Abstract
Immunoglobulin A nephropathy (lgAN) is a common primary glomerulonephritis, but paraneoplastic IgAN has been rarely reported. This current case report describes a 49-year-old male patient that was referred with proteinuria, oedema and hypoproteinaemia after lung cancer surgery and before the first cycle of chemotherapy. Renal biopsy confirmed lgAN. The patient received four cycles of chemotherapy (first cycle: pemetrexed + nedaplatin; second to fourth cycle: pemetrexed + carboplatin). The symptoms of IgAN were gradually relieved with additional cycles of chemotherapy. At the latest follow-up on 10 February 2020, there was no evidence of lung cancer recurrence and all symptoms of lgAN had disappeared. lgAN combined with lung adenocarcinoma is quite rare, which suggests that IgAN might be a paraneoplastic manifestation of lung adenocarcinoma.
Keywords: Chemotherapy, case report, immunoglobulin A nephropathy, lung adenocarcinoma, nephrotic syndrome, proteinuria
Introduction
Immunoglobulin A nephropathy (lgAN) is a common glomerulonephritis, which is characterized by glomerular immune deposits comprising primarily of IgA.1 Paraneoplastic glomerular disease was first reported in 1966 and the incidence rate is approximately 10.9%.2 The most common neoplasms associated with paraneoplastic glomerular disease are carcinomas of the lung and gastrointestinal tract.3 Currently, although lung adenocarcinoma is relatively common, paraneoplastic IgAN due to lung adenocarcinoma has rarely reported. This current case report describes a patient with proteinuria and oedema after surgery for a lung malignancy that was subsequently diagnosed with lgAN.
Case report
A 49-year-old male patient was admitted to the Department of Nephrology, The First Hospital of Jilin University, Changchun, Jilin Province, China on 2 August 2018 with oedema, hypoproteinaemia and proteinuria during the previous 15 days. At 1 month before admission, he had a cough and blood in the sputum. Chest computed tomography (CT) imaging showed a 53 mm × 38 mm tumour in the upper left lobe. His blood test results were as follows: white blood cell count, 15.28 × 109/l; albumin, 40.2 g/l (normal range: 40‒55 g/l). His renal function was normal and he had no history of urinalysis abnormalities or kidney dysfunction. Additionally, urinalysis performed 10 days before surgery was negative for haematuria and proteinuria. The patient immediately underwent wedge resection and lymph node dissection in June 2018 and biopsies revealed lung adenocarcinoma with T2N1M0 stage according to the TNM classification.
On 17 July 2018, urine analysis showed microscopic haematuria (red blood cell [RBC]: 147/per field at high power magnification [HP]) and proteinuria (protein 3+). The 24-h urine analysis revealed proteinuria of 19.38 g/day (normal range: <0.2 g/day) and serum albumin was 31.5 g/l (normal range: 40‒55 g/l). The patient received the first cycle of chemotherapy (1000 mg pemetrexed + 140 mg nedaplatin; intravenous [i.v.] infusion) in July 2018 followed by 800 mg pemetrexed + 450 mg carboplatin i.v. infusion every 21 days for three further cycles (cycles 2–4). Two weeks following the first cycle of chemotherapy, the proteinuria had reduced to 5.62 g/day (normal range: <0.2 g/day), urine analysis showed microscopic haematuria (RBC: 2992/HP) and proteinuria (protein 3+), the serum albumin level was 28.6 g/l (normal range: 40‒55 g/l), the serum creatinine (Scr) level was 92.8 µmol/l (normal range: 54‒106 µmol/l) and multi-deformation of RBCs accounted for 80%, suggesting glomerular haematuria. His blood pressure was 150/84 mmHg and his pulse rate was 76/min. Ultrasound examinations of the heart and lung were normal. The patient had a 1-year history of hypertension and a history of smoking; and had undergone a kidney biopsy in August 2018 due to lower limb oedema, proteinuria (5.62 g/day) and hypoalbuminaemia (albumin: 28.6 g/l; normal range: 40‒55 g/l). Histological analysis of the kidney biopsy suggested lgAN. The kidney biopsy specimen contained 10 glomeruli and immunofluorescence staining showed C3 and IgA deposition in mesangial areas. Staining for IgG, C4 and C1q was negative (Figures 1 and 2). Transmission electron microscopy demonstrated a small number of high electron-dense deposits in the subepithelial region (Figure 3). After the patient completed the second and third cycles of chemotherapy (pemetrexed + carboplatin) from 1 October 2018 to 16 November 2018, the urine analysis results were PRO±/RBC 23.3/HP, proteinuria decreased to 1.02 g/day (normal range: <0.2 g/day) and Scr was 95.3 µmol/l (normal range: 54‒106 µmol/l).
