Abstract
Compression sutures are primarily used to treat atonic postpartum hemorrhage. We herein describe three cases of selective arterial ligation combined with B-Lynch or modified B-Lynch suture for the treatment of intractable postpartum hemorrhage unresponsive to available conservative interventions. Three pregnant women underwent a cesarean section for a macrosomic fetus, fetal distress, and oligohydramnios, respectively. All three women developed intractable postpartum hemorrhage due to uterine atony with no chance of embolization therapy. B-Lynch or modified B-Lynch suture and additional selective arterial ligation were performed using braided absorbable suture. The first woman developed postoperative hematometra and infection without response to drainage and antibiotic therapy. Although laparoscopic exploration was performed to loosen the suture line and drain the hematometra and pyometra, the necrosis and infection could not be controlled. Subtotal hysterectomy was therefore conducted, and the necrotic uterine adnexa was removed. The other two women developed subinvolution of the uterus resulting in prolonged menstruation and amenorrhea, although the uterus was preserved and the bleeding was controlled. Modified B-Lynch suture combined with vascular ligation is an invaluable technique for women with severe intractable postpartum hemorrhage. However, it can lead to serious complications such as uterine necrosis, infection, and subinvolution.
Keywords: Infection, modified B-Lynch suture, postpartum hemorrhage, subinvolution, uterine necrosis, vascular ligation
Introduction
Uterine necrosis is a very rare condition, and only a few cases have been reported to date. Most of these cases occurred after pelvic artery embolization;1,2 uterine necrosis has rarely developed after application of uterine compression sutures.3,4 Uterine compression suture, a technique advocated by the surgeon Christopher B-Lynch, aims to control postpartum hemorrhage.5 A success rate of 91.7% (95% confidence interval, 84.9%–95.5%) for various uterine compression sutures has been reported.6 However, uterine compression suture is also associated with various short-term and long-term complications, such as occlusion of the uterine cavity; thus, infection, pyometra, and synechiae may occur within a short time after this operation.5 In a 5-year retrospective study of 23 women who underwent uterine compression suture, the complication rate was 25%.7 Moreover, other case reports have described uterine necrosis as a severe perioperative complication.8–11 In the longer term, the impairment of fertility and menstrual periods remains controversial because of limited data and lack of long-term follow-up research.5,12
Tadakawa et al.13 reported that 12 of 19 patients who underwent B‐Lynch suture had 14 subsequent pregnancies during a mean follow‐up period of 52.1 months, implying that B‐Lynch sutures for postpartum hemorrhage do not appear to jeopardize fecundity. However, one review showed a risk of impaired fertility in some cases.6 Hence, more analyses of the complications of uterine compression suture are needed. A few cases of uterine necrosis after group A Streptococcus infection have been reported.12,14 In the present report, we describe three women who underwent a combination of B-Lynch or modified B-Lynch suture and vascular ligation to control severe intractable postpartum hemorrhage. After this procedure, the first patient required subtotal hysterectomy, and the right uterine adnexa was removed because of necrosis and infection. The other two women developed long-term subinvolution of the uterus, which caused prolonged menstruation and amenorrhea, respectively. These clinical cases suggest that surgeons should fully consider the complications of combined application of B-Lynch or modified B-Lynch suture and vascular ligation, although such complications are rare.
Case 1
A 28-year-old woman underwent an emergency cesarean section because fetal trial delivery had failed. She developed postpartum hemorrhage because of uterine atony with no response to uterotonics or other conservative interventions. The bleeding volume was 2500 mL, and her hemoglobin concentration was 0.4 g/dL. Bilateral uterine and ovarian artery ligation and B-Lynch suture were performed to treat the severe intractable postpartum hemorrhage because uterine artery embolization was unavailable at the secondary care unit. The procedure was performed using braided absorbable suture (Coated VICRYL® Plus Antibacterial (polyglactin 910) Suture; Ethicon, Inc., Somerville, NJ, USA). The patient also underwent intraoperative transfusion of five units of red blood cells and five units of fresh frozen plasma. On the third day postoperatively, she developed a fever (40°C) associated with diffuse abdominal pain and diarrhea without fetid lochia. B-mode ultrasonography displayed a 68- × 52-mm mass of heterogenous echointensity in the uterine cavity and 59- × 55-mm anechoic area around the uterus. Furthermore, computed tomography showed significant infection in both lungs, enlargement of the uterus with a symmetric density in the uterine cavity, and pelvic infection. Despite antibiotic treatment with cefoperazone sodium + sulbactam sodium and ornidazole, her clinical condition did not improve; therefore, she was referred to a tertiary care hospital for further evaluation.
