Abstract
Predators affect prey through both consumptive and non-consumptive effects (NCEs), and prey typically face threats from multiple simultaneous predators. While different predators have a variety of NCEs on prey, little is known regarding effects of simultaneous multiple predators on demographic habitat selection. Demographic habitat selection is unique among NCEs, especially in discrete habitat patches; decisions directly affect both distribution and abundance of species across habitat patches, rather than simply abundance and performance within patches. Our goal was to determine strength of avoidance responses to multiple species/species combinations of predatory fish, and responses to predator richness. We assessed responses of ovipositing grey treefrogs (Hyla chrysoscelis) to three predatory fish species and substitutive combination of species. In single-species treatments, treefrogs avoided only one species, Notemigonus crysoleucas. All two-species combinations, and the three-species combination, were avoided, including the Fundulus chrysotus × Noturus phaeus combination, of which neither were avoided alone. This suggests emergent properties of multiple predators, with potential interactive effects among cues themselves or in the perception of cues by treefrogs. Our results indicate effects of multiple predators are not predictable based on individual effects, and illustrate the importance and complexity of effects of demographic habitat selection on distribution and abundance.
Keywords: colonization, immigration, non-consumptive effects, patch quality, predation risk, predator-released kairomones
1. Introduction
Organisms often face the threat of multiple predators, and a large literature addresses how exposure risk to multiple simultaneous predators manifests in effects on prey (multi-predator effects, MPEs). The majority of work focuses on lethal effects and whether MPEs can be predicted from the effects of individual predators [1–5]. This derives from a central question in the study of species interactions, which is whether we can use interaction coefficients from pairwise interactions to predict results of multispecies interactions—the community matrix approach [6–8]. Recent work has established that non-consumptive effects (NCEs) of predators, manifested as changes in morphology, life history and behaviour, can have equally strong effects on populations, communities and metacommunities [9–18]. This results partly from consumption affecting only those consumed, whereas NCEs may impact every individual in the population.
Choosing a predator-free patch can be an effective strategy if the absence of predators at the time of colonization is a reliable predictor of long-term absence [19–21]. Unlike most NCEs, effects of predators on immigration behaviour (colonization and oviposition) can affect (meta)populations and resulting (meta)community structure by generating species sorting among patches at the immigration stage [12,15]. Little is known regarding the dynamics of NCEs generated by multiple simultaneous predators on immigration. Consumption and immigration share the characteristic of directly affecting the number of individuals in a habitat patch, consumptive effects by removal and immigration effects by redistribution, but demographic habitat selection, in which habitat choices are long term or irreversible (immigration sensu stricto), is also a metapopulation and metacommunity process. Despite the fact that immigration and consumption both affect abundance, immigration responses probably involve different processes not amenable to simple predictions based on individual predator effects, but rather involve saturation of prey responses, or emergent effects of species combinations [3,5,22,23].
Fish are dominant predators in freshwater systems, yet are heterogeneously distributed across aquatic landscapes due to dispersal limitation. Fish-intolerant species are under intense selective pressure to avoid habitats with fish at the immigration stage, and avoidance of individual fish species has been observed in numerous taxa, including Coleoptera [24–28], Diptera [28–33], Hemiptera [15], Anura [19,24,28,34–36] and Caudata [37,38]. Staats et al. [39] showed that oviposition rate decreases with predator richness for several dipterans, but we know little else about how colonizing species respond to risk from multiple simultaneous predators.
Oviposition site choice is the only form of parental care provided by many organisms, including most aquatic breeding amphibians [40]. Choosing appropriate habitat is crucial for offspring performance and parental fitness, especially with efficient predators. MPEs on oviposition can occur via several mechanisms; predator cues themselves could chemically interact to affect perceived risk, behavioural algorithms for assessing and responding to predation risk could interact, or interactions between the predators themselves, particularly aggression or intraguild predation, could alter chemical cues. Habitat selection is driven by detecting, integrating and responding appropriately to multiple simultaneous environmental (predator) cues. Matching habitat selection, where individuals successfully match their phenotype to habitats where fitness is enhanced, is considered rare because of the diversity and complexity of environmental cues determining expected fitness [41], but in binary situations, such as predator/no predator, it should be more prevalent. Here, we focus on species level response to predators, which is the cumulative result of variable individual intraspecific decisions. The critical point is that how successful the ecological process of habitat selection is in correctly matching phenotype to environment has implications for the evolutionary processes of adaptation and diversification. Thus, the question we address here is what happens when that binary choice of predator versus no predator increases in complexity with multiple simultaneous predators.
We experimentally manipulated composition of the predator ensemble in aquatic mesocosms (habitat patches) in a substitutive design [42], holding initial total predator density and biomass constant while manipulating species composition. Treatments consisted of either one, two or three predatory fish species, and we assayed responses of ovipositing grey treefrogs, Hyla chrysoscelis, to variation in fish species composition. We chose three co-occurring fish covering a range of habitat use patterns; one benthic, one pelagic and one surface species. Predators using different habitats are most likely to stably coexist in a three-species ensemble, least likely to engage in interference competition, and most likely to have synergistic consumptive and NCEs because spatial segregation limits prey refuges.
2. Material and methods
Our experiment was conducted in a large, old field at the University of Mississippi Field Station (UMFS), Lafayette County, MS. We set up five arrays (blocks), each with nine, 1300 l (2.54 m2) cylindrical mesocosms (n = 45) laid out in isosceles trapezoids (figure 1a), crossing the presence/absence of three predatory fish: golden topminnows (Fundulus chrysotus), golden shiners (Notemigonus crysoleucas) and brown madtoms (Noturus phaeus) (figure 1b). These species are among the most frequently encountered fish and co-occur in ponds at UMFS. All three are generalist mesopredators, and each represents a different habitat/foraging strategy; N. crysoleucas is a small, pelagic, omnivorous-planktivorous, gape-limited species, F. chrysotus is a small, surface-feeding topminnow that is also gape-limited, and No. phaeus is a small, benthic foraging catfish with a larger terminal size and size-specific gape than the other two [43]. All three feed primarily on aquatic insects and other invertebrates. Like most predatory fish in North America, they feed opportunistically, and all three represent a significant threat to the eggs and larvae of H. chrysoscelis relative to fish-free controls.
Figure 1.
