Summary
Transactive response DNA binding protein 43 (TDP-43) is a DNA/RNA binding protein involved in transcriptional regulation and RNA processing. It is linked to sporadic and familial amyotrophic lateral sclerosis and frontotemporal lobar degeneration. TDP-43 is predominantly nuclear, but it translocates to the cytoplasm under pathological conditions. Cytoplasmic accumulation, phosphorylation, ubiquitination and truncation of TDP-43 are the main hallmarks of TDP-43 proteinopathies. Among these processes, the pathways leading to TDP-43 fragmentation remain poorly understood. We review here the molecular and biochemical properties of several TDP-43 fragments, the mechanisms and factors mediating their production, and their potential role in disease progression. We also address the presence of TDP-43 C-terminal fragments in several neurological disorders, including Alzheimer's disease, and highlight their respective implications. Finally, we discuss features of animal models expressing TDP-43 fragments as well as recent therapeutic strategies to approach TDP-43 truncation.
Subject areas: Biological sciences, Molecular physiology, Molecular biology, Molecular interaction
Graphical abstract
Highlights
-
•
TDP-43 fragments may contribute to loss- and gain-of-function mechanisms
-
•
Proteolysis as well as alternative splicing can lead to TDP-43 truncation
-
•
Several neurological conditions beyond the ALS/FTLD spectrum display TDP-43 fragments
-
•
The precise biological and pathological roles of TDP-43 fragments are unknown
Biological sciences; Molecular physiology; Molecular biology; Molecular interaction;
Introduction
Transactive response (TAR) DNA binding protein 43 (TDP-43) is involved in multiple aspects of transcriptional regulation and post-transcriptional RNA processing (Buratti and Baralle, 2001; Tollervey et al., 2011) and binds to thousands of target RNA molecules (Tollervey et al., 2011; Sephton et al., 2011 and Xiao et al., 2011). Mutations in the TDP-43-encoding gene TARDBP cause Amyotrophic lateral sclerosis (ALS), a progressive and incurable neurodegenerative condition characterized by loss of motor neurons, disability and death. TDP-43 is also linked to tau-negative frontotemporal lobar degeneration (FTLD) and a significant number of patients with ALS also develop FTLD (Liscic et al., 2008). Pioneering studies in the field found cytosolic translocation and accumulation of phosphorylated, ubiquitinated, and truncated TDP-43 in the brains of patients with FTLD and ALS (Neumann et al., 2006). This and subsequent studies established TDP-43 as a major pathological component of ALS and FTLD, which are also known as “TDP-43 proteinopathies” (Prasad et al., 2019). Notably, the majority of ALS and FTLD cases are sporadic in nature, where wild-type TDP-43 (WT TDP-43) translocates to the cytosol and forms pathological aggregates in neuronal cells (Figure 1). An obvious question here is: what causes the WT TDP-43 protein to take the pathological pathway? What can confer toxicity to native forms of TDP-43? A broader answer to this might be explained directly by protein modifications of TDP-43, such as phosphorylation, ubiquitination, and truncation (Arai et al., 2006; Cairns et al., 2007; Davidson et al., 2007; Neumann et al., 2006; Zhang et al., 2008). Phosphorylation and ubiquitination are widely used as markers of TDP-43 pathology; however the truncated forms of TDP-43 are less appreciated in this regard.
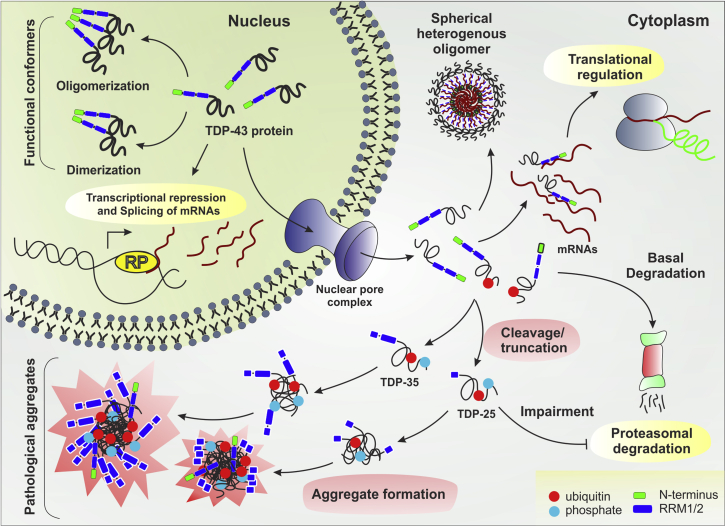
Figure 1.
Effects of TDP-43 truncation in disease pathogenesis
The scheme illustrates that TDP-43 functions normally as dimer or oligomer conformers in the nuclear compartment. However, upon translocation to the cytoplasm, truncation of TDP-43 promotes aggregation (gain-of-function). These aggregates hamper RNA stabilization in the cytoplasm leading to abnormal translational regulation, proteasomal degradation and sequestration/depletion of nuclear TDP-43 (loss-of-function). RP: RNA Polymerase complex.
