Abstract
Objective
To analyze computed tomography (CT) features of symptomatic patients with coronavirus disease 2019 (COVID-19).
Methods
Ninety-five symptomatic patients with COVID-19 confirmed by reverse-transcription polymerase chain reaction from 1 May to 14 July 2020 were retrospectively enrolled. Follow-up CT findings and their distributions were analyzed and compared from symptom onset to late-stage disease.
Results
Among all patients, 15.8% had unilateral lung disease and 84.2% had bilateral disease with slight right lower lobe predilection (47.4%). Regarding lesion density, 49.4% of patients had pure ground glass opacity (GGO) and 50.5% had GGO with consolidation. Typical early-stage patterns were bilateral lesions in 73.6% of patients, diffuse lesions (41.0%), and GGO (65.2%). Pleural effusion occurred in 13.6% and mediastinal lymphadenopathy in 11.5%. During intermediate-stage disease, 47.4% of patients showed GGO as the disease progressed; however, consolidation was the predominant finding (52.6%).
Conclusion
COVID-19 pneumonia manifested on lung CT scans with bilateral, peripheral, and right lower lobe predominance and was characterized by diffuse bilateral GGO progressing to or coexisting with consolidation within 1 to 3 weeks. The most frequent CT lesion in the early, intermediate, and late phases was GGO. Consolidation appeared in the intermediate phase and gradually increased, ending with reticular and lung fibrosis-like patterns.
Keywords: Computed tomography, coronavirus, COVID-19, viral pneumonia, ground glass opacity, consolidation
Introduction
An outbreak of pneumonia of unknown etiology occurred in Wuhan, China in December 2019. A prompt investigation revealed that a novel coronavirus was responsible.1 The World Health Organization christened the disease “coronavirus disease 2019 (COVID-19)” and declared it a pandemic on 11 March 2020. The disease spread rapidly across most countries worldwide during the following weeks, causing a global health emergency.2 As of March 2021, the total number of cases globally has exceeded 118,616,666 with more than 2,630,755 deaths. In Saudi Arabia, the total number of cases has exceeded 381,708 with more than 6556 deaths.
COVID-19 pneumonia is characterized by rapid spread, a wide epidemic range, and major respiratory dysfunction. After the initial outbreak, global attention was soon focused on the situation because of the increasing number of new cases and the high mortality rate.3 The causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been shown to infect human respiratory epithelial cells through an interaction between the viral S protein and the angiotensin-converting enzyme 2 receptor on human cells; thus, SARS-CoV-2 possesses a strong capability to infect humans.4
The clinical features of the initial 41 patients confirmed to be infected with SARS-CoV-2 included lower respiratory tract illness associated with fever, dry cough, and dyspnea.5 These manifestations are similar to those of other diseases caused by coronaviruses, such as severe acute respiratory syndrome and Middle East respiratory syndrome.6,7
Chest computed tomography (CT) has proven to be a useful adjuvant to reverse-transcription polymerase chain reaction and has shown a high sensitivity for the diagnosis of COVID-19. Apart from being a diagnostic tool, CT can be a powerful prognostic tool by facilitating both evaluation of the disease progression and monitoring of the response to therapy.8,9
The imaging features of COVID-19 pneumonia are diverse, ranging from a normal appearance to diffuse changes in the lungs. Different radiological patterns are observed at different times throughout the disease course.10 However, few studies have focused on the dynamic changes of chest CT images. The COVID-19 Reporting and Data System (CO-RADS), which is a categorical assessment scheme for chest CT findings in patients suspected to have COVID-19, was developed to standardize the level of suspicion for pulmonary involvement; the substantial agreement among observers and its discriminatory value make it well-suited for use in clinical practice.11,12 Among the initial patients with COVID-19 pneumonia, the time from symptom onset to the development of acute respiratory distress syndrome was as short as 9 days.5
We conducted the present study to analyze the chest CT imaging features and dynamic changes in 95 symptomatic patients with COVID-19 pneumonia and to compare the imaging features across the disease course from symptom onset to the late stage of the disease.
Materials and methods
This descriptive retrospective study was conducted in our radiology department and approved by the institutional review board of Dallah Hospital, Riyadh, Saudi Arabia. The requirement for informed patient consent was waived by the ethics committee because of the retrospective nature of the study.
