Abstract
Objectives
To describe the clinical, histopathologic, and outcomes data for a cohort of patients with biliary atresia (BA), and to identify the factors affecting survival.
Methods
This was a cross-sectional study of all BA patients diagnosed between 1999 and 2017. Clinical, biochemical, imaging, and histopathologic data were analyzed, and Kaplan–Meier survival rates were compared to identify potential prognostic factors.
Results
We evaluated 23 patients. The median age at the Kasai procedure was 77 ± 34 days, and the median overall survival was 12.5 ± 65 months. Thirteen (56%) patients survived with their native livers, 3 (13%) received a transplant, and 6 died (26%) while awaiting a transplant. Cholangitis and the use of ursodeoxycholic acid were associated with longer survival, while impaired synthetic function was associated with shorter survival.
Conclusions
Most patients presented late for the Kasai procedure. The survival rate with the native liver was comparable to other cohorts. Therefore, clinicians are encouraged to refer for the Kasai procedure even with late presentation (between 60 and 90 days), provided there is no hepatic decompensation.
Keywords: Kasai portoenterostomy, biliary atresia, cholestasis, liver transplantation, pediatric, Saudi Arabia
Introduction
Biliary atresia (BA) is one of the most common non-genetic causes of neonatal cholestasis and the most common indication for pediatric liver transplantation in the USA and Europe.1,2 In the Middle East region, BA is the second most common indication for liver transplantation, following progressive familial intrahepatic cholestasis (PFIC).3 No nationwide data are available to determine the overall incidence in this region; however, most cases of neonatal cholestasis appear to be attributed to BA.4
BA is part of a cluster of hepatobiliary disorders that share a common inciting event in the form of cholangiocytic injury. Subsequently, the end result is either sclerosis and obliteration of the ductal lumens, weakening of the ductal wall resulting in cystic formation (choledochal cysts), or non-specific “giant cell” transformation as giant cell hepatitis.5 BA usually presents in the first to second week of life, with the much less common “syndromic” sub-type presenting much earlier.6 Infants often have a period of normal-colored stools that progress to the classic “clay-colored” stools, with jaundice, in the first few weeks of life. The disease eventually progresses to biliary cirrhosis with portal hypertension, end-stage liver disease, and death, typically within the first 2 years of life if left untreated.5
BA poses a diagnostic challenge to pediatricians, owing in part to its relative rarity, but also because of the perception that jaundice in the neonatal period is usually “physiologic”.7 There is no single best non-invasive testing modality to either diagnose or completely rule out BA, and in most cases, the more invasive “gold standard”, intra-operative cholangiography, or liver biopsy is required. Out of many neonatal cholestasis causes, BA is the only diagnosis that is time-sensitive because early diagnosis can drastically alter outcomes.7
BA treatment depends on the time of diagnosis and the overall liver status. If the diagnosis is made early, typically within the first 60 days of life, then Kasai portoenterostomy is performed in hopes of establishing bile flow and preventing the development of biliary cirrhosis and end-stage liver disease.8,9 However, occasionally, patients require a primary liver transplant when they present with well-established cirrhosis or end-stage liver disease at diagnosis. Furthermore, most patients eventually require liver transplantation following the Kasai procedure, even when it is considered successful.9–11
We present our single-center experience of a cohort of BA patients who underwent Kasai portoenterostomy.
Materials and methods
Patients and data collection
The study was conducted following approval of the Research Committee at the Biomedical Ethics Unit at King Abdulaziz University (KAU), Jeddah, Saudi Arabia (ref # 458-20). This article does not contain any experimental studies on human participants or animals performed by any author. Therefore, the need for patient consent was waived by the committee.
This was a cross-sectional retrospective chart review of all patients diagnosed with BA in a tertiary care center between 1999 and 2017. We used an electronic medical records search engine to identify patients, using “biliary atresia” as a keyword. We evaluated data for all patients with a confirmed diagnosis of BA and who were followed-up at King Abdulaziz University Hospital (KAUH). Patients with missing data for biochemical profiles, intraoperative cholangiography, liver biopsy results, or follow-up data, were excluded. Patients referred for liver transplantation at the time of diagnosis were also excluded.
Data collection comprised demographic, biochemical, imaging, histopathological, management, and follow-up data, including liver transplantation and any complications. Laboratory data, namely alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total and direct bilirubin, and serum albumin, were evaluated at diagnosis and 6 weeks after the Kasai procedure. Hematological data, namely leukocyte count, hemoglobin, platelet count, prothrombin time, partial thromboplastin time, and international normalized ratio (INR), were also evaluated at diagnosis and at follow-up after the Kasai procedure.
Imaging data, namely ultrasonographic (US) findings of a normal or abnormal gallbladder, hepatomegaly, liver echotexture, common bile duct visualization, splenomegaly, signs of portal hypertension, and cord sign, and magnetic resonance cholangiopancreatography (MRCP) and hepatobiliary iminodiacetic acid (HIDA) scan data, were collected when available.
Liver biopsy was obtained either by image-guided percutaneous needle or wedge biopsy, or in some cases, both. Tissue was then stained using hematoxylin and eosin (H & E) and Gömöri trichrome stains and examined by an experienced liver pathologist.
Diagnosing BA
The BA diagnosis was confirmed when either percutaneous or intra-operative cholangiography showed the typical findings of complete obliteration of the common bile duct or the extrahepatic bile duct. Moreover, histopathologic findings of bile plugs, intracanalicular cholestasis, bile ductular proliferation (with and without portal fibrosis), and the absence of giant cell transformation were considered confirmatory for diagnosis in the appropriate clinical context (more particularly, when attempted intra-operative cholangiography failed owing to a contracted or atretic gallbladder).