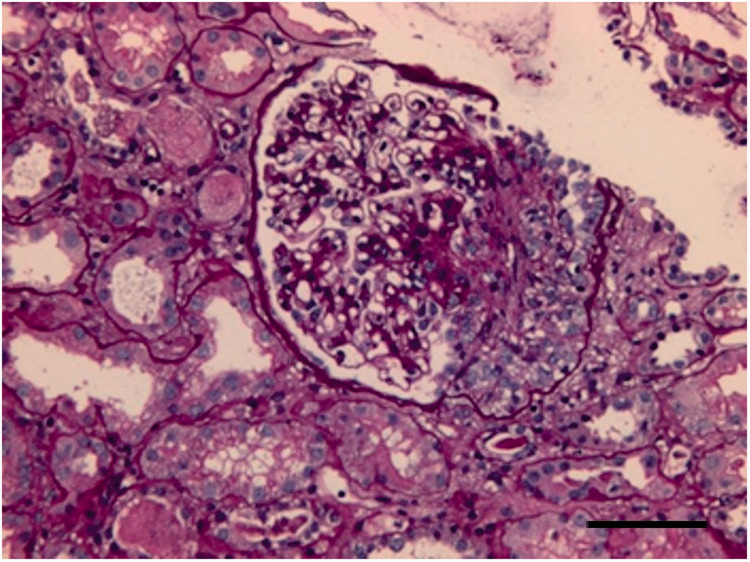
Figure 1.
Representative light photomicrograph from a renal biopsy taken from 49-year-old male patient that presented with oedema, hypoproteinaemia and proteinuria subsequent to the diagnosis and treatment of lung adenocarcinoma. The image shows glomerular mesangial cells and mesangial matrix diffuse hyperplasia. Haematoxylin and eosin staining. Scale bar 20 µm. The colour version of this figure is available at: http://imr.sagepub.com.
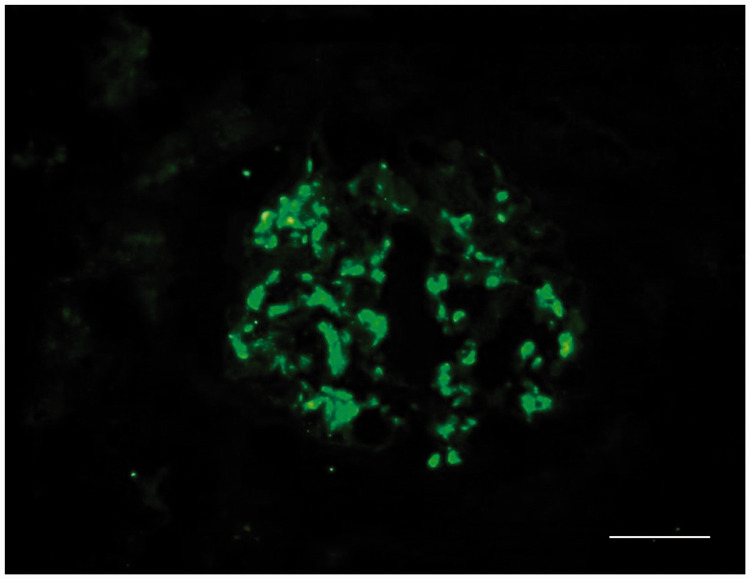
Figure 2.
Representative immunofluorescent photomicrograph from a renal biopsy taken from 49-year-old male patient that presented with oedema, hypoproteinaemia and proteinuria subsequent to the diagnosis and treatment of lung adenocarcinoma. The image shows diffuse glomerular granular capillary deposition of immunoglobulin A. Scale bar 20 µm. The colour version of this figure is available at: http://imr.sagepub.com.
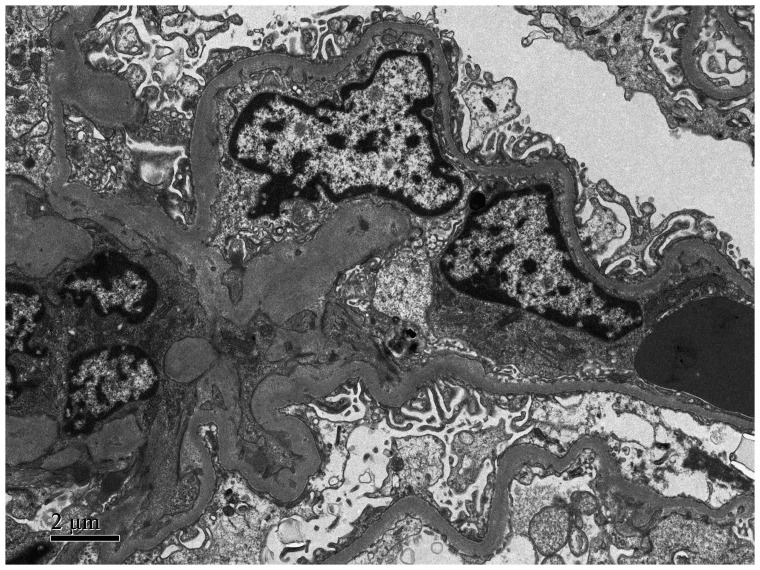
Figure 3.
