Abstract
Background:
Residual rotatory knee laxity at midterm follow-up after isolated anterior cruciate ligament reconstruction (ACLR) versus ACLR with lateral extra-articular tenodesis (LET) remains an issue.
Purpose/Hypothesis:
To evaluate the outcomes of ACLR with or without additional LET at a minimum 2-year follow-up in patients with preoperative high-grade pivot shift (PS). Our hypothesis was that the addition of LET would decrease the risk of secondary meniscal injury and the presence of residual high-grade PS at follow-up.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A retrospective analysis performed at 3 sports medicine centers identified 266 study patients; all had a high-grade PS (grade 2 or 3) preoperatively and underwent isolated ACLR with or without LET. Four different ACLR techniques were used: single-strand quadrupled semitendinosus (ST4) ACLR without LET (ST4 group; n = 55), ST4 with anatomic LET (ST4+LET group; n = 77), bone–patellar tendon and modified Lemaire LET (BTB+LET group; n = 43), and quadriceps tendon and modified Lemaire LET (QT+LET group; n = 91). At follow-up, we evaluated for the presence of high-grade (grade ≥2) PS. Preoperative meniscal tears and their treatment were recorded.
Results:
Overall, 185 (69.5%) patients had at least 1 meniscal tear at index surgery. The mean follow-up period was 44.3 months; 47 (17.7%) patients had a new meniscal tear and 64 (24%) patients had a high-grade PS at follow-up. Compared with meniscal repair, significant predictors for high-grade PS at follow-up were meniscectomy (odds ratio [OR] = 2.65 [95% CI, 1.19-5.63]; P = .02) and nonrepair of preoperative meniscal tear (OR = 3.26 [95% CI, 1.27-9.43]; P = .007). The appearance of a new symptomatic meniscal tear was the strongest significant predictor of high-grade PS at follow-up (OR = 4.31 [95% CI, 2.31-8.06]; P < .001). No significant correlation was observed between the addition of LET and the presence of high-grade PS at follow-up.
Conclusion:
In the current study, 1 in 4 patients with high-grade PS before ACLR with or without LET was at risk of residual rotatory knee laxity at mean 44-month follow-up, regardless of the technique used. Repairing a pre-existing meniscal lesion was more effective than performing LET to decrease the presence of a high-grade PS at follow-up.
Keywords: pivot shift, anterior cruciate ligament reconstruction, lateral extra-articular tenodesis, meniscal tear, rotational laxity
Since Robert Adams described an anterior cruciate ligament (ACL) rupture in 1837, the evolution of surgical ACL reconstruction (ACLR) techniques has been marked by controversy and rediscovery.28,29,32 During the 1970s, 1980s, and 1990s, there were 2 opposing concepts of ACLR. One was anatomic, with an objective of reconstructing the native ACL, and the other was functional, addressing only the pivot shift (PS),20 which was referred to as an extra-articular reconstruction.3,8 This latter technique was effective, but it insufficiently addressed anteroposterior-posterior laxity and was therefore abandoned in favor of anatomic ACLR.27
A 2013 article by Claes et al7 brought back to the forefront an anatomic structure previously described by Paul Segond in the 19th century. This was described as the anterolateral ligament, which is a discrete structure unifying the anterolateral part of the tibia and the femur. This anterolateral complex, as it has been called since the consensus of 2019,11 led to an increasing popularity of lateral extra-articular tenodesis (LET) as an adjunctive procedure to ACLR. Recent biomechanical cadaveric studies18,38 have shown that sectioning of this lateral complex associated with a section of the ACL resulted in an increase in sagittal and rotational laxity. Other studies demonstrated the value of reconstructing the anterolateral complex during ACLR to restore normal knee kinematics.16,31
The clinical results of studies comparing isolated ACLR versus ACLR+LET have shown a significant advantage of ACLR+LET in terms of meniscal tear healing36 and ACL rerupture rates37 in high-risk populations. The effect of ACLR+LET on the control of rotational instability as expressed by the PS at mid- and long-term follow-up remains debatable. This controversy may be related to the many factors that influence rotational instability9,39 and the multitude of LET techniques (anatomic vs nonanatomic) with different biomechanical properties.6,10,12,34,37
The main objective of this study was to evaluate the outcomes of different ACLR techniques with or without additional LET at a minimum 2-year follow-up in a population of patients with high-grade PS before surgery; the secondary objective was to evaluate potential risk factors for high-grade PS and secondary meniscal tears. The primary outcome was the presence of a high-grade PS at follow-up. Our hypothesis was that the addition of LET to primary ACLR would decrease the risk of secondary meniscal injury and the presence of a residual high-grade PS at 2-year follow-up.
Methods
After review board approval was granted, a retrospective analysis was performed from a prospectively collected database in 3 sports medicine centers. We identified 808 patients who underwent isolated ACLR with or without additional LET between March 2013 and May 2017.
Patients aged 18 to 50 years who had isolated ACL injury without collateral ligament injury (magnetic resonance imaging [MRI] grade ≤2) or multiligament injury confirmed by MRI were included in the study. We included only patients with a high-grade PS (grade 2 or 3 according to the International Knee Documentation Committee [IKDC] classification13) identified during examination under anesthesia at the time of surgery. Exclusion criteria included a history of ipsilateral knee surgery, a history of contralateral ACLR, an associated bone or cartilage procedure, pathological hyperlaxity (Beighton score >3), and chronic inflammatory joint disease. Likewise, patients were excluded from the study if a graft rupture was confirmed on MRI, a revision ACLR was performed, or a contralateral ACL rupture had occurred between surgery and the final follow-up visit.
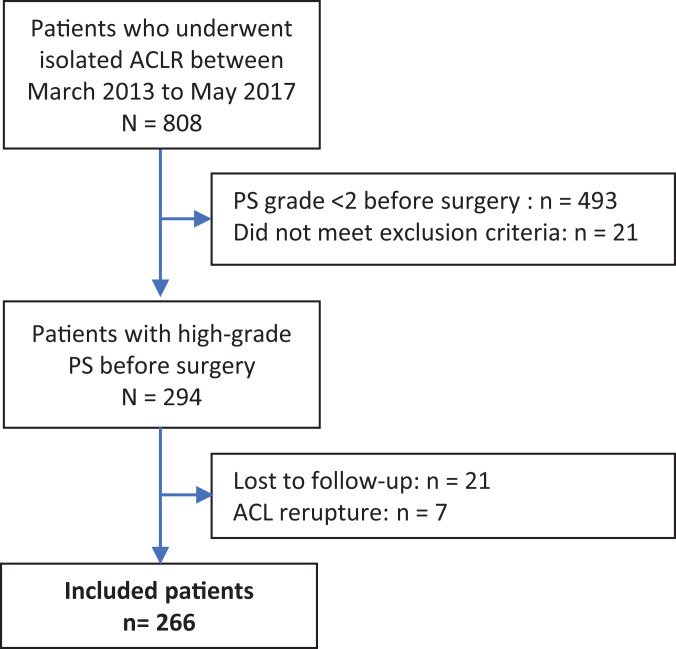
A total of 294 eligible patients were selected (Figure 1). All patients had undergone isolated ACLR either with or without LET. For the follow-up visit, all patients were examined during a dedicated clinical visit. All patients gave valid consent to participate. Twenty-one patients were lost to follow-up (7%). Seven patients suffered from an ACL rerupture which was confirmed by MRI or they had undergone a revision ACLR and were therefore excluded from the study. Finally, 266 patients were included in the study.