This study included 95 symptomatic patients with confirmed COVID-19 who were admitted to our hospital and underwent serial CT studies from 1 May to 14 July 2020. In all patients, COVID-19 was confirmed by next-generation sequencing or real-time reverse-transcription polymerase chain reaction. The patients’ clinical manifestations were recorded at the time of imaging.
Based on the time interval from symptom onset to the CT scan, we collected the CT findings during the early stage (scans performed ≤1 week after symptom onset), intermediate stage (>1 to 2 weeks), and late stage of the disease (>2 to 3 weeks).
Chest CT
All CT scans were performed with patients in the supine position using a LightSpeed™ 7.X CT scanner (GE Healthcare, Chicago, IL, USA). The gantry position rotation period was 0.4 s, with an X-ray tube voltage of 140 kV and a current of 230 to 350 mA. Craniocaudal image acquisition of the entire thorax was carried out with a collimation of 1.25 mm (pitch of 6.0), and the images were reconstructed with a slice thickness of 1 to 5 mm or 1 mm and an interval of 1 to 5 mm or 1 mm, respectively. The mean scan time was 2.30 s. The CT images were evaluated with both lung (width, 1500 Hounsfield units [HU]; level, −600 HU) and mediastinal (width, 400 HU; level, 40 HU) window settings. The reconstructed images were transmitted to a workstation and a picture archiving and communication system for multiplanar reconstruction post-processing. The detailed CT imaging findings and their distribution were retrospectively analyzed using a picture archiving and communication system (Sectra, Linköping, Sweden), compared across the three groups, and recorded.
CT signs were categorized as follows before reviewing the cases:
Distribution: unilateral/bilateral, lobular, subpleural distribution, or diffuse
Number of lesions: single, multiple, or diffuse
Density: ground glass opacity (GGO) or consolidation
Accompanying CT signs included the parallel pleura sign, vascular thickening, crazy paving pattern, subpleural bands, lymphadenopathy, air bronchograms, halo sign, reversed halo sign, cavities, micronodules, a tree-in-bud appearance, and pleural effusion based on the Fleischner Society glossary of terms for thoracic imaging.13 Follow-up chest CT scans were also reviewed and compared with the previous chest CT of the same patient.
Statistical analysis
Statistical analyses were performed with SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± standard deviation. Differences between disease stages were analyzed by Fisher’s exact test.
Results
Of 95 patients confirmed to have COVID-19, 55/95 (57.9%) were female and 40/95 (42.1%) were male. The youngest patient was 18 years old, and the oldest was 84 years old.
The most common symptoms of COVID-19 pneumonia in our study at disease onset were fever in 84 (88.4%) patients, shortness of breath in 69 (72.6%), and dry cough in 65 (68.4%). Other nonspecific symptoms included severe body fatigue in 39 (41.0%) patients, headache in 32 (33.6%), dizziness in 30 (31.5%), sore throat in 10 (10.5%), vomiting in 8 (8.4%), and diarrhea in 11 (11.5%).
All patients had abnormal CT imaging features. Regarding lesion distribution, 15 (15.8%) patients had unilateral lung disease and 80 (84.2%) had bilateral disease, although all lung segments were involved in some patients. There was a slight predilection for the right lower lobe (45 [47.4%) patients).
The lesion distribution was lobular in 70 (73.6%) patients, subpleural in 66 (69.4%), and diffuse in 31 (32.6%).
With respect to the number of lesions, no patients had only a single lesion; however, 55 (57.8%) patients had multiple lesions, and 40 (42.1%) had a diffuse distribution.
Regarding lesion density, 47 (49.4%) patients had pure GGO lesions and 48 (50.5%) had GGO with consolidation.
Accompanying CT signs included vascular signs (vascular thickening) in 20 (21.0%) patients, the crazy paving pattern in 56 (58.9%), parallel pleura sign in 44 (46.3%), halo sign in 29 (30.5%), reversed halo sign in 15 (15.7%), air bronchograms in 48 (50.5%), subpleural bands in 28 (29.4%), pleural effusion in 12 (12.6%), and lymphadenopathy in 13 (13.6%). Only one (1.0%) patient showed cavity formation, and 13 (13.6%) showed a tree-in-bud CT appearance.