Representative transmission electron micrograph from a renal biopsy taken from 49-year-old male patient that presented with oedema, hypoproteinaemia and proteinuria subsequent to the diagnosis and treatment of lung adenocarcinoma. The image shows that the epithelial foot processes are widely fused and there are visible electron dense deposits. Scale bar 2 µm.
The symptoms of oedema were improved during chemotherapy. No immunosuppressants or steroids were used during treatment. The follow-up showed that the 24-h urine protein and urinary RBC count were reduced. Kidney disease was gradually relieved with chemotherapy and the proteinuria was 1.02 g/day after three cycles of chemotherapy. After four cycles of chemotherapy, the urine protein levels returned to normal and the lower limb oedema had resolved, suggesting complete remission. This patient is followed up regularly after discharge (Table 1). At the latest follow-up on 9 June 2020, there was no evidence of lung malignancy recurrence, renal function was normal and urine protein was negative.
Table 1.
Clinical course of a 49-year-old male patient that presented with oedema, hypoproteinaemia and proteinuria subsequent to the diagnosis and treatment of lung adenocarcinoma during ongoing follow-up.
| Parameter | After surgery |
Follow-up, months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 12 | 18 | 24 | ||
| Cycle of chemotherapy | – | First | Second | Third | Fourth | – | – | – | – |
| Serum albumin, g/l | 31.5 | 37.1 | 38.0 | 36.4 | 37.2 | 38.1 | Normal | Normal | Normal |
| Urine protein, g/day | 19.38 | 3.44 | 2.46 | 2.52 | 1.02 | 0.88 | Normal | Normal | Normal |
| Serum creatinine, µmol/l | 77.5 | 112.5 | 108.7 | 89.8 | 95.3 | 87.0 | Normal | Normal | Normal |
The patient provided verbal informed consent for publication of the case. All patient details have been de-identified.
Discussion
Immunoglobulin A nephropathy is the leading form of primary glomerulonephritis associated with end-stage renal failure.4 Its frequent coexistence with chronic liver disease, inflammatory conditions, infections or malignant processes raises the possibility of a pathological rather than coincidental association.5 IgAN is a common disease, so any association may be coincidental rather than causal.
For patients with IgA nephropathy aged older than 60 years, tumour occurrence must be determined. In the previously reported literature, solid malignancies related to IgAN include renal cell carcinoma,6 breast cancer,7 mesothelioma,8 rectal cancer,9 gastric cancer,10 bronchial cancer,11 basaloid squamous cell carcinoma of the oesophagus12 and small cell lung cancer.13 A previous study reported three cases of IgA nephropathy associated with renal cell carcinoma in elderly patients.6 At 1 year after nephrectomy, proteinuria and haematuria were decreased in case 1; and both proteinuria and haematuria had disappeared in case 3.6 Case 2 was started on peritoneal dialysis at 1 year after nephrectomy.6 A report of a case of breast cancer associated with IgA nephropathy noted that the patient’s urine protein was significantly reduced after surgery, chemotherapy and radiation therapy.7 A previous report presented a case of IgAN associated with mesothelioma.8 The development and regression of IgAN during and after treatment with bevacizumab was described in a 68-year-old patient with metastatic rectal malignancy.9 A 45-year-old patient that had been treated for IgA nephritis for 9 months was diagnosed with bronchial carcinoma during treatment.11 After X-ray treatment, the tumour volume was significantly reduced and kidney function was significantly improved.11 A case of IgAN associated with basaloid squamous cell carcinoma of the oesophagus was previously reported.12 A previous study reported a patient with IgA nephropathy associated with small cell lung cancer.13 Based on the literature, it has been well established that, after effective treatment of malignant tumours, most kidney damage can be alleviated, as characterized by decreased urine protein and improved renal function, whereas proteinuria indicates tumour progression or recurrence.14