TDP-43 was initially identified as a transcriptional repressor of the human immunodeficiency virus 1 (Ou et al., 1995). Later, it was found to be involved in alternative splicing of cystic fibrosis transmembrane conductance regulator (CFTR) leading to its exon 9 skipping (Buratti et al., 2001). Despite the initial discovery of the functional role of TDP-43 was made in 1995, its pathological involvement in neurodegeneration was not demonstrated until over a decade later (Neumann et al., 2006). TDP-43 proteinopathies are highly aggressive and patients with ALS reach a fatal outcome within 2–5 years from symptoms onset. The reason why this disease triggers such degree of aggressiveness remains elusive. A possible explanation is that multiple pathways may contribute synergistically to accelerate neuronal loss. For instance, ER stress, oxidative stress, nuclear transport dysfunction, axonal transport defects, and apoptosis are present in cells that display TDP-43 pathology (Ferrari et al., 2011; Goh et al., 2017; Ratti et al., 2020; Walker et al., 2015; Zhang et al., 2009). These and other reports led to a consensus that toxic TDP-43 accumulation is linked to disruption of multiple cascades. However, a couple of pioneer studies suggest that TDP-43 toxicity is primarily driven by cleavage and accumulation of toxic TDP-43 C-terminal fragments (CTFs) (Walker et al., 2015; Zhang et al., 2009). Yet, it is not clear whether truncated forms of TDP-43 are main culprits of the disease and, if so, how they interfere with the cellular machinery. Here, we compile and discuss data regarding the potential pathological role of truncated TDP-43 fragments in TDP-43 proteinopathies beyond the ALS/FTLD spectrum.
TDP-43 fragmentation: gain- and loss-of-function mechanisms
A leading pathological hallmark of TDP-43 proteinopathies is the cytoplasmic accumulation of TDP-43 aggregates in neuronal cells, which seem to present a toxic “gain-of-function” threat in the cytosolic milieu. Conversely, since TDP-43 is primarily a nuclear protein, it is possible that its translocation to the cytosol may have a negative impact in the nucleus, leading to a “loss-of-function” hypothesis (Xu, 2012). Another mechanism of action has been also proposed where a 35kDa truncated form of TDP-43, referred to as TDP-35, confers toxicity via gain-of-function mechanisms (Che et al., 2015). In this case, TDP-35 sequesters TDP-43 through various RNAs and makes cytosolic inclusions with other RNA-binding proteins, resulting in a gain-of-toxic function. However, this sequestration eventually leads to nuclear depletion of TDP-43 and thus TDP-43 CTFs can clearly contribute to both gain- and loss-of-function mechanisms. Consistently, a recent review on the controversial role of TDP-43 aggregates argues that neurotoxicity may not be due merely to TDP-43 aggregation but rather to both loss and gain-of-function processes (Hergesheimer et al., 2019).
TDP-43 functions as a dimer or as homo-oligomer through an N-terminal linkage (Afroz et al., 2017; Kuo et al., 2009; Mompeán et al., 2017) and cleavage in the N-terminus would lead to generation of toxic CTFs. The N-terminal fragments (NTFs) would remain in the nuclei and get quickly degraded, while the CTFs are exported to the cytoplasm, resulting in aggregation (Pesiridis et al., 2011). Aggregation of CTFs may drive the gain-of-toxic function mechanisms, while reduced functional dimerization/oligomerization may contribute to loss-of-function paradigms. Interestingly, the farthest N-terminus of TDP-43 has also been shown to form cytoplasmic aggregates in vivo (Zhang et al., 2013; Sasaguri et al., 2016). However, more work is needed to establish the pathological role of this TDP-43 region.
Characteristics and properties of cleaved TDP-43 fragments
The spectrum of TDP-43 truncated forms is considerably large in terms of their size and properties. Whether or not these differences in size determine the degree of their neurotoxicity is still a major puzzle in the field. To date, most studies have investigated the role of TDP-43 CTFs, and only a few groups are evaluating whether the NTFs contribute to pathogenesis. Nonaka et al. suggested that TDP-43 fragments encompassing residues 1–161 are aggregation-prone species (Nonaka et al., 2009). The N-terminal end of TDP-43 is critically involved in mediating RNA binding and functional assembly, although some reports indicate that the NTFs can be insoluble in nature too (Dammer et al., 2012; Weskamp et al., 2020). Interestingly, recent studies also indicate that the N-terminal domain of TDP-43 retains its capability of being assembled without any aberrant structural deformations (Tsoi et al., 2017). These studies support the idea that C-terminal, but not N-terminal, TDP-43 fragments drive the TDP-43-associated toxicity in pathophysiological conditions. Nonetheless, most studies used antibodies that recognize only the C-terminal region of TDP-43 and the scarcity of data on NTFs may be due to lack of specific detection. We anticipate that the development of new detection tools, such as the recently reported MS-PRM quantification assay (Feneberg et al., 2020), will help to clarify the role of TDP-43 fragments in the future.