The patient continued to have a low fever after admission. Escherichia coli sensitive to moxifloxacin was cultured from a vaginal secretion specimen, and Candida krusei was cultured from a urine specimen. Antimicrobial therapy with moxifloxacin was continuously administered based on the results of the vaginal secretion culture. After 4 days of treatment with moxifloxacin, the patient’s fever persisted. Thus, acetaminophen and teicoplanin were used to strengthen the anti-infection treatment until discharge. Additionally, the uterine cavity was rinsed each day to discharge the hematometra and necrotic tissue. However, these measures were ineffective. Because the patient refused removal of her uterus a laparoscopic exploration was performed to loosen the suture line and drain the hematometra and pyometra 13 days after the cesarean section. During the surgery, widespread abdominal adhesions and encapsulated effusion were found. After pus aspiration and abdominal irrigation, the partially enlarged uterus was exposed and one side of the suture line could be released (Figure 1). The vagina discharged a small amount of odorous pus and blood. A drainage tube was retained in the uterine and abdominal cavities for irrigation, and the patient was treated with intensive antibiotics. Her body temperature slightly decreased with these therapeutic measures, but her high fever returned after 3 days. A computed tomography scan indicated uterine necrosis (Figure 2(a)). Hence, subtotal hysterectomy was conducted, and the necrotic uterine adnexa was removed (Figure 3). Pathological examination revealed that part of the myometrial tissue showed coagulation necrosis, infiltration of large numbers of inflammatory cells, and degeneration and necrosis of the fallopian tube and ovarian artery. The patient’s temperature rapidly decreased and returned to normal on the 10th postoperative day.
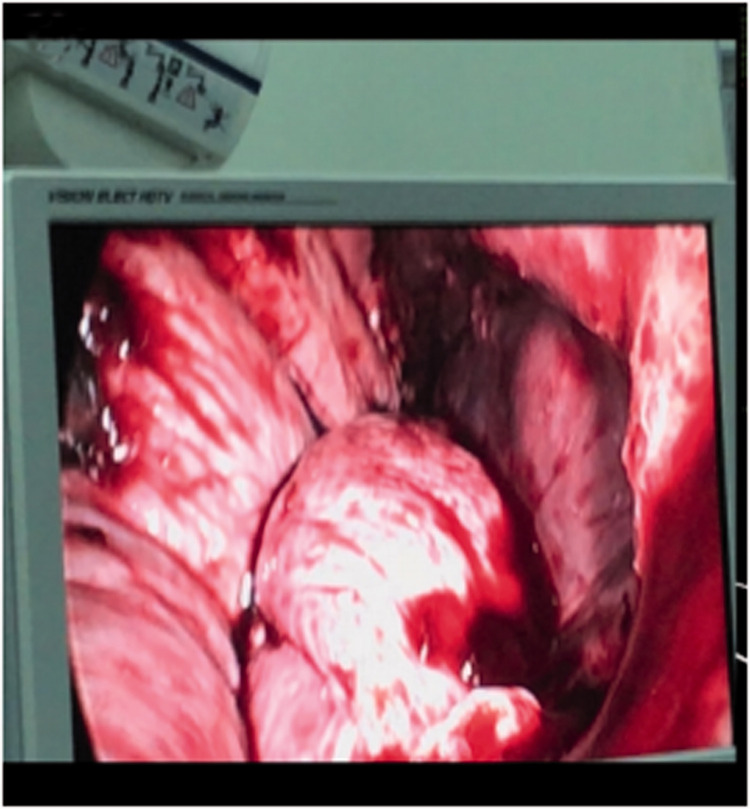
Figure 1.
Laparoscopic exploration in Case 1. The partially enlarged uterus was exposed and one side of the suture line was released.