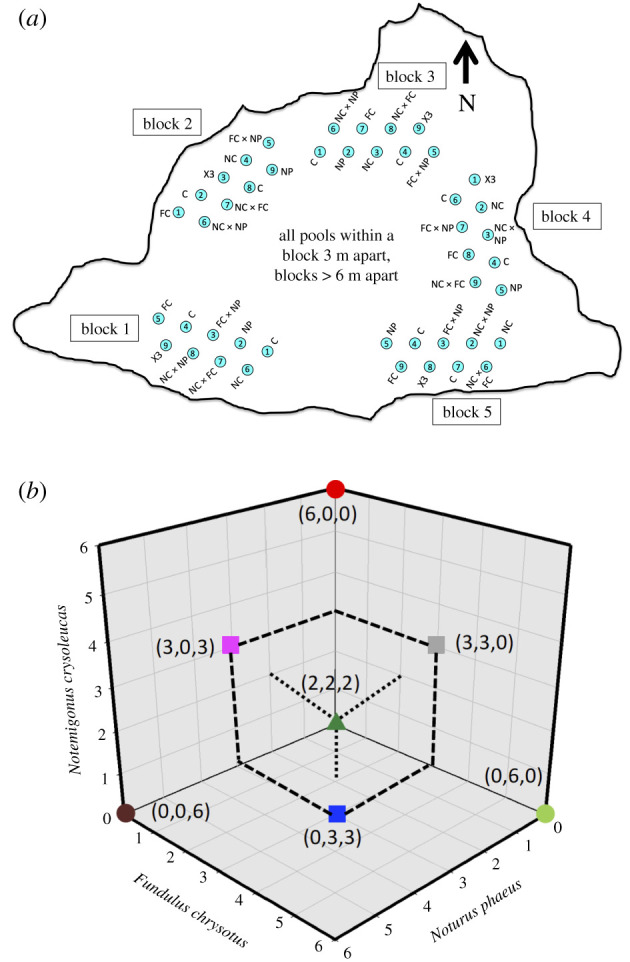
(a) Physical layout of the experiment, not to scale. See photos electronic supplementary material, figure S1. (b) Experimental design. De Wit replacement series crossing the presence/absence of three different fish species in a substitutive design, holding initial total fish density (6 per patch) and biomass constant (see text). Each of seven fish treatments were compared to Controls (not shown—falls at far apex, behind green triangle) using Dunnett's procedure. Three contrasts were conducted among classes of predator richness. Contrasts were (i) single species (circles) versus multiple species treatments (squares and triangle), (ii) single species (circles) versus species pairs (squares) and (iii) single species (circles) versus all three species (triangle) (figure 1b). Key: Notemigonus crysoleucas (NC) (red circle), Fundulus chrysotus (FC) (light green circle), Noturus phaeus (NP) (dark red circle), NC × NP (pink square), NC × FC (grey square), FC × NP (blue triangle), FC × NC × NP (×3 = all three species—dark green triangle). (Online version in colour.)
Mesocosms were filled with well-water from 11 to 13 May 2017, received 1 kg of dried mixed hardwood leaf litter each, and were quickly colonized by zooplankton and numerous small dipterans whose adults and/or eggs/larvae can pass through the screens (1.3 × 1.13 mm mesh used to separate predators and insect colonists/frog eggs) (electronic supplementary material, figure S1), providing the fish a resource base. High overall survival and positive growth of all three fish supports presence of an adequate food base. Six fish were added to each mesocosm on 14 May: 6/species (single species), 3/3 (two-species) and 2/2/2 (three-species). Each block also contained two fishless controls. Density is on the lower end of biomass density from previous experiments and natural ponds, but above the threshold eliciting avoidance in Hyla (0.5 g per 100 l) [44] (W.J.R. 2015, unpublished data). To equalize biomass within blocks, we created pairs composed of one ‘large’ and one ‘small’ individual for each species (by eye to minimize stress), and randomly assigned pairs within blocks, thus establishing initial equal density, approximate biomass and size-structure within blocks. On 15 May the experiment was begun by submerging screen lids to allow oviposition and efficient collection of eggs, and to separate fish from treefrogs—there were no consumptive effects possible here.
Grey treefrogs lay eggs in floating packets of approximately 25–50 eggs spread on the water surface, thus, unlike other species, it is impossible to determine the number of clutches deposited, though we estimate based on mean clutch size. Use of mesocosms and submerged screens (electronic supplementary material, figure S1) is essential, as the eggs begin to sink rapidly as they develop, often overnight. Thus, it is virtually impossible to assay oviposition in individual natural ponds, much less across a gradient of ponds, except by proxy measures that have proved unreliable (i.e. larval abundance). Dead fish (18/210) were replaced until 20 May, after which there was no observed mortality. The experiment was checked each morning for eggs until 1 September 2017; eggs were removed, photographed and counted using ImageJ [45,46], and placed in rearing tanks or fishless ponds. Fish survival from 20 May to the end of the entire experiment (8 December) was 91%: 89% (F. chrysotus), 94% (No. phaeus) and 91% (N. crysoleucas). Only two mesocosms did not fully hold treatment until the end of the experiment; one F. chrysotus × N. phaeus (FC × NP) mesocosm had no surviving F. chrysotus, and one three-species mesocosm (×3) also had no surviving F. chrysotus. We could not track survival during the experiment, so cannot determine when mortality occurred. Since neither mesocosm was an outlier or otherwise unusual, our assumption was that they held their respective treatments for most of the period of treefrog oviposition (see Results). Given the overall low fish mortality, this is not unreasonable. Thus, we included both in the final analyses as their original treatment.
This experiment was also colonized by a diverse assemblage of aquatic insects (5961 insects of 66 species), mostly dytiscid and hydrophilid beetles, and hemipterans [47]. Insects (except the very smallest) were separated from and incapable of interacting with fish due to the screen lids. Many of the colonists, or their larvae, are potentially significant predators of larval anurans, however, just as we removed eggs daily, we removed colonizing insects weekly (from above the screens), so there was no ongoing community assembly (buildup of insect densities) to dampen fish effects [28], or directly drive Hyla oviposition [48], as has been seen with much higher insect densities in much smaller mesocosms. We nevertheless examined possible correlations between insect colonization and Hyla oviposition and present that data in the results.
