Abstract
Pulmonary alveolar proteinosis (PAP) is a rare disorder characterized by the accumulation of excessive surfactant lipids and proteins in alveolar macrophages and alveoli. Oral statin therapy is a novel treatment for PAP with hypercholesterolemia. However, this treatment has never been described in a patient without hypercholesterolemia. Here, we present a case of successful treatment with atorvastatin for a patient with possibly unclassified PAP without hypercholesterolemia who responded poorly to whole lung lavage therapy and inhaled granulocyte-macrophage colony-stimulating factor. After 18 months of atorvastatin treatment, the patient experienced improvements in dyspnea, radiographic abnormalities and pulmonary function. The present case study supports the feasibility of statin therapy for PAP regardless of the level of cholesterol.
Keywords: Pulmonary alveolar proteinosis, statin, therapy, cholesterol, hypercholesterolemia, granulocyte-macrophage colony-stimulating factor
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare and sometimes severe disorder characterized by the accumulation of excessive surfactant lipids and proteins within the alveoli and terminal airways, resulting in impaired gas exchange.1 Clinically, patients with PAP are asymptomatic or present with progressive dyspnea and increased susceptibility to infections.2 PAP is recognized to occur in four distinct clinical forms: (1) autoimmune PAP (primary PAP) associated with elevated levels of autoantibodies against granulocyte-macrophage colony-stimulating factor (GM-CSF); (2) congenital or hereditary PAP due to mutations in GM-CSF receptor alpha and beta genes (CSF2RA and CSF2RB, respectively) or genes involved in surfactant production; (3) secondary PAP caused by various underlying conditions; and (4) unclassified PAP. More than 90% of PAP is autoimmune.3
Whole lung lavage (WLL) therapy is the standard treatment for PAP.4 According to the recent results of a large clinical trial, inhaled GM-CSF is an effective therapy for autoimmune PAP.5 Moreover, plasmapheresis and rituximab infusions have been proposed as alternative therapies for PAP in recent years.6,7 Oral statin therapy, which reduces cholesterol accumulation and ameliorates PAP, is considered a novel pharmacotherapy for PAP with hypercholesterolemia.8 To date, no cases of successful treatment with statins for PAP without hypercholesterolemia have been reported.
Case report
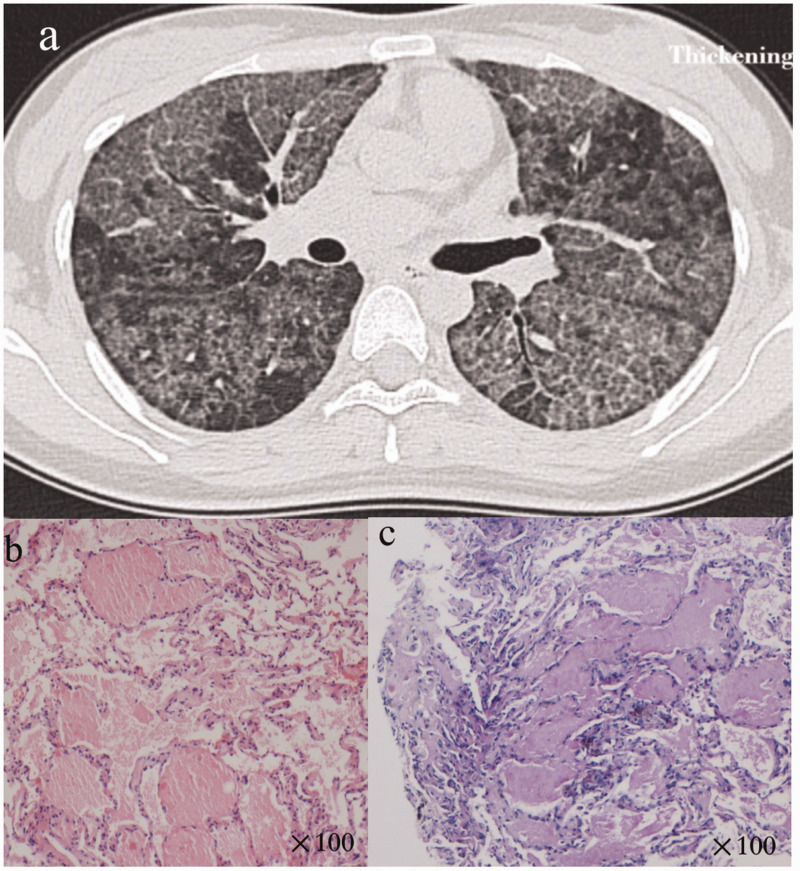
The patient was born in 1988, with no respiratory symptoms before 18 years old. At the age of 21 years old, she was referred to our hospital with a 3-year history of progressive dyspnea and a 2-month history of cough. The patient had no occupational exposure, dust inhalation or smoking history. The patient had no familial history of PAP or preexisting diseases. Physical examination on admission showed a body temperature of 36.5°C, blood pressure of 110/82 mmHg, respiratory frequency of 20 breaths per minute and heart rate of 108 beats per minute. Arterial blood gas measurements were as follows (room air): pH 7.401, partial pressure of carbon dioxide (PCO2) 37.5 mmHg, partial