Definitions:
Successful Kasai procedure: establishment of bile flow as evidenced by total bilirubin concentration ≤20 µmol/L) in the first 6 months after surgery.12
Impaired synthetic function: defined by INR ≥1.5 not responding to vitamin K therapy.
Liver failure: defined by INR ≥2 not responding to vitamin K therapy.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) software, Version 20 (IBM Corp., Armonk, NY, USA) was used to analyze the data. The descriptive analysis incorporated percentages for categorical data and means with standard deviation (SD) for continuous variables. Survival rates were determined using the Kaplan–Meier method; the chi-square test was used to compare categorical variables. The correlation between survival and factors potentially influencing survival was examined using Pearson’s correlation coefficient. A paired samples t-test was used to compare the means of the biochemical variables before and after the Kasai procedure. P < 0.05 was considered statistically significant.
Results
Clinical and demographic characteristics
Thirty-one patients were identified from the medical records. Eight were excluded because of missing cholangiographic, liver biopsy, or clinical data; the remaining 23 patients were included in the analysis. The diagnosis was confirmed in all 23 cases with either intra-operative cholangiography, biopsy, or both. There was a slight female predominance, with a female:male ratio of 1.3:1. The median age of the patients at the time of referral to our center was 60 days (range, 7–138 days).
Nine patients (43.5%) had other associated congenital anomalies, namely heart (atrial septal defect, ventricular septal defect, patent ductus arteriosus, pulmonary artery stenosis, mitral or tricuspid valve regurgitation), kidney, or other system anomalies. One patient had congenital anomalies suggestive of the syndromic type of BA: polysplenia and an absent hepatic portion of the inferior vena cava. Further clinical, demographic, and biochemical data are summarized in Table 1.
Table 1.
Patients’ baseline characteristics.
| Variable | n (%) or mean ± SD |
|---|---|
| Clinical and demographic data: | |
| Saudi citizens | 11 (48) |
| Female sex | 13 (57) |
| Age at diagnosis (days) | 67 ± 37 |
| Positive consanguinity | 12 (52) |
| Family history of biliary atresia | 1 (4) |
| Splenic malformation | 1 (4) |
| Other malformations | 9 (39) |
| Cardiovascular | 5 (21) |
| Renal | 3 (13) |
| Other (inguinal hernia) | 1 (4) |
| Biochemical data: | |
| Total bilirubin (µmol/L) | 165.8 ± 56.4 |
| Direct bilirubin (µmol/L) | 131.6 ± 47.8 |
| Alanine aminotransferase (U/L) | 166 ± 93 |
| Aspartate aminotransferase (U/L) | 240 ± 139 |
| Alkaline phosphatase (U/L) | 815 ± 405 |
| Albumin (g/L) | 33 ± 5 |
| Gamma glutamyl transferase (U/L) | 530 ± 318 |
| Impaired synthetic function | 3 (14) |
| 25-OH Vitamin D (nmol/L) | 19 ± 16.8 |
SD, standard deviation.
Imaging study results
Ultrasonography was performed for 22 patients (97%). The most common abnormal findings were inability to visualize the common bile duct (63.6%) followed by failure to visualize the gallbladder (54.5%) and finding a contracted gallbladder (45.5%); therefore, none of the ultrasonographic variables had high sensitivities. HIDA scan results were available for 16 patients (72.7%). Abnormal findings defined as reduced uptake of the isotope material by the liver and failure of excretion into the small intestine were reported in 94% and 100% of patients, respectively. All patients (100%) who underwent intraoperative cholangiography (n = 18) had abnormal results, confirming the diagnosis of BA. The imaging details are shown in Table 2.
Table 2.
Imaging and radiological characteristics of the study cohort (n = 22).
| Imaging findings | n (%) |
|---|---|
| Ultrasonographic findings (n = 22) | |
| Common bile duct not visualized | 14 (63.6) |
| Gallbladder not visualized | 12 (54.5) |
| Gallbladder contracted | 10 (45.5) |
| Hepatomegaly | 4 (18.2) |
| Splenomegaly | 4 (18.2) |
| HIDA scan findings (n = 16) | |
| Abnormal uptake | 15 (94) |
| No excretion (of the total amount administered) | 16 (100) |
| Intra-operative cholangiography (n = 18) | |
| Abnormal result | 18 (100) |
HIDA, hepatobiliary iminodiacetic acid.
Histopathological findings
Liver biopsy was performed in 19 patients (83%) at presentation. The most common histopathological features were the presence of bile ductular proliferation (94.7%), intra-canalicular cholestasis (89.5%), and portal fibrosis (84.2%). Table 3 summarizes the histopathological findings.
Table 3.
Liver histopathological findings (n=19).
| Biopsy findings | n (%) |
|---|---|
| Bile duct proliferation | 18 (94.7) |
| Intra-canalicular cholestasis | 17 (89.5) |
| Bile plugs | 13 (68.4) |
| Giant cell transformation | 9 (47.4) |
| Portal fibrosis | 16 (84.2) |
Outcomes of Kasai portoenterostomy
All patients (95.7%) except one underwent Kasai portoenterostomy. The median age at the time of surgery was 77 days (range, 18–150 days). The Kasai procedure was successful in six patients (27%), and the average time until normalized bilirubin concentration was 15 weeks (range, 11–20 weeks).