TDP-43 contains two RNA recognition motifs (RRMs) toward the N-terminus, a nuclear localization signal (NLS), and a prion-like glycine-rich domain at the C-terminal end (Figure 2). A putative nuclear export sequence (NES) is recognized by several protein domain finders, but its function is highly controversial. Recent studies imply that this sequence may have little to no role in the export of TDP-43, which is probably driven by its size in a passive manner (Pinarbasi et al., 2018). On the other side, multiple exporter factors including XPO1, XPO7, and NXF1 also contribute to TDP-43 nuclear egress (Archbold et al., 2018), suggesting that its trafficking occurs through partially redundant and overlapping pathways and that other uncharacterized transport motifs may exist within TDP-43.
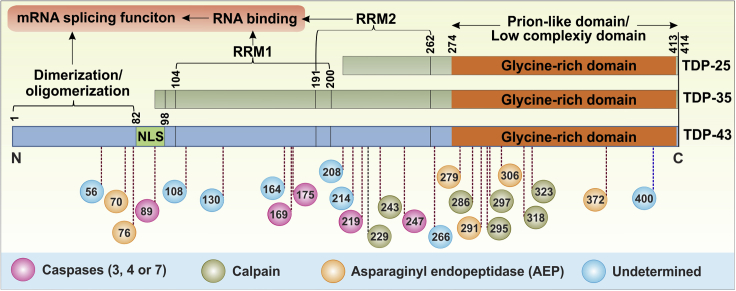
Figure 2.
Schematic representation of TDP-43 protein and its truncation sites
For a Figure360 author presentation of Figure 2, see https://doi.org/10.1016/j.isci.2021.102459.
TDP-43 includes multiple cleavage sites encompassing its NLS (nuclear localizing signal), RNA-binding motifs (RRM1 or RRM2), and Glycine-rich domain. Colored circles represent cleavage type, while the numbers inside indicate the amino acid residue from the N-terminal end. TDP-35 lacks functional NLS, while TDP-25 lacks NLS along with functional RRM domains leading to splicing and RNA-binding abnormalities.
TDP-35 lacks the N-terminus from within NLS and TDP-25 further losses the RRM1 and part of RRM2 in addition to NLS (Figure 2). The presence or absence of certain motifs will have important functional and pathological implications (Li et al., 2015). For instance, TDP-43 participates in the formation of stress granules, which retain RNAs transiently and release them as needed to the ribosome for translation (McDonald et al., 2011). However, the absence of one or both RRMs in TDP-43 CTFs disrupts the equilibrium between stress granules formation and translational regulation and can lead to the formation of proteinaceous aggregates (Wang et al., 2013). To further understand the role of different TDP-43 motifs or domains in protein aggregation, Jiang et al. generated several constructs that express various truncated forms of TDP-43. The authors showed that TDP-43 functions as a 135Å-long homodimer, which was later found to be critical for the splicing activities of TDP-43 (Jiang et al., 2017). Removal of the RRM2 not only leads to disruption of RNA binding capabilities but also makes TDP-43 molecules incapable of forming functional dimers. Moreover, a pathological truncation in TDP-43 within the RRM2 domain (at residue 208) leads to the formation of beta sheet fibrils in neuronal cells but not when the RRM2 is intact. These amyloid beta-like fibrillary structures were apparent in electron microscopy, yet remain undetected by Thioflavin T or amyloid specific antibodies, probably due to amorphous nature of TDP-43 amyloids (Capitini et al., 2014). These studies emphasize the role of beta sheets in pathophysiology through amyloidogenesis. On the other hand, Wang et al. proposed a model where the C-terminal end of TDP-43 forms functional aggregates (larger than oligomers but still well organized) that, after cleavage, transform into pathological aggregates (Wang et al., 2012a) (Figure 1). A separate study defined the thermodynamic and electrostatic characteristics of TDP-43 CFTs through a repulsion model for amyloid formation (Mompeán et al., 2016) and reported that a decreased electrostatic charge in CTFs leads to greater aggregation tendencies. Lastly, TDP-43 CTFs cleaved at different sites exert significant differences in solubility among various fragment sizes (Furukawa et al., 2011). This is consistent with the distinct histopathological properties associated with different TDP-43 fragments. For instance, TDP-25 makes toxic Ub-positive inclusion bodies (IBs), while TDP-35 makes less toxic Ub-negative IBs (Kitamura et al., 2016).
Cell culture paradigms have been instrumental in the study of TDP-43 CTFs. In 2014, Liu et al. generated the first stable cell line expressing TDP-25 and proved that this TDP fragment is preferentially processed through proteasomal degradation (Liu et al., 2014). This may explain the role of CTFs in the secondary TDP-43 pathology seen in various neurodegenerative conditions, where proteasomal degradation is compromised (Chhangani and Mishra, 2013). Structural insights into TDP-43, TDP-35, and TDP-16 (lacking both RRM domains) were recently provided using solid-state nuclear magnetic resonance (Shenoy et al., 2019). TDP-43 and TDP-35 display similar amyloidogenic properties compared to TDP-16. This suggests that the RRM domains play an important role in amyloid formation as TDP-16 tends to make ordered amyloids. In this regard, TDP-25 lacks both RRMs and the NLS. This could help TDP-25 to be retained in the cytosol while absence of RRMs allows amyloid formation to be more ordered, an inherited property of TDP-43 aggregates (Capitini et al., 2014). All these data support the notion that pathological aggregates of TDP-43 in the cytosol comprised full length as well as its various truncated forms.
