Abstract
Trigger point injection (TPI) is commonly administered for myofascial pain syndrome management, but occasionally leads to complications, including bleeding, muscle hematoma, vasovagal syncope, skin infections, and pneumothorax. This report presents a case of TPI-induced iatrogenic spinal cord injury (SCI). A 59-year-old woman received TPI for myofascial pain on both thoracolumbar paraspinal muscles. She experienced an electric shock sensation throughout the lower extremities upon receiving blind TPI in the left thoracolumbar paraspinal muscle, and later complained of weakness (manual muscle test grade: 0–2) and neuropathic pain (numeric rating scale [NRS]: 7) in the lower left extremity. Thoracolumbar magnetic resonance imaging (MRI) 3 days after the TPI revealed a high-intensity T2 signal in the left T12 to L2 spinal cord segments, indicating the presence of edema or inflammation in this region. In concordance with the MRI findings, electrophysiological recordings performed 11 days after the TPI revealed no central motor conduction time response in the left leg. At 7 months post-onset, the patient had partially recovered motor function and neuropathic pain was reduced to NRS 4. Clinicians should be aware of the possibility of needle-induced SCI during paraspinal muscle TPI; imaging guidance may be helpful for accurate needle targeting during the procedure.
Keywords: Trigger point injection, paraspinal, spinal cord injury, motor weakness, myofascial pain syndrome, iatrogenic injury
Introduction
Myofascial pain syndrome is a chronic condition that leads to pain in several areas, including the head, neck, thoracolumbar fascia, back, pelvis, and extremities.1 Local anesthetics, such as lidocaine, bupivacaine, and/or corticosteroids, are used for trigger point injection (TPI).2 After a TPI, the trigger point becomes inactive and the taut bands loosen, which can reduce muscle pain;3 thus, TPI is commonly performed for the management of myofascial pain syndrome. Its effectiveness has been demonstrated in several previous studies.4–6 However, it can induce complications, such as bleeding, muscle hematoma, vasovagal syncope, skin infections, and pneumothorax.7–9
This report describes a patient with TPI-induced iatrogenic spinal cord injury (SCI).
Case report
A 59-year-old Asian woman (body weight: 57 kg, height: 153 cm, body mass index: 24.35 kg/m2, occupation: farmer) received TPI in the thoracolumbar paraspinal muscles at a local medical center because of a 4-month history of persistent middle back pain of a dull and achy nature. Her pain began spontaneously, without any trauma history. She did not have any medical history such as hypertension, diabetes, or neurological disorders. Prior to the TPI, she had not exhibited any neurological symptoms. She had tenderness to palpation on both thoracolumbar paraspinal muscles along with identifiable taut bands. A thoracolumbar spine X-ray image revealed no specific abnormal findings. The physician, who had 25 years of training and experience, diagnosed the patient with myofascial pain syndrome.10 Her pain was not controlled by oral medication (meloxicam 7.5 mg). TPI was performed using a blind technique without imaging guidance (ultrasound or fluoroscopy) in the left thoracolumbar paraspinal muscles, with the patient in a prone position. The patient had never received TPI prior to the injection. The physician used a 24-gauge 60-mm spinal needle and 10 mL of 0.6% lidocaine (3 mL of 2% lidocaine and 7 mL of normal saline solution) as the injection solution. During the procedure, the patient experienced an electric shock sensation throughout the middle back and lower extremities. The physician stopped the procedure immediately, and the solution was not infused.
Immediately after the TPI, the patient developed weakness and severe, sharp, and lancinating pain in the left lower extremity. Three days after the TPI, she was transferred to the rehabilitation department of our hospital, where she provided written informed consent for participation in this report. The study was approved by the institutional review board of Yeungnam University Hospital (2019-05-044).