The typical CT patterns during the early stage of the disease (within the first week after symptom onset) were bilateral lesions in 70 (73.6%) patients, diffuse lesions in 39 (41.0%), and GGO in 62 (65.2%). Pleural effusion was seen in 13 (13.6%) patients, and mediastinal lymphadenopathy was seen in 11 (11.5%).
During the intermediate stage of the disease (second week after symptom onset), 45 (47.4%) patients showed GGO as the disease progressed; however, consolidation was the predominant CT finding in 50 (52.6%) patients.
Follow-up CT was performed to detect pattern changes from the first to second week after symptom onset (Figures 1 and 2). During the late stage of the disease (third week after symptom onset), the predominant imaging patterns were GGO in 17 (17.9%) patients and the reticular pattern in 78 (82.1%) (Figures 3–5).
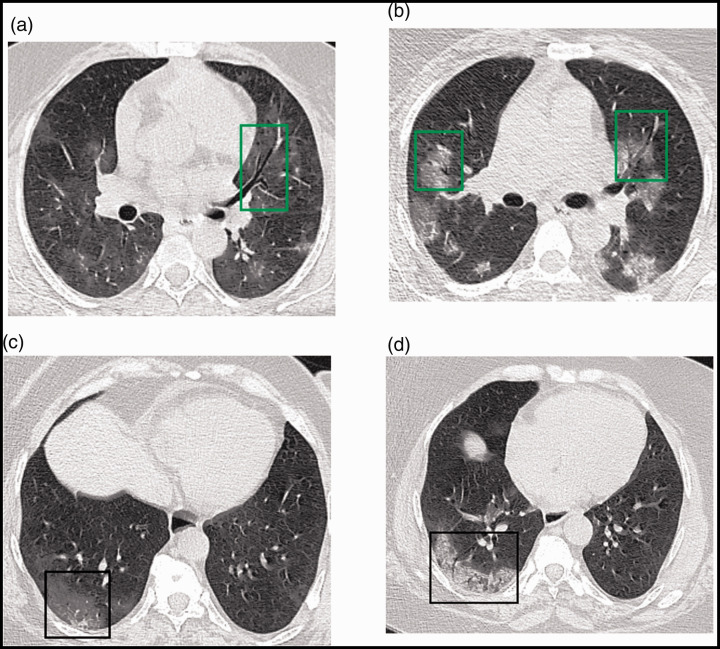
Figure 1.
(a, b) Axial thin-section chest CT images of a 52-year-old woman with COVID-19. (a) During the first week after symptom onset (day 5), multiple scattered bilateral faint ground glass opacities associated with air bronchograms (green square) were observed. (b) the same patient during the second week (day 11). Multiple bilateral central and peripheral mixed patterns with air bronchograms (green squares) were observed. (c, d) Images of a 54-year-old woman. (c) On day 4, a posterior right peripheral ground glass opacity (black square) was observed. (d) The same patient on day 12. Imaging showed a peripheral mixed pattern associated with air bronchograms (black square).
COVID-19, coronavirus disease 2019; CT, computed tomography.
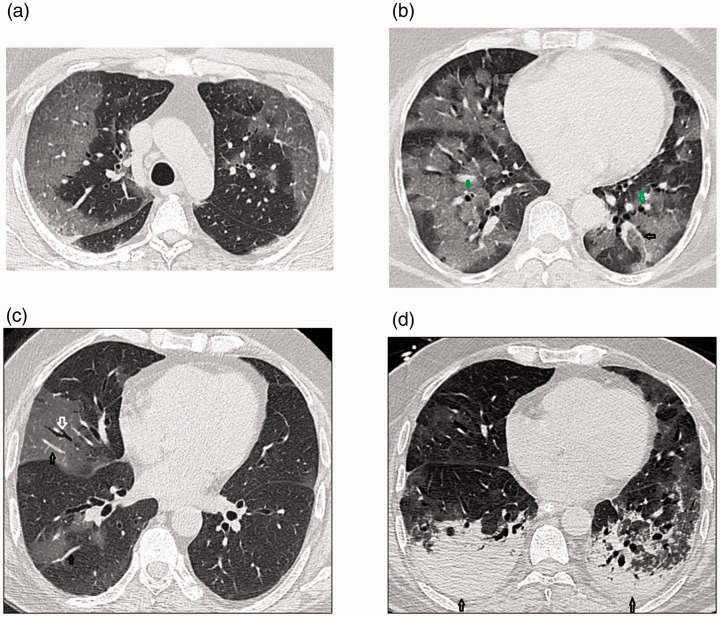
Figure 2.