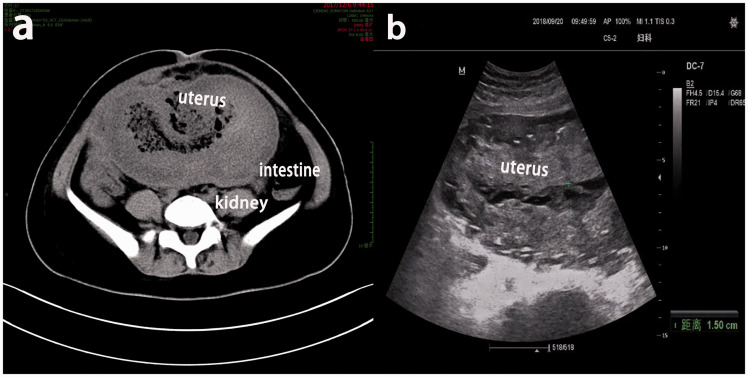
Figure 2.
Imaging findings in Case 1. (a) Computed tomography showed that the volume of the uterus had increased and that the uterine muscular layer was thickened with mixed density. (b) Ultrasound showed that the uterine line was not clear and that the echointensity was inhomogeneous in the uterine cavity.
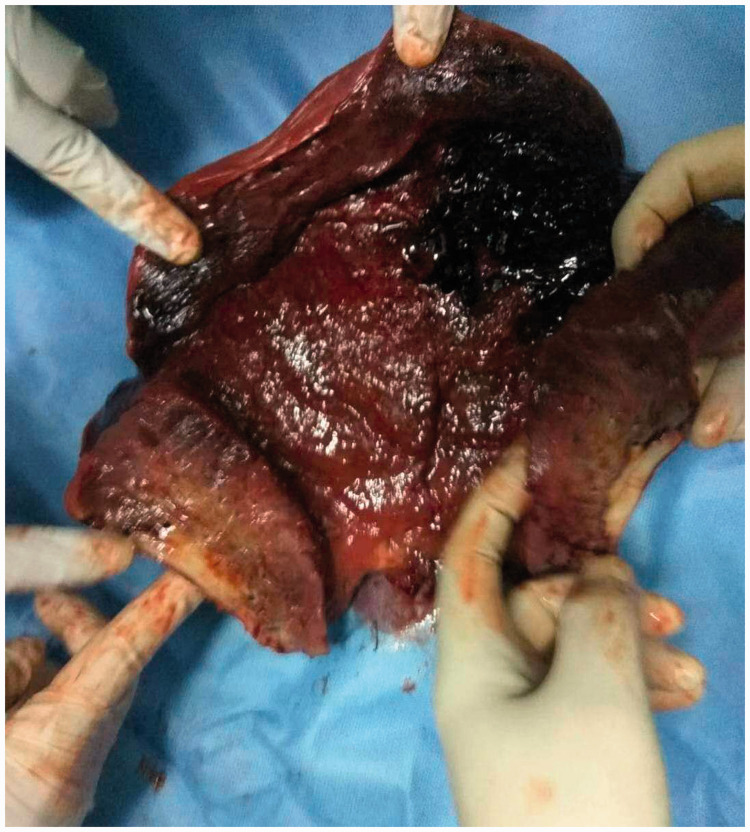
Figure 3.
Subtotal hysterectomy in Case 1. Part of the myometrial tissue showed coagulation necrosis
Case 2
A 35-year-old woman (gravida 2, para 1) with no medical history visited the hospital because of fetal distress at 40 weeks’ gestation. An emergency cesarean section was performed. She also developed postpartum hemorrhage because of uterine atony, as in Case 1. The total estimated blood loss was about 1500 mL, and her hemoglobin concentration decreased to 9.3 g/dL. Bilateral uterine artery ligation was the first procedure conducted; however, the bleeding did not immediately stop. The conventional B-Lynch suture also failed; therefore, modified B-Lynch suture was jointly applied to control the bleeding. The procedure was performed using the same braided absorbable suture as in Case 1. The uterus was then well compressed, and the uterine bleeding significantly decreased. After the surgery, the patient received concomitant treatment with uterotonics and an antimicrobial agent (moxifloxacin) and was discharged 7 days later.