pressure of oxygen (PO2) 72 mmHg and oxygen saturation (SpO2) 95%. Pulmonary function testing revealed a forced vital capacity of 42.5% of the predicted value and a percent predicted diffusion capacity for carbon monoxide (DLCO%pred) of 12.2%. After admission to our hospital, the patient’s laboratory results were as follows: total cholesterol (TC) level = 4.57 mmol/L (normal range, 2.90–6.00 mmol/L), triglyceride level = 0.53 mmol/L (below normal limit, 0.56–1.70 mmol/L), high-density lipoprotein (HDL) level = 1.57 mmol/L (normal range, 0.94–2 mmol/L), low-density lipoprotein (LDL) level = 2.55 mmol/L (normal range, 1.89–3.1 mmol/L), carcinoembryonic antigen level = 3.65 ng/mL (normal range, 0–10 ng/mL) and lactate dehydrogenase (LDH) level = 260 U/L (above normal limit, 109–245 U/L). A high-resolution computed tomogram (HRCT) of the chest revealed diffuse, ground-glass opacification and septal thickening (Figure 1a). The diagnosis of this patient was confirmed by a transbronchial lung biopsy. The bronchoalveolar lavage fluid showed a light milky appearance. Hematoxylin and eosin-stained tissue demonstrated alveolar filling with eosinophilic material (Figure 1b). Periodic acid–Schiff staining was positive (Figure 1c). After excluding infectious or metabolic causes or malignancies, a diagnosis of PAP was confirmed based on a typical chest HRCT and histology (right lower lobe) in 2009.
Figure 1.
Diagnosis of pulmonary alveolar proteinosis was determined on the basis of a typical chest high-resolution computed tomogram (HRCT) and histology. Figure 1a shows HRCT with ground-glass opacification, interstitial thickening and a paving pattern. Hematoxylin and eosin-stained tissue demonstrated alveolar filling with eosinophilic material (Figure 1b, magnification ×100), which was positive for the Periodic acid–Schiff stain (Figure 1c, magnification ×100).
On the basis of the diagnosis of PAP, bilateral WLL treatments (15,000 mL saline/lung) were performed. The symptoms of dyspnea and cough improved, and the degree of pulmonary ground-glass opacification on HRCT was reduced for a short period after WLL treatment. However, because of the persistence of progressive dyspnea, radiographic abnormalities and severely reduced DLCO%pred (11%–12.5%), multiple bilateral WLL treatments were performed 9, 33, 39, 58 and 66 months after the first WLL treatment. Because only a short-term response to WLL was observed and given the lack of a serum GM-CSF autoantibody test, the patient elected to empirically initiate inhaled recombinant human GM-CSF (rhGM-CSF) 150 μg twice daily, three times per week administered 2 weeks per month at age 26 (in 2014). Seven months later, dyspnea and ground-glass opacification on HRCT were persistent. To clarify the specific form of PAP, a serum GM-CSF autoantibody test was performed, and the result was normal (−1.59 μg/mL, the critical threshold is ∼2.39 μg/mL).9 The serum GM-CSF autoantibody test was performed one more time in 2019 and was negative (−0.97 μg/mL). Based on the negative GM-CSF autoantibody tests, this patient could not be classified as autoimmune PAP and was diagnosed with possibly unclassified PAP.
