Abstract
This study aimed to investigate the levels of creatine (Cr) metabolites in the anterior cingulate cortex (ACC), thalamus, and insula of patients with fibromyalgia (FM) using proton magnetic resonance spectroscopy (MRS). The levels of Cr and phosphocreatine (PCr) relative to total Cr (tCr), which includes Cr and PCr, in the ACC, thalamus, and insula were determined using MRS in 12 patients with FM and in 13 healthy controls. The FM group had lower levels of PCr/tCr in the ACC and right insula compared to healthy controls. There was a negative correlation between Cr/tCr in the ACC and total pain levels (McGill Pain Questionnaire-Total; r = −0.579, p = 0.049) and between Cr/tCr in the left insula and affective pain levels (McGill Pain Questionnaire-Affective; r = −0.638, p = 0.047) in patients with FM. In addition, there were negative correlations between stress levels (Stress Response Inventory) and Cr/tCr in the right (r = −0.780, p = 0.005) and left thalamus (r = −0.740, p = 0.006), as well as in the right insula (r = −0.631, p = 0.028) in patients with FM. There were negative correlations between symptom levels of post-traumatic stress disorder (PTSD; PTSD checklist) and Cr/tCr in the right (r = −0.783, p = 0.004) and left thalamus (r = −0.642, p = 0.024) of patients with FM. These findings are paramount to understanding the decisive pathologies related to brain energy metabolism in patients with FM.
Keywords: Fibromyalgia, proton magnetic resonance spectroscopy, creatine, phosphocreatine, energy, stress, post-traumatic stress disorder
Introduction
Fibromyalgia (FM) is characterized by widespread pain that is often accompanied by fatigue, memory problems, and sleep disturbances.1 This disease is also frequently associated with depression, anxiety, and posttraumatic stress disorder (PTSD).2,3 Alterations in both peripheral and central pain processing contribute to central sensitization in FM.4 Chronic pain is maintained, in part, by central sensitization, which is also driven by neuroinflammation in the peripheral (PNS) and central nervous systems (CNS).5
Despite representing only 2% of a person’s total body mass, the brain consumes approximately 20% of the produced energy and is dedicated to cerebral functions.6 Creatine (Cr) is a key regulator of brain energy homeostasis, and a well-balanced Cr metabolism is central in proper brain functioning.7 Creatine facilitates recycling of adenosine triphosphate (ATP) and is a particularly convenient form of energy storage primarily in muscle and brain tissue.8 Creatine also plays a crucial role in rapid energy provision during muscle contraction and involves the transfer of an N-phosphoryl group from phosphorylcreatine to ADP to regenerate ATP through a reversible reaction catalyzed by Cr kinase.9 During periods of low energy demand, ATP can be used to convert Cr to phosphocreatine (PCr), which functions as energy storage. Diet and endogenous synthesis contribute to Cr levels in the brain,7 and deficiencies in Cr metabolism are associated with psychological stress, schizophrenia, and mood and anxiety disorders.10 Creatine supplementation has shown promise as a safe, effective, and tolerable adjunct to medications used to treat neurologic disorders linked with dysfunctional energy metabolism, such as Huntington's Disease and Parkinson's Disease.10 Furthermore, Cr supplementation has been reported to be a useful dietary intervention to improve muscle function in FM patients.9 Alterations in cellular energy metabolism contributes to chronic pain and FM.11 Significantly lower concentrations of PCr in erector spinae were found in FM.12 Thus, to detect the levels of Cr and PCr in brain may contribute to the understanding of pathology related to energy metabolism in FM.
The anterior cingulate cortex (ACC) is critically involved in the response inhibition network in the pain system,13 and ACC links the processes of cognitive interference and parasympathetic modulation with activation in the ACC, a structure critical for the interface between cognition and emotion.14 Patients suffering with chronic pain, often experience more activation of brain regions involved in cognitive and/or emotional pain processing.15 Considering that the clinical features of FM are associated with various psychological symptoms related to stress and PTSD,16,17 ACC related to emotion, cognition and pain is regarded as important region in FM. The somatosensory system involving pain perception includes the thalamus, the primary somatosensory cortex, and the insula.18 Therefore. we hypothesized there would be an abnormal neurometabolites (Cr and PCr) in the ACC, thalamus, and insula involved in the pain processing pathway.19
The essential role of Cr as a natural regulator of energy homeostasis is less known, and Cr metabolism in the brain has been largely unattended in magnetic resonance spectroscopy (MRS) research. This study aimed to investigate the levels of Cr metabolites in the ACC, right and left thalamus, and insula of patients with FM and compare the results with healthy controls using proton MRS. The levels of Cr and PCr relative to total Cr (tCr) levels, which include both Cr and PCr, in the ACC, right and left thalamus, and insula were determined in 12 patients with FM and 13 healthy controls using MRS. Furthermore, we investigated the relationships between the levels of Cr/tCr and PCr/tCr metabolites and pain and psychiatric symptoms in patients with FM.