We used a randomized complete block design crossing the presence/absence of three fish species in a De Wit replacement series [42]. Each of five blocks consisted of nine mesocosms, one replicate of seven treatments and two replicates of fishless controls (figure 1a,b). Controls contain extra replicates because greater precision in estimation of the control disproportionately increases the power of the test, which compares all individual treatments to that control [49,50]. We could not equalize position relative to the forest edge, so we included row (inner versus outer) nested within block as a measure of relative proximity, since treefrogs ultimately arrive from the forest (figure 1). Our primary response variable was mean total eggs per patch and we partitioned mean total eggs into two components, total oviposition nights per patch (eggs in a patch on a night = hits) and mean deposition per patch (eggs per hit). For both mean total eggs and mean deposition we used generalized linear mixed model ANOVA in PROC GLIMMIX (SAS) with treatment as a fixed effect and block, and row nested within block, as random effects, with a quasi-Poisson distribution and a log link function [51], on square root transformed counts . This provided the best fit (minimize χ2/d.f.) to egg count data. For hits we used general linear mixed model ANOVA in PROC MIXED (SAS) with treatment as a fixed effect and block, and row nested within block, as random effects, on squareroot transformed counts , which, again, provided the best fit to the data. All responses to fish by colonizing/ovipositing organisms that use our mesocosms in dozens of prior experiments have been either negative or neutral, which informed our hypothesis that fish effects would manifest as reduced oviposition, hence the more powerful one-tailed test appropriate to a directional hypothesis [52,53]. Treatment means were compared using a one-tailed Dunnett's procedure (with Dunnett–Hsu correction) [49,50], asking whether fish treatments received fewer eggs than controls, and three a priori, non-orthogonal, one-tailed contrasts to examine specific hypotheses relating to multiple predator effects, with the expectation that oviposition would decline with predator richness. Contrasts were (i) single species versus all multiple species treatments, (ii) single species versus species pairs and (iii) single species versus all three species (figure 1b). We also analysed final body size of each of the three fish species. Because this was designed to inform us as to the possible dynamics of the habitat decisions, we used individual mass rather than mesocosm means to gain more insight into the species interactions. We regressed total eggs per patch versus total fish biomass per patch to examine effects of overall fish biomass. All ANOVA-based analyses used SAS v. 9.4 (SAS Institute 2016) with Type III sums of squares and α = 0.05.
3. Results
Grey treefrogs deposited 77 375 eggs in 82 total hits (eggs laid in any patch on a night) spread over 32 of 92 nights, representing the expected output of approximately 100 females. Eggs were laid in 31 of 45 patches, with maximum number of eggs on a single night being 12 969 (8 August), which was the last night of major activity, with less than 2000 eggs total after that date. Maximum number of eggs in a given patch on a single night was 3684 (Control patch), and only 12 of 82 events appeared to represent partial clutches (less than estimated minimum clutch size at UMFS, approx. 300; W.J.R. 2014--2021, personal observation).
Number of treefrog eggs was strongly affected by treatment. Among single species treatments, only the N. crysoleucas (NC) treatment was significantly different from controls, but all multispecies treatments were significantly different from controls, and had effect sizes (Cohen's d) greater than 1.0 (table 1 and figure 2a). All three a priori contrasts—single versus multi, single versus paired (two-species) and single versus X3 (three-species)—were significant (figure 3a). Three of four multispecies effects could be driven by NC (figure 2), but the biomass and density of NC was half to one-third of that in the single species treatment. The significant FC × NP effect, however, involves some sort of cue synergy, as neither individual species was avoided, but avoidance of FC × NP was as strong as any treatment. Any attempt to predict multispecies values by combining, either in an additive or multiplicative manner, single species values is rendered trivial because only one species is avoided, and we have avoidance of a species combination in which neither species in the pair were avoided. Relative preferences among treatment remained constant across the duration of the experiment (electronic supplementary material, figure S2a), with sporadic oviposition in the five avoided treatments (electronic supplementary material, figure S2b).
Table 1.
Main effects, Dunnett's test results (with Dunnett–Hsu correction), and a priori contrasts, for mean total eggs, hits and mean deposition. Significant effects in bold.
| Type III tests of fixed effects | ||||||
|---|---|---|---|---|---|---|
| variable | effect | num d.f. | den d.f. | F-value | Pr > F | |
| mean total eggs | trt | 7 | 32 | 3.46 | 0.0072 | |
| hits | trt | 7 | 32 | 7.57 | <0.0001 | |
| mean deposition | trt | 7 | 32 | 3.46 | 0.0071 | |
| mean total eggs |
hits |
mean deposition |
||||
|---|---|---|---|---|---|---|
| Dunnett's | t-value | adj p | t-value | adj p | t-value | adj p |
| C versus FC | 1.91 | 0.1 | 2.01 | 0.1362 | 1.92 | 0.0984 |
| C versus NC | 3.83 | 0.0012 | 3.5 | 0.0044 | 3.83 | 0.0012 |
| C versus NP | 1.26 | 0.2724 | −0.88 | 0.9955 | 1.25 | 0.2781 |
| C versus FC × NP | 3.2 | 0.0063 | 2.68 | 0.0345 | 3.2 | 0.0062 |
| C versus NC × FC | 4.33 | 0.0003 | 4.98 | <0.0001 | 4.33 | 0.0003 |
| C versus NC × NP | 3.95 | 0.0009 | 4.24 | 0.0006 | 3.96 | 0.0009 |
| C versus X3 | 4 | 0.0008 | 4.53 | 0.0003 | 3.99 | 0.0008 |
| mean total eggs |
hits |
mean deposition |
||||
|---|---|---|---|---|---|---|
| contrast | t-value | Pr > t | t-value | Pr > t | t-value | Pr > t |
| single versus multi | 2.37 | 0.0121 | 4.24 | <0.0001 | 2.36 | 0.0122 |
| single versus paired | 2.2 | 0.0175 | 3.66 | 0.0004 | 2.2 | 0.0174 |
| single versus X3 | 2.23 | 0.0164 | 3.36 | 0.001 | 2.2 | 0.0174 |
Figure 2.
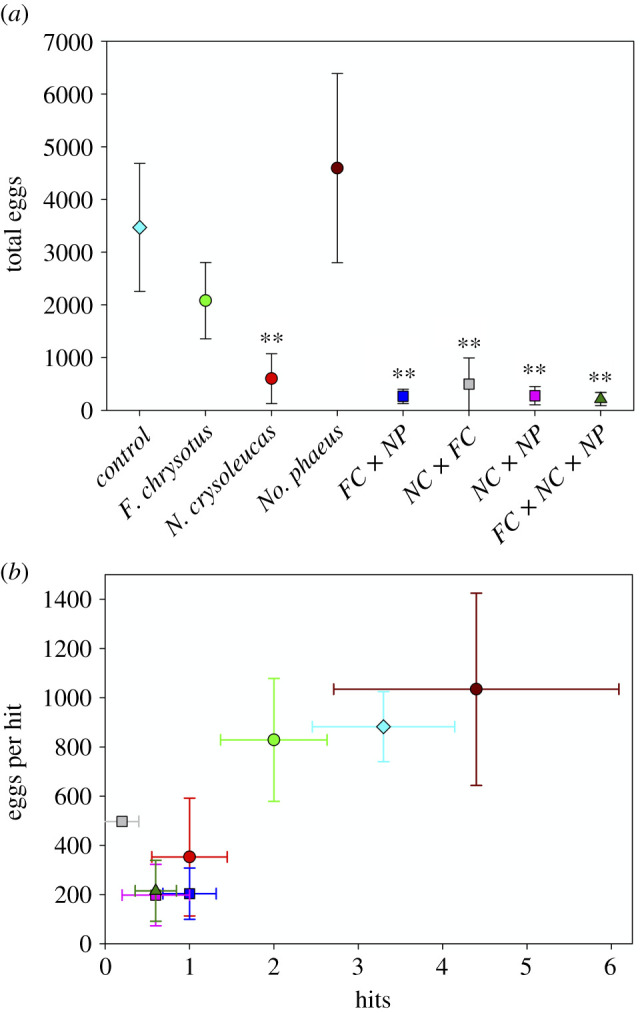
(a) Results of Dunnett's procedure (with Dunnett–Hsu correction) for (a) mean total eggs ± 1 s.e., *p < 0.05, **p < 0.01. (b) Mean total oviposition events (hits) ± 1 s.e. and mean deposition ± 1 s.e., for all individual treatments (table 1 for details). All five treatments in lower left had Dunnett's p < 0.01 for both variables. Both plots show raw treatment means. (Online version in colour.)