Three months later, the patient was readmitted to the hospital because of increased vaginal discharge. Laboratory data showed a white cell count of 9.74 × 109/L with 86.4% neutrophils. Her C-reactive protein concentration was 44.9 mg/L, and her procalcitonin level was normal (<0.25 ng/mL). Her uterus was enlarged with no tenderness. Pus was collected for bacterial culture, which was positive for Gardnerella, methicillin-resistant Staphylococcus aureus, and carbapenem-resistant Enterobacteriaceae. B-mode ultrasonography showed that the uterus was about 9.5 × 7.6 × 6.0 cm3, the uterine cavity line was not clearly displayed, and a hyperechoic stripe was evident in the uterine cavity. Magnetic resonance imaging showed uterine enlargement, uterine cavity expansion, uterine involution, and infection (Appendix Fig. 1). Postpartum uterine closure was considered, and a cervical dilating rod was applied to fully dilate the cervix and facilitate easy discharge of the uterine effusion. At the same time, metronidazole was used as an anti-infection treatment while irrigating the uterine cavity with low pressure. The patient’s clinical symptoms significantly improved. Ten days later, B-mode ultrasonography indicated slightly stronger echo in the uterine cavity with a thickness of 9 mm. At the 20-month follow-up, she had no menstruation, and the infection was considered to have been the cause of the intrauterine adhesions.
Case 3
A 27-year-old pregnant, primiparous woman with no significant medical history visited the hospital. A cesarean section was performed at 37 and 5/7 weeks of gestation because of oligohydramnios. However, the patient developed postpartum hemorrhage. The bleeding did not stop despite treatment with uterotonics and other conservative interventions (e.g., coagulation therapy). After the uterine artery was ligated, the bleeding was controlled without the need for blood transfusion. As additional therapy, hemostasis and methylergonovine for uterine contraction were administered after the surgery. Nevertheless, severe postpartum hemorrhage occurred 13 hours after the surgery, and the patient quickly developed hemorrhagic shock. Her heart rate was 153 beats per minute, respiratory rate was 20 breaths per minute, and blood pressure was 89/58 mmHg. B-mode ultrasonography indicated that the size of the postpartum uterus was about 27.5 × 17.2 × 11.7 cm3, the myometrial echo was not uniform, and the uterine cavity showed a mass of 12.2 × 7.1 cm2 with mixed echointensity.
As soon as the patient went into shock, exploratory laparotomy was performed on an emergency basis. During the surgery, no bleeding occurred in the abdominal cavity. However, the uterus exhibited enlargement, edema, and poor contraction, and the uterine cavity was filled with 2000 mL of blood postoperatively. As in Case 2, conventional B-Lynch suture did not control the bleeding. Modified B-Lynch suture was performed to treat the uncontrollable postpartum hemorrhage; the bleeding was successfully controlled, and the uterus was preserved. The procedure was performed using the same braided absorbable suture as in Cases 1 and 2. During the surgery, the patient was infused with 1800 mL of fresh frozen plasma, 14.5 units of red blood cell suspension, 20 units of cryoprecipitate, and 10 units of platelets. After the surgery, treatment with antibiotics and methylergonovine was continued. Her condition improved significantly, and she was discharged 10 days later. However, she was hospitalized again for an enlarged uterus and irregular menstruation more than 3 months after the cesarean section. Ultrasound showed that the uterine line was not clear and that the echointensity was inhomogeneous in the uterine cavity (Figure 2(b)). We considered that the modified B-Lynch suture might have caused segmental dilatation and stricture of the uterine cavity, resulting in menstrual blood retention. Hysteroscopy was performed to remove the protuberant part of the endometrial surface. After 20 months of follow-up, she developed oligomenorrhea.
Discussion
In the first case, conventional B-Lynch suture and bilateral uterine and ovarian artery ligation (uterine devascularization) were applied for treatment of postpartum hemorrhage. The greatly reduced blood supply of the uterus and complication with infection led to necrosis of the uterine body and uterine adnexa on the right side. In the other two cases, the bleeding could not be controlled by conventional B-Lynch suture, and both modified B-Lynch suture and bilateral uterine artery ligation were used to control the bleeding. This resulted in subinvolution of the uterus, and the patients developed complications such as menorrhagia and amenorrhea.
