Abstract
For mitigating the COVID-19 pandemic, much emphasis is made on implementing non-pharmaceutical interventions to keep the reproduction number below one. However, using that objective ignores that some of these interventions, like bans of public events or lockdowns, must be transitory and as short as possible because of their significant economic and societal costs. Here, we derive a simple and mathematically rigorous criterion for designing optimal transitory non-pharmaceutical interventions for mitigating epidemic outbreaks. We find that reducing the reproduction number below one is sufficient but not necessary. Instead, our criterion prescribes the required reduction in the reproduction number according to the desired maximum of disease prevalence and the maximum decrease of disease transmission that the interventions can achieve. We study the implications of our theoretical results for designing non-pharmaceutical interventions in 16 cities and regions during the COVID-19 pandemic. In particular, we estimate the minimal reduction of each region’s contact rate necessary to control the epidemic optimally. Our results contribute to establishing a rigorous methodology to design optimal non-pharmaceutical intervention policies for mitigating epidemic outbreaks.
Keywords: epidemic outbreak, non-pharmaceutical interventions, COVID-19, optimal control
1. Introduction
Since the seminal work of May & Anderson [1], the design of interventions to eradicate infectious diseases has the objective of achieving a basic (R0) or effective reproduction number below one [2,3]. The underlying assumption here is that it is possible to maintain interventions for long periods, such as long-term vaccination programmes. During the COVID-19 pandemic, this same objective is guiding the design of non-pharmaceutical interventions (NPIs) [4]. However, maintaining NPIs like bans of public events or lockdowns for long periods of time is infeasible because of their substantial economic and societal costs [5,6]. Actually, instead of aiming for eradication, NPIs aim to mitigate the economic and social costs of an epidemic outbreak [7]. Nevertheless, we still lack simple guidelines to design NPIs for mitigating epidemic outbreaks, analogous to the R0 < 1 condition for eradication.
Here, we use the classic Susceptible–Infected–Removed (SIR) epidemiological model to fully characterize the design of NPIs for mitigating epidemic outbreaks. With this aim, we consider that NPIs should achieve an optimal tradeoff between two objectives [8]. First, optimal NPIs must minimize the period in which they need to be applied, consequently minimizing their associated economic and societal costs. Second, optimal NPIs must guarantee that the disease prevalence does not exceed a specified maximum level, which for example can represent health services’ capacity for that particular disease outbreak [9]. We obtain a full analytical characterization of such optimal NPIs for mitigating epidemic outbreaks, specifying the optimal intervention at each state that the epidemic can be. This characterization yields the necessary and sufficient criterion for the existence of optimal NPIs for mitigation, analogous to the R0 < 1 condition for eradication. We find that reducing the reproduction number below one is sufficient but not necessary for their existence. Instead, for mitigation, we show that the desired maximum disease prevalence determines the necessary reduction in the reproduction number. The consequence of not reducing the reproduction number below one is that interventions must start before the disease prevalence reaches the specified maximum level. We also demonstrate numerically that the derived optimal NPIs for mitigation are robust to uncertainties in the model parameters and unmodelled epidemic dynamics (e.g. undetected infections). Finally, we explore the implications of our theoretical result by analysing the response of 16 cities and regions across the globe to the COVID-19 pandemic, finding that most regions achieved a larger-than-necessary reduction in transmission. Our results contribute to designing NPIs to optimally and robustly mitigate epidemic outbreaks.
2. Characterizing optimal non-pharmaceutical interventions
2.1. Optimal epidemic mitigation using non-pharmaceutical interventions
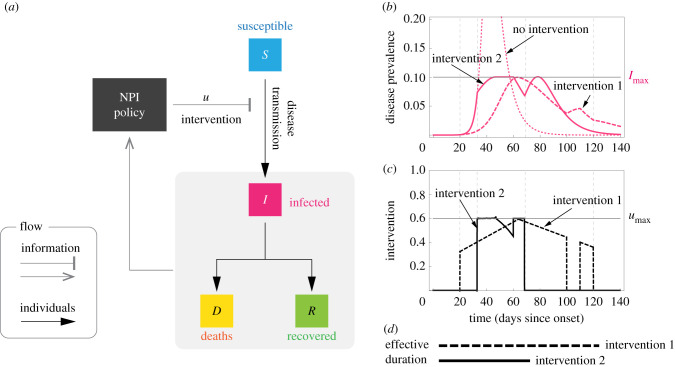
Our objective is to characterize the reduction in the disease transmission that is optimal for each state in which the epidemic outbreak can be. For this, we leverage on the mathematical tractability of the SIR model [10], where the state can be characterized by the pair . Here, S is the proportion of the population that is susceptible to the disease, and I is the disease prevalence (i.e. the proportion of the population that is infected); see figure 1a. We discuss later other more detailed epidemic models. The epidemic state changes with time t as the disease is transmitted, producing the trajectory (S(t), I(t)) for t ≥ 0. For epidemic mitigation, we consider that the goal is keeping the disease prevalence below a specified level Imax ∈ (0, 1]. A main factor determining this constant is health services’ capacity in the sense that a prevalence above Imax causes higher mortality due to hospital saturation [9]. In general, the selection of Imax could depend on other social and economic factors of the specific population where the outbreak occurs. To keep I(t) ≤ Imax, we assume we can apply one or several NPIs that reduce disease transmission by the factor (1 − u), for some u ∈ [0, 1]; see figure 1a. The NPIs achieve no reduction when u = 0, and they completely stop transmission when u = 1. Different NPIs correspond to particular values of u. For instance, a study of NPIs during the COVID-19 pandemic [11] found that closing most non-essential business corresponds to u ≈ 0.25, closing schools and universities to u ≈ 0.37, and limiting gatherings to at most 10 people to u ≈ 0.42. In practice, it can be unfeasible to fully stop the disease transmission (i.e. u < 1) because of inherent challenges like asymptomatic transmission [12], or because of the necessity of maintaining a working economy [13]. Therefore, we upper-bound the reduction by umax ∈ (0, 1). We say that u is admissible if u ∈ [0, umax].