Full length TDP-43 and its aggregation-prone CTFs have also been detected in exosomes in cellular models of TDP-43 proteinopathies, as well as in human brain samples from patients with ALS and FTLD (Nonaka et al., 2013; Riku, 2020). Exosomal detection of TDP-43 in blood plasma is linked to disease progression in ALS (Chen et al., 2020). Since insoluble aggregates of CTFs are enriched in these exosomes, it has been suggested that truncated forms of TDP-43 may serve as seeds for prion-like cell-to-cell propagation (Riku, 2020). But, spreading of TDP-43 can also happen through transsynaptic means (Jamshidi et al., 2020). Thus, even though increasing data support the anatomical spreading of TDP-43, the molecular and cellular mechanisms of this spreading as well as the potential role of CTFs in this phenomenon are unclear and require further investigation.
Factors mediating proteolytic cleavage of TDP-43
The mechanisms underlying TDP-43 truncation are still poorly understood (Berning and Walker, 2019). Several enzymes are known to cleave TDP-43 at various sites along its 414-amino acid length (Figure 2 and Table 1), but many sites are still undetermined. For instance, Igaz et al. observed TDP-43 CTFs ranging from 22 to 24kDa in brain but not in the spinal cord of patients with ALS and FTLD-U and identified Arg208 as a pathological cleavage site after sequencing these truncated forms (Igaz et al., 2008); however, it is still uncertain what causes this cleavage. Caspase 3-mediated cleavage is considered as an important step in TDP-43 toxicity, though Caspase 3-independent cleavage of TDP-43 at various sites has also been reported (Table 1). Caspase 7, but not Caspase 8, can also cleave recombinant TDP-43 into 25 and 35 kDa fragments (Shenoy et al., 2019). Apoptotic conditions can activate caspases due to several stressors such as lower calcium levels (De Marco et al., 2014), leading to TDP-43 truncation. When caspase-cleaved TDP-25 and TDP-35 fragments were comparatively analyzed with full-length TDP-43 in motor neurons, TDP-25 demonstrated to be more aggregation prone (Cicardi et al., 2018). In this report, TDP-43 remains in the nuclei, TDP-35 partially mislocalizes to the cytoplasm making small aggregates, and TDP-25 (which lacks NLS) remains exclusively in the cytosol forming large, irregular and insoluble aggregates associated with autophagy impairment in neuronal cells. Interestingly, over-expression of TDP-25 does not alter the functionality of TDP-43 in nuclei, as evidenced by performing typical CFTR exon 9 skipping assays. This suggests that endogenous TDP-43 retains its activity in this context and that TDP-25 toxicity appears to be due to its own toxic gain-of-function in the cytosol. On the other hand, mutant TDP-43M337V was expressed in the brain of monkeys (Rhesus macaque) and mice to compare its distribution and fragmentation (Yin et al., 2019). Mutant TDP-43 translocates to the cytoplasm in the monkey brain but stays primarily in the nuclei of the mouse brain. The primate-specific ER-associated Caspase 4, which is absent in mice, was responsible for cleavage and accumulation of TDP-25 and TDP-35 in the neuronal cytosol. Importantly, when human Caspase 4 was over-expressed in the brain of old TDP-43M337V mice, these animals successfully produced the anticipated CTFs, which confirmed the critical role of Caspase 4 in TDP-43 cleavage. This is relevant because the authors also observed increased levels of Caspase 4 in patients with ALS.
Table 1.