Three patients (13.6%) received corticosteroid treatment post-operatively. Nine (41%) patients developed immediate post-operative complications, namely ascending cholangitis, sepsis, intestinal obstruction, or perforation at the anastomotic site. The most common long-term complications following the Kasai procedure were recurrent ascending cholangitis (n = 10), portal hypertension (n = 8), and impaired synthetic function (n = 8) (Table 4).
Table 4.
Treatment and outcomes data post-Kasai procedure (n=22).
| Variable | n(%) or mean ± SD |
|---|---|
| Number of successful Kasai procedures | 6 (26%) |
| Weeks until normal bilirubin concentration | 15 ± 4 |
| Median age at Kasai (in days) | 76 ± 34 |
| Post-operative treatment | |
| Prednisolone | 3 (14) |
| Ursodeoxycholic acid | 18 (90) |
| Antibiotics (cotrimoxazole) | 4 (18) |
| Immediate post-op complications | |
| Ascending cholangitis | 3 (13) |
| Intestinal obstruction | 2 (8) |
| Sepsis | 4 (17) |
| Perforation at the anastomotic site | 1 (4) |
| Long-term complications | |
| Ascending cholangitis | 10 (43) |
| Number of cholangitis episodes | 2 ± 1 |
| Portal hypertension | 8 (36) |
| Impaired synthetic function | 8 (36) |
| Long-term outcomes | |
| Liver transplantation | 3 (15) |
| Surviving patients with native liver | 13 (56) |
| Died | 6 (26) |
| Lost to follow-up | 1 (4) |
SD, standard deviation.
A paired-samples t-test comparing biochemical parameters prior to and 6 weeks following the Kasai procedure showed a significant decline in ALP, GGT, and albumin concentrations (Table 5).
Table 5.
Paired-samples t tests for biochemical data before and 6 weeks after the Kasai procedure.