Diverse factors can cause complications when performing modified B-Lynch suture and uterine devascularization. The blood supply to the uterus originates mainly from the main trunk of the uterine artery and the ascending branch and lower branch of the vaginal artery. In patients with uterine devascularization, the ovarian artery and the upward branch of the uterine artery are ligated, and necrosis can occur if the collateral circulation is not abundant or the round uterine ligament arteries do not supply enough blood. After the uterine devascularization, as in Case 1, it is impossible for the collateral circulation to establish the blood supply within a short period of time. Under these circumstances, the uterine compression suture has a high chance of causing hematometra, infection, and local ischemia of the uterus. In fact, these were the key factors that led to necrosis of the uterus and adnexa in Case 1. In the other two cases, the bleeding could not be completely controlled by the routine B-Lynch suture because of extremely poor uterine contractility and excessive stretching of the uterus. Thus, we decided to perform a self-created technique to preserve the patients’ uterus. On the basis of conventional B-Lynch suture (Appendix. Fig. 2(a)), transverse sutures were added to strengthen the compression of the uterus and thus more effectively stop the bleeding; this is called modified B-Lynch suture (Appendix. Fig. 2(b)). However, modified B-Lynch suture resulted in incomplete involution of the uterus and partial uterine coagulation necrosis.
The normal physiological volume in the uterus is 5 mL, and menstrual blood is discharged normally under these conditions. After uterine enlargement, the menstrual blood cannot be discharged, leading to secondary infection as observed in Cases 2 and 3. In Case 2, the patient developed pyometra and recurrent vaginal infection. Although these complications improved after uterine drainage and lavage, the infection led to intrauterine adhesions and secondary amenorrhea. In Case 3, the uterus was overstretched because of the postpartum hemorrhage, and the folding and suturing of the uterine wall caused poor drainage of the menstrual blood. This led to rehospitalization of the patient because of menorrhagia.
Managing intractable postpartum hemorrhage due to uterine atony may be challenging. Various uterine compression sutures and balloon tamponade techniques have been introduced for the treatment of postpartum hemorrhage. A controversial issue in the use of uterine compression sutures is concomitant arterial ligation. The uterine artery, the uterine branch of the ovarian artery, and/or the internal iliac artery are commonly unilaterally or bilaterally ligated in patients with uterine atony.15 Wohlmuth et al.16 performed B-Lynch suture in 22 women with drug-resistant uterine atony from 1997 to 2005. B-Lynch suture was performed alone and in combination with arterial ligation in 12 and 10 women, respectively. The success rate of the B-Lynch procedure (alone or in combination with ligation of the bilateral uterine arteries and the uterine branch of the ovarian artery) was 77%.
Combined application of uterine compression suture and vascular ligation is an invaluable technique for women with severe intractable postpartum hemorrhage. However, the three women in the present study developed complications after undergoing this procedure. Fotopoulou and Dudenhausen17 suggested that the combination of compression sutures and additional vessel ligation was more often associated with complications than was the use of compression sutures alone, probably because of the development of uterine ischemia and inflammation. Therefore, the blood supply must be fully considered when combining uterine compression sutures and arterial ligation for women with intractable postpartum hemorrhage. In addition, the blood supply must be closely monitored after the surgery, and once infection occurs, it must be controlled in time to prevent uterine necrosis. If uterine compression sutures and uterine devascularization cannot stop intraoperative bleeding or if they cause severe complications, hysterectomy is required to prevent septicemia, embolism, and other complications that may endanger the patient’s life.
The success rate of uterine compression sutures depends on various factors, including the patient’s hemodynamic stability, the severity and duration of the hemorrhage, the experience of the surgeon and staff, and the availability of blood and blood products. It has been suggested that B-Lynch suture may cause defects in the anterior wall of the uterus. Thus, excessive folding of the uterine wall due to suture compression may have unpredictable effects on the uterine myometrium and endometrial vessels.18 Considering these adverse gynecological outcomes, uterine compression suture should not be employed concomitantly with vascular ligation.