TDP-43 cleavage sites and responsible enzymes
| Enzyme | Cleavage site | Fragment size (kDa) and type | Experimental system | References |
|---|---|---|---|---|
| Caspase 3 | 89, 219, 247 | 25, 35, 42 CTF | Cell culture | (Zhang et al., 2007) |
| Caspase 4 | 174 | 25 CTF | in vitro and in monkey | (Li et al., 2015) |
| Caspase 7 | 89, 219 | 25, 35, 42 CTF | Cell culture | (Zhang et al., 2007) |
| Caspase (unknown) | 169 | 27 CTF | Cell culture | (Suzuki et al., 2011) |
| Calpain 1 and Calpain 2 | 229, 243, 246, 286, 295, 297, 318, 324 | 32, 33, 36 CTF and NTF | Cell culture, mouse and human ALS specimens | (Yamashita et al., 2012; Yang et al., 2014b) |
| Asparaginyl endopeptidase (AEP) | 70, 76, 279, 291, 285, 306, 372 | 22-37 CTF | Cell culture and Mouse | (Herskowitz et al., 2012) |
| Undetermined | 55, 108, 208, 130, 164, 214, 266, 400 | 15-43 NTF and CTF | Mouse | (Igaz et al., 2008; Kametani et al., 2016) |
In addition to caspase-mediated cleavage, other important classes of proteases have also been reported to cleave TDP-43 (Table 1). The Ca2+-dependent cysteine protease Calpain specifically cleaves mouse and human TDP-43 in Neuro2a cells and such cleavage can be blocked with the specific Calpain inhibitors MDL28170 and ALLN, but not with caspase inhibitors (Yamashita et al., 2012). Interestingly, ALS-linked TDP-43M337V and TDP-43A315T mutant isoforms are highly vulnerable to Calpain cleavage compared to TDP-43 WT. Calpain-dependent TDP-43 fragments were also observed in the spinal cords of patients with ALS, a finding consistent with increased levels of activated Calpain-I and Calpain-II in these specimens (Yamashita et al., 2012). On the other hand, a mass spectrometry analysis in brain samples from patients with FTLD led to the identification of 32 and 35 kDa TDP-43 fragments truncated at N291 and N306. These fragments were cleaved by asparaginyl endopeptidase (AEP) and were absent in AEP null mice (Herskowitz et al., 2012), which validates specificity. AEP can cleave TDP-43 at seven asparagine residues in vitro, but the biological relevance of this finding remains to be elucidated. Taken together, these findings indicate that caspase-dependent and -independent processes (Figure 2) participate in the generation of TDP-43 fragments. Additional studies will be required to fully understand the interplay between these cleavage mechanisms.
The role of phosphorylation in TDP-43 cleavage
TDP-43 hyperphosphorylation is widely accepted as hallmark of TDP-43 pathology. Indeed, phosphorylation of TDP-43 in C. elegans drives TDP-43 proteinopathy, and inhibition of phosphorylation prevents neurodegeneration in this model (Liachko et al., 2010, 2013). However, the role of TDP-43 phosphorylation in the cleavage process is controversial. For instance, hyperphosphorylation of TDP-43 seems to modulate its aggregation in a cooperative manner through phosphorylation at five different serine residues (Li et al., 2011). Mutations that mimic a hyperphosphorylated state in these five residues reduce aggregation in vitro and in vivo. Since TDP-43 phosphorylation occurs post-truncation in this work, it was suggested that hyperphosphorylation may serve as a defensive mechanism to reduce TDP-43 aggregation and also to increase solubility of TDP-43 and its truncated forms. Another study reported that, after heat-shock induction, TDP-43 is double phosphorylated (T153,Y155) by MEK, a heat shock response-dependent kinase that contributes to protein homeostasis. Interestingly, this phosphorylated form does not aggregate and remains soluble under protein misfolding conditions, suggesting that this particular modification might also be non-pathogenic and rather critical to regulate TDP-43 function (Li et al., 2017). A different group proved that phosphorylation at positions 403/404 and 409/410 makes TDP-43 more resistant to Calpain cleavage (Yamashita et al., 2016). Phosphorylation of these sites by enzymes like Casein kinases (CK1/CK2) or Tau tubulin kinases (TTBK1/TTBK2) helps retaining non-cleaved forms resulting in lower toxicity. These results suggest that the phosphorylated forms of TDP-43 might be the consequence of a cellular strategy to overcome proteotoxic stress. Lastly, even if the CTFs are more likely to get phosphorylated, their inclusion formation properties seem to be independent of such modification (Zhang et al., 2009). This suggests that TDP-43 truncation and phosphorylation may occur independently, yet phosphorylation can influence aggregation of TDP-43 and its truncated forms in a cytoprotective manner.
Role of pathogenic mutations in TDP-43 fragmentation
To date, more than 50 mutations in TDP-43 have been found to cause ALS (Buratti, 2015). Numerous studies confirm the toxic effects of mutant forms of TDP-43 and their different truncated products, but only a few reports correlate TDP-43 mutations with its truncation. For instance, expression analysis in Neuro2a cells indicated that TDP-43 with mutation D169G is more susceptible to caspase 3 cleavage than the wild-type version, resulting in high levels of TDP-35 with increased thermal stability (Chiang et al., 2016). Nonaka et al. also expressed two GFP-tagged TDP-43 fragments, GFP-TDP162-414 and GFP-TDP218-414, with and without pathological mutations to investigate their effect on TDP-43-mediated toxicity (Nonaka et al., 2009). The authors demonstrated that 14 different mutations linked to ALS pathology significantly enhance the aggregation of GFP-TDP162-414. However, no significant effect was found on GFP-TDP218-414, probably due to a masking effect of the higher aggregation propensity of this shorter form. Interestingly, no aggregation was observed in cells expressing full length TDP-43 with the same mutations. This suggests that truncation is a prerequisite for TDP-43 aggregation, which in turn can be potentiated through pathological mutations. Furthermore, a recent study in cultured neuroblastoma cells showed that TDP-43 disrupts the nuclear membrane structure, nuclear pore complex functionality and RNA export (Chou et al., 2018). This effect was significantly higher with CTFs compared to WT or even ALS-linked mutant TDP-43. In addition, CTFs co-aggregate with nucleoporin proteins (Nups) through their similar low complexity domains (prion-like GC-rich domain in CTFs and FG-rich domain in Nups). This information expands the functional features of CTFs and supports their role in influencing the overall nucleo-cytoplasmic transport within cells. Also, compared to full-length wild-type or mutant TDP-43 overexpression, CTFs led to high levels of cell death through apoptotic pathways (Chou et al., 2018). Lastly, TDP-43 can spread intercellularly and, therefore, extracellular levels of TDP-43 and its truncated forms may have an impact on this phenomenon. To test this hypothesis, wild-type TDP-43 as well as two ALS-linked mutants (M337V and A315T) were targeted to the extracellular space (Zhao et al., 2015). In this study, mutant TDP-43 activates microglia through upregulating NOX2, TNF-α, and IL-1β. The wild-type isoform also activates microglia in similar fashion but at lesser extent. Interestingly, when these mutations were tested in the context of TDP-25, activation of microglia was significantly higher compared to the wild type counterpart. This suggests that disease-associated mutations have the ability to enhance cytotoxicity of truncated TDP-43.