Figure 3.
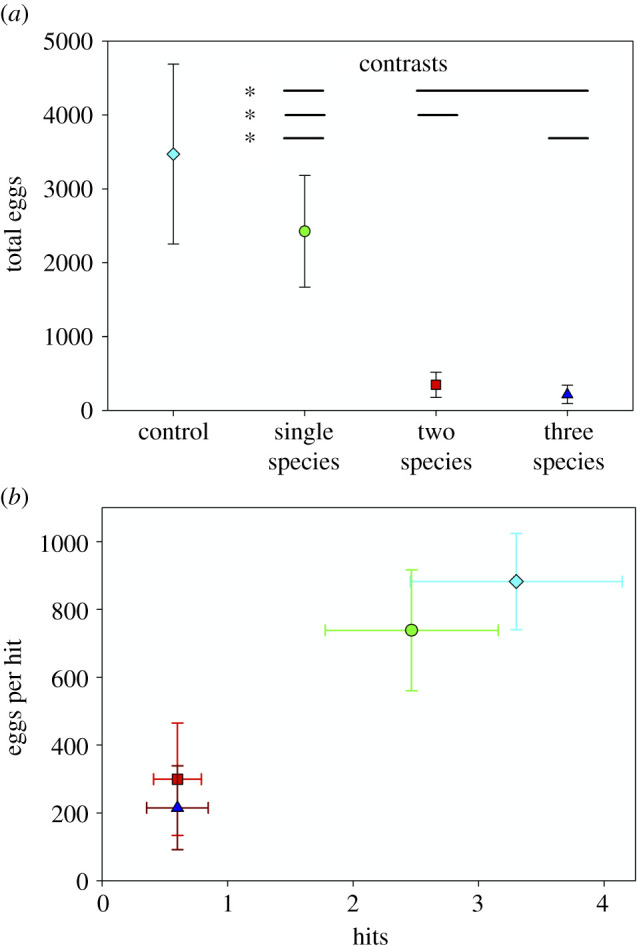
(a) Mean total eggs ± 1 s.e., (b) mean total oviposition events (hits) ± 1 s.e. and mean deposition ± 1 s.e., by contrast group (control shown for reference only). Bars indicate contrasts, *p < 0.05, **p < 0.01. Contrasts were (i) single species versus multiple species, (ii) single species versus species pairs and (iii) single species versus all three species (figure 1b). Both plots show raw contrast group means. (Online version in colour.)
Partitioning the overall response to individual treatments into its two components, oviposition events (eggs in a patch on a night = hits) and mean deposition (eggs per hit), results largely mirrored those for mean total eggs. Thus, variation in mean total eggs was driven by both variation in activity (hits), and variation in the mean number of eggs laid per hit, both of which were lower in avoided treatments (figure 2b). The same is true for a priori contrasts (figure 3b).
We only have final body size (mass) data for the three fish species that was taken after the end of insect sampling on 8 December, whereas approximately 95% of oviposition was completed by 8 August. However, we can explore that data for clues about how the species interact, but only in the context of this experiment which limited the range of available prey and thus the opportunity to partition resources by prey type. With that caveat, our original expectations with regard to species interactions are supported; all three species showed negative intraspecific density-dependent growth, with smallest body size in single species treatments. For N. crysoleucas and No. phaeus the largest body size was in three species treatments, and for F. chrysotus it was the FC × NP treatment. There was significant variation in final total biomass among fish treatments (F6,187 = 14.26, p < 0.0001), but regression of total number of eggs per patch against total mass per patch (excluding controls) showed no relationship (r = 0.0101, p = 0.5640, n = 35) (electronic supplementary material, figure S3), indicating that species identity and species combinations drove observed differences, not fish biomass.
There was also no correlation between the total number of colonizing insects per patch and total eggs per patch (r = 0.049, p = 0.751, n = 45) and, most instructive, no correlation within the fishless controls (despite a large outlier) (r = 0.422, p = 0.224, n = 10). If higher insect abundance was impacting Hyla oviposition decisions we would expect a negative correlation within the controls, since controls had the highest mean insect abundance and the greatest range of abundance (electronic supplementary material, figure S4). The overall patterns for both insects and Hyla are more indicative of shared avoidance responses than any interaction between the two taxa in this experiment where insects were removed weekly (electronic supplementary material, figure S5).
4. Discussion
It has become increasingly clear that predators can generate NCEs whose impacts equal or exceed consumptive effects on prey, ranging from changes in individual morphology to effects on ecosystem dynamics [9–12,16,17]. Despite this, surprisingly little is known regarding the dynamics of NCEs of exposure to multiple simultaneous predator species, and this is especially true for effects on immigration behaviour (but see [39]).
We focus on a common form of habitat selection, oviposition site choice. Ovipositing species may vary in their ability to detect relevant predator cue or cues, or may vary in whether or not they respond to the cue(s); we can only determine whether cues are detected by the receiver by observing a response. Although consumption and immigration both directly affect the number of individuals, immigration responses to multiple predators are more difficult to predict, and may involve saturation of responses or emergent effects of species combinations. Emergent responses may be mediated by interactions among the cues themselves, by an interactive effect on behavioural algorithms (driven by simultaneous detection of multiple predators), or variation in the actual cues present because of interactions between the predators themselves. Based on widespread behavioural responses of prey to olfactory cues produced by predatory fish [54], workers hypothesized a generalized fish kairomone(s), and any species that detects this kairomone signature avoids all predatory fish, though there has always been some variation in the observed strength of responses. Many responding species avoid fish as disparate as Esox, which are highly effective predators, and largely planktivorous Pimephales and Notemigonus, which certainly suggested generalized, rather than species-specific, cues [34,55]. However, as the taxonomic breadth of both prey and fish species tested increases, we see variation in which species avoid fish, and which fish and fish combinations they avoid. If fish produce species-specific cues, ovipositing female Hyla may vary in either the detection or response to each of them, and possibly to combinations of species. The presence and strength of responses often does not reflect our anthropocentric perception of predation risk, which typically focuses on adult predators preying on adult prey; we rarely have a complete picture of predation across all life stages of an organism [56–58]. Thus, any perceived mismatch may derive from our expectations rather than reality. The amount and/or detectability of the cue(s) produced may vary independently of biomass or trophic position.