Appendix figures
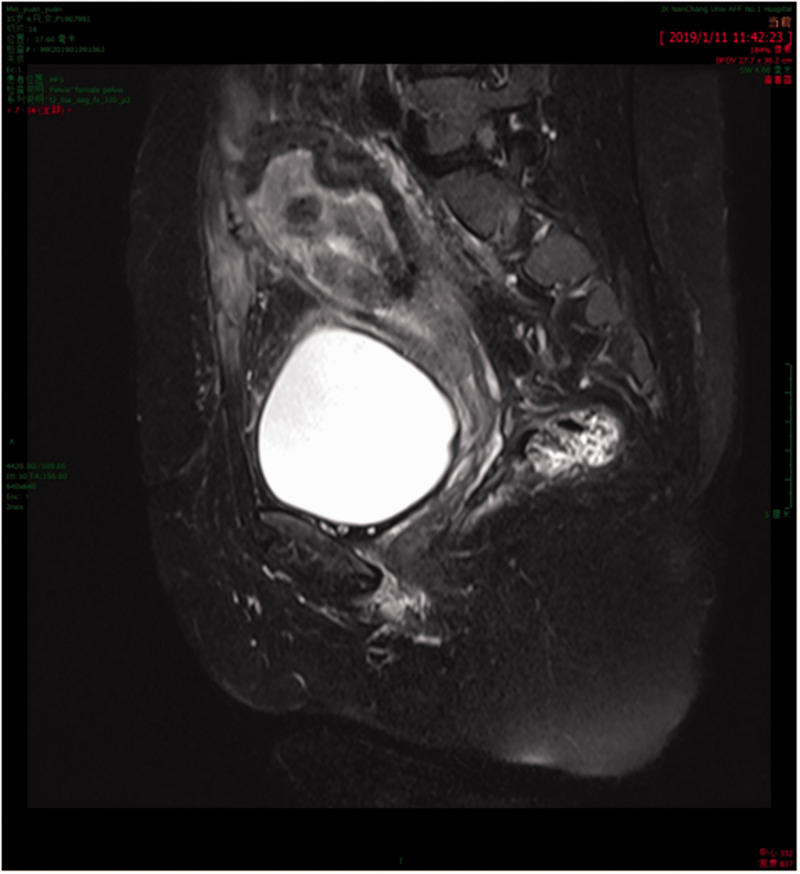
Appendix Figure 1.
Magnetic resonance imaging in Case 2 showed uterine enlargement, uterine cavity expansion, uterine involution, and infection
Appendix Figure 2.
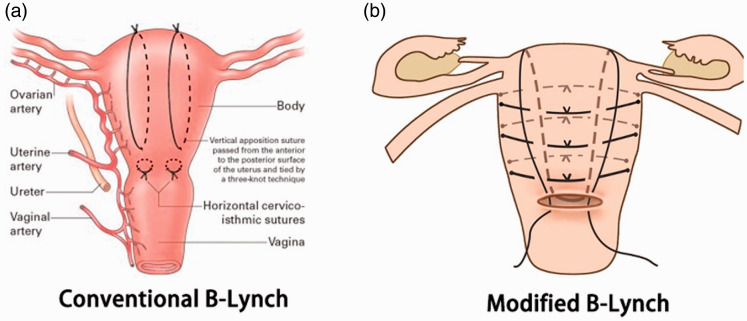
(a) Conventional B-Lynch suture. This is a form of compression suture used in obstetrics to mechanically compress an atonic uterus in the face of severe postpartum hemorrhage. (b) Modified B-Lynch suture. This technique was devised by the authors to preserve the patients’ uterus. On the basis of conventional B-Lynch suture, transverse sutures were added to strengthen the compression of the uterus and thus more effectively stop the bleeding.
Footnotes
Ethics approval and consent: This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (approved on June 4, 2020; approval no. 2017075). All patients’ details have been deidentified. The authors obtained patient consent for treatment. The authors also obtained written informed consent from the patients for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Author contributions: LL and QC conceived the review. XC appraised the quality of the paper. JW and HZ read and checked the case report before submission. LL, QC, and MC wrote, reviewed, and edited the paper. All authors have read and approved the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was funded by the National Natural Science Foundation of Jiangxi Province (grant number 81560247) and the Science and Technology Research Project of Jiangxi Education Department (grant number GJJ180006).