Transverse thin-section CT scans in patients with COVID-19 pneumonia. (a) A 61-year-old woman on day 5 after symptom onset. Bilateral large peripheral subpleural ground glass opacities were observed; they were more severe on the right side, with smooth interlobular and intralobular septal thickening (crazy paving pattern). (b) The same patient on day 13. Extensive ground glass opacities were seen in both lungs, involving almost all of the lower lobes, giving a white lung appearance. A prominent vascular sign (black arrow) and bronchiectatic changes (green arrows) were also evident. (c) A 74-year-old man 6 days after symptom onset. Right lung ground glass opacities associated with air bronchograms (white arrow) and vascular signs (black arrows) were observed. (d) The same patient on day 14. Imaging demonstrated bilateral ground glass and mixed-density opacities, bronchiectatic changes, and mild bilateral pleural effusion (black arrows).
COVID-19, coronavirus disease 2019; CT, computed tomography.
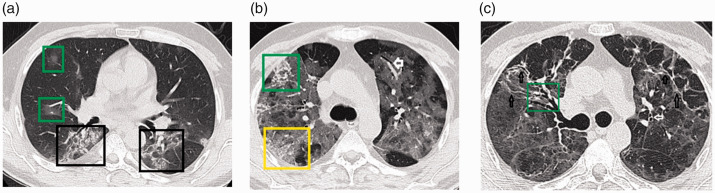
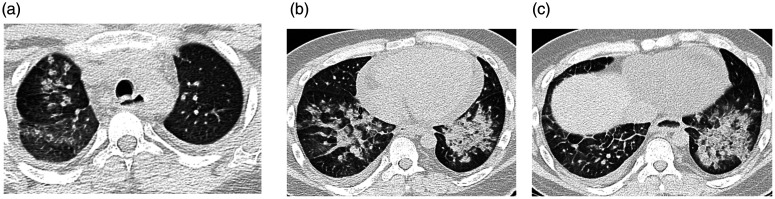
Figure 3.
Transverse thin-section serial CT scans of a 55-year-old man with COVID-19 pneumonia. (a) Day 7 after symptom onset. Imaging showed patchy ground glass (green squares) and bilateral mixed-density opacities affecting the posterior lung parenchyma with air bronchograms and some reticular densities (black square). (b) Day 16: Extensive bilateral ground glass opacities giving a white lung appearance. Also present were vascular signs (white arrow), reticular opacities (green square), and the crazy paving pattern (yellow square). (c) Day 20: Diffuse ground glass opacities with bilateral reticular densities mainly at the anterior aspect (black arrows). Also present are air bronchograms (green square) and bronchiectatic changes (white arrow).
COVID-19, coronavirus disease 2019; CT, computed tomography.
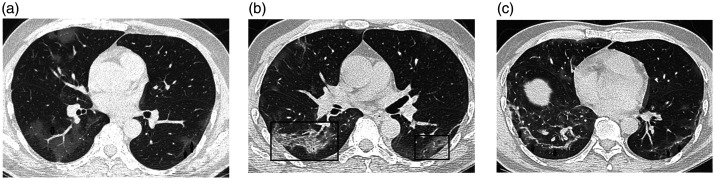
Figure 4.
Transverse thin-section serial CT scans of a 54-year-old man with COVID-19 pneumonia. (a) Day 4 after symptom onset: Bilateral (more severe on right) patchy ground glass opacities affecting the lung parenchyma; vascular signs (arrow) and subpleural bands (double arrows) were also seen (b) Day 11: bilateral peripheral ground glass opacities associated with smooth interlobular and intralobular septal thickening (crazy paving pattern) (black square). (c) Day 19: Bilateral basal reticular opacities (arrows).