Methods
Participants
This study recruited 12 patients from the National University Hospital who fulfilled the criteria of the American College of Rheumatology for FM.20 Thirteen individuals of comparable age and gender with the FM patients, and who exhibited no pain or neurological symptoms, were used as healthy controls. Subjects who exhibited leukocytosis or high levels of high-sensitivity C-reactive protein (hs-CRP) were excluded. The inclusion criteria for FM subjects were as follows: diagnosed with FM; between 21 and 63 years of age; and not taking benzodiazepine or will discontinue benzodiazepine 2 weeks before the study. Patients who 1) were diagnosed with a major neuropsychiatric disorder before the diagnosis of FM, 2) have concurrent neurological disease (cerebrovascular disease or brain tumors), 3) have a history of brain trauma, 4) have leukocytosis or high levels of hs-CRP, or 5) could not undergo PET/magnetic resonance imaging (MRI) were excluded. The sensory and affective dimensions of each patient’s current level pain were assessed using the Short-Form McGill pain Questionnaire (SF-MPQ), which contains 11 McGill Pain Questionnaire-Sensory (MPQ-S) and four McGill Pain Questionnaire-Affective (MPQ-A) pain items.21 The visual analogue scale (VAS) was used to measure pain intensity. Stress levels in FM patients were assessed using the Stress Response Inventory (SRI), which consists of 39 items (score range, 0–156) categorized into seven factors: fatigue, tension, frustration, anger, depression, somatization, and aggression.22 The PTSD checklist was used to assess the development of PTSD in respondents after exposure to a traumatic event.23 This study was approved by the Institutional Review Board of the Seoul National University Hospital. All data were obtained under written informed consent that was granted by all subjects after receiving a full explanation of the experimental methods.
1H-MRS data acquisition and processing
All magnetic resonance (MR) data were collected using a 3.0-T human MR scanner with a 16-channel head and neck coil (Siemens Trio system; Siemens Medical Solutions, Erlangen, Germany). For 1H-MRS volume localization, anatomical images were collected using a T2-weighted fast spin echo sequence along the axial (axi), sagittal (sag), and coronal (cor) planes (repetition time [TR] = 6090 ms [axi], 5910 ms [sag and cor], echo time [TE] = 89 ms, flip angle = 90°/130°, field of view = 220 × 199 mm2 [axi and cor], 220 × 220 mm2 [sag], matrix size = 256 × 180, echo train length = 5, echo spacing = 9.93 ms, receiver bandwidth [BW] = 271 Hz/pixel, number of slices = 30 [no gap], slice thickness = 5 mm, number of signal averages [NSA] = 128).
Afterwards, based on scout images, five volumes of interest (VOIs) were selected in the ACC (2 × 2 × 2 cm3), right and left thalamus (2 × 2 × 1.5 cm3), and right and left insula (2 × 1.5 × 2 cm3) of each subject. The VOIs in the right and left thalamus were placed along the axis of the thalamus to cover maximum volume. Following auto shimming over the VOI, 1H-MRS data were acquired using a point-resolved spectroscopy pulse sequence (PRESS)24 with TR/TE = 2000/30 ms, 2048 data points, BW = 2500 Hz, NSA = 128, four dummy scans, and four-step phase cycling. The main PRESS sequence was preceded by water and outer-volume suppression modules. The carrier frequency was adjusted to −2.3 ppm from the water resonance to minimize voxel displacement.
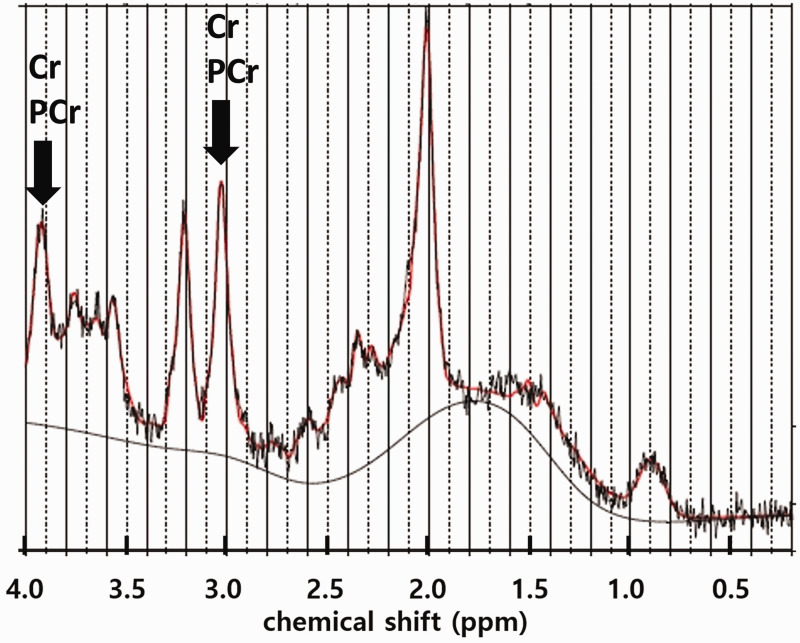
The 1H-MRS data were analyzed using LC Model25 (ver. 6.3.1J) in the range of 4.2 ppm to 1.0 ppm. The final data analysis included only those metabolites with a Cramer–Rao lower bound (CRLB) < 30%. Because of this cutoff, the number of samples used in each metabolite was different for each result. An example of the MRS spectrum acquired from the right insula of a patient is shown in Figure 1.