Figure 1.
Optimal non-pharmaceutical interventions. (a) Susceptible–Infected–Removed (SIR) model with non-pharmaceutical interventions (NPIs) reducing disease transmission. For the optimal NPI design problem, the objective is to design the intervention u*(t) with minimal effective duration such that u*(t) ∈ [0, umax] and I(t) ≤ Imax for all t ≥ 0. (b,c) The response of the SIR model for two interventions (parameters are β = 0.52, γ = 1/7, I0 = 8.855 × 10−7 and S0 = 1 − I0). Both interventions 1 and 2 satisfy u(t) ≤ umax and guarantee that I(t) ≤ Imax. Actually, intervention 2 is the optimal one derived using our analysis: it is the intervention with minimal effective duration satisfying I(t) ≤ Imax. (d) The effective duration of an intervention measures the interval between the start of the outbreak and the last time that a non-zero intervention is applied. In this example, the effective duration of intervention 1 is 120 days, while the effective duration of intervention 2 is 69 days.
Different admissible NPIs can keep the disease prevalence below Imax. For instance, ‘intervention 1’ in the example of figure 1b,c keeps this restriction and it has an ‘effective duration’ of 120 days. Here, the effective duration of an intervention is the time interval between the start of the outbreak and the last time that a non-zero intervention is applied (figure 1d). ‘Intervention 2’ of figure 1b,c also keeps the restriction I(t) ≤ Imax, but its effective duration is only 69 days. To design the optimal NPI for mitigating epidemic outbreaks, we ask for the intervention with minimal effective duration. Specifically, we ask for the admissible intervention u*(S(t), I(t)) required now (i.e. at the current state) such that: (1) it minimizes the effective duration of the intervention and (2) it ensures that the prevalence can be maintained below Imax for all future time by using some admissible intervention. If the optimal NPI problem has a solution u*, then u*(S, I) characterizes the optimal reduction in the disease transmission that the NPIs should achieve if the epidemic state is (S, I). In particular, u* gives the optimal way to start and stop the NPIs.
2.2. Optimal non-pharmaceutical interventions for mitigation exist without reducing the reproduction number below one
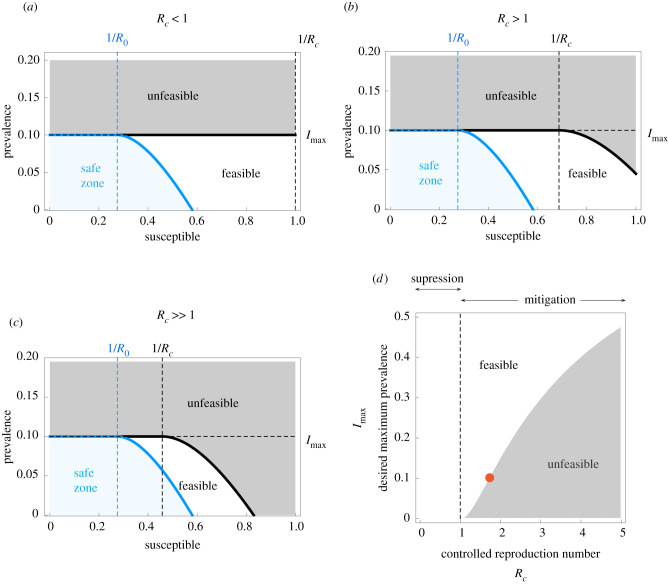
Our first main result is a complete analytical characterization of the optimal NPIs for outbreak mitigation in the SIR model (see box 1 for a summary and electronic supplementary material, note S1, for details). To understand how these optimal NPIs work, note that the SIR model predicts a safe zone of states (S, I) where, without any further interventions, the disease prevalence will not exceed Imax (blue zone in figure 2a–c). The safe zone is characterized by the inequality , where R0 is the basic reproduction number of the outbreak in the population, and the function is defined in equation (2.1) of box 1. The goal of the optimal NPIs is thus to reach this safe zone as fast as possible without violating the restriction I(t) ≤ Imax. The ability to achieve this goal depends on the epidemic state. That is, we can partition the plane (S, I) in two regions: those states from which it is possible to reach the safe zone without exceeding Imax (feasible states), and those where it is impossible (unfeasible states). We find these two regions are characterized by the separating curve , where we call Rc := (1 − umax)R0 the controlled reproduction number (figure 2a–c). Note that Rc describes the maximum reduction in the basic reproduction number that (constant) admissible interventions can achieve. Therefore, Rc < 1 is the necessary and sufficient condition that a constant and permanent admissible intervention (i.e. u(t) ≡ const. for all t ≥ 0) needs to satisfy to eradicate a disease outbreak in the SIR model. However, for outbreak mitigation, our analysis shows that feasible states exists without achieving disease eradication (white regions in figure 2b,c). This result is important because it proves that optimal NPIs for epidemic mitigation do not require reducing the basic reproduction number below one (i.e. without achieving Rc < 1).