ORCID iD: Qi Chen https://orcid.org/0000-0002-4153-2367
References
- 1.Poujade O, Ceccaldi PF, Davitian C, et al. Uterine necrosis following pelvic arterial embolization for post-partum hemorrhage: review of the literature. Eur J Obstet Gynecol Reprod Biol 2013; 170: 309–314. [DOI] [PubMed] [Google Scholar]
- 2.Belghiti J, Tassin M, Raiffort C, et al. [Uterine necrosis after arterial embolization for postpartum hemorrhage]. Gynecol Obstet Fertil 2014; 42: 126–128. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki S, Jitsumori M, Hara T, et al. Systematic review on the needle and suture types for uterine compression sutures: a literature review. BMC Surg 2019; 19: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodhi W, Golara M, Karangaokar V, et al. Uterine necrosis following application of combined uterine compression suture with intrauterine balloon tamponade. J Obstet Gynaecol 2012; 32: 30–31. [DOI] [PubMed] [Google Scholar]
- 5.Saroja CSM, Nankani A, El-Hamamy E. Uterine compression sutures, an update: review of efficacy, safety and complications of B-Lynch suture and other uterine compression techniques for postpartum haemorrhage. Arch Gynecol Obstet 2010; 281: 581–588. [DOI] [PubMed] [Google Scholar]
- 6.Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv 2007; 62: 540–547. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Mathur M, Tagore S. Complications and pregnancy outcome following uterine compression suture for postpartum haemorrhage: a single centre experience. J Obstet Gynaecol 2014; 34: 383–386. [DOI] [PubMed] [Google Scholar]
- 8.Ochoa M, Allaire AD, Stitely ML. Pyometria after hemostatic square suture technique. Obstet Gynecol 2002; 99: 506–9. [DOI] [PubMed] [Google Scholar]
- 9.Treloar EJ, Anderson RS, Andrews HS, et al. Uterine necrosis following B‐Lynch suture for primary postpartum haemorrhage. BJOG 2006; 113: 486–488. [DOI] [PubMed] [Google Scholar]
- 10.Joshi VM, Shrivastava M. Partial ischemic necrosis of the uterus following a uterine brace compression suture. BJOG 2004; 111: 279–280. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb AG, Pandipati S, Davis KM, et al. Uterine necrosis: a complication of uterine compression sutures. Obstet Gynecol 2008; 112: 429–431. [DOI] [PubMed] [Google Scholar]
- 12.Boie S, Krog J, Tørring S, et al. Life-threatening necrotizing myometritis, due to Group A streptococcus - still a life-threatening condition. Clin Case Rep 2015; 3: 291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadakawa M, Sugawara J, Saito M, et al. Fertility and pregnancy outcomes following B-Lynch sutures for post-partum hemorrhage. J Obstet Gynaecol Res 2015; 41: 559–564. [DOI] [PubMed] [Google Scholar]
- 14.Satia M, More V. Uterine necrosis in a case of B-Lynch brace suture. Int J Reprod Contracept Obstet Gynecol 2016; 5: 2466–2469. [Google Scholar]
- 15.Verit FF, Etin O, Keskin S, et al. Does bilateral uterine artery ligation have negative effects on ovarian reserve markers and ovarian artery blood flow in women with postpartum hemorrhage? Clin Exp Reprod Med 2019; 46: 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohlmuth CT, Gumbs J, Quebral-Ivie J. B-Lynch suture: a case series. Int J Fertil Womens Med 2005; 50: 164–173. [PubMed] [Google Scholar]
- 17.Fotopoulou C, Dudenhausen JW. Uterine compression sutures for preserving fertility in severe postpartum haemorrhage: an overview 13 years after the first description. J Obstet Gynaecol 2010; 30: 339–349. [DOI] [PubMed] [Google Scholar]
- 18.Roman H, Sentilhes L, Cingotti M, et al. Uterine devascularization and subsequent major intrauterine synechiae and ovarian failure. Fertil Steril 2005; 83: 755–757. [DOI] [PubMed] [Google Scholar]