Our assumption is that treefrogs responded to the treatments we imposed, and these are innate, rather than learned responses, since female treefrogs visit ponds at most two nights per year (only to breed), are relatively short-lived, and would seem to have no mechanism for assessing the success of prior breeding events. The alternative is that, given variation in presence/absence, species identity and species richness of fish, the background communities may have differed and treefrogs are responding to those differences. We removed the larger insects weekly, so there was no community assembly or buildup of densities in the patches to affect treefrog oviposition [28,48]. Thus, only organisms small enough to pass through the screens remained, and most of these (zooplankton and aquatic insects) are grazers on periphyton and phytoplankton. While there was probably subtle variation in assemblages across treatments (since we chose fish of very different habitat use patterns), the most obvious difference is in the amount of plant (periphyton and phytoplankton) biomass between fish and fishless treatments. Because of predation pressure on grazers there is greater plant biomass with fish than in fishless treatments. This should actually bias against any fish avoidance, because larval treefrogs are grazers on this same resource. However, H. chrysoscelis does not respond to variation in productivity in the presence or absence of fish [24,59], so the parsimonious explanation is that H. chrysoscelis responded directly to fish cues and combinations of fish cues.
Here we used a substitutive, rather than the more typical additive design used in studies of consumptive MPEs, because our interest was in predator identity and richness, not density. We set our density above the typical observed threshold for observed effects in treefrogs (0.5 g per 100 l) [44,55]. Of course, this may vary with prey and predator species. Our key finding is that all multiple predator treatments were strongly avoided, although only one of three fish was avoided when alone. Avoidance of all multi-predator treatments was also the general pattern for insects (electronic supplementary material, figure S5), but with considerable variation among taxa [47]. The major difference was that while H. chrysoscelis only avoided N. chrysoleucus among the single species treatments, insects as a group avoided both N. chrysoleucus and F. chrysotus, with stronger response to the latter (electronic supplementary material, figure S5). For Hyla, three multiple predator treatments might be explained solely by effects of N. crysoleucas, which was strongly avoided, but the biomass density of N. crysoleucas was halved in two-species treatments, and reduced by two-thirds in the three-species treatment, and our biomass densities were not high relative to either previous experiments or natural systems. Nonetheless, it is possible that two N. crysoleucas is above the critical detection threshold, as we see a similar pattern with the hemipteran genus Sigara (water boatmen), which avoid only F. chrysotus, and all treatments containing F. chrysotus, but not the remaining two-species treatment [47]. Nothing in single species responses predicts that the FC × NP treatment would be avoided, and as avoided as strongly as those containing N. crysoleucas. Given the unpredictable response to FC × NP, it is possible that emergent effects of the type seen in the FC × NP treatment, not simply the presence of NC, played a role in all multi-predator treatments.
We have clear emergent effects of multiple predators; however, the mechanism is unknown. Chemical cues typically drive observed immigration responses in anurans and insects via predator-released kairomones (PRKs). Fish species may simply vary in the amount of cue produced, however, limited characterization of fish kairomones suggests they are very difficult to identify and may be taxonomically unique [60,61], and work on PRKs of other taxa has suggested they are both sender and receiver specific [62–66]. Avoidance of Aphredoderus sayanus, which is chemically cryptic, cannot be induced by increasing the amount of cue (fish biomass) (W.J.R. 2015, 2016, unpublished data), and here we see that oviposition activity is not correlated with total fish biomass. Variation in both amount of cue and its specific signature are potential contributors, but based on this experiment, it is likely the three fish do not produce the same PRK. We can reasonably conclude that ovipositing treefrogs detect and identify N. crysoleucas, both alone and in mixtures, but whether treefrogs cannot detect No. phaeus and F. chrysotus, or simply ignore their presence, except in a mixed assemblage, is unknown. This would be surprising, because eggs and early larvae are vulnerable to gape-limited F. chrysotus, and a wider range of larval body sizes are vulnerable to No. phaeus. Numerous species of colonizing insects detected and avoided these two-species in the same experiment; No. phaeus generated the weakest response among insects, but, unlike treefrogs, F. chrysotus generated the strongest insect response among single species treatment (electronic supplementary material, figure S5) [47]. So, clearly PRKs are present at detectable levels for these two species, and treefrogs are at least as sensitive to cue intensity as many aquatic insects [44,55]. Strong avoidance of the FC × NP treatment suggests that treefrogs, as well, are detecting both species, choosing to ignore them when alone, and avoid them when together. Alternatively, the fishes themselves may interact to produce unique cues, but F. chrysotus and No. phaeus are the least likely pair to interact, either by exploitation or interference, one being benthic and one surface-dwelling, and that is borne out in the fish body size data. Aspects of predator behaviour (e.g. changes in foraging dynamics and dietary breadth) or predator physiology (e.g. metabolic rates or cue production), may change in response to other predators, which potentially alters predator cues or other components of the habitat, such as nutrient turnover, and algal and zooplankton abundance. However, in the context of this experiment, final fish body size data confirms our expectation regarding niche separation, with all three showing negative intraspecific density-dependent growth. Nonetheless, three F. chrysotus with three conspecifics may still behave differently than three F. chrysotus with three No. phaeus, and vice versa, but the most intense competitive interactions here are intraspecific.
Whatever the mechanism, the combination of PRKs, or the combination of interacting predators, generates an emergent response. This suggests that reduced oviposition in two-species and three-species treatments is not driven simply by presence of N. crysoleucas, rather, species combinations produce a unique kairomone signature themselves. Alternatively, treefrogs and others may possess not just a cue intensity threshold, but also a cue diversity threshold—a simple behavioural algorithm that says ‘if more than one species of fish (or perhaps predator species in general), avoid’.
In addition to affecting species distribution and abundance, and processes of community assembly [12,15], habitat selection plays a potentially critical role in a variety of evolutionary processes [67,68]. Habitat selection that successfully matches individual phenotype to environments (directed gene flow), while considered less prevalent and flexible than in situ adaptive plasticity [41], has greater adaptive potential than either adaptive plasticity or divergent natural selection in generating local adaptation and preventing local maladaptation [69]. Thus, habitat selection, if sufficiently strong, can both reinforce local adaptation, or adaptation to a habitat type, and limit future niche shifts by restricting the opportunity for adaptation to alternative habitats [67,69,70].