Box 1. Optimal NPIs for the Susceptible–Infected–Removed (SIR) model.
The SIR model with interventions u(t) ∈ [0, umax] reducing disease transmission takes the form
Here, S(t) and I(t) are the proportion of the population that is susceptible or infected at time t ≥ 0, respectively. We denote by (S0, I0) the initial state at t = 0. The parameters of the SIR model are the (effective) contact rate β ≥ 0, and the mean residence time of infected individuals γ ≥ 0 (in units of day−1). By assuming S0 ≈ 1, these two parameters yield the basic reproduction number R0 = β/γ.
We are interested in reaching the safe zone
where
| 2.1 |
The safe zone is the largest set with the following property: if, for any given time t1, the state (S1, I1) belongs to , we can set u = 0 henceforth and still have I(t) ≤ Imax for all t ≥ t1. That is, when is reached, we can terminate the intervention with the assurance that a possible rebound in the disease prevalence will not exceed Imax.
Our goal is to steer an arbitrary initial state (S0, I0) to the safe zone in minimal time without violating the constraint I(t) ≤ Imax. We say that an intervention achieving this goal is an optimal intervention.
In electronic supplementary material, note S1, we prove that the existence of an optimal intervention is characterized by the separating curve as follows:
1. An optimal intervention exists if and only if the initial state (S0, I0) lies below this separating curve (i.e. ).
Above, Rc : = (1 − umax)R0 is the controlled reproduction number. Moreover:
2. If it exists, the optimal intervention u* at the state (S, I) is
| 2.2 |
with
Above, the curve is defined in electronic supplementary material, note S1, while S* denotes the intersection of and .
Code to calculate the optimal interventions is provided as electronic supplementary material.
Figure 2.
Existence of non-pharmaceutical interventions in the Susceptible–Infected–Removed model. Parameters are γ = 1/7, β = 0.52 (i.e. R0 = 3.64) and Imax = 0.1. The safe zone (in blue) consists of all states that do not exceed Imax without interventions. This zone is characterized by the inequality . The plane is further divided into feasible states that can reach the safe zone without exceeding Imax (white), and unfeasible states that cannot (grey). Feasible and unfeasible states are separated by the separating curve (black line). (a) For ‘strong’ interventions with umax = 0.8, the controlled reproduction number is Rc = (1 − umax)R0 = 0.728 < 1. Here, the separating curve is the straight line Imax, implying that all states below Imax are feasible. Note this case corresponds to eradication. (b) For ‘intermediate’ interventions with umax = 0.6, the controlled reproduction number is Rc = (1 − umax)R0 = 1.456 > 1. Here, the separating curve is nonlinear, and some states below Imax are unfeasible. (c) For ‘weak’ interventions with umax = 0.4 we obtain Rc = 2.184 > 1. In this case, states with S(0) ≈ 1 are unfeasible. (d) For S(0) → 1, our design criterion for NPIs prescribes the values of Rc that a given Imax can manage.
2.3. A design criterion for optimal non-pharmaceutical interventions
We demonstrated above that optimal NPIs for outbreak mitigation exist even when Rc > 1. However, how large can Rc be before NPIs keeping I(t) ≤ Imax do not exist? When S(0) → 1, our characterization shows that such NPIs exists if and only if
| 2.3 |
The above inequality is our second main result, connecting the specified maximum disease prevalence Imax with the outbreak’s controlled reproduction number Rc = (1 − umax)R0 (electronic supplementary material, note S2). The inequality (2.3) governs the existence of NPIs for mitigating epidemic outbreaks, in analogy to how the condition Rc < 1 works for disease eradication. Note that Rc < 1 is a sufficient condition for the existence for NPIs, but the inequality (2.3) shows that this condition is far from necessary. If Imax > 0, there exists Rc > 1 for which NPIs exist (figure 2d). Note also that the maximum feasible Rc increases with Imax.
We can use (2.3) to design NPIs for outbreak mitigation as follows. Consider an infectious disease outbreak with a given R0 and that the specified maximum prevalence is Imax. Then, the inequality (2.3) gives the criterion to design NPIs by providing the range of disease transmission reduction umax that the NPIs should attain. In particular, it provides the minimal reduction in the disease transmission required for the existence of NPIs. For example, if Imax = 0.1 then is the maximum admissible controlled reproduction number (orange point in figure 2d). Therefore, if an outbreak in the population has R0 = 3, then the minimal required reduction is because .