In 2017, at age 29, bilateral WLL treatment was performed repeatedly to relieve the symptom of dyspnea and reduce the oxygen requirement. Until the patient reached the age of 30 (on 11 October 2018), we attempted to increase the yield of lipoproteins recovered from the lungs with bilateral high-volume WLL treatment under the support of cardiopulmonary bypass. Moreover, slim-type gene sequencing of all exons was performed on the patient and her parents. A mutation in the deleted in lung and esophageal cancer 1 (DLEC1) gene (c.3487C>T, the 3487th nucleotide in the coding region changed from C to T) was detected and caused an amino acid substitution from arginine to tryptophan at position 1160 (p.Arg1160Trp). A hybrid nucleotide mutation in the coatomer protein subunit alpha (COPA) gene (c.2977A>C, the 2977th nucleotide in the coding region changed from A to C) was detected and caused an amino acid substitution from asparagine to histidine at position 993. Mutations in the DLEC1 gene are associated with various cancers, and COPA gene mutation is observed in patients with COPA syndrome. However, the patient had no clinical manifestations or imaging features of related tumors and COPA syndrome. Additionally, mutations in the genes encoding surfactant protein B and surfactant protein C or alpha and beta genes (CSF2RA and CSF2RB) were not detected. Forty days after the last WLL treatment, she presented with progressive breathlessness, and HRCT revealed diffuse ground-glass opacification.
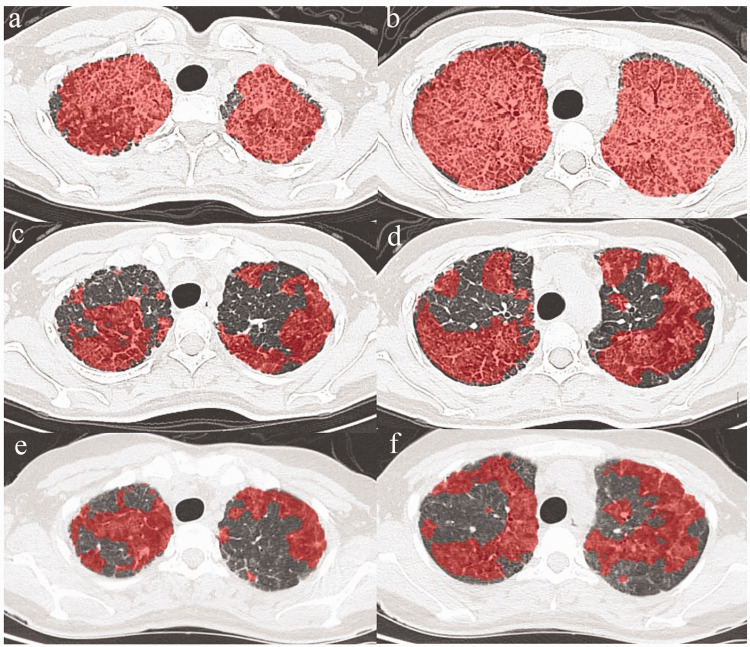
Oral statin was initiated on 27 November 2018 after the patient provided written informed consent for statin therapy. At statin initiation, the patient’s laboratory results were as follows: TC level = 4.59 mmol/L (normal range, 2.90–6.00 mmol/L), TG level = 0.41 mmol/L (below normal limit, 0.56–1.70 mmol/L), HDL level = 1.42 mmol/L (normal range, 0.94–2 mmol/L), LDL level = 2.62 mmol/L (normal range, 1.89–3.1 mmol/L) and LDH level = 382 U/L (above normal limit, 109–245 U/L). SpO2 was 87% in ambient air. During the first 2 weeks, the patient received 20 mg of oral atorvastatin daily. Later, the dose of atorvastatin was increased to 30 mg daily for 4 weeks. From week 6 onward, she received 40 mg atorvastatin daily. After 8 weeks of atorvastatin therapy, clinical and radiological improvements in PAP were clearly observed. After 18 months of atorvastatin treatment, chest HRCT revealed reduced pulmonary ground-glass opacification. Quantitative analysis of the computed tomography scans using an artificial intelligence tool provided an