| Laboratory parameter | n | Value before Kasai Mean (±SD) | Value after Kasai Mean (±SD) | P value |
|---|---|---|---|---|
| Total bilirubin (µmol/L) | 18 | 171 (63.2) | 148.7 (171) | 0.5 |
| Direct Bilirubin (µmol/L) | 14 | 133.3 (44.4) | 124.8 (131.6) | 0.7 |
| Alanine aminotransferase (U/L) | 18 | 160 (97) | 137 (98) | 0.14 |
| Aspartate aminotransferase (U/L) | 18 | 218 (132) | 181 (115) | 0.13 |
| Alkaline phosphatase (IU/L) | 18 | 708 (240) | 524 (307) | 0.02 |
| Gamma glutamyl transferase (U/L) | 17 | 538 (347) | 235 (204) | 0.006 |
| Albumin (g/L) | 18 | 32 (4.8) | 27 (9.8) | 0.01 |
P-values in bold are significant. SD, standard deviation.
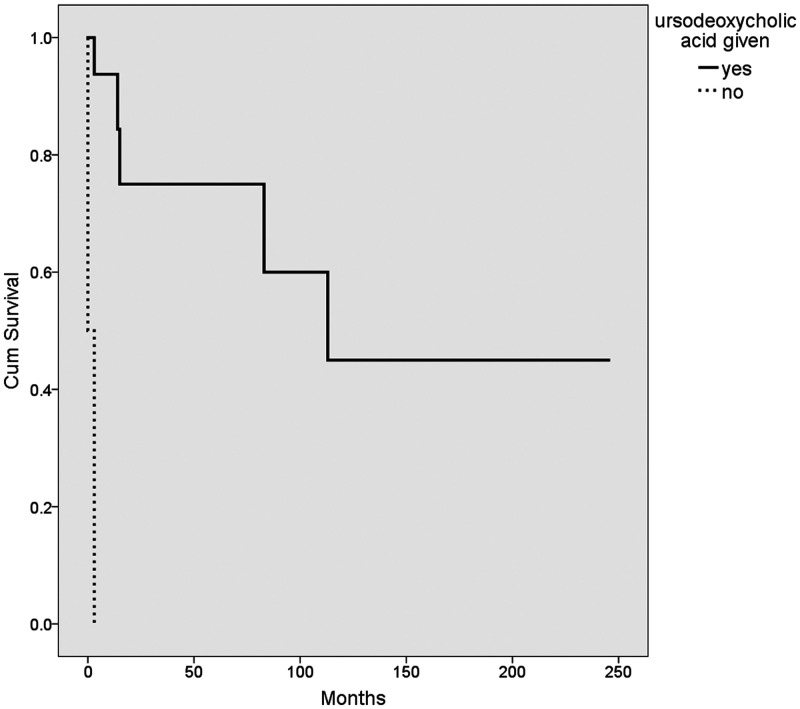
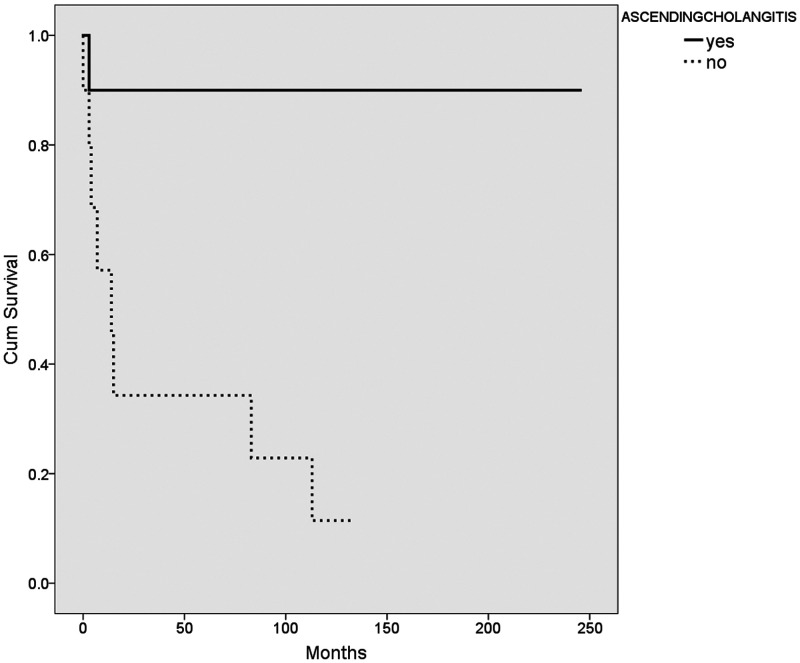
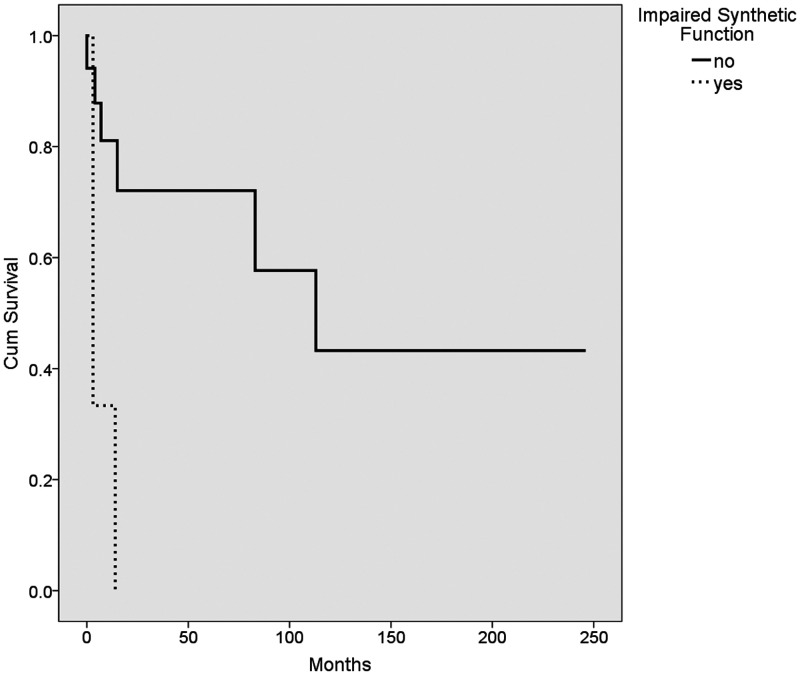
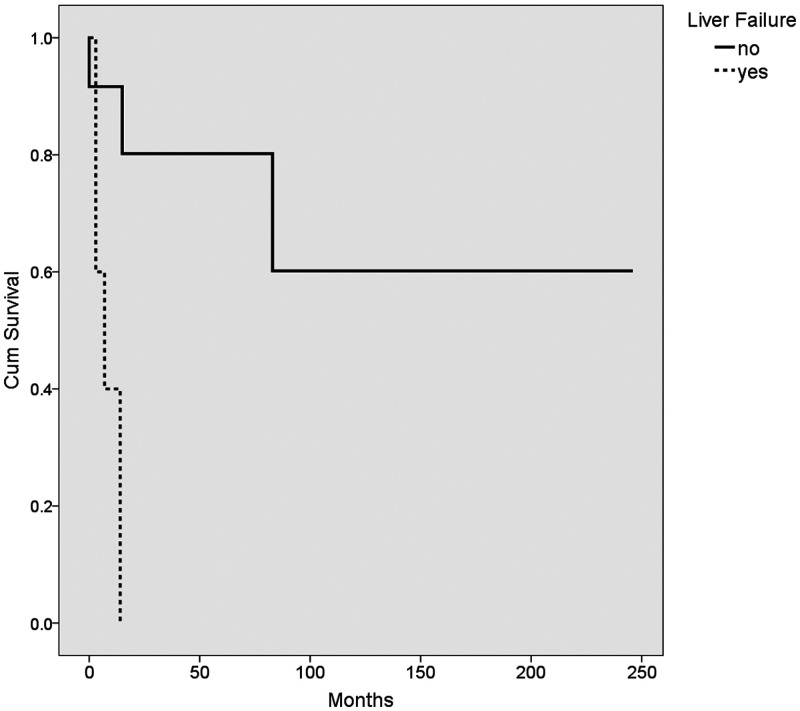
The median survival age in our cohort was 12.5 months (range, 0.5–246 months). Of particular note, none of the patients who had success with the Kasai procedure subsequently died or required a transplant. The median survival for patients who underwent a successful Kasai procedure was 45.5 ± 56 months (range 11–141 months). Kaplan–Meier survival analysis of the different variables was performed using the log rank method (Table 6, Figures 1–4). The use of ursodeoxycholic acid, and ascending cholangitis (any number of episodes), were significantly associated with longer survival (P = 0.0001 and P = 0.01, respectively), whereas impaired synthetic function at presentation and liver failure following the Kasai procedure were associated with shorter survival (P = 0.003 and P = 0.004, respectively). Additionally, almost none of our cases without a cholangitis episode (n = 13) had a successful Kasai procedure (n = 12/13, p = 0.052).
Table 6.
