-A., Huang X., S N., Hu Z., Li H., Ji X., et al. (2019). Thioredoxin1 Regulates Conidia Formation, Hyphal Growth, and Trap Formation in the Nematode-Trapping Fungus Arthrobotrys Oligospora . Ann. Microbiol. 69, 1267–1274. 10.1007/s13213-019-01511-5 [DOI] [Google Scholar]
- Wietse D. B., Wagenaar A. M., Klein Gunnewiek P. J. A., van Veen J. A. (2007). In Vitro Suppression of Fungi Caused by Combinations of Apparently non-Antagonistic Soil Bacteria. FEMS Microbiol. Ecol. 59, 177–185. 10.1111/j.1574-6941.2006.00197.x [DOI] [PubMed] [Google Scholar]
- Wolstenholme A. J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. (2004). Drug resistance in veterinary helminths. Trends Parasitol. 20, 469–476. [DOI] [PubMed] [Google Scholar]
- Xie M., Bai N., Yang J., Jiang K., Zhou D., Zhao Y., et al. (2020). Protein Kinase Ime2 is Required for Mycelial Growth, Conidiation, Osmoregulation, and Pathogenicity in Nematode-Trapping Fungus Arthrobotrys Oligospora . Front. Microbiol. 10, 3065. 10.3389/fmicb.2019.03065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Wang Y., Tang L., Yang L., Zhou D., Li Q., et al. (2019). AoStuA, an APSES Transcription Factor, Regulates the Conidiation, Trap Formation, Stress Resistance and Pathogenicity of the Nematode-Trapping Fungus Arthrobotrys Oligospora . Environ. Microbiol. 21, 4648–4661. 10.1111/1462-2920.14785 [DOI] [PubMed] [Google Scholar]
- Xu C., Minghe M., Leming Z., Keqin Z. (2004). Soil Volatile Fungistasis and Volatile Fungistatic Compounds. Soil Biol. Biochem. 36, 1997–2004. 10.1016/j.soilbio.2004.07.020 [DOI] [Google Scholar]
- Yadav S. K., Singla-Pareek S. L., Ray M., Reddy M. K., Sopory S. K. (2005). Methylglyoxal Levels in Plants Under Salinity Stress are Dependent on Glyoxalase I and Glutathione. Biochem. Biophys. Res. Commun. 337, 61–67. 10.1016/j.bbrc.2005.08.263 [DOI] [PubMed] [Google Scholar]
- Yang J., Wang L., Ji X., Feng Y., Li X., Zou C., et al. (2011). Genomic and Proteomic Analyses of the Fungus Arthrobotrys Oligospora Provide Insights Into Nematode-Trap Formation. PloS Pathog. 7, e1002179. 10.1371/journal.ppat.1002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yu Y., Li J., Zhu W., Geng Z., Jiang D., et al. (2013). Characterization and Functional Analyses of the Chitinase-Encoding Genes in the Nematode-Trapping Fungus Arthrobotrys Oligospora . Arch. Microbiol. 195, 453–462. 10.1007/s00203-013-0894-6 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li S., Li H., Wang R., Xu J. (2020). Fungi-Nematode Interactions: Diversity, Ecology, and Biocontrol Prospects in Agriculture. J. Fungi (Basel) 6, 206. 10.3390/jof6040206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Zheng Y., Ma Y., Yang L., Xie M., Zhou D., et al. (2019). The Velvet Proteins VosA and VelB Play Different Roles in Conidiation, Trap Formation, and Pathogenicity in the Nematode-Trapping Fungus Arthrobotrys Oligospora . Front. Microbiol. 10, 1917. 10.3389/fmicb.2019.01917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C. S., Mo M. H., Gu Y. Q., Zhou J. P., Zhang K. Q. (2007). Possible Contributions of Volatile-Producing Bacteria to Soil Fungistasis. Soil Biol. Biochem. 39, 2371–2379. 10.1016/j.soilbio.2007.04.009 [DOI] [Google Scholar]
- Zuin A., Vivancos A. P., Sansó M., Takatsume Y., Ayté J., Inoue Y., et al. (2005). The Glycolytic Metabolite Methylglyoxal Activates Pap1 and Sty1 Stress Responses in Schizosaccharomyces Pombe . J. Biol. Chem. 280, 36708–36713. 10.1074/jbc.M508400200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.