Perhaps the most surprising recent revelation with regard to predator–prey systems is that consumption is not the most important driver of prey dynamics in all systems [9–13,15]. NCEs may equal or exceed those of consumption on the population as a whole, partly because consumption directly affects only those consumed, whereas non-consumptive direct and indirect effects may affect every individual in a population [71,72]. Changes in immigration rates can affect all individuals in a metapopulation, because habitat selection, especially in mosaic aquatic systems, redistributes individuals among populations—an individual that colonizes or oviposits in one habitat patch, cannot colonize or oviposit those same offspring in another patch. The fact that treefrogs avoid combinations of species, without avoiding the individual species, increases the frequency of such NCEs across the range of predator assemblages and the range of responding prey. Detecting strong, and complex, non-consumptive MPEs on oviposition site choice in treefrogs further illustrates how prevalent and important NCEs can be on the distribution and abundance of species, and in the assembly of natural communities.
Supplementary Material
Acknowledgements
B. McDaniel, T. Breech, R. Scott, S. McNamara, K. Potts and T. Chavez assisted with fieldwork, support was provided by the University of Mississippi, and the UM Field Station. This is publication no. 025 from the Center for Biodiversity and Conservation Research.
Ethics
Use of animals was approved by UM's IACUC Committee (protocol no. 14-027) and the Mississippi Department of Wildlife, Fisheries and Parks (permit no. 052517).
Data accessibility
Raw data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.t76hdr80m [73].
Authors' contributions
W.J.R.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing-original draft, writing-review and editing; J.R.B.: conceptualization, data curation, investigation, methodology, supervision, visualization, writing-review and editing; M.R.P.: conceptualization, data curation, investigation, methodology, supervision, writing-review and editing
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Support was provided by the Henry L. and Grace Doherty Foundation.
References
- 1.Soluk DA. 1993. Multiple prey effects; predicting combined functional response of stream fish and invertebrate predators. Ecology 74, 219–225. ( 10.2307/1939516) [DOI] [Google Scholar]
- 2.Vance-Chalcraft HD, Soluk DA. 2005. Multiple predator effects result in risk reduction for prey across multiple prey densities. Oecologia 144, 472–480. ( 10.1007/s00442-005-0077-5) [DOI] [PubMed] [Google Scholar]
- 3.McCoy MW, Stier AC, Osenberg CW. 2012. Emergent effects of multiple predators on prey survival: the importance of depletion and the functional response. Ecol. Lett. 15, 1449–1456. ( 10.1111/ele.12005) [DOI] [PubMed] [Google Scholar]
- 4.Wilbur HM, Fauth JE. 1990. Experimental aquatic food webs: interactions between two predators and two prey. Am. Nat. 135, 176–204. ( 10.1086/285038) [DOI] [Google Scholar]
- 5.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355. ( 10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 6.Vandermeer JH. 1970. The community matrix and the number of species in a community. Am. Nat. 104, 73–83. ( 10.1086/282641) [DOI] [PubMed] [Google Scholar]
- 7.Vandermeer JH. 1969. The competitive structure of communities: an experimental approach with protozoa. Ecology 50, 362–371. ( 10.2307/1933884) [DOI] [Google Scholar]
- 8.Novak M, Yeakel JD, Noble AE, Doak DF, Emmerson M, Estes JA, Jacob U, Tinker MT, Wootton JT. 2016. Characterizing species interactions to understand press perturbations: what is the community matrix? Annu. Rev. Ecol. Evol. Syst. 47, 409–432. ( 10.1146/annurev-ecolsys-032416-010215) [DOI] [Google Scholar]
- 9.Werner EE, Peacor SD. 2003. A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100. ( 10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2) [DOI] [Google Scholar]
- 10.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 11.Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163. ( 10.1111/j.1461-0248.2003.00560.x) [DOI] [Google Scholar]
- 12.Resetarits WJ, Pintar MR. 2016. Functional diversity of non-lethal effects, chemical camouflage, and variation in fish avoidance in colonizing beetles. Ecology 97, 3517–3529. ( 10.1002/ecy.1593) [DOI] [PubMed] [Google Scholar]
- 13.McCauley SJ, Rowe L, Fortin M-J. 2011. The deadly effects of ‘nonlethal’ predators. Ecology 92, 2043–2048. ( 10.1890/11-0455.1) [DOI] [PubMed] [Google Scholar]
- 14.Trekels H, Vanschoenwinkel B. 2016. When fear kicks in, predator cues initially do not but eventually do affect insect distribution patterns in a new artificial pond cluster. Hydrobiologia 790, 1–10. ( 10.1007/s10750-016-3027-9) [DOI] [Google Scholar]
- 15.Resetarits WJ, Pintar MR, Bohenek JR, Breech TM. 2019. Patch size as a niche dimension: aquatic insects behaviorally partition enemy-free space across gradients of patch size. Am. Nat. 194, 776–793. ( 10.1086/705809) [DOI] [PubMed] [Google Scholar]
- 16.Schmitz OJ, Beckerman A, O'Brien KM. 1997. Behaviorally mediated trophic cascades: effects of predation risk on food web interactions. Ecology 78, 1388–1399. ( 10.1890/0012-9658(1997)078[1388:BMTCEO]2.0.CO;2) [DOI] [Google Scholar]
- 17.Breviglieri CPB, Oliveira PS, Romero GQ. 2017. Fear mediates trophic cascades: nonconsumptive effects of predators drive aquatic ecosystem function. Am. Nat. 189, 490–500. ( 10.1086/691262) [DOI] [PubMed] [Google Scholar]
- 18.Peckarsky BL, et al. 2008. Revisiting the classics: considering nonconsumptive effects in textbook examples of predator–prey interactions. Ecology 89, 2416–2425. ( 10.1890/07-1131.1) [DOI] [PubMed] [Google Scholar]
- 19.Resetarits WJ, Wilbur HM. 1989. Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology 70, 220–228. ( 10.2307/1938428) [DOI] [Google Scholar]