Figure 1.
A representative 1H-MRS spectrum acquired from the right insula of a patient. The co-resonating Cr and PCr signals at ∼3.0 and ∼3.9 ppm are marked with arrows. Cr: creatine; PCr: phosphocreatine.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 21.0 (SPSS, Chicago, IL). The differences in MRS between healthy controls and patients with FM were assessed using a two-tailed Student’s t-test. After the data were tested for normality, the Mann-Whitney U test was used to analyze data that were not normally distributed. Pearson’s correlation analysis was used to evaluate the association between psychological test scores and the levels of neurometabolites measured using MRS. P-values <0.05 were considered statistically significant without correction for multiple comparisons.
Results
Study participants and basic information
A total of 12 FM patients and 13 healthy controls completed the study; their demographics and clinical characteristics are presented in Table 1. Age, gender ratio, and education level were not significantly different between the two groups. The average duration of pain in patients with FM was 4.4 years.
Table 1.
Participant's demographic characteristics.
| FM patients | Healthy controls | p-value | |
|---|---|---|---|
| N | 12 | 13 | |
| Age | 41.7 ± 14.0 | 40.1 ± 6.3 | p = 0.723 |
| Gender | 5M, 7 F | 9M, 4 F | p = 0.165 |
| Education (years) | 14.4 ± 2.6 | 16.2 ± 2.1 | p = 0.068 |
| Duration of illness (years) | 4.4 ± 3.1 | N/A |
Data are mean ± standard deviation. FM: Fibromyalgia, M: male, F: female.
Differences in Cr/tCr and PCr/tCr levels between FM patients and controls
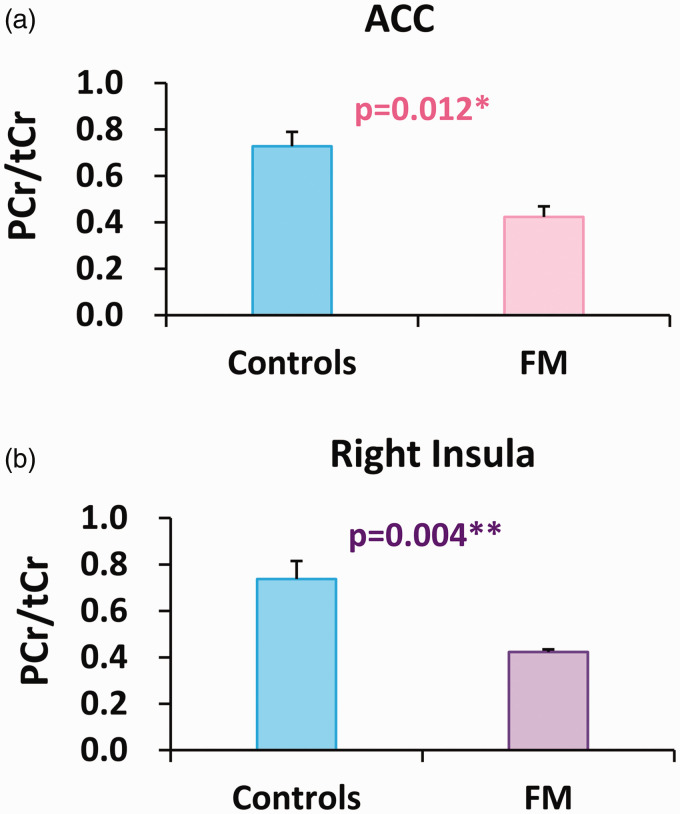
Lower levels of PCr/tCr were observed in the ACC (controls: n = 7, FM: n = 5, p = 0.012; Figure 2(a)) and the right insula (controls: n = 9, FM: n = 4, p = 0.004; Figure 2(b)) of FM patients compared with controls. Higher levels of Cr/tCr were observed in the right insula of FM patients compared with controls (controls: n = 6, FM: n = 12, p = 0.037; Table 2).