Figure 1.
Study flowchart. ACL, anterior cruciate ligament; ACLR, ACL reconstruction; PS, pivot shift.
Surgical Technique
All patients underwent surgery by 4 different senior surgeons (S.P., N.P., C.S., M.O.) from 3 sports medicine centers using 4 ACLR techniques with different types of grafts and LET. The choice of surgical techniques was based on individual surgeons’ preferences. It was therefore possible to separate the patients into 4 groups: (1) single-strand quadrupled semitendinosus ACLR without LET (ST4 group) (n = 55), (2) ST4 with additional anatomic LET using the same hamstring graft (ST4+LET group) (n = 77) , (3) bone–patellar tendon and LET (BTB+LET group) (n = 43), and (4) quadriceps tendon and LET (QT+LET group) (n = 91).24
ST4 Group
In this group, the ST4 technique4 was performed using a 3-cm vertical incision.5 The semitendinosus tendon was identified and harvested using an open stripper. The tendon was folded into 4 strands, and the ends were secured using a No. 2 FiberWire suture (Arthrex). The quadrupled graft was then preconditioned by traction at 150 N for a minimum of 10 minutes. Bone tunnels were made using femoral and tibial outside-in drill guides, aiming at the respective anatomic footprints of the ACL. Both tibial and femoral ACL tunnels were prepared with a cannulated drill that matched the graft diameter. Graft fixation was performed in the tibia and femur using a PEEK Cage System (Sacimex) with the knee at 20° of flexion and the tibia at 0° of rotation. No LET was performed in this group of patients.
ST4+LET Group
This ACLR+LET technique was previously described by Boutsiadis et al.2 It entailed the same skin incision and approach as for the ST4 group. The gracilis and semitendinosus tendons were harvested and completely detached from their tibial attachment. The combined part of the graft was composed of 4 strands: 1 strand from the gracilis and 3 strands from the semitendinosus. Two additional strands of the gracilis were used for LET. An outside-in femoral guide was placed proximally and posteriorly to the lateral epicondyle at the femoral origin of the anterolateral ligament and the intra-articular target at the ACL femoral origin. The tibial tunnel was drilled in an outside-in fashion. A drill bit was used to prepare full-length tunnels with a diameter that matched the graft diameter. A second, independent tibial tunnel was made for the anatomic LET. The second tunnel started medially (2 cm anterior to the tibial ACL tunnel) and exited laterally, immediately posterior to the Gerdy tubercle.
The graft was shuttled through the tibial and femoral ACL tunnel. The ACL was initially fixed in the tibia by a loop button (BTB TightRope; Arthrex) and in the femoral tunnel by an absorbable interference screw (Biosteon; Stryker) with the knee at 20° of flexion and the tibia at 0° of rotation. The LET graft was shuttled underneath the iliotibial band (ITB), under the lateral collateral ligament, and through its tibial tunnel and fixed by securing an adjustable-loop button (BTB TightRope) in 20° of knee flexion and neutral rotation.
BTB+LET Group
Two longitudinal skin incisions of 3 cm each were made for BTB graft harvesting: the first incision over the distal patella and the second over the anterior tibial tuberosity (ATT). A double-blade scalpel with a distance between the blades of 10 mm was used to section the tendon subcutaneously from the distal pole of the patella to the ATT. The patellar bone block (2 cm long × 1 cm wide × 1 cm deep) was cut first by using an oscillating saw. The patellar bone block was then passed from the proximal to the distal skin incision. The second bone block (2 cm long × 1 cm wide × 1 cm deep) was obtained from the ATT in a similar fashion. After calibration of the graft, a 30-mm, inside-out femoral tunnel was drilled. The tibial tunnel was drilled in an outside-in fashion using a trephine. The excess tibial bone was used to fill the harvesting site of the ATT. Graft fixation was achieved with 2 absorbable interference screws (Biosure; Smith & Nephew).
For the LET, a modified Lemaire technique was used.22 An oblique skin incision was made between the lateral femoral epicondyle and the Gerdy tubercle, measuring approximately 5 cm. A tendon strip of 1 × 8 cm was harvested from the ITB, leaving the attachment at the Gerdy tubercle intact. The proximal end of the ITB graft was whipstitched using a No. 1 Vicryl (Ethicon; Johnson and Johnson) suture. Once the fibular collateral ligament (FCL) had been identified, small capsular incisions were made anteriorly and posteriorly to the proximal portion of the ligament. Metzenbaum scissors were then placed deep along the FCL to bluntly dissect a tract for graft passage. The ITB graft was fixed to the distal femur just anteriorly to the intermuscular septum and proximally to the femoral attachment site of the FCL using a 25-mm—deep blind tunnel with a resorbable interference screw (Biosure; Smith & Nephew). Fixation was performed with the knee at 20° of flexion and the tibia at 0° of rotation.
QT+LET Group
The quadriceps tendon graft was exposed via a longitudinal 2- to 3-cm skin incision over the proximal patella. A regular scalpel was used to prepare and harvest the quadriceps tendon graft. The bone block (2 cm long × 1 cm wide × 1 cm deep) was cut using an oscillating saw. The dissection was then extended proximally, with care taken not to enter the suprapatellar pouch, and only the superficial and the intermediate layers of the quadriceps tendon were harvested. After graft calibration, a 30-mm inside-out femoral tunnel was drilled. The tibial tunnel was drilled in an outside-in fashion. Femoral fixation was carried out via an adjustable loop button (BTB TightRope). The bone block of the graft was placed on the tibial side and fixed with a resorbable interference screw (Biosteon). Fixation was performed with the knee at 20° of flexion and the tibia at 0° of rotation. The LET was performed using the same modified Lemaire technique as used in the BTB+LET group.
Rehabilitation
The choice of rehabilitation protocol was based on individual surgeon preferences. In all cases, immediate full weightbearing and progressive range of motion exercises were recommended. The main focus of the early rehabilitation period was on quadriceps activation exercises and regaining full extension. A gradual return to sports was allowed after surgeons’ approval, between 4 and 6 months for noncontact sports and between 9 and 12 months for contact sports.