- 20.Kershenbaum A, Spencer M, Blaustein L, Cohen JE. 2012. Modelling evolutionarily stable strategies in oviposition site selection, with varying risks of predation and intraspecific competition. Evol. Ecol. 26, 955–974. ( 10.1007/s10682-011-9548-9) [DOI] [Google Scholar]
- 21.Blaustein L. 1999. Oviposition site selection in response to risk of predation: evidence from aquatic habitats and consequences for population dynamics and community structure. In Evolutionary theory and processes: modern perspectives (ed. Wasser SP), pp. 441–456. Dordrecht, the Netherlands: Kluwer. [Google Scholar]
- 22.Davenport JM, Chalcraft DR. 2013. Nonconsumptive effects in a multiple predator system reduce the foraging efficiency of a keystone predator. Ecol. Evol. 3, 3063–3072. ( 10.1002/ece3.691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz OJ. 2007. Predator diversity and trophic interactions. Ecology 88, 2415–2426. ( 10.1890/06-0937.1) [DOI] [PubMed] [Google Scholar]
- 24.Binckley CA, Resetarits WJ. 2008. Oviposition behavior partitions aquatic landscapes along predation and nutrient gradients. Behav. Ecol. 19, 552–557. ( 10.1093/beheco/arm164) [DOI] [Google Scholar]
- 25.Pintar MR, Bohenek JR, Eveland LL, Resetarits WJ. 2018. Colonization across gradients of risk and reward: nutrients and predators generate species-specific responses among aquatic insects. Funct. Ecol. 32, 1589–1598. ( 10.1111/1365-2435.13086) [DOI] [Google Scholar]
- 26.Binckley CA, Resetarits WJ. 2005. Habitat selection determines abundance, richness and species composition of beetles in aquatic communities. Biol. Lett. 1, 370–374. ( 10.1098/rsbl.2005.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodin T, Johansson F, Bergsten J. 2006. Predator related oviposition site selection of aquatic beetles (Hydroporus spp.) and effects on offspring life-history. Freshw. Biol. 51, 1277–1285. ( 10.1111/j.1365-2427.2006.01563.x) [DOI] [Google Scholar]
- 28.Kraus JM, Vonesh JR. 2010. Feedbacks between community assembly and habitat selection shape variation in local colonization. J. Anim. Ecol. 79, 795–802. ( 10.1111/j.1365-2656.2010.01684.x) [DOI] [PubMed] [Google Scholar]
- 29.Petranka JW, Fakhoury K. 1991. Evidence of a chemically-mediated avoidance response of ovipositing insects to blue-gills and green frog tadpoles. Copeia 1991, 234–239. ( 10.2307/1446271) [DOI] [Google Scholar]
- 30.Eveland LL, Bohenek J, Silberbush A, Resetarits WJ. 2016. Detection of fish and newt kairomones by ovipositing mosquitoes. In Chemical signals in vertebrates 13 (eds Schulte BA, Goodwin T, Ferkin MH), pp. 247–259. Berlin, Germany: Springer. [Google Scholar]
- 31.Shaalan EAS, Canyon DV. 2009. Aquatic insect predators and mosquito control. Trop. Biomed. 26, 223–261. [PubMed] [Google Scholar]
- 32.Silberbush A, Blaustein L. 2008. Oviposition habitat selection by a mosquito in response to a predator: are predator-released kairomones air-borne cues? J. Vector Ecol. 33, 208–211. ( 10.3376/1081-1710(2008)33[208:OHSBAM]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 33.Blaustein L, Kiflawi M, Eitam A, Mangel M, Cohen JE. 2004. Oviposition habitat selection in response to risk of predation in temporary pools: mode of detection and consistency across experimental venue. Oecologia 138, 300–305. ( 10.1007/s00442-003-1398-x) [DOI] [PubMed] [Google Scholar]
- 34.Binckley CA, Resetarits WJ. 2003. Functional equivalence of non-lethal effects: generalized fish avoidance determines distribution of gray treefrog, Hyla chrysoscelis, larvae. Oikos 102, 623–629. ( 10.1034/j.1600-0706.2003.12483.x) [DOI] [Google Scholar]
- 35.Resetarits WJ, Bohenek JR, Breech T, Pintar MR. 2018. Predation risk and patch size jointly determine perceived patch quality in ovipositing treefrogs, Hyla chrysoscelis. Ecology 99, 661–669. ( 10.1002/ecy.2130) [DOI] [PubMed] [Google Scholar]
- 36.Kloskowski J. 2020. Better desiccated than eaten by fish: distribution of anurans among habitats with different risks to offspring. Freshw. Biol. 65, 2124–2134. ( 10.1111/fwb.13608) [DOI] [Google Scholar]
- 37.Kats LB, Sih A. 1992. Oviposition site selection and avoidance of fish by streamside salamanders (Ambystoma barbouri). Copeia 2, 468–473. ( 10.2307/1446206) [DOI] [Google Scholar]
- 38.Davenport JM, Hampson ME, King AB, Bishir SC. 2017. The effects of sunfish on spotted salamander oviposition, hatching time, and larval survival. Amphib.-Reptil. 38, 327–337. ( 10.1163/15685381-00003113) [DOI] [Google Scholar]
- 39.Staats EG, Agosta SJ, Vonesh JR. 2016. Predator diversity reduces habitat colonization by mosquitoes and midges. Biol. Lett. 12, 3–6. ( 10.1098/rsbl.2016.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duellman WE, Trueb L. 1986. Biology of amphibians. New York, NY: McGraw-Hill. [Google Scholar]
- 41.Edelaar P, Jovani R, Gomez-Mestre I. 2017. Should I change or should I go? Phenotypic plasticity and matching habitat choice in the adaptation to environmental heterogeneity. Am. Nat. 190, 506–520. ( 10.1086/693345) [DOI] [PubMed] [Google Scholar]
- 42.De Wit CT. 1960. On competition. Versl. Landbouwkd. Onderz. 66, 1–82. [Google Scholar]
- 43.Chan MD, Parsons GR. 2000. Aspects of brown madtom, Noturus phaeus, life history in Northern Mississippi. Copeia 2000, 757–762. ( 10.1643/0045-8511(2000)000[0757:AOBMNP]2.0.CO;2) [DOI] [Google Scholar]
- 44.Rieger JF, Binckley CA, Resetarits WJ. 2004. Larval performance and oviposition site preference along a predation gradient. Ecology 85, 2094–2099. ( 10.1890/04-0156) [DOI] [Google Scholar]
- 45.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohenek JR, Resetarits WJ. 2017. An optimized method to quantify large numbers of amphibian eggs. Herpetol. Notes 10, 573–578. [Google Scholar]
- 47.Resetarits WJ, Bohenek JR, Pintar MR. In press. Complex multi-predator effects on demographic habitat selection and community assembly in colonizing aquatic insects. Ecol. Monogr. [Google Scholar]
- 48.Pintar MR, Resetarits WJ Jr. 2020. Aquatic beetles influence colonization of disparate taxa in small lentic systems. Ecol. Evol. 10, 12 170–12 182. ( 10.1002/ece3.6845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunnett CW. 1955. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 50, 1096–1121. ( 10.1080/01621459.1955.10501294) [DOI] [Google Scholar]