Nephropathy may develop before, in parallel with or following malignancy.15 The molecular mechanisms of carcinoma-associated nephropathy remain poorly understood.16 One of the possible mechanisms for the development of nephropathy in patients with malignant disease is the deposition of tumour-specific antibodies or antibody complexes in the glomeruli.17 The deposition of immune complexes may cause inflammation, release of reactive oxygen species and complement activation, possibly leading to glomerular damage.18 However, establishing a pathophysiological link between tumours and glomerulopathy is not always necessary for clinical diagnosis.19 The diagnosis of paraneoplastic nephropathy is based on the entire clinical process and the characteristic symptoms in the presence of malignancy.20 The diagnosis of paraneoplastic nephropathy is based on several criteria: (i) no alternative aetiology other than neoplastic disease; (ii) the correlation of the diagnosis of glomerulopathy and diagnosis of neoplastic disease; (iii) the improvement of glomerulopathy after surgical removal or chemotherapy or radiotherapy of the tumour; (iv) the worsening of glomerulopathy after tumour recurrence.19
Systemic steroid administration is the main treatment for primary nephrotic syndrome.21 However, for paraneoplastic nephrotic syndrome, the priority is the treatment of the malignancy and an improvement in the nephrotic syndrome can be obtained by successful cancer treatment.22 Notably, both neoplastic diseases and their treatment can cause renal damage.23 Thus, chemotherapy and other therapeutic drugs with low nephrotoxicity must be chosen.
Complete surgical resection of a lung adenocarcinoma was reported to prevent the progression of the nephrotic syndrome and decrease the excretion of urinary protein.24 The current case had proteinuria and hypoalbuminaemia symptoms after surgery and before chemotherapy. The renal biopsy pathology was consistent with IgAN and the patient was not administered active steroid or immunosuppressive therapy. Chemotherapy was administered following surgical resection and the nephropathy resolved subsequently. The strong time association and remission of kidney involvement following successful cancer treatment suggested a causal relationship between lung adenocarcinoma and IgAN in the current case.
In conclusion, evidence from the literature and clinical practice suggests that a strong pathogenetic link exists between glomerulus nephritis and malignancies. Further research is needed on the pathophysiological association between IgAN and malignancy. For cancer patients with renal dysfunction, regular follow-up is important. In particular, for middle-aged and elderly patients, more attention should be given to the treatment and prognosis of tumour-related renal damage.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060521996868 for Paraneoplastic immunoglobulin A nephropathy in a patient with lung adenocarcinoma: A case report and literature review by Jing Wang, Yang Liu, Nian Liu, Menghan Gao and Hang Yuan in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060521996868 for Paraneoplastic immunoglobulin A nephropathy in a patient with lung adenocarcinoma: A case report and literature review by Jing Wang, Yang Liu, Nian Liu, Menghan Gao and Hang Yuan in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jing Wang https://orcid.org/0000-0002-3715-1174
References
- 1.Perše M andVečerić-Haler Ž.. The Role of IgA in the Pathogenesis of IgA Nephropathy. Int J Mol Sci 2019; 20: 6199. DOI: 10.3390/ijms20246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JC Yamauchi H andHopper J Jr.. The association of cancer and the nephrotic syndrome. Ann Intern Med 1966; 64: 41–51. DOI: 10.7326/0003-4819-64-1-41. [DOI] [PubMed] [Google Scholar]
- 3.Wagrowska-Danilewicz M andDanilewicz M.. Nephrotic syndrome and neoplasia: our experience and review of the literature. Pol J Pathol 2011; 62: 12–18. [PubMed] [Google Scholar]
- 4.Robert T, Berthelot L, Cambier A, et al. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trends Mol Med 2015; 21: 762–775. DOI: 10.1016/j.molmed.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Saha MK, Julian BA, Novak J, et al. Secondary IgA nephropathy. Kidney Int 2018; 94: 674–681. DOI: 10.1016/j.kint.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimura I, Tojo A, Kinugasa S, et al. Renal cell carcinoma in association with IgA nephropathy in the elderly. Am J Med Sci 2009; 338: 431–432. DOI: 10.1097/MAJ.0b013e3181ae1b12. [DOI] [PubMed] [Google Scholar]