Preoperative Data Collection
Preoperative data including age, sex, body mass index, preoperative sagittal laxity assessed using the objective IKDC score, and PS grade under general anesthesia (IKDC grade 2 or 3) were collected from the routine preoperative questionnaire and operative report. Data regarding the type of sport practiced (contact or noncontact pivoting sport) prior to the ACL injury and the level of competition (professional or amateur) were also collected from the routine preoperative questionnaire and confirmed with the patient during the clinical follow-up appointment.
Preoperative meniscal assessment on MRI was performed by a radiologist and an independent orthopaedic surgeon (C.J.), neither of whom were involved in the surgical procedures. In cases of differing opinions, interpretation by third surgeon (C.P.) was sought to determine the decision. All of these assessors were blinded to the other data of the study and the type of ACLR performed. Meniscal tears were classified as follows:
For the medial meniscus: (1) posterior meniscosynovial separations or ramp lesions43; (2) bucket-handle and vertical lesions involving at least the posterior and middle segments; (3) vertical, horizontal, or radial tears of the middle segment; and (4) posterior root lesions.
For the lateral meniscus: (1) bucket-handle and vertical lesions involving at least the posterior and middle segments; (2) vertical, horizontal, or radial tears of the middle segment; and (3) posterior root lesions.
Data on treatment strategy were also collected, and treatments were classified as repair, meniscectomy, or nonrepair.
Follow-up Consultation
The follow-up consultation was performed by a single fellowship-trained orthopaedic surgeon (C.J.) who was independent from the surgeons who performed the ACLRs. The examiner was blinded for information on the presence or absence of meniscal tears, the grade of preoperative PS, and the identity of the surgeon.
During this consultation, the following factors were evaluated: sagittal laxity using a Telos or Genourob arthrometer; the presence or absence of high-grade PS (IKDC grade ≥2)13; functional outcomes using the Knee injury and Osteoarthritis Outcome Score (KOOS) and Lysholm questionnaires; and the ability to return to preinjury sport.
If a new symptomatic meniscal tear was suspected on clinical examination (a history of knee pain or mechanical symptoms such as meniscal blockage with joint line tenderness), a new MRI was performed. The same double-blinded interpretation method as described previously was performed by 2 examiners (1 radiologist and 1 orthopaedic surgeon [C.P.], neither of whom were involved in the surgical procedure or the patient visit). Only new meniscal tears that were not found on preoperative MRI were considered. If patients had sustained subsequent knee surgery after the initial ACLR, the operative report was collected to identify the meniscal status.
Statistical Analysis
The descriptive statistics are presented as mean ± SD for quantitative variables. Means and standard deviations were determined for each of the measurements made for the population. Normal (Gaussian) distributions were determined. Univariate analysis was performed using either parametric or nonparametric testing to estimate difference between groups. Multiple linear regression models were developed to establish the determinants for each of the variables. For each model, variables with a P value of <.1 were kept in the final model. A sample-size calculation showed that 50 patients per group would allow us to demonstrate a >3-fold difference in terms of PS presence at a minimum of 2 years of follow-up after surgery (expected rate 30%) between groups.35 For all statistical analysis, PASW Statistics Version 20 (SPSS, IBM) was used. The threshold for statistical significance was set at P < .05.
Results
Patients
Of the initial 294 study patients, 21 (7.1%) patients were lost to follow-up, and 7 (2.4%) patients experienced an ACL rerupture or underwent revision ACLR (1 patient in the ST4 group, 3 patients in the ST4+LET group, 2 patients in the BTB+LET group, and 1 patient in the QT+LET group). Ultimately, 266 patients were included in the study (55 patients in the ST4 group, 77 patients in the ST4+LET group, 43 patients in the BTB+LET group, and 91 patients in the QT+LET group). Patient characteristics are summarized in Table 1. The mean overall follow-up was 44.3 months. Preoperatively, 63.2% of patients displayed a grade 2 PS, 61.7% practiced a contact sport, and 17.3% were professionals. Patients had a mean preoperative sagittal laxity of 6.8 ± 2.5 mm. The mean follow-up, distribution of PS grade, and type of sport practiced were significantly different between the 4 groups.
Table 1.
Patient Characteristicsa
| Overall (N = 266) |
ST4 (n = 55) |
ST4+LET (n = 77) |
BTB+LET (n = 43) |
QT+LET (n = 91) |
Pb | |
|---|---|---|---|---|---|---|
| Age, y | 30.4 ± 8.4 | 30.5 ± 7.9 | 30.7 ± 8.4 | 30.8 ± 8.9 | 29.9 ± 8.0 | .8 |
| Sex, % male | 71.3 | 72.7 | 75.4 | 74.4 | 71.8 | .3 |
| Body mass index, kg/m2 | 24.0 ± 2.6 | 23.9 ± 1.9 | 24.6 ± 2.3 | 23.8 ± 2.2 | 24.1 ± 2.6 | .8 |
| Differential preoperative laxity, mm | 6.8 ± 2.5 | 6.9 ± 3.3 | 6.7 ± 3.6 | 6.7 ± 3.5 | 6.8 ± 2.9 | .7 |
| Pivot shift, n (%) | .02* | |||||
| Grade 2 | 168 (63.2) | 29 (52.7) | 50 (64.9) | 22 (51.2) | 67 (73.6) | |
| Grade 3 | 98 (36.8) | 26 (47.3) | 27 (35.1) | 21 (48.8) | 24 (26.4) | |
| Type of sport, n (%)c | .01** | |||||
| Contact | 164 (61.7) | 34 (61.8) | 51 (66.2) | 24 (55.8) | 55 (60.4) | |
| Noncontact | 102 (38.3) | 21 (38.2) | 26 (33.8) | 19 (44.2) | 36 (39.6) | |
| Professional athlete, n (%) | 46 (17.3) | 9 (16.3) | 14 (18.2) | 7 (16.3) | 16 (17.6) | .7 |
| Mean follow-up, mo | 44.3 | 47.1 | 50.2 | 45.1 | 37.3 | <.001*** |
aData are reported as mean ± SD unless otherwise indicated. BTB+LET, bone–patellar tendon and modified Lemaire LET; LET, lateral extra-articular tenodesis; QT+LET, quadriceps tendon and modified Lemaire LET; ST4, single-strand quadrupled semitendinosus; ST4+LET, ST4 with LET using the same hamstring graft.
bGlobal analysis of variance between the 4 groups.
cPivoting sports with contact: soccer, handball, basketball, rugby, motocross. Pivoting sports without contact: alpine skiing, fitness, gymnastics, tennis.