Alternative splicing also creates neurotoxic TDP-43 fragments
A very intriguing study proposed that a truncated form of TDP-43 can be also generated via alternative splicing mechanisms (Xiao et al., 2015). The authors unraveled a new splice variant of TDP-43 that is upregulated four-fold in the spinal cord of patients with ALS. The corresponding transcript, known as TDP-43 r.[106-196del], has 91-nt spliced out in exon 2 and uses the third ATGMet85 of full-length TDP-43 as initiation codon to generate Met85-TDP-35 (Figure 3). Interestingly, ATGMet85 is located immediately upstream of the caspase-3 recognition site involved in the generation of cleaved TDP-35, which complicates the precise identification of TDP-35 species. To address this problem, neo-epitope antibodies against the N-terminus sequences of the Met85-or caspase 3-cleaved TDP-35 were generated (Xiao et al., 2015). The use of these two antibodies revealed that the TDP-35 species found in motor neurons of ALS tissues are only recognized with the Met85-TDP-35 antibody. The authors also demonstrated that expression of Met85-TDP-35 in primary motor neurons leads to cytosolic aggregates and aggressive neuronal death, highlighting the pathological relevance of this splice variant.
More recently, iPSC-derived neuronal cells were used to demonstrate that hyperexcitability triggers TDP-43 pathology through upregulation of two atypical, shortened TDP-43 (sTDP-43) isoforms (Weskamp et al., 2020). These truncated isoforms are the result of an intriguing alternative splicing event, where the majority of the exon 6 is eliminated through a downstream splice acceptor at the TARDBP 3′ UTR. Both sTDP-43 transcripts (sTDP43-1 and −2) produce proteins that differ only by three amino acid residues and lack the entire glycine-rich domain. The involvement of the TARDBP 3′UTR in the alternative splicing leads to the inclusion of a new C-terminal sequence encoding for 18-amino acids that are absent in full-length TDP-43 (Figure 3). This distinct C-terminal tail contains a putative NES (TSLKV) that contributes to the accumulation of insoluble sTDP43 aggregates in the cytosol. Over-expression of sTDP43 in rodent primary cortical neurons is neurotoxic and accompanied by nuclear depletion and cytosolic sequestration of endogenous TDP-43. Of note, sTDP43-1 transcripts are substantially enriched in human spinal neurons and an antibody against the 18-amino acid C-terminal end of sTDP43 detected a remarkable accumulation of cytosolic sTDP43 deposits in ALS spinal cord and cortex. Interestingly, the authors found that some ALS patient cells display cytosolic sTDP43 deposits despite normal distribution of nuclear TDP-43, suggesting that sTDP43 deposition may play an early pathological role (Weskamp et al., 2020). Unfortunately, the mechanisms that control the sTDP43-linked transcriptional splicing are currently unknown.
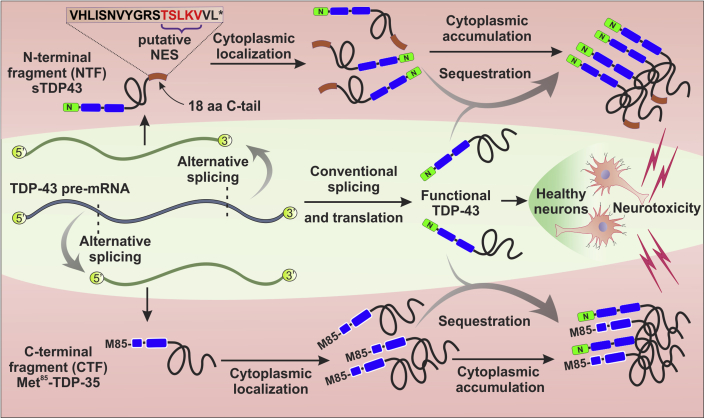
Figure 3.
