Abstract
Rhodococcosis is a serious infection specially affecting immunocompromised populations. We report a case of disseminated infection by Rhodococcus equi in a renal transplant patient, that was initially diagnosed as histoplasmosis, highlighting the potential for confusion between rhodococcosis and other infections. Clinicians and pathologists should correlate histopathology findings with the clinical and microbiological data.
Keywords: Rhodococcus equi, Transplant, Immunocompromise
Introduction
Rhodococcus equi is an emerging zoonotic infection, especially among immunocompromised hosts. Diagnosis of R. equi can be difficult because of its clinical similarities with other opportunistic infections, diphtheroid appearance on Gram stain, and positive acid-fast, periodic acid-Schiff (PAS) and Gomori methenamine silver (GMS) stains, leading to delayed or missed diagnosis. We report a case of disseminated rhodococcosis in a renal transplant patient who was initially thought to have histoplasmosis.
Case presentation
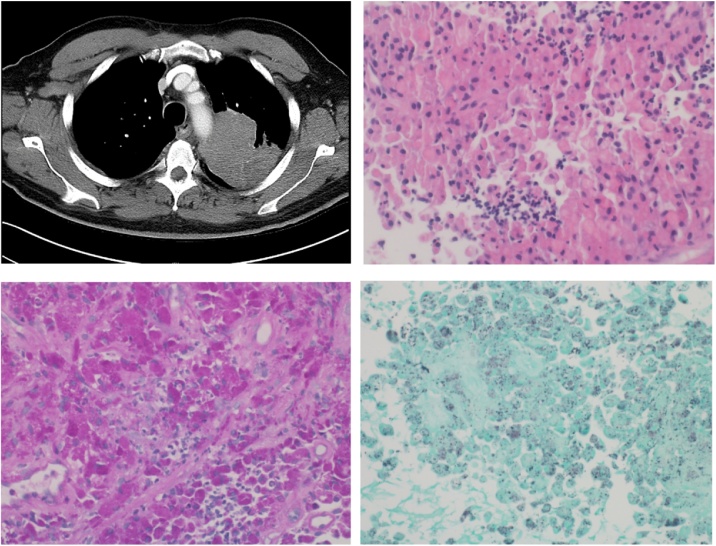
A 60-year-old African-American male with type 2 diabetes mellitus (A1C 7.6 %), hypertension, end stage renal disease requiring kidney transplant from a living donor 9 years prior presented with night sweats, decreased appetite, and weight loss for six months. A left upper lobe mass (8.1 × 7.4 × 8.5 cm) was found on computed tomography (CT) of the chest (Fig. 1). His anti-rejection regimen consisted of mycophenolic acid 360 mg twice daily, tacrolimus 2 mg twice daily, and prednisone 5 mg once daily, with no recent episodes of rejection or opportunistic infection reported. A transbronchial biopsy was negative for malignancy. The bronchoalveolar fluid (BAL) bacterial culture grew penicillin-susceptible Streptococcus pneumoniae for which he received amoxicillin. BAL cultures for acid fast bacilli (AFB) or fungi were negative. His serum and urine antigens for Blastomyces, Coccidioides, and Histoplasma were negative, as well as the Cryptococcus serum antigen. He later underwent a CT-guided biopsy of the lung mass, with histopathology revealing organisms suggestive of Histoplasma within histiocytes (Fig. 1, lower left). Bacterial culture grew Kocuria species, deemed not to be pathogenic. AFB and fungal cultures were negative. Blastomyces, Coccidioides and Histoplasma antibodies by immunodiffusion and fungal blood culture were negative. Liposomal amphotericin B 3 mg/kg/day was given for one week followed by oral itraconazole tablets (200 mg three times a day for 3 days and then 200 mg twice a day). Itraconazole levels were not available.
Fig. 1.
Upper left: CT of the chest showing the mass in left upper lobe. Upper right: Core biopsy shows a sheetlike histiocytic proliferation with abundant eosinophilic foamy cytoplasm and eccentric nuclei. Scattered collections of neutrophlic infiltrates around the histiocytes are present. These features are consistent with malakoplakia. No evidence of Michaelis-Gutmann bodies is identified. Lower left: PAS stain with diastase performed on the core biopsy shows the cytoplasms of the histiocytes is packed with PAS positive organisms (400X). Lower right: GMS stain performed on the core biopsy show scattered intracytoplasmic small organisms, some have diploid cocobacilli appearance (400×).
Six weeks later, patient presented with weight loss, hemoptysis and intermittent fevers for about four weeks. He was noted to have decreased breath sounds in the left upper and middle lobes. Physical exam was otherwise normal. A complete blood count showed WBC 7720 cells/μL (7000 neutrophils/μL, 410 lymphocytes/μL), hemoglobin 9.2 g/dL, and platelets 313 × 109/L. Complete metabolic profile was significant for creatinine 2 mg/dL (baseline 2–2.6 mg/dL). Chest imaging showed a worsening left upper lobe consolidation with central cavitation.
A left upper lobectomy revealed a mass surrounded by purulent material and invading the aorta and the second rib, which impeded resection. The purulent material, pleural and pericardial fluid were drained and incisional biopsy of the mass was performed. Gram stain on the lung tissue showed gram-positive cocci in pairs, chains, and clusters. Empiric intravenous vancomycin was initiated. Blood cultures grew Rhodococcus equi, which was also isolated from lung tissue, lymph node and pericardial fluid. Meropenem and ciprofloxacin were added empirically. The isolate was found to be susceptible to amikacin, ceftriaxone, imipenem, linezolid, moxifloxacin, tobramycin and intermediate to ciprofloxacin. Histopathology of lung tissue and lymph node showed necrotizing granulomatous inflammation and organisms on PAS and GMS suggestive of Histoplasma (Fig. 1, lower left and lower right). Urine Histoplasma antigen in this second hospitalization and all the operative fungal cultures were negative.