2.4. Optimal non-pharmaceutical interventions for outbreak mitigation are simple
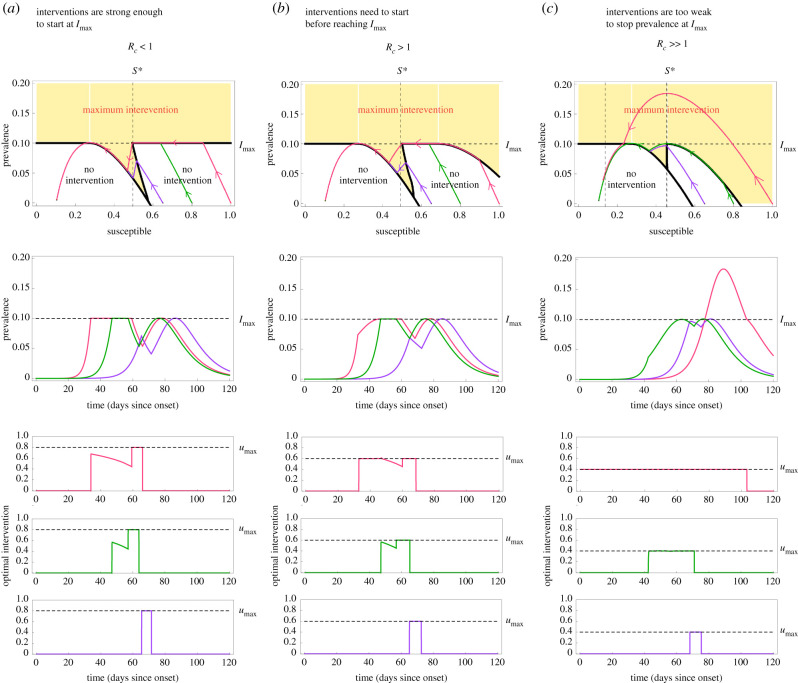
For any epidemic state, the optimal transmission reduction takes a simple form which can be described by colouring the (S, I) plane; see top row of figure 3. Here, for all states in the white region the optimal intervention is no intervention; for all states in the yellow region the optimal intervention is u*(S, I) = umax. There are regions (specifically lines) where the optimal intervention switches frequently between u* = 0 and u* = umax producing a so-called ‘singular arc’ that slides along the two regions, leading to an ‘average’ intervention u* ∈ [0, umax]. In general, we find that the optimal NPIs have four phases: a first one where no intervention is needed, a second phase where interventions start with maximum strength, a third phase of gradual decrease of interventions, and a ‘final push’ where the maximum interventions are re-applied for a short period to reach the safe zone faster.
Figure 3.
Optimal non-pharmaceutical interventions in the Susceptible–Infected–Removed model. For all panels, the parameters of the SIR model are γ = 1/7, β = 0.52 (i.e. R0 = 3.64) and Imax = 0.1. We consider a population of N = 8.855 × 106 individuals (like in Mexico City) and I0 = 1/N. Panels shows trajectories for three initial proportions of the susceptible population: large S0 = 1 − I0 ≈ 1 (pink), medium S0 = 0.8 (green) and small S0 = 0.65 (purple). (a) For umax = 0.8, we have Rc = (1 − umax)R0 = 0.728 ≤ 1. In this case, the optimal intervention starts when the disease prevalence reaches Imax. Afterwards, the intervention decreases in a hyperbolic arc until reaching the point S = S*. At that time, the intervention becomes maximum in the ‘final push’ to reach the safe zone. (b) For umax = 0.58, the controlled reproduction number is Rc = (1 − umax)R0 = 1.52 > 1. Here, , implying that the epidemic still can be mitigated for initial states with S0 ≈ 1 and I0 ≈ 0 (pink trajectory). In this case, the optimal intervention starts when the initial condition hits the separating curve below Imax at t = 35. At that instant, the intervention starts with the maximum value umax, and continues in that form until the trajectory reaches Imax. (c) Choosing umax = 0.4 yields Rc = 2.184 > 1. In this case, the optimal intervention problem does not have a solution for all initial states S0 > 0.85. This is illustrated by pink trajectory: even when applying the maximum intervention from the start, I(t) will grow beyond Imax.
We illustrate the above behaviour in three qualitatively different cases. The first case is when the optimal intervention starts just when the disease prevalence reaches Imax (figure 3a). This case occurs when the interventions are strong enough to stop the rise in prevalence at Imax regardless of the remaining fraction the population that is still susceptible to the disease. Our analysis shows that this case occurs if and only if umax is large enough to render Rc = (1 − umax)R0 ≤ 1. When the initial susceptible population is close to 1 (pink trajectory in figure 3a), the optimal intervention first waits until the disease prevalence reaches Imax. At that time, the optimal NPI stops the disease prevalence exactly at Imax, and then it gradually decreases its magnitude to ensure that the disease prevalence slides along Imax as the susceptible population decreases. When the susceptible population reaches the threshold S*, the optimal intervention is again the maximum one (figure 3a). This ‘final push’ allows reaching the safe zone faster, releasing the interventions sooner. The middle and bottom panels of figure 3a show the resulting disease prevalence and optimal interventions as a function of time. Note that a smaller initial susceptible population yields other trajectories (green and purple in figure 3a).
The second case is when an ‘early’ intervention is necessary before the disease prevalence reaches Imax (figure 3b). This case happens when the admissible reduction in the contact rate cannot immediately stop the disease prevalence at Imax if the susceptible population is large at that time. We find this case occurs if and only if umax is small in the sense that Rc = (1 − umax)R0 > 1. Here, a trajectory may hit the yellow region before reaching Imax (pink trajectory in figure 3b). When that happens, the optimal intervention starts with the maximum reduction u* = umax. Then it maintains this maximum reduction to ‘slide’ the trajectory between the yellow and white regions. Once the trajectory reaches Imax, the magnitude of the optimal intervention decreases to slide the trajectory along Imax. Again, the final push occurs when the susceptible population reaches the point S*.
The third case is when the initial state (S0, I0) lies in the unfeasible region (figure 3c). This case occurs when umax is so small that, even if the maximum admissible intervention u = umax is applied from the start of the outbreak, the disease prevalence will exceed Imax (pink trajectory in figure 3c). In this case, the optimal intervention problem is unfeasible because it is impossible to achieve I(t) ≤ Imax. However, note that using u* = umax yields the smallest prevalence peak. Other trajectories that start with a smaller proportion of susceptible individuals remain feasible (green and purple in figure 3c). In particular, note that the threshold S* decreases as Rc increases.