Kaplan–Meier survival analysis of the variables related to survival, using the log rank method.
| Variable | Value Mean survival in months ± SD | Value Mean survival in months ± SD | P value |
|---|---|---|---|
| Gender | male 175 ± 42 | female 57 ± 17 | 0.09 |
| Nationality | Saudi 94 ± 17 | non-Saudi 111 ± 37 | 0.4 |
| Impaired synthetic function at presentation | no 136 ± 32 | yes 6.6 ± 3.6 | 0.003 |
| Liver fibrosis on biopsy | Stages 1 and 2 117 ± 21 | Stages 3 and 4 12 ± 4 | 0.35 |
| Ursodeoxycholic acid | given 143 ± 33 | not given 1.5 ± 1.5 | 0.0001 |
| All post-operative complications | yes 30 ± 7.8 | no 119 ± 36 | 0.4 |
| Ascending cholangitis | yes 221 ± 23 | no 42 ± 16.7 | 0.01 |
| Post-operative liver failure | yes 8 ± 2.5 | no 166 ± 37 | 0.004 |
P-values in bold are significant. SD, standard deviation.
Figure 1.
Kaplan–Meier survival plot showing the mean survival of patients (n = 18) who received ursodeoxycholic acid (solid line) and those who did not (dashed line).
Figure 2.
Kaplan–Meier survival plot showing the mean survival of patients (n = 20) with cholangitis (solid line) and those without (dashed line).
Figure 3.
Kaplan–Meier survival plot showing the mean survival of patients (n = 20) who had impaired synthetic function (dashed line) and those with normal synthetic function (solid line).
Figure 4.
Kaplan–Meier survival plot showing the mean survival of patients (n = 19) with liver failure post-Kasai (dashed line) and those without (solid line)
A bivariate survival analysis of age, number of cholangitis episodes, and biochemical variables was performed using Pearson’s correlation coefficient (Table 7). The number of cholangitis episodes after the Kasai procedure showed a significant positive correlation with survival (r = 0.69, P = 0.02).
Table 7.
Bivariate analysis of survival according to age, laboratory variables, and the number of cholangitis episodes, using Pearson’s correlation coefficient.
| Variable | Pearson’s correlation coefficient | P value |
|---|---|---|
| Age at Kasai | −0.2 | 0.4 |
| Total serum bilirubin (µmol/L) | 0.06 | 0.7 |
| Direct serum bilirubin (µmol/L) | 0.01 | 0.9 |
| Alanine aminotransferase (U/L) | −0.3 | 0.1 |
| Aspartate aminotransferase (U/L) | −0.4 | 0.07 |
| Alkaline phosphatase (U/L) | −0.2 | 0.3 |
| Albumin (g/L) | 0.3 | 0.08 |
| Gamma glutamyl transferase (U/L) | 0.02 | 0.9 |
| Number of cholangitis episodes | 0.69 | 0.02 |
| 25-hydroxyvitamin D (nmol/L) | 0.4 | 0.2 |
P-values in bold are significant.
Discussion
Various theories have been postulated regarding the pathogenesis of BA, such as viral infection, defective embryogenesis, abnormal fetal or prenatal circulation, genetic factors, environmental toxins, abnormal inflammatory response, autoimmunity, and susceptibility factors.6 The presence of seasonal variation in the incidence of BA and a higher rate of cytomegalovirus (CMV) antibody detected in infants with BA may support a viral etiology.13 Additionally, an association between BA and CMV may negatively influence outcomes following Kasai portoenterostomy.6,13
Regardless of the exact mechanisms involved, the condition may be acquired rather than inherited, according to the disease evolution after birth, evidence from monozygotic and dizygotic twin studies that have shown discordance in the diagnosis of BA, and a lack of family history of this condition.6 BA may be present at birth, or shortly after birth, in a small subset (10%) of infants with associated splenic malformations, which is referred to as syndromic or congenital BA. The pathogenesis of this entity could be the product of an in utero insult.6,14 Only one patient in the present cohort was diagnosed with the syndromic subtype of BA.
Irrespective of the subtype, BA is occasionally associated with various other congenital anomalies, such as those involving cardiovascular and gastrointestinal systems.15,16 The reported incidence in previous studies varies between 15% and 44%, similar to our findings. The association with splenic malformations is considered a subtype of BA with a different pathogenesis, as well as a poor response to the Kasai procedure.6,14–16
One finding in the present study concerns the older age at diagnosis and late referrals, which is a common issue worldwide.9,17,18 Delayed diagnosis can be attributed to a lack of knowledge of normal stool color because pale stool color may be considered normal by some inexperienced mothers.19 This issue has led some health authorities to implement national screening programs using ‘stool cards’, which has been successful in Taiwan and Canada.20,21 Another reason for delayed diagnosis is dismissing jaundice as physiological in the absence of the required blood testing.7 Referral from remote towns and villages may also contribute to delayed diagnosis. Finally, visualizing the common bile duct on US has been described in BA and could contribute to delayed diagnosis when perceived as a finding that excludes BA.22
Imaging studies have been used to aid in the diagnosis of BA for a number of decades. However, imaging modalities are currently considered to have insufficiently high accuracy to be a gold standard test. Therefore, intra-operative cholangiography with liver biopsy is still the predominant tool used for analysis.7 This was shown in the present cohort, where none of the US findings had high sensitivities. Studies of US in BA have shown low sensitivities but very high specificity, with the “triangular cord sign” having the highest specificity.23,24 In contrast to US, a HIDA scan is a good tool for excluding BA because this test has a very high negative predictive value and sensitivity, but low specificity.25 In the present cohort, a HIDA scan showing a lack of radioisotope excretion in the small bowel was 100% sensitive.