Figure 2.
Decreases in PCr/tCr levels in ACC and right insula in FM compared with controls. FM: fibromyalgia; PCr: phosphocreatine; tCr: total creatine (creatine + PCr); ACC: anterior cingulate cortex. *p < 0.05, **p < 0.01. (a) controls: n = 7, FM: n = 5. (b) controls: n = 9, FM: n = 4.
Table 2.
Comparison of neurometabolites between FM patients and healthy controls.
| Metabolites | Groups | N | mean | SD | p-value |
|---|---|---|---|---|---|
| Anterior cingulate cortex | |||||
| Cr/tCr | CON | 9 | 0.634 | 0.264 | p = 0.434 |
| FM | 12 | 0.718 | 0.199 | ||
| PCr/tCr | CON | 7 | 0.728 | 0.164 | p = 0.012 |
| FM | 5 | 0.477 | 0.103 | ||
| Right thalamus | |||||
| Cr/tCr | CON | 13 | 0.853 | 0.223 | p = 0.616 |
| FM | 11 | 0.817 | 0.233 | ||
| Left thalamus | |||||
| Cr/tCr | CON | 10 | 0.884 | 0.141 | p = 0.971 |
| FM | 12 | 0.876 | 0.169 | ||
| Right insula | |||||
| Cr/tCr | CON | 6 | 0.580 | 0.155 | p = 0.037 |
| FM | 12 | 0.780 | 0.185 | ||
| PCr/tCr | CON | 9 | 0.737 | 0.234 | p = 0.004 |
| FM | 4 | 0.423 | 0.023 | ||
| Left insula | |||||
| Cr/tCr | CON | 8 | 0.681 | 0.234 | p = 0.668 |
| FM | 10 | 0.728 | 0.225 | ||
| PCr/tCr | CON | 8 | 0.661 | 0.220 | p = 0.364 |
| FM | 8 | 0.553 | 0.240 | ||
Bold letter: significant results. CON: healthy controls; FM: fibromyalgia.
Correlations between Cr/tCr and PCr/tCr levels and pain, stress, and PTSD symptoms in FM patients
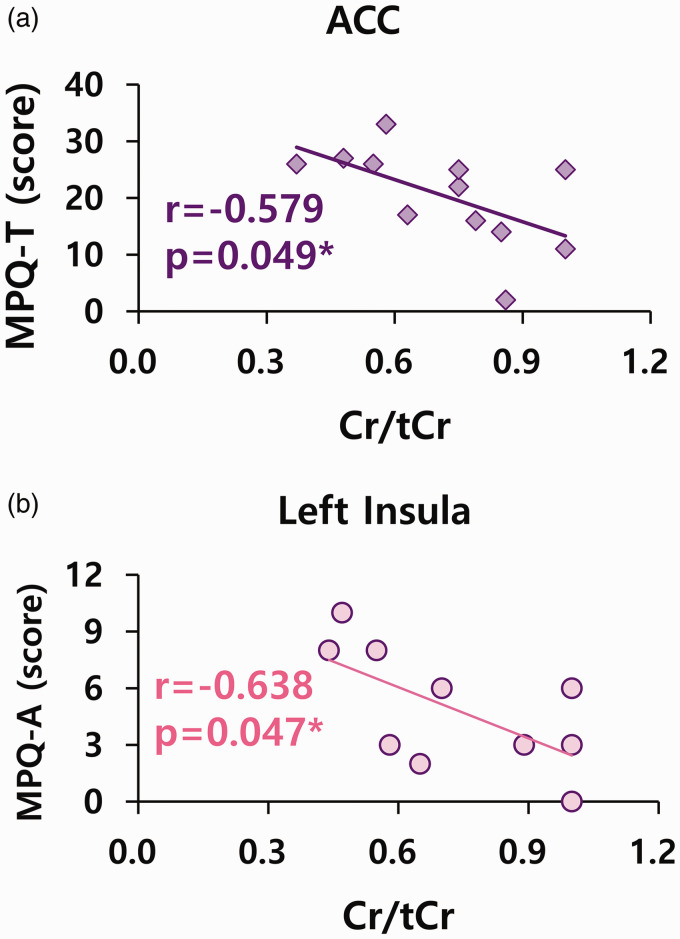
First, there was a significant correlation between Cr/tCr in the ACC and pain levels in patients with FM. There was a negative correlation between Cr/tCr in the ACC and total pain levels (MPQ-T) in FM patients (n = 12, r = −0.579, p = 0.049; Figure 3(a)). There was also a negative correlation between Cr/tCr in the left insula and affective pain levels (MPQ-A) in FM patients (n = 10, r = −0.638, p = 0.047; Figure 3(b)). A negative correlation was also observed between pain intensity (VAS) and Cr/tCr in the right (n = 10, r = −0.749, p = 0.005) and left insula (n = 12, r = −0.713, p = 0.021) of FM patients. Additionally, a negative correlation was found between PCr/tCr in the left insula and sensory pain levels (MPQ-S) (n = 8, r = −0.764, p = 0.027).