* Pairwise comparison exhibited significant differences between the BTB+LET group vs the ST4+LET (P = .01) and QT+LET (P = .02) groups and between the ST4 group vs the ST4+LET (P = .01) and QT+LET (P = .03) groups.
** Pairwise comparison exhibited significant differences between the ST4+LET vs BTB+LET (P = .008) groups.
*** Pairwise comparison exhibited significant differences between the QT+LET group vs the ST4+LET (P = .0009), ST4 (P = .0005), and BTB+LET (P = .01) groups.
Meniscal Status
A total of 185 (69.5%) patients had at least 1 meniscal tear at the time of the index surgery, 49 (26.5%) of which were bimeniscal. The distribution of types of meniscal tear in the 4 groups is summarized in Table 2. The cohort entailed 127 repairs (68.6%), 32 partial and subtotal meniscectomies (17.3%), and 26 nonrepaired tears (14.1%) (Table 3). No significant difference was observed between the 4 groups concerning the distribution of meniscal tears, but a significant difference (P = .01) was observed between the groups concerning their therapeutic management, with more patients in the QT+LET group undergoing repair.
Table 2.
Meniscal Preoperative Data and Therapeutic Managementa
| Overall (N = 266) |
ST4 (n = 55) |
ST4+LET (n = 77) |
BTB+LET (n = 43) |
QT+LET (n = 91) |
Pb | |
|---|---|---|---|---|---|---|
| Meniscal tear | 185 | 33 | 58 | 26 | 68 | .09 |
| Bimeniscal tear | 49 (26.5) | 10 (30.3) | 14 (24.1) | 4 | 21 (30.8) | .29 |
| Medial meniscal tear | 121 (65.4) | 23 (69.7) | 34 (58.6) | 16 (61.5) | 48 (70.5) | .053 |
| Bucket-handle | 11 | 2 | 2 | 4 | 3 | |
| Posterior root | 3 | 0 | 1 | 0 | 2 | |
| Ramp lesion | 57 | 11 | 17 | 7 | 22 | |
| Midsegment | 50 | 10 | 14 | 5 | 21 | |
| Lateral meniscal tear | 64 (34.6) | 10 (30.3) | 24 (41.4) | 10 (38.5) | 20 (29.5) | |
| Bucket-handle | 10 | 1 | 6 | 2 | 1 | |
| Posterior root | 36 | 8 | 10 | 5 | 13 | |
| Midsegment | 18 | 1 | 8 | 3 | 6 | |
| Therapeutic management | .01 | |||||
| Repair* | 127 (68.6) | 21 (63.6) | 37 (63.8) | 18 (69.2) | 51 (75) | |
| Meniscectomy** | 32 (17.3) | 6 (18.2) | 12 (20.7) | 4 (15.4) | 10 (14.7) | |
| Nonrepair*** | 26 (14.1) | 6 (18.2) | 9 (17.2) | 4 (15.4) | 7 (10.3) |
aData are reported as n or as n (%). BTB+LET, bone–patellar tendon and modified Lemaire LET; LET, lateral extra-articular tenodesis; QT+LET, quadriceps tendon and modified Lemaire LET; ST4, single-strand quadrupled semitendinosus; ST4+LET, ST4 with LET using the same hamstring graft.
bGlobal analysis of variance between the 4 groups.
* Pairwise comparison exhibited significant differences between QT+LET vs ST4 (P = .006) and QT+LET vs ST4+LET (P = .002).
** Pairwise comparison exhibited significant differences between QT+LET vs ST4+LET (P = .006) and BTB+LET vs ST4+LET(P = .008).
*** Pairwise comparison exhibited significant differences between QT+LET vs ST4 (P = .004) and QT+LET vs ST4+LET (P = .006).
Table 3.
Therapeutic Management of Meniscal Tearsa
| Repair | Meniscectomy | Nonrepair | |
|---|---|---|---|
| Medial meniscal tear | |||
| Ramp | 40 | 0 | 17 |
| Posterior root | 2 | 1 | 0 |
| Bucket-handle | 9 | 2 | 0 |
| Midsegment | 27 | 21 | 2 |
| Lateral meniscal tear | |||
| Posterior root | 33 | 3 | 0 |
| Bucket-handle | 8 | 2 | 0 |
| Midsegment | 8 | 3 | 7 |
aData are reported as No. of patients.
Functional Outcomes
No significant differences were observed between the groups in the functional outcomes of the KOOS and Lysholm scores (Table 4). The mean postoperative differential sagittal laxity was 2.3 ± 2.6 mm with no significant difference between groups. In total, 165 (62.0%) patients were able to return to their preinjury sport, and no significant difference was observed between groups for this parameter. A total of 64 (24.0%) patients presented with a high-grade PS at follow-up (29.1% for ST4, 26.0% for ST4+LET, 20.9% for BTB+LET, and 20.8% for QT+LET) (Table 4).
Table 4.
Postoperative Outcomesa
| Overall (N = 266) |
ST4 (n = 55) |
ST4+LET (n = 77) |
BTB+LET (n = 43) |
QT+LET (n = 91) |
P | |
|---|---|---|---|---|---|---|
| KOOS | ||||||
| Symptoms | 83.9 ± 14.6 | 84.2 ± 15.0 | 83.4 ± 13.5 | 82.0 ± 16.1 | 85.4 ± 14.8 | .76 |
| Pain | 87.5 ± 14.2 | 89.2 ± 12.2 | 86.6 ± 12.2 | 87.7 ± 18.6 | 87.8 ± 13.9 | .73 |
| Quality of Life | 75.4 ± 20.9 | 76.3 ± 16.3 | 73.2 ± 18.4 | 73.7 ± 22.3 | 78.1 ± 20.7 | .49 |
| Sport/Recreation | 75.9 ± 18.6 | 77.9 ±15.1 | 70.3 ± 17.6 | 81.4 ± 16.1 | 77.3 ± 20.8 | .08 |
| Activities of Daily Living | 90.6 ± 11.5 | 93.0 ± 9.3 | 90.2 ± 10.0 | 88.2 ± 15.7 | 91.9 ± 10.7 | .45 |
| Global | 85.2 ± 12.5 | 87.3 ± 10.1 | 84.3 ± 10.6 | 84.2 ± 15.8 | 86.4 ± 12.7 | .51 |
| Lysholm score | 89.3 ± 11.3 | 87.9 ± 9.9 | 86.2 ± 10.4 | 89.5 ± 14.8 | 90.1 ± 13.3 | .67 |
| Differential postoperative laxity, mm | 2.3 ± 2.6 | 2.3 ± 2.3 | 1.92 ± 2.1 | 2.2 ± 2.1 | 2.2 ± 2.6 | .73 |
| Return to preinjury sport | 165 (62.0) | 33 (60.0) | 46 (59.7) | 27 (63.7) | 59 (64.8) | .41 |
| High-grade pivot shift at follow-up | 64 (24.0) | 16 (29.1) | 20 (26.0) | 9 (20.9) | 19 (20.8) | .17 |
| New symptomatic meniscal tear | 47 (17.7) | 14 (25.5) | 13 (16.9) | 6 (14.0) | 14 (15.4) | .04* |
| Infection | 5 (1.4) | 1 (1.8) | 1 (1.3) | 1 (2.3) | 2 (2.2) | .33 |
aData are reported as mean ± SD or n (%). BTB+LET, bone–patellar tendon and modified Lemaire LET; LET, lateral extra-articular tenodesis; QT+LET, quadriceps tendon and modified Lemaire LET; ST4, single-strand quadrupled semitendinosus; ST4+LET, ST4 with LET using the same hamstring graft.