Mechanisms of TDP-43 truncation through alternative splicing
Center: The TDP-43 primary transcript generates a conventional mRNA splicing variant that encodes full-length TDP-43 in healthy neurons. However, under unknown conditions, at least two different kind of TDP-43 alternative transcripts can translate into N- or C-terminally truncated forms.
Top: An alternative splicing event that removes most of the TARDBP exon 6 and integrates part of the 3′UTR leads to the generation of shortened TDP-43 (sTDP-43) isoforms. These splice variants lack the conventional C-terminus and acquire a distinct 18 amino acids C-terminal tail (brown rectangle). This new sequence contains a functional putative nuclear export sequence (NES) that promotes its cytosolic accumulation as well as sequestration of normal TDP-43, resulting in neurotoxicity.
Bottom: A different splicing event gives rise to a C-terminal fragment through the use of an alternative translation initiation codon at Methionine-85 (M85) of the full-length TDP-43. The resulting isoform, referred to as Met85-TDP-35, accumulates in cytoplasm where it can sequester endogenous TDP-43 and lead to neurotoxicity.
Truncated TDP-43 – A common player in various proteinopathies
Several studies indicate that TDP-43 abnormalities are not limited to ALS and are common in Alzheimer's disease (AD) and other neurodegenerative conditions (Prasad et al., 2019). Soon after reporting the pathological role of TDP-43 in ALS and FTLD, Neumann's group found TDP-43 proteinopathy in nearly one-fourth of corticobasal degeneration (CBD) cases and demonstrated the presence of TDP-25 in CBD brains (Uryu et al., 2008). Another earlier report documented TDP-43 pathology (aggregation and phosphorylation) in almost 50% of AD cases (Wilson et al., 2011). A more recent study evaluating 14 different cerebral regions from 193 AD brains reported that almost all the specimens were positive for TDP-43 inclusions in later stages of AD (Josephs et al., 2016). Unfortunately, the use of an antibody that recognizes C-terminal region of TDP-43 did not distinguish the truncated forms from the full length TDP-43 in this work. However, a very recent report classifies AD pathology based on the presence of full length or CTFs of TDP-43 (Tomé et al., 2020). Here, AD pathology is categorized as AD without TDP-43 (ADTDP−), AD with TDP-43 full length (ADFL) and AD with CTFs of TDP-43 (ADCTF). Interestingly, most AD cases (60%) in this work showed truncated TDP-43 inclusions. In this regard, Herman et al. made an interesting finding that could, in part, explain the increased TDP-43 deposition associated with disease progression (Herman et al., 2012). The authors demonstrated that lentiviral-mediated expression of Aβ42 in rats induces phosphorylation of TDP-43 and promotes its cleavage to 35kDa forms. The authors also found that TDP-43 expression triggers abnormalities in APP processing, resulting in higher production of amyloidogenic fragments. These findings uncovered a molecular cross talk between TDP-43 and APP metabolism and highlighted the importance of studying TDP-43 truncation in AD pathogenesis. In addition to AD, TDP-43 CTFs are also present in other neurodegenerative conditions (Table 2). For instance, TDP-25 and TDP-35 were found associated with Lewy bodies and Hirano bodies in postmortem cases of Parkinson's disease (PD) and dementia with Lewy bodies (DLB) (Kokoulina and Rohn, 2010). Interestingly, truncated fragments of TDP-43 co-localize with alpha-synuclein within the substantia nigra, suggesting that TDP-43 cleavage may play a key role in both of these alpha-synucleinopathies. On the other hand, it has been shown that polyglutamine expansions in ataxin 2 activate the Caspase 3 pathway, leading to TDP-43 truncation and subsequent toxicity (Hart and Gitler, 2012). Caspase inhibitors rescue this pathological condition, which supports the toxic contributions of TDP-43 fragmentation. Secondary TDP-43 pathology has been also documented in other polyglutamine disorders including Huntington Disease (Tada et al., 2012) and Spinocerebellar ataxia (SCA) forms such as SCA2, SCA3, and SCA7 (Alves et al., 2016; Tan et al., 2009; Toyoshima et al., 2011). However, the presence of TDP-43 truncation in these diseases remains elusive. Taken together, these studies highlight the presence of TDP-43 fragments in multiple neurological conditions (Figure 4), but their direct contributions to disease pathogenesis remains to be clarified.
Table 2.