Figure 3.
Correlations between Cr/tCr in ACC and left insula and pain levels in FM. Cr: creatine; tCr: total creatine (Cr + phosphocreatine); ACC: anterior cingulate cortex. *p < 0.05. (a) n = 12. (b) n = 10.
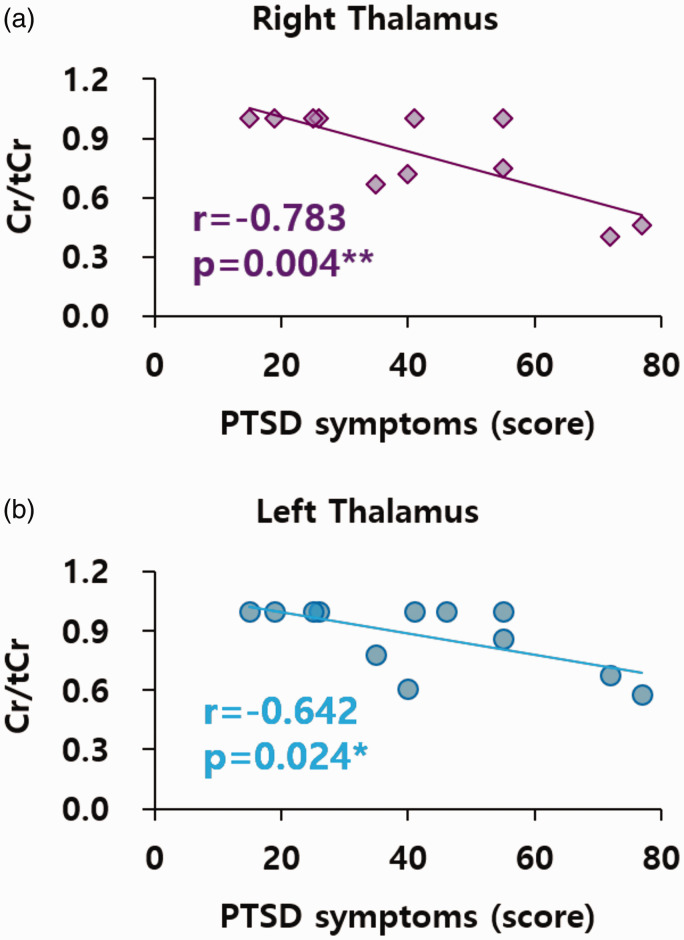
Second, there were significant correlations between Cr/tCr and stress and PTSD in FM patients. There was a negative correlation between stress (SRI) and Cr/tCr in the right (n = 11, r = −0.780, p = 0.005) and left thalamus (n = 12, r = −0.740, p = 0.006) of FM patients. In addition, a negative correlation was observed between stress (SRI) and Cr/tCr in the right insula of the FM group (n = 12, r = −0.631, p = 0.028). There was a negative correlation between PTSD and Cr/tCr in the right (n = 11, r = −0.783, p = 0.004; Figure 4(a)) and left thalamus (n = 12, r = −0.642, p = 0.024; Figure 4(b)) of FM patients. Additionally, there was a positive correlation between the duration of pain and Cr/tCr in the ACC (n = 11, r = 0.694, p = 0.018) and left insula (n = 9, r = 0.705, p = 0.034) of the FM group.
Figure 4.
Correlations between Cr/tCr in right and left thalamus and PTSD symptoms in FM. Cr: creatine; tCr: total creatine (Cr + phosphocreatine); PTSD: post-traumatic stress disorder. *p < 0.05, **p < 0.01. (a) n = 11. (b) n = 12.
Discussion
Lower levels of PCr/tCr were found in the ACC and right insula of the FM group compared with healthy controls. Low levels of Cr/tCr were associated with high pain levels in FM patients. In addition, low levels of Cr/tCr were correlated with high stress (SRI) and PTSD levels in FM patients.