- 50.Hsu JC. 1992. The factor analytic approach to simultaneous inference in the general linear model. J. Comput. Graph. Stat. 1, 151–168. ( 10.2307/1390839) [DOI] [Google Scholar]
- 51.Steele RGD, Torrie JH, Dickey D. 1997. Principles and procedures of statistics: A biometrical approach. New York, NY: McGraw-Hill. [Google Scholar]
- 52.Rice WR, Gaines SD. 1994. ‘Heads I win, tails you lose’: testing directional alternative hypotheses in ecological and evolutionary research. Trend Ecol. Evol. 9, 235–237. ( 10.1111/j.1558-5646.1996.tb02381.x) [DOI] [PubMed] [Google Scholar]
- 53.Ruxton GD, Neuhäuser M. 2010. When should we use one-tailed hypothesis testing? Methods Ecol. Evol. 1, 114–117. ( 10.1111/j.2041-210X.2010.00014.x) [DOI] [Google Scholar]
- 54.Wisenden BD. 2000. Olfactory assessment of predation risk in the aquatic environment. Phil. Trans. R. Soc. Lond. B 355, 1205–1208. ( 10.1098/rstb.2000.0668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resetarits WJ, Binckley CA. 2013. Is the pirate really a ghost? Evidence for generalized chemical camouflage in an aquatic predator, pirate perch Aphredoderus sayanus. Am. Nat. 181, 690–699. ( 10.1086/670016) [DOI] [PubMed] [Google Scholar]
- 56.Wilbur HM. 1988. Interactions between growing predators and growing prey. In Size-structured populations (eds Ebenmann B, Persson L), pp. 157–170. Berlin, Germany: Springer. [Google Scholar]
- 57.Krenek L, Rudolf VHW. 2014. Allometric scaling of indirect effects: body size ratios predict non-consumptive effects in multi-predator systems. J. Anim. Ecol. 83, 1461–1468. ( 10.1111/1365-2656.12254) [DOI] [PubMed] [Google Scholar]
- 58.Rudolf VHW, Rasmussen NL, Dibble CJ, Van Allen BG.. 2014. Resolving the roles of body size and species identity in driving functional diversity. Proc. R. Soc. B 281, 20133203. ( 10.1098/rspb.2013.3203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNamara SC, Pintar MR, Resetarits WJ Jr. 2021. Temperature but not nutrient addition affects abundance and assemblage structure of colonizing aquatic insects. Ecology 102, e03209. ( 10.1002/ecy.3209) [DOI] [PubMed] [Google Scholar]
- 60.Akkas SB, Kepenek AO, Beklioglu M, Severcan F. 2009. Molecular approach to the chemical characterization of fish-exuded kairomone: a Fourier transform infrared spectroscopic study. Aquat. Sci. 72, 71–83. ( 10.1007/s00027-009-0114-2) [DOI] [Google Scholar]
- 61.Wisenden BD. 2014. Chemical cues that indicate risk of predation. In Fish pheromones and related cues (eds Sorenson PW, Wisenden BD), pp. 131–148. Ames, IA: John Wiley & Sons. [Google Scholar]
- 62.Weiss LC, et al. 2018. Identification of Chaoborus kairomone chemicals that induce defences in Daphnia. Nat. Chem. Biol. 14, 1133–1139. ( 10.1038/s41589-018-0164-7) [DOI] [PubMed] [Google Scholar]
- 63.Poulin RX, Lavoie S, Siegel K, Gaul DA, Weissburg MJ, Kubanek J. 2018. Chemical encoding of risk perception and predator detection among estuarine invertebrates. Proc. Natl Acad. Sci. USA 115, 662–667. ( 10.1073/pnas.1713901115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hahn MA, Effertz C, Bigler L, von Elert E. 2019. 5α-cyprinol sulfate, a bile salt from fish, induces diel vertical migration in Daphnia. eLife 8, 1–15. ( 10.7554/eLife.44791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selander E, Kubanek J, Hamberg M, Andersson MX, Cervin G, Pavia H. 2015. Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proc. Natl Acad. Sci. USA 112, 6395–6400. ( 10.1073/pnas.1420154112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silberbush A, Markman S, Lewinsohn E, Bar E, Cohen JE, Blaustein L. 2010. Predator-released hydrocarbons repel oviposition by a mosquito. Ecol. Lett. 13, 1129–1138. ( 10.1111/j.1461-0248.2010.01501.x) [DOI] [PubMed] [Google Scholar]
- 67.Fry JD. 1996. The evolution of host specialization: are trade-offs overrated? Am. Nat. 148, S84–S107. ( 10.1086/285904) [DOI] [Google Scholar]
- 68.Edelaar P, Siepielski AM, Clobert J. 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 2462–2472. ( 10.1111/j.1558-5646.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- 69.Nicolaus M, Edelaar P. 2018. Comparing the consequences of natural selection, adaptive phenotypic plasticity, and matching habitat choice for phenotype–environment matching, population genetic structure, and reproductive isolation in meta-populations. Ecol. Evol. 8, 3815–3827. ( 10.1002/ece3.3816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Resetarits WJ. 1996. Oviposition site choice and life history evolution. Am. Zool. 36, 205–215. ( 10.1093/icb/36.2.205) [DOI] [Google Scholar]
- 71.Resetarits WJ, Binckley CA, Chalcraft DR. 2005. Habitat selection, species interactions, and processes of community assembly in complex landscapes: a metacommunity perspective. In Metacommunities: spatial dynamics and ecological communities (eds Holyoak M, Leibold MA, Holt RD), pp. 374–398. Chicago, IL: University of Chicago Press. [Google Scholar]
- 72.Orrock JL, Dill LM, Sih A, Grabowski JH, Peacor SD, Peckarsky BL, Preisser EL, Vonesh JR, Werner EE. 2010. Predator effects in predator-free space: the remote effects of predators on prey. Open Ecol. 3, 22–30. ( 10.2174/1874213001003030022) [DOI] [Google Scholar]
- 73.Resetarits WJ Jr, Bohenek JR, Pintar MR. 2021. Data from: Predator-specific responses and emergent multi-predator effects on oviposition site choice in grey treefrogs, Hyla chrysoscelis. Dryad Digital Repository. ( 10.5061/dryad.t76hdr80m) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Resetarits WJ Jr, Bohenek JR, Pintar MR. 2021. Data from: Predator-specific responses and emergent multi-predator effects on oviposition site choice in grey treefrogs, Hyla chrysoscelis. Dryad Digital Repository. ( 10.5061/dryad.t76hdr80m) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.t76hdr80m [73].