COVID-19, coronavirus disease 2019; CT, computed tomography.
Figure 5.
Transverse thin-section serial CT scans of a 77-year-old man with COVID-19 pneumonia. (a) Day 6 after symptom onset: patchy ground glass/consolidation opacities affecting the right upper lung parenchyma. (b) Day 13: Expansion of mixed-density pattern with a small round cystic lesion on the right side (arrow). (c) Day 20: Enlarged and higher-density left basal pulmonary consolidation with right basal reticular opacities (arrows).
COVID-19, coronavirus disease 2019; CT, computed tomography.
Of all 95 patients confirmed to have COVID-19 viral pneumonia, 25 showed respiratory deterioration with an elevated serum D-dimer concentration. CT angiography was performed for 25 patients suspected to have pulmonary embolism. Eleven of these 25 patients showed CT evidence of acute pulmonary embolism. Unilateral acute pulmonary embolism was seen in 3 of 11 patients, and 8 of 11 patients showed a bilateral distribution. Three patients showed pulmonary embolism at the lobar level, three at the segmental level, and five at the lobar, segmental, and subsegmental levels.
Discussion
The outbreak of COVID-19 pneumonia has resulted in a global health emergency similar to the outbreaks of severe acute respiratory syndrome in 2003 and Middle East respiratory syndrome in 2012, both of which were also caused by viruses belonging to the family Coronaviridae.14
We conducted the present study to analyze the chest CT features in 95 symptomatic patients with COVID-19 pneumonia and to compare the imaging findings across the disease course with the aim of facilitating early diagnosis in future patients.
In this study, the most common symptoms of COVID-19 pneumonia at disease onset were fever, shortness of breath, and dry cough, consistent with the manifestations of lower respiratory tract infections. A previous study showed that the virus mainly invades lung interstitial tissue and bronchioles, causing bronchiolitis and peripheral inflammation before it spreads and invades the lung tissue.11 The right lower lobe was the most commonly affected lobe (47.4%). This is consistent with the findings of previous studies.10–15
We suggest that this finding is likely due to the anatomical structure of the trachea and bronchi. The right bronchus is short and straight, and the causative virus might tend to prefer the right lower lobe. In one study, the median time from onset of symptoms to mechanical ventilation was 10.5 days, and the time to intensive care unit admission was 10.5 days (range, 8–17 days).5 In the present study, the extent of the disease on CT scans markedly increased during the first and second weeks after symptom onset and then gradually decreased in the third week. Therefore, we can conclude that the radiological course is consistent with the clinical course of COVID-19 pneumonia. This indicates the importance of serial CT imaging during follow-up of the disease because it helps in continuous monitoring of disease changes (either progression or improvement of lung lesions) during the treatment of these patients.
Our results showed that the most frequent distribution of COVID-19 lesions in chest CT images was bilateral and peripheral with middle/lower lobe predominance, and the predominant pattern was GGO that progressed to or coexisted with consolidations within 1 to 3 weeks. Many studies have shown that these imaging characteristics are nonspecific10 and bear some resemblance to those of severe acute respiratory syndrome coronavirus16,17 and Middle East respiratory syndrome coronavirus infections.18,19 Those studies also revealed that none of the CT features of COVID-19 seem to be specific or diagnostic and that COVID-19 pneumonia shares CT features with other noninfectious conditions that present as subpleural airspace disease.10,20,21
During the first week after symptom onset, chest CT showed bilateral diffuse GGO and consolidation; some patients also showed pleural effusion and lymphadenopathy. During the second week after symptom onset, the GGO lesions decreased and consolidation became the most common pattern. A reticular pattern and irregular interlobular or septal thickening progressively increased from the second week, indicating the development of interstitial changes and a fibrosis-like pattern. As the disease progressed in the third week after symptom onset, consolidation and mixed patterns became more common, while GGO lesions decreased at this stage.