Lower levels of PCr/tCr were observed in the ACC and right insula of FM patients compared with healthy controls; this deficiency in PCr/tCr may contribute to abnormal Cr energy metabolism and deficient energy levels, which might be related to the brain pathology in FM. Creatine supplementation increases intramuscular phosphorylcreatine content and improves lower- and upper-body muscle function in patients with FM.9 Patients with FM have reduced intramuscular ATP and PCr content compared to healthy subjects.26–28 The platelets of FM patients had significantly lower ATP levels, suggesting that disturbances in the homeostasis of platelet ATP metabolism may play a role in the pathogenesis of FM.29 One explanation for the lower concentrations of ATP and PCr in FM patients is the presence of mitochondrial alterations.28 Mitochondrial dysfunction has been demonstrated in muscle cells of FM patients30; if mitochondrial dysfunction is also present in the central neurons, this could result in lower ATP levels leading to generalized hypersensitivity and chronic widespread pain.30 Inflammation is implicated in the pathophysiology of FM and may be a mitochondrial dysfunction-dependent event.31 Considering that Cr modulates GABAergic and glutamatergic cerebral pathways,8 lower PCr/tCr levels may cause dysfunctional pain modulation in the cerebral pain pathway, particularly in those two pathways. Considering that the glutamate/Cr ratios within the bilateral ventrolateral prefrontal cortex were significantly higher in patients with FM compared to the controls,32 low PCr levels may cause dysfunctions in glutamate neurotransmission in the brains of FM patients.
In a previous report, pain was inversely correlated with ATP and PCr levels27; in this study, low levels of Cr/tCr were associated with high pain levels in patients with FM. Increased levels of inflammation are related to pain in FM, suggesting that oxidative stress, mitochondrial dysfunction, and inflammation may have a role in its pathophysiology.33 Considering the analgesic potential of Cr and its effect on inflammation-based nociception,34 lower levels of Cr may contribute to mitochondrial dysfunction, inflammation, and pain in this disease.33
Low levels of Cr/tCr were correlated with high stress and PTSD levels in FM patients in this study. Considering that a single, prolonged stress event decreased glutamate, glutamine, and Cr concentrations in the medial prefrontal cortex of rats,35 high stress levels may lead to low Cr/tCr levels in FM patients. Patients with FM reported a higher percentage of exposure to trauma, more traumatic experiences, and more PTSD symptoms compared to the control group.36 Furthermore, reduced Cr levels were found in the left hippocampus and bilateral occipital white matter in the PTSD group.37 Thus, high stress levels and PTSD may decrease Cr/tCr in FM patients. Creatine can act as a “spatial energy buffer” by transferring energy from the mitochondria to the cytosol.9 Psychological stress induces metabolic and neuroendocrine changes that lead to structural and functional recalibrations of the mitochondria.38 Because mitochondrial dysfunction has also been observed in patients with FM,39 it is possible that, in these patients, stress and mitochondrial dysfunction may lead to decreased energy levels secondary to decreased Cr, ultimately contributing to the pathophysiology of FM. If mitochondrial dysfunction is also present in central neural cells, this could result in lower ATP pools in these cells and lead to generalized hypersensitivity and chronic widespread pain.30 Cognitive stress may have acute negative effects on pain modulation in patients with FM.40 Therefore, high stress and mitochondrial dysfunction may lead to deficiencies in Cr-derived energy and high pain levels. Oral Cr administration may improve short-term memory and intelligence/reasoning in healthy individuals.41 Considering that cognitive dysfunction and poorer memory functions are found in patients with FM,42,43 Cr supplementation may be a useful intervention to alleviate these effects.9 However, there was a positive correlation between pain duration and Cr/tCr in the ACC and left insula of patients with FM. The ACC is a critical structure for the interface between cognition and emotion14 and is related to the response inhibition network in the pain system.13 An increase in Cr/tCr, which depends on the duration of pain, may be associated with an increase in protective mechanisms in cognition, emotion, and the pain inhibition network of patients with FM.
This study has a few limitations. Our study examined only a small number of patients with FM. Thus, future studies with larger sample sizes will be required to generalize these findings for FM patients.
The FM group was demonstrated to have lower energy levels compared to the control group due to decreased PCr/tCr in the ACC and right insula. Low Cr/tCr was associated with high pain, stress, and PTSD levels in FM. That is, high stress may contribute to low Cr/tCr, and energy deficiency from low Cr/tCr and PCr/tCr may be associated with high pain levels in FM. These findings represent a decisive pathology related to energy metabolism in the brains of patients with FM.
Acknowledgements
The authors thank the technical contribution of Da Ye Lee. The authors would also like to thank all the participants for their valuable time in being involved in this research.
Footnotes
Authors’ Contributions: YHJ analyzed and interpreted the data, and conceived and designed the analysis. YHJ, SHC, DHK wrote the manuscript. HK provided imaging preprocessing, and wrote the methods. DL and JYL collected the data. JYM and SHC contributed to revising the manuscript. SHC obtained funding. DHK contributed to the study concept and design. SHC and DHK approved the final content of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. NRF- 2019R1A2C1086581).