2.5. Optimal non-pharmaceutical interventions for outbreak mitigation are robust
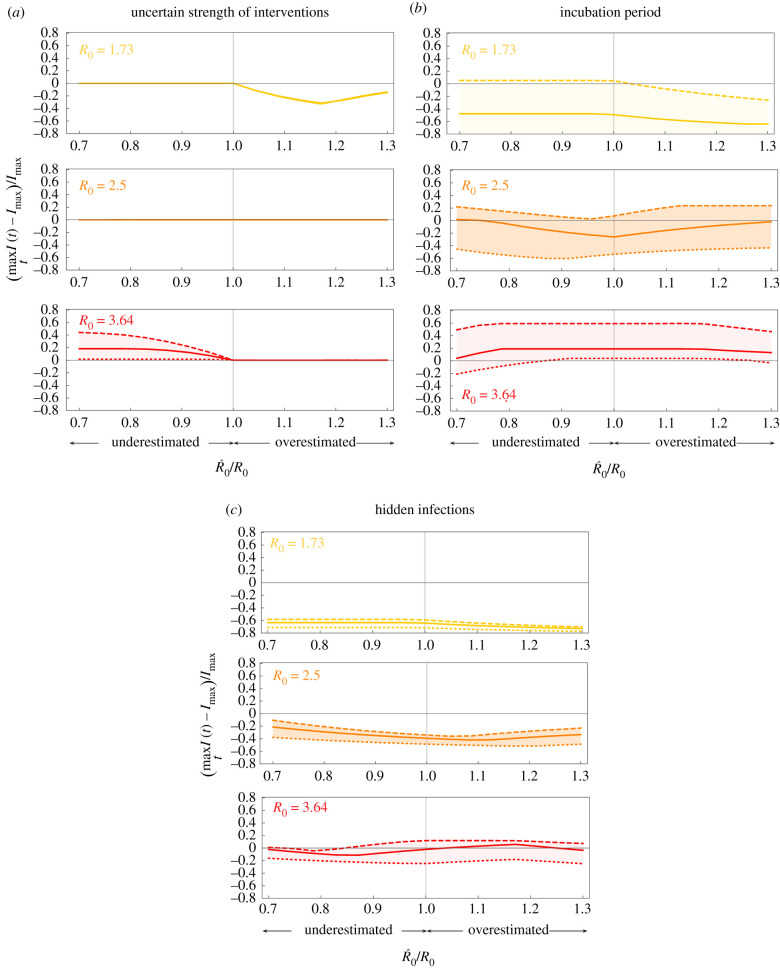
To evaluate the optimal NPIs for outbreak mitigation in more realistic scenarios, we numerically analysed their performance in three epidemic models with uncertain epidemic parameters and more detailed epidemic dynamics (see details in electronic supplementary material, note S3). In all cases, we consider that the basic reproduction number has been estimated as using an SIR model, and that the optimal NPIs for mitigation are designed using this estimate. Then, these optimal NPIs are applied to an outbreak with possibly different epidemic dynamics and possibly different R0. Note that estimation errors in R0 will affect the correct start of the NPIs and the ‘final push’ for reaching the safe zone.
In the first scenario, we consider an outbreak with SIR dynamics where the strength of the NPIs is uncertain. We model this uncertainty replacing u by ku in the model equations, where k ∈ (0, 1). Then, for example, k = 0.9 (resp. k = 1.1) represents a 10% underestimation (resp. overestimation) of the NPI strength. Across outbreaks with different R0’s and an uncertainty of 10% in the intervention’s strength, we find that the disease prevalence is maintained below Imax as long as R0 is not underestimated (figure 4a). In the second scenario, we consider an SEIR outbreak with an incubation period for the disease. For an incubation period of 7 days, as typical for a COVID-19 infection, the optimal NPIs maintain the disease prevalence below Imax if R0 < 2.5 and its value is estimated with an error of below 30% (solid yellow and orange in figure 4b). For larger R0 or a larger incubation period, the disease prevalence may exceed Imax (red in figure 4b).
Figure 4.
Optimal non-pharmaceutical interventions are robust. For all panels, the estimated parameters used for constructing the optimal NPIs are , , Imax = 0.1, umax = 0.6. We consider a population of N = 8.855 × 106 as in Mexico City, and the initial conditions I(0) = 1/N and S(0) = 1 − 1/N. If the models contain other state variables, they were initialized at zero. The optimal NPIs are constructed assuming , while the actual epidemic dynamics has a possibly different R0 = β/γ. Panels show results for outbreaks with three values of R0: low (yellow), medium (orange) and large (red). (a) SIR model where the reduction in the disease transmission by the NPIs is uncertain. We model this case replacing u by ku in the model equations. Panel shows the results for k = 1.1 (dotted), k = 1 (solid) and k = 0.9 (dashed). (b) SEIR model where exposed individuals do not transmit the infection, with λ > 0 the incubation period. Panel shows the results for λ = 1/5 (dotted), λ = 1/7 (solid) and λ = 1/11 (dashed). (c) SEIIR model with λ = 1/7 and two classes of infected individuals (symptomatic and asymptomatic). Here, p ∈ [0, 1] is the proportion of exposed individuals that become asymptomatic. The vertical axis denotes the disease prevalence for symptomatic individuals. The panel shows the results for p = 0.55 (dotted), p = 0.7 (solid) and p = 0.8 (dashed).