- 7.Jiang D, Zhang X, Liu J, et al. Triple negative breast cancer and immunoglobulin A nephropathy: A case report and literature review. Oncol Lett 2018; 15: 979–983. DOI: 10.3892/ol.2017.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawole A, Daw H, Taylor H, et al. Immunoglobulin A nephropathy associated with mesothelioma. WMJ 2012; 111: 29–32. [PubMed] [Google Scholar]
- 9.Yahata M, Nakaya I, Sakuma T, et al. Immunoglobulin A nephropathy with massive paramesangial deposits caused by anti-vascular endothelial growth factor therapy for metastatic rectal cancer: a case report and review of the literature. BMC Res Notes 2013; 6: 450. DOI: 10.1186/1756-0500-6-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocyigit I, Dortdudak S, Eroglu E, et al. Immunoglobulin A nephropathy could be a clue for the recurrence of gastric adenocarcinoma. Nefrologia 2013; 33: 853–855. DOI: 10.3265/Nefrologia.pre2013.Sep.12266. [DOI] [PubMed] [Google Scholar]
- 11.Schütte W, Ohlmann K, Koall W, et al. Paraneoplastic IgA nephritis as the initial symptom of bronchial carcinoma. Pneumologie 1996; 50: 494–495 [Article in German, English abstract]. [PubMed] [Google Scholar]
- 12.Lam KY, Law SY, Chan KW, et al. Glomerulonephritis associated with basaloid squamous cell carcinoma of the oesophagus. A possible unusual paraneoplastic syndrome. Scand J Urol Nephrol 1998; 32: 61–63. DOI: 10.1080/003655998750014738. [DOI] [PubMed] [Google Scholar]
- 13.Yacoub G, Kosseifi SG, Shah LS, et al. IgA nephropathy and small cell lung carcinoma. Tenn Med 2008; 101: 35–37, 40. [PubMed] [Google Scholar]
- 14.Crawford AR, Dworkin L, Leonard K, et al. Recurrence of paraneoplastic membranous glomerulonephritis following chemoradiation in a man with non-small-cell lung carcinoma. Rare Tumors 2013; 5: 62–64. DOI: 10.4081/rt.2013.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int 1999; 56: 355–377. DOI: 10.1046/j.1523-1755.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 16.Cambier JF andRonco P.. Onco-nephrology: glomerular diseases with cancer. Clin J Am Soc Nephrol 2012; 7: 1701–1712. DOI: 10.2215/cjn.03770412. [DOI] [PubMed] [Google Scholar]
- 17.Lewis MG Loughridge LW andPhillips TM.. Immunological studies in nephrotic syndrome associated with extrarenal malignant disease. Lancet 1971; 2: 134–135. DOI: 10.1016/s0140-6736(71)92305-1. [DOI] [PubMed] [Google Scholar]
- 18.Pani A, Porta C, Cosmai L, et al. Glomerular diseases and cancer: evaluation of underlying malignancy. J Nephrol 2016; 29: 143–152. DOI: 10.1007/s40620-015-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanaji N, Watanabe N, Kita N, et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol 2014; 5: 197–223. DOI: 10.5306/wjco.v5.i3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadokoro A, Ishii T, Takahama T, et al. Paraneoplastic focal segmental glomerulosclerosis in a patient with lung adenocarcinoma. Intern Med 2013; 52: 1953–1956. DOI: 10.2169/internalmedicine.52.0485. [DOI] [PubMed] [Google Scholar]
- 21.Maixnerova D andTesar V.. Emerging Modes of Treatment of IgA Nephropathy. Int J Mol Sci 2020; 21: 9064. DOI: 10.3390/ijms21239064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ota S, Fujigaki Y, Tamura Y, et al. Significance of Earlier Initiation of Chemotherapy for Lung Cancer Complicated with Primary or Secondary Nephrotic Syndrome following Its Appropriate Differential Diagnosis. Case Rep Oncol 2019; 12: 53–58. DOI: 10.1159/000493851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rombola G, Vaira F, Trezzi M, et al. Pemetrexed induced acute kidney injury in patients with non-small cell lung cancer: reversible and chronic renal damage. J Nephrol 2015; 28: 187–191. DOI: 10.1007/s40620-014-0117-5. [DOI] [PubMed] [Google Scholar]
- 24.Coltharp WH, Lee SM, Miller RF, et al. Nephrotic syndrome complicating adenocarcinoma of the lung with resolution after resection. Ann Thorac Surg 1991; 51: 308–309. DOI: 10.1016/0003-4975(91)90810-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060521996868 for Paraneoplastic immunoglobulin A nephropathy in a patient with lung adenocarcinoma: A case report and literature review by Jing Wang, Yang Liu, Nian Liu, Menghan Gao and Hang Yuan in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_0300060521996868 for Paraneoplastic immunoglobulin A nephropathy in a patient with lung adenocarcinoma: A case report and literature review by Jing Wang, Yang Liu, Nian Liu, Menghan Gao and Hang Yuan in Journal of International Medical Research