ORCID iD: Do-Hyung Kang https://orcid.org/0000-0002-8741-5748
References
- 1.Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311: 1547–1555. [DOI] [PubMed] [Google Scholar]
- 2.Chinn S, Caldwell W, Gritsenko K. Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep 2016; 20: 25. [DOI] [PubMed] [Google Scholar]
- 3.Yavne Y, Amital D, Watad A, Tiosano S, Amital H. A systematic review of precipitating physical and psychological traumatic events in the development of fibromyalgia. Semin Arthritis Rheum 2018; 48: 121–133. [DOI] [PubMed] [Google Scholar]
- 4.Rahman A, Underwood M, Carnes D. Fibromyalgia. BMJ 2014; 348: g1224. [DOI] [PubMed] [Google Scholar]
- 5.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018; 129: 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 2011; 14: 724–738. [DOI] [PubMed] [Google Scholar]
- 7.Hjelmervik H, Hausmann M, Craven AR, Hirnstein M, Hugdahl K, Specht K. Sex- and sex hormone-related variations in energy-metabolic frontal brain asymmetries: a magnetic resonance spectroscopy study. Neuroimage 2018; 172: 817–825. [DOI] [PubMed] [Google Scholar]
- 8.Joncquel-Chevalier Curt M, Voicu PM, Fontaine M, Dessein AF, Porchet N, Mention-Mulliez K, Dobbelaere D, Soto-Ares G, Cheillan D, Vamecq J. Creatine biosynthesis and transport in health and disease. Biochimie 2015; 119: 146–165. [DOI] [PubMed] [Google Scholar]
- 9.Alves CR, Santiago BM, Lima FR, Otaduy MC, Calich AL, Tritto AC, de Sá Pinto AL, Roschel H, Leite CC, Benatti FB, Bonfá E, Gualano B. Creatine supplementation in fibromyalgia: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken) 2013; 65: 1449–1459. [DOI] [PubMed] [Google Scholar]
- 10.Allen PJ. Creatine metabolism and psychiatric disorders: does creatine supplementation have therapeutic value? Neurosci Biobehav Rev 2012; 36: 1442–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Tilburg MAL, Parisien M, Boles RG, Drury GL, Smith-Voudouris J, Verma V, Khoury S, Chabot-Doré AJ, Nackley AG, Smith SB, Whitehead WE, Zolnoun DA, Slade GD, Tchivileva I, Maixner W, Diatchenko L. A genetic polymorphism that is associated with mitochondrial energy metabolism increases risk of fibromyalgia. Pain 2020; 161: 2860–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerdle B, Ghafouri B, Lund E, Bengtsson A, Lundberg P, Ettinger-Veenstra HV, Leinhard OD, Forsgren MF. Evidence of mitochondrial dysfunction in fibromyalgia: deviating muscle energy metabolism detected using microdialysis and magnetic resonance. J Clin Med 2020; 9: 3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt-Wilcke T, Kairys A, Ichesco E, Fernandez-Sanchez ML, Barjola P, Heitzeg M, Harris RE, Clauw DJ, Glass J, Williams DA. Changes in clinical pain in fibromyalgia patients correlate with changes in brain activation in the cingulate cortex in a response inhibition task. Pain Med 2014; 15: 1346–1358. [DOI] [PubMed] [Google Scholar]
- 14.Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage 2004; 22: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 15.Henry DE, Chiodo AE, Yang W. Central nervous system reorganization in a variety of chronic pain states: a review. PM&R 2011; 3: 1116–1125. [DOI] [PubMed] [Google Scholar]
- 16.Malin K, Littlejohn GO. Psychological factors mediate key symptoms of fibromyalgia through their influence on stress. Clin Rheumatol 2016; 35: 2353–2357. [DOI] [PubMed] [Google Scholar]
- 17.Toussaint LL, Whipple MO, Vincent A. Post-traumatic stress disorder symptoms may explain poor mental health in patients with fibromyalgia. J Health Psychol 2017; 22: 697–706. [DOI] [PubMed] [Google Scholar]
- 18.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9: 463–463. [DOI] [PubMed] [Google Scholar]
- 19.Linnman C, Becerra L, Borsook D. Inflaming the brain: CRPS a model disease to understand neuroimmune interactions in chronic pain. J Neuroimmune Pharmacol 2013; 8: 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010; 62: 600–610. [DOI] [PubMed] [Google Scholar]
- 21.Melzack R. The short-form McGill pain questionnaire. Pain 1987; 30: 191–197. [DOI] [PubMed] [Google Scholar]
- 22.Koh KB, Park JK, Kim CH, Cho S. Development of the stress response inventory and its application in clinical practice. Psychosom Med 2001; 63: 668–678. [DOI] [PubMed] [Google Scholar]
- 23.Blevins CA, Weathers FW, Davis MT, Witte T, Domino JL. The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): development and initial psychometric evaluation. J Trauma Stress 2015; 28: 489–498. [DOI] [PubMed] [Google Scholar]