Liver biopsy findings described in BA are bile ductular proliferation, bile plugs, and fibrosis, with an overall high accuracy ranging from 88% to 90%.26,27 Bile plugs, absence of bile duct paucity, and absent to few giant cell transformations are the highest predictors of BA in descending order.27 In the present cohort, ductular proliferation was seen in almost all cases. Interestingly, a significant number of patients had giant cell transformation, which may indicate that some cases fall into a “gray zone” between BA and giant cell hepatitis.5
When faced with cholestasis, significant clinical attention is first typically directed towards either diagnosing or excluding BA. Of all cholestatic conditions, BA is the only one that is “time-sensitive” because early diagnosis significantly improves outcomes, and if missed, can have adverse consequences.1,28–31 The benefit of early intervention on survival continues to show positive correlation with younger age at surgery.28,32 Traditionally, the first 60 days of life are critical in establishing bile flow to prevent or ameliorate liver-related morbidity and mortality in BA patients. However, the cutoff of 60 days is somewhat arbitrary because establishing bile flow with Kasai portoenterostomy even beyond that age may still result in favorable outcomes.11,33,34 In the present cohort, almost all cases underwent surgery at or beyond the age of 60 days. Accordingly, the success and survival rates are comparative to those in previous reports.11,33,34
In addition to age at the Kasai procedure, other predictors of success have been examined. The presence of fibrosis on biopsy, portal hypertension, cholangitis episodes following surgery, small ductular size, duration of jaundice, need for phototherapy in the neonatal period, and non-clearance of jaundice after surgery are associated with the need for liver transplantation following the Kasai procedure.9,10,35–37
The optimum treatment for late BA presentation remains controversial, especially considering the difficulty in predicting Kasai procedure prognosis.7 On the one hand, survival with the native liver for those undergoing the Kasai procedure beyond 60 days appears to be more favorable than previously suggested.11,32–34 Early transplantation (in the first year of life) is associated with higher morbidity and mortality.38 Moreover, organ availability is limited and even more so for patients younger than 1 year, making transplantation a significant challenge as a first-line treatment.39 On the other hand, liver transplantation within the first year of life following a failed Kasai procedure is associated with poorer outcomes compared with primary transplantation, possibly attributable to the increased incidence of sepsis and cholangitis associated with the Kasai procedure, although this is disputed.40–42 Moreover, the Kasai procedure itself may render a future transplant procedure technically more difficult.43,44
The most common complication with Kasai portoenterostomy is ascending cholangitis and recurrent ascending cholangitis, but portal hypertensive complications, intestinal obstruction, sepsis, and liver failure are also possible.45 The present cohort showed comparable complication rates, but an unusual finding was the favorable survival associated with cholangitis. This may have been confounded by Kasai success, as almost none of our cases without a cholangitis episode (n = 13) had a successful Kasai procedure, indicating they had complete obliteration to begin with, and hence, worse survival. Cholangitis incidence is higher with clearance of jaundice following Kasai.46 However, this finding is controversial because cholangitis and recurrent cholangitis are negative predictors of survival owing to the effect of repeated inflammation and progressive fibrosis on duct patency.46–48
Our study may be limited by the relatively small sample size, despite it being one of the largest cohorts in the region. Liver biopsy was not performed in all cases; therefore, calculating predictive values for specific histopathological signs was not feasible. In addition, survival among different BA subtypes was not assessed as it was not possible to make an accurate distinction based on imaging alone. Finally, post-operative antibiotic and steroid use was not standardized across all cases undergoing Kasai portoenterostomy.
Conclusions
Patient age at diagnosis of BA falls under the “late” category for most of the cohort; however, overall survival with the native liver is comparable to other cohorts. Clinicians are encouraged to refer for Kasai portoenterostomy even after the classic “60-day window” has passed, especially considering the comparatively higher transplant mortality rate before 1 year of age vs after 1 year of age. Finally, the factors associated with longer survival in BA are Kasai procedure success and the use of ursodeoxycholic acid, whereas factors associated with shorter survival are impaired synthetic function at presentation and post-operative liver failure.
Acknowledgement
The authors acknowledge Dr Trevor Rawbone, Cardiff, UK, for English editing and proofreading of the manuscript.
Footnotes
Author Contributions: Guarantor of the article: OS
Development of study concept and design: AK, OS.
Acquisition, analysis, and interpretation of the data: AK, AA, OS.
Statistical analysis: AK, OS.
Drafting of the manuscript: AK, OS.