automatic and objective estimation of the disease burden of PAP. We evaluated the percentage of lung ground-glass opacification and the density of ground glass at baseline and on follow-up scans. Percent changes in the quantitative analysis of lung ground-glass opacification and the density of ground glass at baseline, 11 months and 18 months were shown in Table 1 and Figure 2. We observed that the quantitative percentages of ground-glass opacification of the whole lung and per lobe were lower on follow-up scans compared with the baseline HRCT. Furthermore, the density of ground glass decreased to almost normal levels. After 18 months of atorvastatin treatment, the levels of TC, LDL and LDH had decreased. The definite laboratory results were as follows: TC level = 3.33 mmol/L (normal range, 2.90–6.00 mmol/L), TG level = 0.55 mmol/L (below normal limit, 0.56–1.70 mmol/L), HDL level = 1.31 mmol/L (normal range, 0.94–2 mmol/L), LDL level = 1.63 mmol/L (below normal limit, 1.89–3.1 mmol/L) and LDH level = 239 U/L (normal range, 109–245 U/L). SpO2 was 96% in ambient air. In addition, at the beginning of oral statin therapy, the dyspnea of the patient was too severe to complete the pulmonary function test. During the long-term follow-up, the pulmonary function of the patient improved rapidly, especially the levels of DLCO%pred (Table 2). No other therapies were performed during the 18-month period that the patient was on statin therapy.
Table 1.
Percent changes in the quantitative analysis of lung ground-glass opacification and the density of ground glass at baseline, 11-months and 18-months.
| Percent changes (%) | Baseline | 11-month follow-up | 18-month follow-up |
|---|---|---|---|
| Total opacification of whole lung | 96.4 | 67.4 | 64.6 |
| Opacification of right upper lobe | 100 | 44.7 | 66.6 |
| Opacification of right middle lobe | 100 | 94.1 | 88.4 |
| Opacification of right lower lobe | 100 | 74.2 | 58.6 |
| Opacification of left upper lobe | 98.2 | 71.9 | 70.7 |
| Opacification of left lower lobe | 94.6 | 93.4 | 59.5 |
| HU between –∞ and −750 | 8.2 | 14 | 8.2 |
| HU between −750 and −300 | 41.6 | 35.5 | 49.5 |
| HU between −300 and 49 | 31.2 | 13.3 | 6.4 |
| HU of 50+ | 13.2 | 4.2 | 0.5 |
HU, Hounsfield unit.
Figure 2.
High-resolution computed tomogram (HRCT) scans showing changes in ground-glass opacification. Scans at the initiation of oral atorvastatin therapy (Figure 2a and Figure 2b). HRCT revealed reduced pulmonary ground-glass opacification at 11-months (Figure 2c and Figure 2d) and 18-months (Figure 2e and Figure 2f) of follow-up.
Table 2.