* Pairwise comparison exhibited significant differences between BTB+LET, QT+LET, and ST4+LET groups vs the ST4 group (P = .04).
Pivot Shift
A multivariate analysis was performed to determine the factors that were predictive of the presence of high-grade PS at follow-up (Table 5). Among the examined variables, no significant association was observed between age, sex, type of sport, professional sports practice, and high-grade PS identified at follow-up. A preoperative grade 3 PS significantly correlated with the presence of high-grade PS at follow-up (odds ratio [OR] = 3.23 [95% CI, 1.81-5.75]; P < .001). The length of follow-up was significantly correlated with the presence of high-grade PS at follow-up (P < .001). The presence of a preoperative meniscal tear also significantly correlated with the presence of a high-grade PS at follow-up (OR = 3.47 [95% CI, 1.62-7.43]; P < .001). Meniscectomy was a significant predictor for the presence of a high-grade PS compared with meniscal repair (OR = 2.65 [95% CI, 1.19-5.63]; P = .02). Similarly, a nonrepaired meniscal tear was a significant predictor for the presence of high-grade PS at follow-up compared with meniscal repair (OR = 3.26 [95% CI, 1.27-9.43]; P = .007). The appearance of a new meniscal tear was the strongest significant predictor of the presence of high-grade PS at follow-up (OR = 4.31 [95% CI, 2.31-8.06]; P < .001). No significant correlation was observed between the addition of LET and the presence of high-grade PS at follow-up (OR = 0.71 [95% CI, 0.37-1.39]; P = .31).
Table 5.
Multivariate Analysis of High-Grade Pivot Shift at Follow-upa
| Adjusted OR | 95% CI | P | |
|---|---|---|---|
| Age | NA | NA | .51 |
| Sex ratio | 1.21 | 0.62-2.03 | .22 |
| Body mass index | NA | NA | .64 |
| Follow-up | NA | NA | <.001 |
| Preoperative pivot shift: grade 3 vs grade 2 | 3.23 | 1.81-5.75 | <.001 |
| Type of sport: contactb | 1.45 | 0.59-2.76 | .345 |
| Professional athlete | 0.96 | 0.46-2.01 | .69 |
| Preoperative meniscal tear | 3.47 | 1.62-7.43 | <.001 |
| Preoperative bimeniscal tear | 1.66 | 0.84-3.27 | .14 |
| Therapeutic management | |||
| Meniscectomy vs repair | 2.65 | 1.19-5.63 | .02 |
| Repair vs nonrepair | 3.26 | 1.27-9.43 | .007 |
| Meniscectomy vs nonrepair | 1.71 | 0.64-4.44 | .43 |
| Lateral extra-articular tenodesis | 0.71 | 0.37-1.39 | .31 |
| New symptomatic meniscal tear | 4.31 | 2.31-8.06 | <.001 |
aBolded P values indicate statistical significance. NA, not applicable; OR, odds ratio.
bPivoting sports with contact: soccer, handball, basketball, rugby, motocross.
New Symptomatic Meniscal Injury
A total of 47 (17.7%) patients sustained a new symptomatic meniscal tear during the follow-up (25.5% in the ST4 group, 16.9% in the ST4+LET group, 15.4% in the QT+LET group, and 14.0% in the BTB+LET group). After univariate analysis, a significant difference was observed between these 4 groups (P = .04). A multivariate analysis was performed to determine the predictors of a new symptomatic meniscal tear (Table 6). Among the examined variables, no significant association was observed for preoperative grade of PS, age, sex, type of sport, professional sports practice, and the occurrence of a new meniscal tear. The addition of LET was not protective against the occurrence of a new symptomatic meniscal tear (OR = 1.22 [95% CI, 0.54-2.23]; P = .27). The mean follow-up time was significantly associated with the risk of a new symptomatic meniscal tear (P < .001). Not repairing a meniscal tear at index surgery, compared with a repair, was a significant predictor of a new symptomatic meniscal tear (OR = 2.13 [95% CI, 1.11-4.55]; P = .02). The presence of a high-grade PS at follow-up was the strongest significant predictor of a new symptomatic meniscal tear, with an OR of 4.31 (95% CI, 2.31-8.06; P < .001).
Table 6.
Multivariate Analysis of Predictive Factors for New Symptomatic Meniscal Teara
| Adjusted OR | 95% CI | P | |
|---|---|---|---|
| Age | NA | NA | .33 |
| Sex ratio | 1.23 | 0.54-2.07 | .64 |
| Body mass index | NA | NA | .25 |
| Follow-up | NA | NA | .01 |
| Preoperative pivot shift: grade 3 vs grade 2 | 1.84 | 0.80-2.91 | .61 |
| Type of sport: contactb | 1.13 | 0.55-2.11 | .33 |
| Professional athlete | 1.75 | 0.87- 3.56 | .11 |
| Preoperative meniscal tear | 1.07 | 0.56-2.03 | .83 |
| Preoperative bimeniscal tear | 1.20 | 0.58-2.49 | .54 |
| Therapeutic management | |||
| Meniscectomy vs repair | 1.79 | 0.79-3.32 | .29 |
| Repair vs nonrepair | 2.13 | 1.11-4.55 | .02 |
| Meniscectomy vs nonrepair | 1.07 | 0.31-3.7 | .87 |
| Lateral extra-articular tenodesis | 1.22 | 0.54-2.23 | .27 |
| High-grade pivot shift at follow-up | 4.31 | 2.31-8.06 | <.001 |
aBolded P values indicate statistical significance. NA, not applicable; OR, odds ratio.
bPivoting sports with contact: soccer, handball, basketball, rugby, motocross.