For the final scenario, we consider an SEIIR model with an incubation period of 7 days and with a fraction p ∈ [0, 1] of infected individuals that are asymptomatic and thus remain hidden to the epidemic surveillance system. The goal is to maintain the prevalence of symptomatic individuals below Imax, without knowing the fraction of asymptomatic individuals. This situation occurs during the COVID-19 pandemic, where between p = 0.55 and p = 0.8 of infections are asymptomatic [14]. For p < 0.7 and R0 < 3.64, the optimal NPIs maintain the disease prevalence of symptomatic individuals below or very close to Imax if the estimation error for R0 is below 30% (dotted and solid lines in figure 4c). An outbreak with low R0 produces a maximum disease prevalence of symptomatic individuals below Imax, which may result in a larger effective duration of the interventions. Overall, these numerical results show that the optimal NPIs are robust against a wide range of parameter uncertainty and unmodelled dynamics, provided that the estimation error in the outbreak’s basic reproduction number does not exceed 30%.
3. Designing optimal non-pharmaceutical interventions for mitigating the COVID-19 pandemic
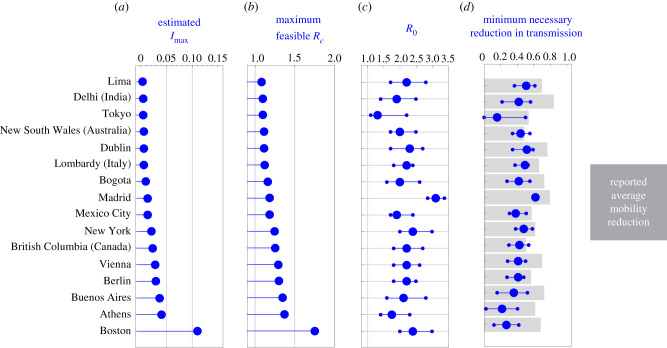
To explore the implications of our simple criterion for designing NPIs for outbreak mitigation, we analysed how 16 cities and regions implemented NPIs during the COVID-19 pandemic. For each region or city, we constructed Imax using the number of available intensive care beds during the first months of the pandemic, considering that a fraction of the infected individuals will require them (electronic supplementary material, note S4). The values for Imax that we obtain range from 2.87 × 10−3 for Lima (Peru) to 109.78 × 10−3 for Boston (USA), reflecting the large heterogeneity of the available health services across the globe (figure 5a). With this information, we calculated the maximum feasible for each region using our design criterion of inequality (2.3). Since is a monotone function of Imax, we find that follows the same trend as Imax (figure 5b). The smallest occurs for Lima and the largest for Boston. Note that in both cases . This result implies that, for the R0 of a region’s disease outbreak, a successful mitigation of the outbreak requires NPI policies that achieve at least a reduction such that .
Figure 5.
Minimum necessary reduction in disease transmission for NPIs in the COVID-19 pandemic. (a) Calculated Imax according to the proportion of available intensive care beds in each region or city and the estimated fraction of infected individuals requiring intensive care. (b) Maximum controlled reproduction number Rc that each region or city can handle according to its Imax. Larger Imax allows a larger Rc. (c) Basic reproduction number R0 per region or city before interventions started. Median (blue big dot), and 95% confidence interval (smaller dots) are shown. (d) Minimum umax necessary for feasibility for each region or city (blue) according to the R0 of (c). Grey bars denote the reported average mobility reduction in each region between 19 March and 30 April 2020.
Next, we investigated the minimal reduction in transmission required to achieve those upper bounds for the COVID-19 pandemic. For this, we first collected information for the R0 in each region calculated at the start of the pandemic and when the NPIs were inactive (electronic supplementary material, note S3). We find a median nominal R0 of 2.2, with Tokyo having the smallest one (R0 = 1.3) and Madrid having the largest one (R0 = 3.11); see figure 5c. From these values of R0, we calculated the minimal required reduction per region or city (blue in figure 5d). For the nominal R0’s per region or city, we find that a median reduction of of 0.42 is necessary. However, this minimal necessary reduction is heterogeneous across regions. For example, Tokyo just requires while Madrid requires . These two cities have the smallest and largest R0, respectively. If two cities have similar R0, then the city with large Imax ends up requiring a smaller (e.g. Boston with and Lima with ).
To evaluate the feasibility of achieving the minimal reduction predicted by our analysis, we collected data for the average mobility reduction in each region during the NPIs (grey in figure 5d; electronic supplementary material, note S4). Considering this average mobility reduction as a proxy for the reduction in disease transmission, we find that all regions achieved a greater than necessary reduction. For example, Delhi attained a mobility reduction of 0.84, while the minimal necessary reduction in transmission according to our analysis is . Other regions are in the boundary. For example, New South Wales attained a mobility reduction of 0.48, while the minimal necessary reduction in transmission was . Overall, across regions, we find a median excess of 0.22 in the reduction of mobility compared to the minimal reduction in transmission predicted by our analysis.
4. Discussion
Our results provide a complete analytical characterization of the optimal NPIs for mitigating epidemic outbreaks in the SIR model. We also show that these optimal NPIs are robust as they can ‘work’ in epidemic models with more complicated dynamics. The SIR model is a minimal strategic model of the general population dynamics of a disease. Although this model ignores critical epidemiological phenomena, using the SIR model allows us to leverage on its mathematical tractability to obtain a complete characterization of the optimal NPIs for outbreak mitigation. The feedback form u*(S, I) of the optimal intervention reflects such complete characterization, prescribing the optimal action to perform if the epidemic is at any state (S, I). This feedback strategy should be contrasted to most other studies applying optimal control to epidemic outbreaks, where the derived optimal intervention u*(t, S0, I0) is an open-loop function of time [15–18]. The open-loop intervention gives the optimal action at any time given a particular initial state (S0, I0). However, it does not tell us the optimal action if the epidemic is not in the exact state predicted by the model.