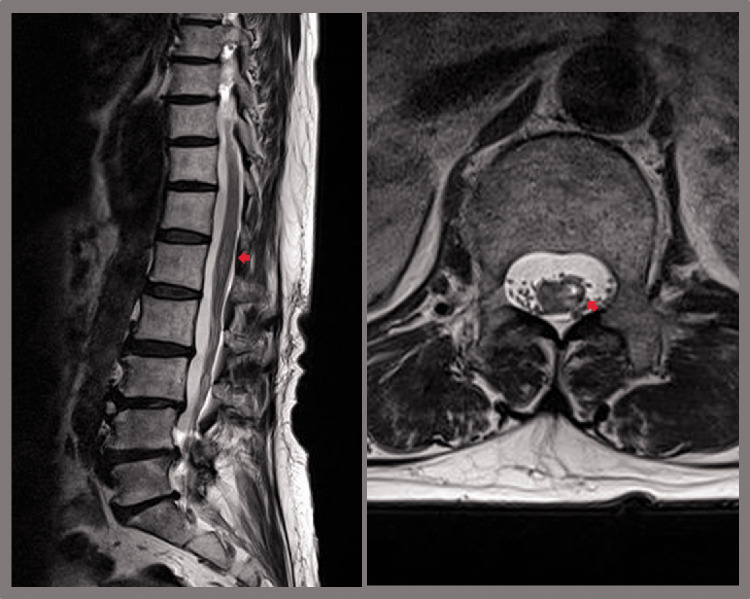
On physical examination, the patient exhibited severe weakness in the lower left extremity: her hip flexor and knee extensor manual muscle testing (MMT) scores were grade 2, and her ankle dorsiflexor, long toe extensor, and ankle plantarflexor MMT scores were grade 0. Furthermore, impaired light touch sensation was observed in the L2 to S5 dermatomes on the left side, and she had impaired pain perception in dermatomes L3 to S5 on the right side of the body. Based on the International Standards for Neurological Classification of Spinal Cord Injury,11 her neurological level of injury was L1, and she was classified as grade D according to the American Spinal Cord Injury Association Impairment Scale (AIS). She was unable to walk and required a wheelchair to move around. Her tendon reflexes and muscle tone were unaltered, and bladder and bowel symptoms were absent. The patient complained of neuropathic pain (tingling, sharp, and lancinating; numeric rating scale [NRS]: 7) in the left lower extremity. Thoracolumbar spine magnetic resonance imaging (MRI) revealed a high-intensity T2 signal in the left T12 to L2 segments of the spinal cord 3 days after TPI (Figure 1). Electrophysiological recordings, which were performed 11 days after TPI, showed no response in central motor conduction time (CMCT) in the left tibialis anterior muscle, whereas the biceps brachii, adductor pollicis brevis, and right tibialis anterior muscle CMCTs were within the normal range. The patient’s compound motor action potentials and sensory nerve action potentials had normal latencies and amplitudes during a nerve conduction test 11 days after TPI, and electromyography revealed no active denervation potentials in the lumbar paraspinal muscles or those of the bilateral lower extremities. Spine MRI revealed a skin-to-epidural space depth of 4.6 cm.
Figure 1.
Sagittal (left) and axial (right) T2-weighted thoracolumbar spine magnetic resonance imaging performed 3 days after the trigger point injection, showing T2 high signal intensity (red arrows) in the left T12 to L2 segments of the spinal cord.
The patient underwent comprehensive rehabilitative management, including neuromuscular electrical simulation therapy for the knee extensors and ankle dorsiflexors (20 minutes, twice per day, 7 days per week). In addition, movement therapy was administered 6 days per week (Monday to Friday: 2.5 hours per day, Saturday: 1 hour per day), primarily to improve motor function and postural control. Seven months after the onset of SCI, the patient’s left hip flexor and knee extensor motor functions had improved to MMT grade 3, and the left ankle plantarflexor muscle function had improved to MMT grade 2. Conversely, motor functions in the left ankle dorsiflexor and long toe extensor muscles had not improved (MMT grade: 0) (Table 1). The patient’s pain perception remained impaired, but her ability to sense light touch in the left lower extremity was nearly completely recovered. Additionally, her neuropathic pain was reduced from an NRS score of 7 to an NRS score of 4 by treatment with 450 mg pregabalin and 200 mg tapentadol, and she was able to walk independently using a walker.
Table 1.
Changes in manual muscle testing scores in the patient.
| Three days after injury onset | Seven months after injury onset | |
|---|---|---|
| Hip flexor | 2 | 3 |
| Knee extensor | 2 | 3 |
| Ankle dorsiflexor | 0 | 1 |
| Long toe extensor | 0 | 1 |
| Ankle plantarflexor | 0 | 2 |
Manual muscle testing scores were as follows: 0, total paralysis; 1, palpable or visible contraction; 2, active movement, full range of motion with gravity eliminated; 3, active movement, full range of motion against gravity; 4, active movement, full range of motion against gravity and moderate resistance in a muscle-specific position; 5, normal active movement, full range of motion against gravity and full resistance in a muscle-specific position, as would be expected from an otherwise unimpaired person.
Discussion
The present case report describes a patient who developed SCI after TPI in the left thoracolumbar paraspinal muscles. MRI and electrophysiological studies were performed at 3 and 11 days after TPI, respectively. On T2-weighted MRI, the high-intensity T2 signal in the left side of spinal cord segments T12 to L2 indicated the presence of edema or inflammation in these regions.12 Electrophysiological studies revealed that CMCT from the left tibialis anterior had no response, but there were no other abnormal findings, suggesting that the patient had thoracolumbar myelopathy on the left side only. Based on the MRI and electrophysiological results, the patient’s report of experiencing an electric shock sensation throughout the middle back and lower extremities. and the onset of motor weakness soon after the procedure, the patient was diagnosed with SCI caused by a needle injury during a TPI in the thoracolumbar paraspinal muscles.