Discussion
At approximately 3.5 years postoperatively, a residual high-grade PS could be identified in a quarter of patients who had primary ACLR, regardless of the presence or absence of an additional LET. Grade 3 PS at index surgery, meniscal injury status, and type of meniscal tear treatment were strong predictors of the presence of a high-grade PS at follow-up. The addition of LET during ACLR was not protective against the occurrence of new meniscal tears.
The PS test is a complex clinical sign assessing internal rotation and anterior tibial translation. Despite the recent development of objective and standardized evaluation methods,17,19,42 clinical examination remains the gold standard. The exact origin of the PS phenomenon is controversial and not completely understood.9 A multifactorial background involving different anatomic knee structures seems to be the most likely hypothesis. In their literature review, Tanaka et al39 highlighted the role of the anterolateral complex,23 meniscal lesions (posterolateral26 and posteromedial25 specifically), iliotibial band or Kaplan fibers,34 and the bone morphology of the tibial plateau in the occurrence of the PS after an ACL injury. In the present study, a high-grade PS could be identified at follow-up in approximately 1 in 4 patients who had a high-grade PS at the time of surgery. This is different from a general population of ACL-injured individuals in the sense that only a minority of patients with primary ACLR usually present with a high-grade PS (26.5% in a recent study by Magnussen et al21). A previous clinical study highlighted risk factors for residual PS after ACLR without LET. In their analysis, Ueki et al41 reported that only a high-grade preoperative PS was a risk factor for residual PS. However, that study was carried out with a maximum of 1 year of follow-up and on a very small proportion of their series: 48 of 368 patients (13%) had a high-grade PS preoperatively,41 compared with all 266 patients in the present study.
To improve rotational control in patients with ACL injury, various biomechanical studies have emphasized the need to add LET to ACLR when a high-grade PS is present. Inderhaug et al16 demonstrated that LET allowed restoration of knee kinematics to normal. Similarly, Geeslin et al10 reported in a cadaveric study that sequential ACLR and LET reconstructions significantly reduced rotational knee laxity. The clinical interest of LET continues to be debated, and its results differ significantly from one study to the next.30 However, in a recent meta-analysis14 of 14 studies comparing isolated ACLR with ACLR associated with LET, only 2 studies reported a statistically significant advantage of the addition of LET on the presence of a postoperative PS. A second recent systematic review30 showed that the addition of LET compared with an isolated ACLR significantly reduced the risk of a postoperative PS but with a low relative risk of 0.91 to 0.99. These 2 studies demonstrate the difficulty of concluding with certainty a causal relationship between LET and a reduced risk of postoperative PS. However, the small number of comparative trials included in these studies, the interobserver (and interstudy) variability of the PS test, and the lack of information regarding meniscal status and related procedures make it almost impossible to interpret these results. In the current study, no statistically significant relationship could be identified between the 2 LET techniques and the presence of a high-grade PS at follow-up.
One of the hypotheses put forward regarding the origin of PS, other than that of the anterolateral complex, is based on the existence of an injury to other secondary restraints, including meniscal injuries. Some studies highlighted the role of ramp25 and posterior meniscus root lesions40 in the occurrence of increased rotational laxity after an ACL injury. These findings correlate with those observed in the current study, where only patients with preoperative high-grade rotational laxity were included. Indeed, of 266 patients, 185 had at least 1 meniscal tear. Half of these tears involved the posteromedial complex (ramp lesion + root of the medial meniscus) and the posterolateral part of the lateral meniscus (root of the lateral meniscus).
Regarding the meniscal procedures associated with ACLR (nonrepair, repair, or meniscectomy) according to the type of tear, 17 of 57 medial meniscal ramp lesions (29.8%) were not repaired, and 4 of 39 medial and lateral root lesions (10.3%) were treated by meniscectomy. Given the current clinical and biomechanical evidence, as well as the multifactorial origin of the PS, the associated meniscal injuries and their intraoperative management may provide an explanation for the high incidence of postoperative high-grade PS in this patient cohort.
Another objective of this study was to assess the risk of sustaining a new symptomatic meniscal tear and determine the predictive factors thereof. A strong association was observed between new meniscal tears and the presence of high-grade PS at follow-up (OR = 4.31 [95% CI, 2.31-8.06]; P < .001). However, given the study method, it was not possible to determine whether the occurrence of a new symptomatic meniscal tear is the consequence or cause of the presence of a PS at follow-up. Similar to the presence of high-grade PS at follow-up, meniscal tears left unrepaired were significant predictors of a new symptomatic meniscal tear in comparison with meniscal repairs. It was interesting to note that 17 of the 25 meniscal lesions that were not repaired in this series were ramp lesions. Therefore, the persistence of a nonrepaired ramp lesion may be a predictor of a new meniscal injury and a high-grade PS at follow-up. Indeed, the rate of new symptomatic meniscal tears in this subgroup was 36% (9/25) and the presence of a high-grade PS at follow-up was 64% (16/25). This would confirm the previously described role of ramp lesions for high-grade rotational laxity.25 The retrospective design of this study did not allow us to differentiate between stable and unstable ramp lesions. Therefore, no conclusions can be drawn as to whether repair of ramp lesions would have improved the clinical results or whether all types of ramp lesions should be repaired.1
This study has several strengths that are important to highlight. Only patients with a preoperative high-grade PS were included in the study. The overall series included 266 patients with primary ACL injury who had a high-grade PS at index surgery, and these patients were selected using rigorous inclusion criteria at a minimal follow-up of 2 years. Although the multicentric design of a study is often a weakness, the fact that the respective surgical techniques were used in a routine manner by experienced surgeons in the different centers allowed us to rule out individual learning curves, which may jeopardize studies with higher levels of evidence.33
The rigorous determination of meniscal status at index surgery (intact vs injured, type and zone of injury) and the subsequent therapeutic management (repair vs meniscectomy vs nonrepair) has provided additional evidence about the role of the meniscus in the origin of the PS.15 No significant relationship was found between the main outcomes of the present study (evidence of high-grade PS and/or new meniscal tears at follow-up) and LET. Because this study did not investigate the success or failure of meniscal repair performed at index surgery, the previously described protective effect of LET on the failure of meniscal repair36 cannot be confirmed. This suggests that in this high-risk population, the underlying biomechanical process for secondary meniscal injuries was highly related to the presence of rotational laxity at the final follow-up, which was found to be almost equivalent in the LET and non-LET groups.
Repair of meniscal tears, particularly posterolateral and posteromedial meniscal lesions, seems to be a major factor in reducing the risk of rotational laxity after ACLR in this high-risk population. Therefore, this type of advanced meniscal repair in association with primary ACL reconstruction needs further investigation in the future.