Understanding the optimal action to perform at any state has the crucial advantage of allowing us to apply this knowledge to any model, and to reality. Feedback can give control strategies the required robustness to work on real systems despite large uncertainties and unknown dynamics [19,20], and we numerically confirmed that our optimal NPIs for outbreak mitigation have such robustness. Indeed, other works have also found that interventions derived from the SIR model can work in detailed agent-based models of epidemic outbreaks [21]. Future work could analyse the robustness of optimal interventions when the epidemic state is not entirely known. This situation may happen when significant delays exist in reporting new infections, or when tests for identifying infected individuals are limited. For example, control-theoretical techniques like the construction of observers and predictors allow applying our optimal interventions when the only available information is the disease prevalence, and when this information is obtained with a significant delay [22].
Our framework could also guide the complete characterization of optimal NPIs for mitigating epidemic outbreaks using more detailed models or more detailed optimization objectives, but this is likely very challenging. Indeed, deriving such complete characterization for very detailed models can be unreasonable, considering the tradeoff between how detailed is a model and how much we can trust its predictions [23]. Note also that our approach could be applied to calculate the optimal NPIs in the presence of a constant vaccination rate by modifying the SIR model accordingly (e.g. [24]).
The optimal intervention resulting from our analysis can take a continuum of values that may be infeasible to implement in practice. We can use an averaging approach to circumvent this problem. Namely, consider a time window of T days (e.g. a week). Suppose that the average reduction prescribed by the optimal intervention over a certain window is . We can realize this reduction on average by combining days of maximum reduction with (T − d) days without intervention. This approach yields an intervention similar to Karin et al. [25], with the difference that the periods of intervention and activity are optimally balanced. Quantifying the errors produced by such approximations, in particular over the singular arc [26], deserves further study.
Our criterion to design optimal NPIs for mitigating epidemic outbreaks is obtained by characterizing the necessary and sufficient conditions for the existence of solutions to an optimal control problem. Specifically, the low dimensionality of the SIR model allowed us to apply Green’s theorem to compare the cost of any two interventions analytically (electronic supplementary material, note S1.4). In this sense, the method we use to derive the optimal NPIs is closer to our previous work on optimal control for bioreactors [27]. In general, deriving such complete characterization of optimal control problems is challenging because it involves solving an infinite-dimensional optimization [28]. Indeed, computational methods cannot produce such a characterization [29], and established analytical methods like Pontryagin’s maximum principle only yield necessary conditions for optimality [28]. Several works have applied these and other optimal control methods to the SIR model (e.g. [30,31]). The COVID-19 pandemic has produced a surge in the development of numerical and analytical methods to design optimal NPIs minimizing diverse criteria, including the infection peak [32], number of infections [33,34] and economic costs [35,36].
We will inevitably face new epidemics where NPIs are the only option to control the outbreaks. Rather counterintuitively, we find that for ‘ending’ an epidemic outbreak as fast as possible using NPIs, it is not always optimal to apply the maximum intervention. This observation illustrates the need for developing a better scientific understanding that informs the design of optimal NPIs and planning the required health services capacity.
Data accessibility
The data analysed are reported in electronic supplementary material, notes S3 and S4. Code supporting the results is archived in the Github repository: https://github.com/SyntheticDynamics/OptimalOutbreakMitigation.
Authors' contributions
M.T.A. conceived the project. F.C. did the mathematical analysis with the help of J.A.M. R.M.M., M.T.A. and F.C. did the numerical analysis. M.T.A. wrote the manuscript. All authors revised the manuscript.
Competing interests
We declare we have no competing interests.
Funding
M.T.A. acknowledges the financial support provided by CONACyT grant no. A1-S-13909. J.X.V.-.H. acknowledges support from DGAPA-PAPIIT-UNAM grant no. IV100220 (convocatoria especial COVID-19) and DGAPA-PAPIIT-UNAM grant no. IN115720.
References
- 1.Anderson RM, May RM. 1985. Vaccination and herd immunity to infectious diseases. Nature 318, 323-329. ( 10.1038/318323a0) [DOI] [PubMed] [Google Scholar]
- 2.Anderson RM, May RM. 1992. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Fraser C, Riley S, Anderson RM, Ferguson NM. 2004. Factors that make an infectious disease outbreak controllable. Proc. Natl Acad. Sci. USA 101, 6146-6151. ( 10.1073/pnas.0307506101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupferschmidt K. 2020. Ending coronavirus lockdowns will be a dangerous process of trial and error. Science—AAAS. ( 10.1126/science.abc2507) [DOI] [Google Scholar]
- 5.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. 2006. Strategies for mitigating an influenza pandemic. Nature 442, 448-452. ( 10.1038/nature04795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollingsworth TD, Klinkenberg D, Heesterbeek H, Anderson RM. 2011. Mitigation strategies for pandemic influenza A: balancing conflicting policy objectives. PLoS Comput. Biol. 7, e1001076. ( 10.1371/journal.pcbi.1001076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson N et al. 2020. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College COVID-19 Response Team.