TPI is commonly used for the treatment of myofascial pain syndrome,4,13 and complications from TPI are rare. However, in the patient described in the current report, it appears that the TPI needle passed through the T12 to L1 interlaminar and epidural space and subsequently into the spinal cord, resulting in iatrogenic SCI caused by needle trauma during TPI on the paraspinal muscles.
In previous studies, the mean depths from the skin to the lumbar epidural space and from the skin to the thoracic epidural space have been described as within the range of 4.6 to 5.3 cm14,15 and 4.1 to 6.0 cm, repectively.16 Therefore, careful attention should be paid when needles are inserted deeper than these distances. Additionally, shallow paraspinal muscles increase the probability of SCI. To prevent SCI during TPI, clinicians should have adequate anatomical knowledge, and a needle of 1.5 inches (3.8 cm) or less in length is recommended for TPI in the thoracolumbar paraspinal muscles. Furthermore, ultrasonographic guidance may be helpful to improve the safety of the procedure.
To conclude, we presented a case of iatrogenic SCI in a patient who received TPI in the thoracolumbar paraspinal muscles by blind injection and subsequently developed motor weakness and sensory deficits. To the best of our knowledge, only one previous study has reported SCI after TPI. In 2011, Rathmell et al.17 described a patient who experienced SCI after TPI in the cervical spinal muscles when a 22-gauge 3-inch spinal needle was used under fluoroscopic guidance. Clinicians should be aware of the possibility of needle-induced SCI during this procedure, and should exert caution when performing TPI.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a National Research Foundation of Korea Grant funded by the Korean government (grant no. NRF-2021R1A2C1013073).
ORCID iD: Min Cheol Chang https://orcid.org/0000-0002-7629-7213
References
- 1.Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol 2007; 21: 427–445. [DOI] [PubMed] [Google Scholar]
- 2.Staal JB, De Bie R, De Vet HC, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev 2008; 3: CD001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sciotti VM, Mittak VL, DiMarco L, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain 2001; 93: 259–266. [DOI] [PubMed] [Google Scholar]
- 4.Han SC, Harrison P. Myofascial pain syndrome and trigger-point management. Reg Anesth 1997; 22: 89–101. [DOI] [PubMed] [Google Scholar]
- 5.Tough EA, White AR, Cummings TM, et al. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomized controlled trials. Eur J Pain 2009; 13: 3–10. [DOI] [PubMed] [Google Scholar]
- 6.Giamberardino MA, Affaitati G, Fabrizio A, et al. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol 2011; 25: 185–198. [DOI] [PubMed] [Google Scholar]
- 7.Hong CZ. Considerations and recommendations regarding myofascial trigger point injection. J Musculoskelet Pain 1994; 2: 29–59. [Google Scholar]
- 8.Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician 2002; 65: 653–660. [PubMed] [Google Scholar]
- 9.Criscuolo CM. Interventional approaches to the management of myofascial pain syndrome. Curr Pain Headache Rep 2001; 5: 407–411. [DOI] [PubMed] [Google Scholar]
- 10.Simons DG. New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil 2008; 89: 157–159. [DOI] [PubMed] [Google Scholar]
- 11.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spine Cord Med 2011; 34: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery 1990; 26: 217–226. [DOI] [PubMed] [Google Scholar]
- 13.Sciotti VM, Mittak VL, DiMarco L, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain 2001; 93: 259–266. [DOI] [PubMed] [Google Scholar]
- 14.Clinkscales CP, Greenfield MLVH, Vanarase M, et al. An observational study of the relationship between lumbar epidural space depth and body mass index in Michigan parturients. Int J Obstet Anesth 2007; 16: 323–327. [DOI] [PubMed] [Google Scholar]
- 15.Watts RW. The influence of obesity on the relationship between body mass index and the distance to the epidural space from the skin. Anaesth Intensive Care 1993; 21: 309–310. [DOI] [PubMed] [Google Scholar]
- 16.Lai HC, Liu TJ, Peng SK, et al. Depth of the thoracic epidural space in paramedian approach. J Clin Anesth 2005; 17: 339–343. [DOI] [PubMed] [Google Scholar]
- 17.Rathmell JP, Michna E, Fitzgibbon DR, et al. Injury and liability associated with cervical procedures for chronic pain. Anesthesiology 2011; 114: 918–926. [DOI] [PubMed] [Google Scholar]