There are several limitations to the present study. First, the main judgment criterion of the study is based on a subjective assessment of PS. To limit this bias as much as possible, a single trained examiner (C.J.), who was not involved in the surgical procedures, performed all PS tests. Although it was impossible to perform a blinded evaluation of surgical techniques due to the presence of different skin scars, no information regarding preoperative meniscal status, surgical management of these lesions, and the existence of new meniscal tears was transmitted to the examiner. Second, with respect to the retrospective character of this study, it is impossible to say whether the documented PS at the follow-up examination was the persistence of an initially unaddressed PS or a recurrence that came about gradually over time. Given the high surgical volume of the 4 involved surgeons, the anatomic ACL positioning during reconstruction, and manual intra- and postoperative PS control, it is reasonable to assume that the PS was completely restored at the end of the surgical procedure. In that case, a recurrence of high-grade PS during the follow-up period would have been a result of rotational laxity gradually decompensating over time, probably explained by decompensation of the LET or stretching of the ACL graft (given the 2-mm increase in sagittal laxity for all the 4 groups). Nevertheless, an incomplete restoration or normalization of the preoperative high-grade PS during surgery cannot be totally excluded. Third, graft reruptures were excluded from this analysis before the inclusion of patients because the aim of the study was to assess the risk of high-grade PS at follow-up in patients with a fully functional ACLR. We decided to exclude those patients from the study protocol following the sample size analysis of Getgood et al.12 Those authors found that 255 patients per group would be needed to detect a relative risk reduction in the rates of clinical failure in the LET group of 40% or greater. With the very selective patient population analyzed in this study, the sample size was insufficient to evaluate ACLR rerupture as a primary or secondary outcome. Therefore, no information was available on the relationship between the presence of high-grade PS at follow-up and the risk of graft ruptures.21,37 In the same manner, some patients may have experienced meniscal repair failure. However, this possibility was not specifically investigated as one of the principal outcome parameters because repair failures are rare and difficult to diagnose without invasive arthro-computed tomography or arthro-MRI examinations. Therefore, the clinical impact of persistent meniscal tears after repair was impossible to quantify on MRI. Fourth, the sample size of this study was not calculated to compare the different LET techniques. Although there were significant differences between the LET groups, no conclusion could be formally drawn.
Conclusion
In this study, 1 in 4 of patients with high-grade PS before ACLR, with or without LET, were at risk of residual rotatory knee laxity at a mean follow-up of 44 months regardless of the technique used. Repair of a preexisting meniscal lesion may be more effective than performing LET to decrease the presence of a high-grade PS at minimum of 2-year follow-up.
Footnotes
Final revision submitted November 7, 2020; accepted December 4, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: S.P. has received consulting fees from Zimmer Biomet. C.S. has received consulting fees from Zimmer Biomet and Smith & Nephew. N.P. has received consulting fees from Zimmer Biomet and Smith & Nephew. M.O. has received consulting fees from Arthrex, Stryker, and Newclip. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Comite de Protection des Personnes Sud Méditerranée I (study ID: 2016-015724-11)
References
- 1. Balazs GC, Greditzer HG, Wang D, et al. Non-treatment of stable ramp lesions does not degrade clinical outcomes in the setting of primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2020;28(11):3576–3586. [DOI] [PubMed] [Google Scholar]
- 2. Boutsiadis A, Brossard P, Panisset J-C, Graveleau N, Barth J. Minimally invasive combined anterior and anterolateral stabilization of the knee using hamstring tendons and adjustable-loop suspensory fixation device: surgical technique. Arthrosc Tech. 2017;6(2):e419–e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnett QM, Fowler PJ. Reconstruction of the anterior cruciate ligament: historical overview. Orthop Clin North Am. 1985;16(1):143–157. [PubMed] [Google Scholar]
- 4. Calas P, Dorval N, Bloch A, Argenson J-N, Parratte S. A new anterior cruciate ligament reconstruction fixation technique (quadrupled semitendinosus anterior cruciate ligament reconstruction with polyetheretherketone cage fixation). Arthrosc Tech. 2012;1(1):e47–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavaignac E, Marot V, Faruch M, et al. Hamstring graft incorporation according to the length of the graft inside tunnels. Am J Sports Med. 2018;46(2):348–356. [DOI] [PubMed] [Google Scholar]
- 6. Christel P, Djian P. Anterilateral extra-articular tenodesis of the knee using a short strip of fascia lata [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2002;88(5):508–513. [PubMed] [Google Scholar]
- 7. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clancy WG, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352–359. [PubMed] [Google Scholar]
- 9. Fu FH, Herbst E. Editorial commentary: the pivot-shift phenomenon is multifactorial. Arthroscopy. 2016;32(6):1063–1064. [DOI] [PubMed] [Google Scholar]
- 10. Geeslin AG, Moatshe G, Chahla J, et al. Anterolateral knee extra-articular stabilizers: a robotic study comparing anterolateral ligament reconstruction and modified Lemaire lateral extra-articular tenodesis. Am J Sports Med. 2018;46(3):607–616. [DOI] [PubMed] [Google Scholar]
- 11. Getgood A, Brown C, Lording T, et al. The anterolateral complex of the knee: results from the International ALC Consensus Group Meeting. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):166–176. [DOI] [PubMed] [Google Scholar]
- 12. Getgood AMJ, Bryant DM, Litchfield R, et al. Lateral extra-articular tenodesis reduces failure of hamstring tendon autograft anterior cruciate ligament reconstruction: 2-year outcomes from the STABILITY Study randomized clinical trial. Am J Sports Med. 2020;48(2):285–297. [DOI] [PubMed] [Google Scholar]
- 13. Hefti F, Müller W, Jakob RP, Stäubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226–234. [DOI] [PubMed] [Google Scholar]
- 14. Hewison CE, Tran MN, Kaniki N, Remtulla A, Bryant D, Getgood AM. Lateral extra-articular tenodesis reduces rotational laxity when combined with anterior cruciate ligament reconstruction: a systematic review of the literature. Arthroscopy. 2015;31(10):2022–2034. [DOI] [PubMed] [Google Scholar]