- 8.Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. 2020. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 395, 931-934. ( 10.1016/S0140-6736(20)30567-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elston J, Cartwright C, Ndumbi P, Wright J. 2017. The health impact of the 2014–15 Ebola outbreak. Public Health 143, 60-70. ( 10.1016/j.puhe.2016.10.020) [DOI] [PubMed] [Google Scholar]
- 10.Kermack WO, McKendrick AG. 1927. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A 115, 700-721. ( 10.1098/rspa.1927.0118) [DOI] [Google Scholar]
- 11.Brauner JM et al. 2020. Inferring the effectiveness of government interventions against COVID-19. Science 371, eabd9338. ( 10.1126/science.abd9338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, Galvani AP. 2020. The implications of silent transmission for the control of COVID-19 outbreaks. Proc. Natl Acad. Sci. USA 117, 17 513-17 515. ( 10.1073/pnas.2008373117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKee M, Stuckler D. 2020. If the world fails to protect the economy, COVID-19 will damage health not just now but also in the future. Nat. Med. 26, 640-642. ( 10.1038/s41591-020-0863-y) [DOI] [PubMed] [Google Scholar]
- 14.For Evidence-Based Medicine OC. COVID-19: What proportion are asymptomatic? Available from: https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/.
- 15.Behncke H. 2000. Optimal control of deterministic epidemics. Optimal Control Appl. Methods 21, 269-285. ( 10.1002/oca.678) [DOI] [Google Scholar]
- 16.Yan X, Zou Y. 2008. Optimal and sub-optimal quarantine and isolation control in SARS epidemics. Math. Comput. Model. 47, 235-245. ( 10.1016/j.mcm.2007.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowthorn RE, Laxminarayan R, Gilligan CA. 2009. Optimal control of epidemics in metapopulations. J. R. Soc. Interface 6, 1135-1144. ( 10.1098/rsif.2008.0402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caetano MAL, Yoneyama T. 2001. Optimal and sub-optimal control in Dengue epidemics. Optimal Control Appl. Methods 22, 63-73. ( 10.1002/oca.683) [DOI] [Google Scholar]
- 19.Åström KJ, Murray RM. 2010. Feedback systems: an introduction for scientists and engineers. Princeton, NJ: Princeton University Press. [Google Scholar]
- 20.Angulo MT, Velasco-Hernandez JX. 2018. Robust qualitative estimation of time-varying contact rates in uncertain epidemics. Epidemics 24, 98-104. ( 10.1016/j.epidem.2018.03.001) [DOI] [PubMed] [Google Scholar]
- 21.Di Lauro F, Kiss IZ, Rus D, Della Santina C. 2020. COVID-19 and flattening the curve: a feedback control perspective. IEEE Control Syst. Lett. 5, 1435-1440. ( 10.1109/LCSYS.2020.3039322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castaños F, Mondié S. In press. Observer-based predictor for a susceptible-infectious-recovered model with delays: an optimal-control case study. Int. J. Robust Nonlinear Control. ( 10.1002/rnc.5522) [DOI]
- 23.Saltelli A et al. 2020. Five ways to ensure that models serve society: a manifesto. London, UK: Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 24.Shulgin B, Stone L, Agur Z. 1998. Pulse vaccination strategy in the SIR epidemic model. Bull. Math. Biol. 60, 1123-1148. ( 10.1016/S0092-8240(98)90005-2) [DOI] [PubMed] [Google Scholar]
- 25.Karin O et al. 2020. Adaptive cyclic exit strategies from lockdown to suppress COVID-19 and allow economic activity. medRxiv. ( 10.1101/2020.04.04.20053579) [DOI] [Google Scholar]
- 26.Fridman LM. 2002. Singularly perturbed analysis of chattering in relay control systems. IEEE Trans. Autom. Control 47, 2079-2084. ( 10.1109/TAC.2002.805672) [DOI] [Google Scholar]
- 27.Moreno J. 1999. Optimal time control of bioreactors for the wastewater treatment. Optimal Control Appl. Methods 20, 145-164. () [DOI] [Google Scholar]
- 28.Lenhart S, Workman JT. 2007. Optimal control applied to biological models. New York, NY: CRC Press. [Google Scholar]
- 29.Bonnans F, Martinon P, Grélard V. 2012. Bocop: a collection of examples.
- 30.Hansen E, Day T. 2011. Optimal control of epidemics with limited resources. J. Math. Biol. 62, 423-451. ( 10.1007/s00285-010-0341-0) [DOI] [PubMed] [Google Scholar]
- 31.Di Giamberardino P, Iacoviello D. 2017. Optimal control of SIR epidemic model with state dependent switching cost index. Biomed. Signal Process. Control 31, 377-380. ( 10.1016/j.bspc.2016.09.011) [DOI] [Google Scholar]
- 32.Morris DH, Rossine FW, Plotkin JB, Levin SA. 2020. Optimal, near-optimal, and robust epidemic control. (http://arxiv.org/abs/200402209)
- 33.Tsay C, Lejarza F, Stadtherr MA, Baldea M. 2020. Modeling, state estimation, and optimal control for the US COVID-19 outbreak. (http://arxiv.org/abs/200406291) [DOI] [PMC free article] [PubMed]
- 34.Bliman PA, Duprez M. 2021. How best can finite-time social distancing reduce epidemic final size? J. Theor. Biol. 511, 110557. ( 10.1016/j.jtbi.2020.110557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez FE, Argente D, Lippi F. In press. A simple planning problem for COVID-19 lockdown, testing, and tracing. Am. Econ. Rev. Insights. [Google Scholar]
- 36.Piguillem F, Shi L. 2020. Optimal COVID-19 quarantine and testing policies. EIEF Working Papers Series 2004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analysed are reported in electronic supplementary material, notes S3 and S4. Code supporting the results is archived in the Github repository: https://github.com/SyntheticDynamics/OptimalOutbreakMitigation.