Changes in pulmonary function during the 18 months of follow-up.
| Pulmonary function test | Baseline | 11-month follow-up | 18-month follow-up |
|---|---|---|---|
| Percent of predicted forced vital capacity (FVC%pred) | NA | 45.4 | 51.2 |
| Percent of predicted forced expiratory volume in one second (FEV1%pred) | NA | 49.7 | 53.4 |
| Percent of predicted diffusion capacity for carbon monoxide (DLCO%pred) | NA | 11.1 | 31.2 |
Discussion
This report described a case of successful treatment with atorvastatin for a patient with possibly unclassified PAP without hypercholesterolemia who responded poorly to WLL and inhaled GM-CSF supplementation. The patient received repeated WLL treatments, but the symptom of dyspnea was improved for only a short period. WLL is associated with adverse effects, such as infections, fever, convulsions, pneumothorax, pleural effusion, hypoxemia or even death. Inhaled GM-CSF therapy in this patient had no effect on dyspnea, and the serum GM-CSF autoantibody test was negative. GM-CSF plays an important role in the pathogenesis of autoimmune PAP, and some prospective multicenter studies of inhaled GM-CSF therapy showed improvements in gas exchange, especially in patients with severe PAP.10,11 In a recent trial by Trapnell et al.,5 daily administration of inhaled recombinant GM-CSF resulted in greater improvements in pulmonary gas transfer and functional health status. In a further mechanistic study, GM-CSF was shown to stimulate cholesterol clearance in macrophages in a reversible and concentration-dependent manner. Loss of GM-CSF stimulation altered lung surfactant composition by increasing the relative proportion of cholesterol.12
A previous study reported that oral statin therapy was associated with clinical and radiological improvements in PAP patients with hypercholesterolemia.8 In our study, statin therapy was identified as a novel treatment for possibly unclassified PAP without hypercholesterolemia and even for critically ill patients with PAP. Pulmonary surfactant is composed of 80% polar lipids (primarily phosphatidylcholine and multiple less-abundant phospholipid species), 10% neutral lipids (primarily free cholesterol with small amounts of triglycerides and free fatty acids) and 10% surfactant proteins. Surfactant homeostasis is maintained by the secretion and recycling of surfactant components by alveolar type II epithelial cells and the catabolism of these components by alveolar macrophages.13 Statins inhibit 3-hydroxy-3-methylglutaryl-CoA reductase, thereby reducing endoplasmic reticulum cholesterol levels and facilitating cholesterol efflux.14 In a study by McCarthy et al.,8 ex vivo statin treatment reduced cholesterol accumulation in alveolar macrophages by 40%, which demonstrated that statin therapy had a direct effect on alveolar macrophages. Compared with the cost, complications and short-term effectiveness of WLL, statin therapy is simple, inexpensive and safe, providing important benefits for these purposes. In the future, the feasibility of oral statin therapy will be determined in larger samples of patients with PAP.
Mutations in the DLEC1 gene are associated with various cancers, and COPA gene alterations have been observed in patients with COPA syndrome. The COPA gene is also associated with autoimmune diseases. Patients with COPA syndrome typically present with interstitial lung disease with pulmonary hemorrhage and subsequently developed arthritis.15 However, the patient in this study did not show these clinical features, excluding the diagnosis of COPA syndrome. The DLEC1 gene is related to the cilia and flagella of epithelial cells. DLEC1 was initially discovered in 1999 as a candidate tumor suppressor gene in lung, esophageal and renal cancers.16 DLEC1 is located at chromosome 3p22-p21.3, a region recognized as a hot spot likely to contain tumor suppressor genes with frequent genetic abnormalities. The overexpression of DLEC1 significantly suppresses the clonogenicity of tumor cells.17 The downregulation or loss of DLEC1 expression has been observed in multiple cancers, including lung cancer. However, mutations in the DLEC1 gene have not been detected in any type of cancer.18 In this study, a mutation in the DLEC1 gene was observed. The patient had no clinical manifestations or imaging features of related cancers. Currently, the association of mutations in DLEC1 and COPA genes with PAP is unknown. Moreover, the relationship between cholesterol metabolism and mutations in COPA and DLEC1 genes remains to be clarified.
Although PAP can wane or exhibit spontaneous remission, the clinical manifestations and imaging features of this patient were persistent. In this study, a case of successful treatment with statin therapy for possibly unclassified PAP without hypercholesterolemia was presented. No other therapies were performed during the 18-month period that the patient was on statin therapy. After 18 months of atorvastatin treatment, improvements in dyspnea, radiographic abnormalities and pulmonary function were observed. Statin therapy for PAP, regardless of the cholesterol level, may provide a more simple and inexpensive treatment approach and warrants further investigation.