Critical revision of the manuscript for important intellectual content: AK, AA, OS.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ammar Khayat https://orcid.org/0000-0002-4819-9552
References
- 1.Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis”? Clin Liver Dis 2006; 10: 27–53, v. DOI: 10.1016/j.cld.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant 2019; 19: 184–283. DOI: 10.1111/ajt.15276. [DOI] [PubMed] [Google Scholar]
- 3.Al Sebayel M, Abaalkhail F, Al Abbad S, et al. Liver transplantation in the Kingdom of Saudi Arabia. Liver Transpl 2017; 23: 1312–1317. DOI: 10.1002/lt.24803. [DOI] [PubMed] [Google Scholar]
- 4.Dehghani SM, Efazati N, Shahramian I, et al . Evaluation of cholestasis in Iranian infants less than three months of age. Gastroenterol Hepatol Bed Bench 2015; 8: 42–48. [PMC free article] [PubMed] [Google Scholar]
- 5.Landing BH. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst–the concept of infantile obstructive cholangiopathy. Prog Pediatr Surg 1974; 6: 113–139. [PubMed] [Google Scholar]
- 6.Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol 2015; 12: 342–352. DOI: 10.1038/nrgastro.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawaz R, Baumann U, Ekong U, et al. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64: 154–168. DOI: 10.1097/mpg.0000000000001334. [DOI] [PubMed] [Google Scholar]
- 8.Kasai M. Treatment of biliary atresia with special reference to hepatic porto-enterostomy and its modifications. Prog Pediatr Surg 1974; 6: 5–52. [PubMed] [Google Scholar]
- 9.Chardot C, Carton M, Spire-Bendelac N, et al. Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology 1999; 30: 606–611. DOI: 10.1002/hep.510300330. [DOI] [PubMed] [Google Scholar]
- 10.Baerg J, Zuppan C, Klooster M. Biliary atresia–a fifteen-year review of clinical and pathologic factors associated with liver transplantation. J Pediatr Surg 2004; 39: 800–803. DOI: 10.1016/j.jpedsurg.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Chardot C, Carton M, Spire-Bendelac N, et al. Is the Kasai operation still indicated in children older than 3 months diagnosed with biliary atresia? J Pediatr 2001; 138: 224–228. DOI: 10.1067/mpd.2001.111276. [DOI] [PubMed] [Google Scholar]
- 12.Hadzic N. Medical management of the ‘failing' Kasai portoenterostomy. S Afr Med J 2012; 102: 868–871. [DOI] [PubMed] [Google Scholar]
- 13.Zani A, Quaglia A, Hadzić N, et al. Cytomegalovirus-associated biliary atresia: an aetiological and prognostic subgroup. J Pediatr Surg 2015; 50: 1739–1745. DOI: 10.1016/j.jpedsurg.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Davenport M, Savage M, Mowat AP, et al. Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery 1993; 113: 662–668. [PubMed] [Google Scholar]
- 15.Schwarz KB, Haber BH, Rosenthal P, et al. Extrahepatic anomalies in infants with biliary atresia: results of a large prospective North American multicenter study. Hepatology 2013; 58: 1724–1731. DOI: 10.1002/hep.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang MC, Chang MH, Chiu SN, et al. Implication of early-onset biliary atresia and extrahepatic congenital anomalies. Pediatr Int 2010; 52: 569–572. DOI: 10.1111/j.1442-200X.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 17.Shneider BL, Brown MB, Haber B, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148: 467–474. DOI: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 18.Wildhaber BE, Majno P, Mayr J, et al. Biliary atresia: Swiss national study, 1994-2004. J Pediatr Gastroenterol Nutr 2008; 46: 299–307. DOI: 10.1097/MPG.0b013e3181633562. [DOI] [PubMed] [Google Scholar]
- 19.Bakshi B, Sutcliffe A, Akindolie M, et al. How reliably can paediatric professionals identify pale stool from cholestatic newborns? Arch Dis Child Fetal Neonatal Ed 2012; 97: F385–F387. DOI: 10.1136/fetalneonatal-2010-209700. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber RA, Masucci L, Kaczorowski J, et al. Home-based screening for biliary atresia using infant stool colour cards: a large-scale prospective cohort study and cost-effectiveness analysis. J Med Screen 2014; 21: 126–132. DOI: 10.1177/0969141314542115. [DOI] [PubMed] [Google Scholar]
- 21.Chen SM, Chang MH, Du JC, et al. Screening for biliary atresia by infant stool color card in Taiwan. Pediatrics 2006; 117: 1147–1154. DOI: 10.1542/peds.2005-1267. [DOI] [PubMed] [Google Scholar]
- 22.Azuma T, Nakamura T, Nakahira M, et al. Pre-operative ultrasonographic diagnosis of biliary atresia–with reference to the presence or absence of the extrahepatic bile duct. Pediatr Surg Int 2003; 19: 475–477. DOI: 10.1007/s00383-003-0962-0.] [DOI] [PubMed] [Google Scholar]
- 23.Mittal V, Saxena AK, Sodhi KS, et al. Role of abdominal sonography in the preoperative diagnosis of extrahepatic biliary atresia in infants younger than 90 days. AJR Am J Roentgenol 2011; 196: W438–W445. DOI: 10.2214/ajr.10.5180. [DOI] [PubMed] [Google Scholar]
- 24.Humphrey TM, Stringer MD. Biliary atresia: US diagnosis. Radiology 2007; 244: 845–851. DOI: 10.1148/radiol.2443061051. [DOI] [PubMed] [Google Scholar]
- 25.Kianifar HR, Tehranian S, Shojaei P, et al. Accuracy of hepatobiliary scintigraphy for differentiation of neonatal hepatitis from biliary atresia: systematic review and meta-analysis of the literature. Pediatr Radiol 2013; 43: 905–919. DOI: 10.1007/s00247-013-2623-3. [DOI] [PubMed] [Google Scholar]