- 24.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann NY Acad Sci 1987; 508: 333–348. [DOI] [PubMed] [Google Scholar]
- 25.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson A, Henriksson KG, Larsson J. Reduced high-energy phosphate levels in the painful muscles of patients with primary fibromyalgia. Arthritis Rheum 1986; 29: 817–821. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Phothimat P, Oates CT, Hernanz-Schulman M, Olsen NJ. Use of P-31 magnetic resonance spectroscopy to detect metabolic abnormalities in muscles of patients with fibromyalgia. Arthritis Rheum 1998; 41: 406–413.9506567 [Google Scholar]
- 28.Gerdle B, Forsgren MF, Bengtsson A, Leinhard OD, Sören B, Karlsson A, Brandejsky V, Lund E, Lundberg P. Decreased muscle concentrations of ATP and PCR in the quadriceps muscle of fibromyalgia patients – a 31P-MRS study. Eur J Pain 2013; 17: 1205–1215. [DOI] [PubMed] [Google Scholar]
- 29.Bazzichi L, Giannaccini G, Betti L, Fabbrini L, Schmid L, Palego L, Giacomelli C, Rossi A, Giusti L, De Feo F, Giuliano T, Mascia G, Bombardieri S, Lucacchini A. ATP, calcium and magnesium levels in platelets of patients with primary fibromyalgia. Clin Biochem 2008; 41: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 30.Meeus M, Nijs J, Hermans L, Goubert D, Calders P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: peripheral and central mechanisms as therapeutic targets? Expert Opin Ther Targets 2013; 17: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 31.Cordero MD, Díaz-Parrado E, Carrión AM, Alfonsi S, Sánchez-Alcazar JA, Bullón P, Battino M, de Miguel M. Is inflammation a mitochondrial dysfunction-dependent event in fibromyalgia? Antioxid Redox Signal 2013; 18: 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feraco P, Bacci A, Pedrabissi F, Passamonti L, Zampogna G, Pedrabissi F, Malavolta N, Leonardi M. Metabolic abnormalities in pain-processing regions of patients with fibromyalgia: a 3T MR spectroscopy study. AJNR Am J Neuroradiol 2011; 32: 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez-Domínguez B, Bullón P, Román-Malo L, Marín-Aguilar F, Alcocer-Gómez E, Carrión AM, Sánchez-Alcazar JA, Cordero MD. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with fibromyalgia. Mitochondrion 2015; 21: 69–75. [DOI] [PubMed] [Google Scholar]
- 34.Izurieta Munoz H, Gonzales EB, Sumien N. Effects of creatine supplementation on nociception in young male and female mice. Pharmacol Rep 2018; 70: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knox D, Perrine SA, George SA, Galloway MP, Liberzon I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett 2010; 480: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miró E, Martínez MP, Sánchez AI, Cáliz R. Clinical manifestations of trauma exposure in fibromyalgia: the role of anxiety in the association between posttraumatic stress symptoms and fibromyalgia status. J Trauma Stress 2020; 33: 1082–1092. [DOI] [PubMed] [Google Scholar]
- 37.Villarreal G, Petropoulos H, Hamilton DA, Rowland LM, Horan WP, Griego JA, Moreshead M, Hart BL, Brooks WM. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: preliminary results. Can J Psychiatry 2002; 47: 666–670. [DOI] [PubMed] [Google Scholar]
- 38.Picard M, McEwen BS. Psychological stress and mitochondria: a conceptual framework. Psychosom Med 2018; 80: 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordero MD, de Miguel M, Moreno-Fernández AM. Mitochondrial dysfunction in fibromyalgia and its implication in the pathogenesis of disease. Med Clin (Barc) 2011; 136: 252–256. [DOI] [PubMed] [Google Scholar]
- 40.Coppieters I, Cagnie B, Nijs J, van Oosterwijck J, Danneels L, De Pauw R, Meeus M. Effects of stress and relaxation on central pain modulation in chronic whiplash and fibromyalgia patients compared to healthy controls. Pain Physician 2016; 19: 119–130. [PubMed] [Google Scholar]
- 41.Avgerinos KI, Spyrou N, Bougioukas KI, Kapogiannis D. Effects of creatine supplementation on cognitive function of healthy individuals: a systematic review of randomized controlled trials. Exp Gerontol 2018; 108: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell T, Trost Z, Buelow MT, Clay O, Younger J, Moore D, Crowe M. Meta-analysis of cognitive performance in fibromyalgia. J Clin Exp Neuropsychol 2018; 40: 698–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartkowska W, Samborski W, Mojs E. Cognitive functions, emotions and personality in woman with fibromyalgia. Anthropol Anz 2018; 75: 271–277. [DOI] [PubMed] [Google Scholar]