Acute pulmonary embolism was an important thromboembolic complication in this study and was diagnosed mainly in the third week after onset of symptoms in 11 (11.6%) patients. This proportion was lower than that in other studies, which reported acute pulmonary embolism in almost 25% of patients with COVID-19.22 Another study suggested that patients with COVID-19 who have severe clinical features such as respiratory deterioration and an elevated serum D-dimer concentration may have associated acute pulmonary embolism, requiring contrast-enhanced CT angiography for diagnosis.23 Additionally, the study indicated that whenever a CT evaluation for parenchymal involvement of COVID-19 pneumonia is performed, a simultaneous evaluation of the pulmonary arteries is also essential to identify early signs of associated pulmonary embolism.23
Limitations
The main limitations of this study are the relatively small number of patients, the absence of a prognostic analysis, and the lack of long-term follow-up CT findings.
Conclusion
COVID-19 pneumonia manifested on lung CT scans as bilateral peripheral lesions with right lower lobe predominance; the lesions were characterized by diffuse bilateral GGOs that progressed to or coexisted with consolidations within 1 to 3 weeks. The most frequent CT lesions in patients with COVID-19 in the early, intermediate, and late phases were GGOs, whereas consolidation appeared in the intermediate phase and gradually increased over time. In the late stage of the disease, however, the reticular pattern was predominant.
Footnotes
Author contributions: Conceived and designed the experiments: HD and MY. Analyzed the data: HD. Wrote the first draft: HD. Contributed to the writing of the manuscript: MY and WY. Agree with study findings and conclusions: All authors. Jointly developed the structure and arguments for the paper: MY and WY. Made critical revisions and approved final version: HD.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor: The scientific guarantor of this publication is the corresponding author.
Statistics and biometry: The corresponding author and first author have significant statistical expertise. No complex statistical methods were necessary for this paper.
ORCID iD: Hoda Salah Darwish https://orcid.org/0000-0002-6597-1209
References
- 1. Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. DOI: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novel coronavirus (2019-nCoV) situation reports. [cited 2020 Apr 8]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 3.Coronavirus disease 2019. (COVID-19). World Health Organization. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/novel-coronavirus-2019-ncov. [PubMed]
- 4.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63: 457–460. DOI: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. DOI: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsang KW, Ho PL, Ooi GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003; 348: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 7.Assiri A, Al-Tawfq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology 2020; 19: 200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabatabaei SMH, Talari H, Moghaddas F, et al. CT features and short-term prognosis of COVID-19 pneumonia: a single-center study from Kashan, Iran. Radiol Cardiothorac Imaging 2020; 2: e200130. DOI: 10.1148/ryct.2020200130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20: 425–434. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020; 295: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prokop M, Everdingen WV, Vellinga TVR, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19—definition and evaluation. Radiology 2020; 296: E97–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo HJ, Lim S, Choe J, et al. Radiographic and CT features of viral pneumonia. Radiographics 2018; 38: 719–739. 10.1148/rg.2018170048 [DOI] [PubMed] [Google Scholar]
- 14.Yoon HS, Lee KH, Kim Y, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol 2020; 21: 494–500. 10.3348/kjr.2020.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Zhang Z, Shi Y, et al. Emerging H7N9 influenza A (novel reassortant avian-origin) pneumonia: radiologic findings. Radiology 2013; 268: 882–889. [DOI] [PubMed] [Google Scholar]
- 16.Muller NL, Ooi GC, Khong PL, et al. Severe acute respiratory syndrome: radiographic and CT findings. AJR Am J Roentgenol 2003; 181: 3–8. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z, Liang C, Zhang J, et al. Clinical and imaging findings in patients with severe acute respiratory syndrome. Chin Med J (Engl) 2003; 116: 1104–1105. [PubMed] [Google Scholar]
- 18.Das KM, Lee EY, Enani MA, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol 2015; 204: 736–742. [DOI] [PubMed] [Google Scholar]
- 19.Ajlan AM, Ahyad RA, Jamjoom LG, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol 2014; 203: 782–787. [DOI] [PubMed] [Google Scholar]
- 20.Ujita M, Renzoni EA, Veeraraghavan S, et al. Organizing pneumonia: perilobular pattern at thin-section CT. Radiology 2004; 232: 757–761. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 2020; 56: 2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darwish HS, Habash MY, Habash WY. COVID-19 viral pneumonia complicated with acute pulmonary embolism: a descriptive study. Radiol Res Pract 2021; 2021: 6649086. [DOI] [PMC free article] [PubMed] [Google Scholar]