The day after the lobectomy, patient acutely decompensated with shock requiring vasopressors and also developed abdominal distension. Computed tomography showed pneumomediastinum with pneumopericardium, as well as diffuse small bowel gaseous distension with pneumatosis intestinalis. With concern for small bowel obstruction and abdominal compartment syndrome, the patient was taken for exploratory laparotomy, which revealed extensively gangrenous, inoperable small bowel. At this point, it was determined that the patient would not survive this significant amount of ischemia and gangrene. Family decided to proceed with comfort care measures and the patient passed away.
Discussion
Rhodococcosis is a zoonosis seen among land and water animals, such as goats, fouls, cattle, horses, and seals [1,2]. In humans, R. equi is an opportunistic pathogen among patients with impaired cell-mediated immunity, often presenting as necrotizing pneumonia with cavitary lesions and consolidation. An association with other recent opportunistic infection and diabetes mellitus has been reported in transplant recipients [3,4].
R. equi is a non-spore forming, non-motile, obligate aerobic, intracellular gram positive coccobacillus that can be easily mistaken for a diphtheroid such as Corynebacterium species [5]. The colonies produce a very distinct salmon color at four to seven days of incubation [6].
Although rhodococcosis-histoplasmosis co-infection is possible in immunocompromised patients, we believe that our patient did not have histoplasmosis: multiple fungal cultures from blood, respiratory samples and lung tissue were negative for Histoplasma; Histoplasma antigens in serum and urine were negative; Histoplasma antibodies by immunodiffusion were negative. Additionally, patient deteriorated while on antifungal therapy. In rhodococcosis, histopathology shows histiocytic infiltration with intracellular bacteria that stain with PAS [7], and also with GMS, which can lead to confusion with fungal organisms. Histopathology from our patient is seen in Fig. 1. Rhodococcus organisms are smaller and have coccoid and coccobacillary appearance without budding.
Surgical debridement and catheter removal due to biofilm formation are as important as the use of effective antimicrobials [3,4]. Vancomycin, imipenem, ciprofloxacin, rifampin, and erythromycin have all been shown to have good activity against R. equi [8]. For transplant patients, combination therapy with 2–3 antimicrobial drugs is recommended (vancomycin, fluoroquinolone, +/− carbapenem); trimethoprim-sulfamethoxazole and clindamycin should be avoided due to variable susceptibility and interactions with anti-rejection drugs should be considered before choosing macrolides or rifampin [4]. The optimal duration of therapy is unknown; however after two to four weeks of IV therapy, chronic suppression with oral antimicrobials is advised [3,9,10].
In conclusion, rhodococcosis is a serious opportunistic infection in immunocompromised patients. Diagnosis can be missed or delayed because rhodococcosis can masquerade as other infections. Clinicians and pathologists should always have an expanded differential diagnosis in immunocompromised patients.
Funding source
None.
Ethical approval
University of Missouri IRB #2004691 HS.
HS Case Report Form #212024.
Consent
Consent waiver was requested as patient is deceased and patient’s family not easily accessible.
No identifiers utilized in this case report.
Author contribution
All authors contributed equally.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Giguere S., Cohen N., Keith Chaffin M. Rhodococcus equi: clinical manifestations, virulence, and immunity. J Vet Intern Med. 2011;25(6):1221–1230. doi: 10.1111/j.1939-1676.2011.00804.x. [DOI] [PubMed] [Google Scholar]
- 2.Walsh R.D., Schochd P.E., Cunha B.A. Rhodococcus. Infect Control Hosp Epidemiol. 1993;14(05):282–287. doi: 10.1086/646738. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock D.M., Brown A.E. Rhodococcus equi: an emerging pathogen. Clin Infect Dis. 2002;34(10):1379–1385. doi: 10.1086/340259. [DOI] [PubMed] [Google Scholar]
- 4.Vergidis P., Ariza-Heredia E.J., Nellore A. Rhodococcus infection in solid organ and hematopoietic stem cell transplant recipients. Emerg Infect Dis. 2017;23(3):510–512. doi: 10.3201/eid2303.160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott J.F. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4(1):20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton M., Hughes K. Corynebacterium equi: a review. Vet Bull. 1980;50:65–80. [Google Scholar]
- 7.Yamshchikov A.V., Schuetz A., Lyon G.M. Rhodococcus equi infection. Lancet Infect Dis. 2010;10(5):350–359. doi: 10.1016/S1473-3099(10)70068-2. [DOI] [PubMed] [Google Scholar]
- 8.Jacks S.S., Giguère S., Nguyen A. In vitro susceptibilities of Rhodococcus equi and other common equine pathogens to azithromycin, clarithromycin, and 20 other antimicrobials. Antimicrob Agents Chemother. 2003;47(5):1742–1745. doi: 10.1128/AAC.47.5.1742-1745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahamat-Langendoen J.C., van Meurs M., Zijlstra J.G., Lo-Ten-Foe J.R. Disseminated Rhodococcus equi infection in a kidney transplant patient without initial pulmonary involvement. Diagn Microbiol Infect Dis. 2009;65(4):427–430. doi: 10.1016/j.diagmicrobio.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Tse K., Tang S., Chan T., Lai K. Rhodococcus lung abscess complicating kidney transplantation: successful management by combination antibiotic therapy. Transpl Infect Dis. 2008;10(1):44–47. doi: 10.1111/j.1399-3062.2007.00231.x. [DOI] [PubMed] [Google Scholar]