Footnotes
Ethics statement: This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital of Medical School of Nanjing University (No. 2019-106-01), and the patient provided written informed consent for publication of the case report.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethics statement: This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital of Medical School of Nanjing University (No. 2019-106-01), and the patient provided written informed consent for publication of the case report.
Funding: This work received financial support from the National Nature Science Foundation of China (81570061), the National Key Research and Development Program of China (2016YFC0901502) and the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (2017-12 M-2-001). Serum GM-CSF autoantibody tests and slim-type gene sequencing of all exons were funded by these projects.
ORCID iD: Shenyun Shi https://orcid.org/0000-0002-2022-121X
References
- 1.Suzuki T andTrapnell BC.. Pulmonary Alveolar Proteinosis Syndrome. Clin Chest Med 2016; 37: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Abdelmalak B, Inoue Y, et al. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir Med 2018; 6: 554–565. [DOI] [PubMed] [Google Scholar]
- 3.Trapnell BC, Nakata K, Bonella F, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers 2019; 5: 16. [DOI] [PubMed] [Google Scholar]
- 4.Beccaria M, Luisetti M, Rodi G, et al. Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J 2004; 23: 526–531. [DOI] [PubMed] [Google Scholar]
- 5.Trapnell BC, Inoue Y, Bonella F, et al. Inhaled Molgramostim Therapy in Autoimmune Pulmonary Alveolar Proteinosis. N Engl J Med 2020; 383: 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonnell MJ, Reynolds C, Tormey V, et al. Pulmonary alveolar proteinosis: report of two cases in the West of Ireland with review of current literature. Ir J Med Sci 2014; 183: 123–127. [DOI] [PubMed] [Google Scholar]
- 7.Soyez B, Borie R, Menard C, et al. Rituximab for Auto-Immune Alveolar Proteinosis, a Real Life Cohort Study. Respir Res 2018; 19: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy C, Lee E, Bridges JP, et al. Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis. Nat Commun 2018; 9: 3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao YL, Xu KF, Li Y, et al. Occupational inhalational exposure and serum GM-CSF autoantibody in pulmonary alveolar proteinosis. Occup Environ Med 2015; 72: 504–512. [DOI] [PubMed] [Google Scholar]
- 10.Tazawa R, Hamano E, Arai T, et al. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2005; 171: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 11.Wylam ME, Ten R, Prakash UB, et al. Aerosol granulocyte-macrophage colony-stimulating factor for pulmonary alveolar proteinosis. Eur Respir J 2006; 27: 585–593. [DOI] [PubMed] [Google Scholar]
- 12.Sallese A, Suzuki T, McCarthy C, et al. Targeting cholesterol homeostasis in lung diseases. Sci Rep 2017; 7: 10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Gil J andWeaver TE.. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda) 2010; 25: 132–141. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res 2006; 47: 1339–1351. [DOI] [PubMed] [Google Scholar]
- 15.Kumrah R, Mathew B, Vignesh P, et al. Genetics of COPA syndrome. Appl Clin Genet 2019; 12: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daigo Y, Nishiwaki T, Kawasoe T, et al. Molecular cloning of a candidate tumor suppressor gene, DLC1, from chromosome 3p21.3. Cancer Res 1999; 59: 1966–1972. [PubMed] [Google Scholar]
- 17.Imreh S Klein G andZabarovsky ER.. Search for unknown tumor-antagonizing genes. Genes Chromosomes Cancer 2003; 38: 307–321. [DOI] [PubMed] [Google Scholar]
- 18.Seven D, Yavuz E, Kilic E, et al. DLEC1 is not silenced solely by promoter methylation in head and neck squamous cell carcinoma. Gene 2015; 563: 83–86. [DOI] [PubMed] [Google Scholar]