- 26.Rastogi A, Krishnani N, Yachha SK, et al. Histopathological features and accuracy for diagnosing biliary atresia by prelaparotomy liver biopsy in developing countries. J Gastroenterol Hepatol 2009; 24: 97–102. DOI: 10.1111/j.1440-1746.2008.05737.x. [DOI] [PubMed] [Google Scholar]
- 27.Russo P, Magee JC, Anders RA, et al. Key histopathologic features of liver biopsies that distinguish biliary atresia from other causes of infantile cholestasis and their correlation with outcome: a multicenter study. Am J Surg Pathol 2016; 40: 1601–1615. DOI: 10.1097/pas.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokol RJ, Shepherd RW, Superina R, et al. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46: 566–581. DOI: 10.1002/hep.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lally KP, Kanegaye J, Matsumura M, et al. Perioperative factors affecting the outcome following repair of biliary atresia. Pediatrics 1989; 83: 723–726. [PubMed] [Google Scholar]
- 30.Chittmittrapap S, Chandrakamol B, Poovorawan Y, et al . Factors influencing outcome after hepatic portoenterostomy for biliary atresia: a logistic regression analysis. J Med Assoc Thai 2005; 88: 1077–1082. [PubMed] [Google Scholar]
- 31.Nio M, Ohi R, Miyano T, et al. Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg 2003; 38: 997–1000. DOI: 10.1016/s0022-3468(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 32.Serinet MO, Wildhaber BE, Broue P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123: 1280–1286. DOI: 10.1542/peds.2008-1949. [DOI] [PubMed] [Google Scholar]
- 33.Tagge DU, Tagge EP, Drongowski RA, et al. A long-term experience with biliary atresia. Reassessment of prognostic factors. Ann Surg 1991; 214: 590–598. DOI: 10.1097/00000658-199111000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen BT, Lee H, Sullivan K, et al. The Kasai portoenterostomy: when is it too late? J Pediatr Surg 2001; 36: 97–99. DOI: 10.1053/jpsu.2001.20020. [DOI] [PubMed] [Google Scholar]
- 35.Lykavieris P, Chardot C, Sokhn M, et al. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41: 366–371. DOI: 10.1002/hep.20547. [DOI] [PubMed] [Google Scholar]
- 36.Ohya T, Miyano T, Kimura K. Indication for portoenterostomy based on 103 patients with Suruga II modification. J Pediatr Surg 1990; 25: 801–804. DOI: 10.1016/s0022-3468(05)80025-9. [DOI] [PubMed] [Google Scholar]
- 37.Subramaniam R, Doig CM, Bowen J, et al. Initial response to portoenterostomy determines long-term outcome in patients with biliary atresia. J Pediatr Surg 2000; 35: 593–597. DOI: 10.1053/jpsu.2000.0350593. [DOI] [PubMed] [Google Scholar]
- 38.Carceller A, Blanchard H, Alvarez F, et al. Past and future of biliary atresia. J Pediatr Surg 2000; 35: 717–720. DOI: 10.1053/jpsu.2000.6034. [DOI] [PubMed] [Google Scholar]
- 39.Parikh ND, Hutton D, Marrero W, et al. Projections in donor organs available for liver transplantation in the United States: 2014-2025. Liver Transpl 2015; 21: 855–863. DOI: 10.1002/lt.24136. [DOI] [PubMed] [Google Scholar]
- 40.Alexopoulos SP, Merrill M, Kin C, et al. The impact of hepatic portoenterostomy on liver transplantation for the treatment of biliary atresia: early failure adversely affects outcome. Pediatr Transplant 2012; 16: 373–378. DOI: 10.1111/j.1399-3046.2012.01677.x. [DOI] [PubMed] [Google Scholar]
- 41.Ryckman FC, Alonso MH, Bucuvalas JC, et al. Biliary atresia–surgical management and treatment options as they relate to outcome. Liver Transpl Surg 1998; 4: S24–S33. [PubMed] [Google Scholar]
- 42.Ryckman F, Fisher R, Pedersen S, et al. Improved survival in biliary atresia patients in the present era of liver transplantation. J Pediatr Surg 1993; 28: 382–385; discussion 386. DOI: 10.1016/0022-3468(93)90236-e. [DOI] [PubMed] [Google Scholar]
- 43.Sandler AD, Azarow KS, Superina RA. The impact of a previous Kasai procedure on liver transplantation for biliary atresia. J Pediatr Surg 1997; 32: 416–419. DOI: 10.1016/s0022-3468(97)90594-7. [DOI] [PubMed] [Google Scholar]
- 44.Wood RP, Langnas AN, Stratta RJ, et al. Optimal therapy for patients with biliary atresia: portoenterostomy (“Kasai” procedures) versus primary transplantation. J Pediatr Surg 1990; 25: 153–160; discussion 160-152. DOI: 10.1016/s0022-3468(05)80183-6. [DOI] [PubMed] [Google Scholar]
- 45.Jung E, Park WH, Choi SO. Late complications and current status of long-term survivals over 10 years after Kasai portoenterostomy. J Korean Surg Soc 2011; 81: 271–275. DOI: 10.4174/jkss.2011.81.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar R, Lal BB, Sood V, et al. Predictors of successful Kasai portoenterostomy and survival with native liver at 2 years in infants with biliary atresia. J Clin Exp Hepatol 2019; 9: 453–459. DOI: 10.1016/j.jceh.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramachandran P, Safwan M, Balaji MS, et al. Early cholangitis after portoenterostomy in children with biliary atresia. J Indian Assoc Pediatr Surg 2019; 24: 185–188. DOI: 10.4103/jiaps.JIAPS_96_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SY, Lin CC, Tsan YT, et al. Number of cholangitis episodes as a prognostic marker to predict timing of liver transplantation in biliary atresia patients after Kasai portoenterostomy. BMC Pediatr 2018; 18: 119. DOI: 10.1186/s12887-018-1074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]