- 15. Hoshino Y, Hiroshima Y, Miyaji N, et al. Unrepaired lateral meniscus tears lead to remaining pivot-shift in ACL-reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 202;28(11):3504–3510. [DOI] [PubMed] [Google Scholar]
- 16. Inderhaug E, Stephen JM, Williams A, Amis AA. Anterolateral tenodesis or anterolateral ligament complex reconstruction: effect of flexion angle at graft fixation when combined with ACL reconstruction. Am J Sports Med. 2017;45(13):3089–3097. [DOI] [PubMed] [Google Scholar]
- 17. Ishibashi Y, Tsuda E, Yamamoto Y, Tsukada H, Toh S. Navigation evaluation of the pivot-shift phenomenon during double-bundle anterior cruciate ligament reconstruction: is the posterolateral bundle more important? Arthroscopy. 2009;25(5):488–495. [DOI] [PubMed] [Google Scholar]
- 18. Kittl C, Inderhaug E, Williams A, Amis AA. Biomechanics of the anterolateral structures of the knee. Clin Sports Med. 2018;37(1):21–31. [DOI] [PubMed] [Google Scholar]
- 19. Labbe DR, de Guise JA, Godbout V, et al. Accounting for velocity of the pivot shift test manoeuvre decreases kinematic variability. Knee. 2011;18(2):88–93. [DOI] [PubMed] [Google Scholar]
- 20. Lane CG, Warren R, Pearle AD. The pivot shift. J Am Acad Orthop Surg. 2008;16(12):679–688. [DOI] [PubMed] [Google Scholar]
- 21. Magnussen RA, Reinke EK, Huston LJ, et al. Effect of high-grade preoperative knee laxity on 6-year anterior cruciate ligament reconstruction outcomes. Am J Sports Med. 2018;46(12):2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathew M, Dhollander A, Getgood A. Anterolateral ligament reconstruction or extra-articular tenodesis: why and when? Clin Sports Med. 2018;37(1):75–86. [DOI] [PubMed] [Google Scholar]
- 23. Monaco E, Maestri B, Labianca L, et al. Navigated knee kinematics after tear of the ACL and its secondary restraints: preliminary results. Orthopedics. 2010;33(10 suppl):87–93. [DOI] [PubMed] [Google Scholar]
- 24. Mouarbes D, Dagneaux L, Olivier M, et al. Lower donor-site morbidity using QT autografts for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2558–2566. [DOI] [PubMed] [Google Scholar]
- 25. Mouton C, Magosch A, Pape D, Hoffmann A, Nührenbörger C, Seil R. Ramp lesions of the medial meniscus are associated with a higher grade of dynamic rotatory laxity in ACL-injured patients in comparison to patients with an isolated injury. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1023–1028. [DOI] [PubMed] [Google Scholar]
- 26. Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38(8):1591–1597. [DOI] [PubMed] [Google Scholar]
- 27. O’Brien SJ, Warren RF, Wickiewicz TL, et al. The iliotibial band lateral sling procedure and its effect on the results of anterior cruciate ligament reconstruction. Am J Sports Med. 1991;19(1):21–24; discussion 24-25. [DOI] [PubMed] [Google Scholar]
- 28. Ollivier M. Anterior cruciate ligament reconstruction: the long road from science to clinical relevance. Knee Surg Relat Res. 2018;30(2):93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reider B. ACL or ACL [editorial]. Am J Sports Med. 2020;48(2):281–284. [DOI] [PubMed] [Google Scholar]
- 30. Rezende FC, de Moraes VY, Martimbianco ALC, Luzo MV, da Silveira Franciozi CE, Belloti JC. Does combined intra- and extraarticular ACL reconstruction improve function and stability? A meta-analysis. Clin Orthop Relat Res. 2015;473(8):2609–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Samuelson M, Draganich LF, Zhou X, Krumins P, Reider B. The effects of knee reconstruction on combined anterior cruciate ligament and anterolateral capsular deficiencies. Am J Sports Med. 1996;24(4):492–497. [DOI] [PubMed] [Google Scholar]
- 32. Schindler OS. Surgery for anterior cruciate ligament deficiency: a historical perspective. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):5–47. [DOI] [PubMed] [Google Scholar]
- 33. Seil R. In high tibial osteotomy, closing and opening wedges did not differ for clinical outcomes at up to two years. J Bone Joint Surg Am. 2018;100(10):882. [DOI] [PubMed] [Google Scholar]
- 34. Smith PA, Thomas DM, Pomajzl RJ, Bley JA, Pfeiffer FM, Cook JL. A biomechanical study of the role of the anterolateral ligament and the deep iliotibial band for control of a simulated pivot shift with comparison of minimally invasive extra-articular anterolateral tendon graft reconstruction versus modified Lemaire reconstruction after anterior cruciate ligament reconstruction. Arthroscopy. 2019;35(5):1473–1483. [DOI] [PubMed] [Google Scholar]
- 35. Song G-Y, Hong L, Zhang H, Zhang J, Li Y, Feng H. Clinical outcomes of combined lateral extra-articular tenodesis and intra-articular anterior cruciate ligament reconstruction in addressing high-grade pivot-shift phenomenon. Arthroscopy. 2016;32(5):898–905. [DOI] [PubMed] [Google Scholar]
- 36. Sonnery-Cottet B, Saithna A, Blakeney WG, et al. Anterolateral ligament reconstruction protects the repaired medial meniscus: a comparative study of 383 anterior cruciate ligament reconstructions from the SANTI Study Group with a minimum follow-up of 2 years. Am J Sports Med. 2018;46(8):1819–1826. [DOI] [PubMed] [Google Scholar]
- 37. Sonnery-Cottet B, Saithna A, Cavalier M, et al. Anterolateral ligament reconstruction is associated with significantly reduced ACL graft rupture rates at a minimum follow-up of 2 years: a prospective comparative study of 502 patients from the SANTI Study Group. Am J Sports Med. 2017;45(7):1547–1557. [DOI] [PubMed] [Google Scholar]
- 38. Spencer L, Burkhart TA, Tran MN, et al. Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(9):2189–2197. [DOI] [PubMed] [Google Scholar]
- 39. Tanaka M, Vyas D, Moloney G, Bedi A, Pearle AD, Musahl V. What does it take to have a high-grade pivot shift? Knee Surg Sports Traumatol Arthrosc. 2012;20(4):737–742. [DOI] [PubMed] [Google Scholar]
- 40. Tang X, Marshall B, Wang JH, et al. Lateral meniscal posterior root repair with anterior cruciate ligament reconstruction better restores knee stability. Am J Sports Med. 2019;47(1):59–65. [DOI] [PubMed] [Google Scholar]
- 41. Ueki H, Nakagawa Y, Ohara T, et al. Risk factors for residual pivot shift after anterior cruciate ligament reconstruction: data from the MAKS group. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3724–3730. [DOI] [PubMed] [Google Scholar]
- 42. Vaidya RK, Yoo CW, Lee J, Han H-S, Lee MC, Ro DH. Quantitative assessment of the pivot shift test with smartphone accelerometer. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2494–2501. [DOI] [PubMed] [Google Scholar]
- 43. Yeo Y, Ahn JM, Kim H, et al. MR evaluation of the meniscal ramp lesion in patients with anterior cruciate ligament tear. Skeletal Radiol. 2018;47(12):1683–1689. [DOI] [PubMed] [Google Scholar]