Detection of TDP-43 fragments in human neurological conditions
| Disease | TDP-43 truncated forms | Reference |
|---|---|---|
| Amyotrophic lateral sclerosis | 23-37 kDa fragments | (Neumann et al., 2006) |
| Frontotemporal lobar degeneration | 23-27 kDa fragments | (Igaz et al., 2008) |
| Alzheimer's disease | TDP-25 | (Rohn, 2008) |
| Corticobasal degeneration | TDP-25 | (Uryu et al., 2008) |
| Pick disease | TDP-25 and TDP-35 | (Rohn and Kokoulina, 2009) |
| Parkinson's disease | TDP-25 (in Lewy bodies) | (Kokoulina and Rohn, 2010) |
| Traumatic brain injury | 35 kDa fragments | (Yang et al., 2014b) |
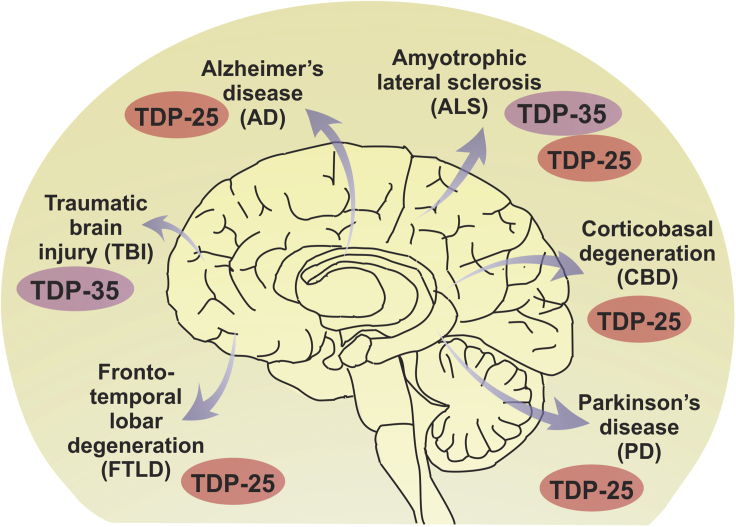
Figure 4.
TDP-43 fragments accumulate in various neurodegenerative disorders
Cytoplasmic accumulation of TDP-35 and TDP-25 CTFs can be found in primary (ALS and FTLD) and secondary (AD, CBD, PD, TBI) TDP-43 proteinopathies. Although TDP-43 pathology is also present in Huntington Disease and several Spinocerebellar ataxia (SCA) forms such as SCA2, SCA3 and SCA7, the presence of TDP-43 fragments in these polyglutamine disorders remain elusive.
Do TDP-43 fragments interact with other truncated proteins involved in neurodegeneration?
This is an open question in the field. Fragmentation of proteins into smaller truncated forms has been observed in other proteinopathies associated with neurodegenerative diseases. However, whether TDP-43 CTFs can interact with those truncated peptides to alter neuropathology has not been investigated yet. As mentioned above, TDP-43 CTFs co-localize with alpha-synuclein in Lewy bodies and Hirano bodies within the substantia nigra in Parkinson's disease and dementia with Lewy bodies (Kokoulina and Rohn, 2010), suggesting a potential direct interaction between the two proteins. Alpha-synuclein exhibits extensive post-translational modifications within pathological inclusions and approximately 15–20% is C-terminally truncated (Sorrentino and Giasson, 2020). C-terminal truncations of alpha-synuclein enhance amyloid aggregation in vitro and in vivo (Sorrentino and Giasson, 2020). However, the study of their interactions with TDP-43 fragments is prevented by lack of reliable truncation-specific tools. Another limitation is that removal of exon 5 in the alpha-synuclein mRNA through alternative splicing generates splice variants lacking C-terminal residues, which may behave similarly to C-terminally truncated isoforms (Gámez-Valero and Beyer, 2018).
Cumulative evidence suggest that Tau proteolysis can also influence neurodegeneration in a fragment-dependent manner and more than 50 proteolytic tau fragments have been reported (Quinn et al., 2018). Among them, Tau45-230 was recently found at high levels in motor neurons in patients with ALS (Vintilescu et al., 2016). This 17kDa fragment triggers neuronal death and behavioral deficits when expressed in the hippocampus of transgenic mice (Lang et al., 2014), suggesting that its presence in ALS may alter the course of neuropathology. However, the potential interaction of this fragment with full-length and/or truncated TDP-43 remains to be determined. On the other hand, the potential formation of hybrid TDP-43/amyloid beta and TDP-43/alpha-synuclein oligomers has been documented in AD brains (Guerrero-Muñoz et al., 2014), but the precise nature of these structures is unclear, as well as the involvement of the respective truncated products. The presence of multiple neurotoxic protein fragments in a variety of conditions provides an opportunity to investigate their potential pathological associations and molecular interactions. Thus, implementation of new diagnostic reagents and tools is critical to reliably address this fundamental question in the field.
Insights from animal models carrying TDP-43 CTFs
Numerous animal models, ranging from yeast to primates, have been generated to study TDP-43 proteinopathies in vivo. Overexpression of both mouse and human WT TDP-43 in mice induces neurodegeneration (Tsai et al., 2010; Wils et al., 2010), whereas loss of function of TDP-43 leads to embryonic lethality (Lutz, 2018; Philips and Rothstein, 2015). Partial loss of function of TDP-43 also appears to be neurodegenerative, but mice still die within a few months (Yang et al., 2014a). It is worth mentioning that detection of TDP-43 fragments correlates with very aggressive phenotypes in mice over-expressing both WT and mutant TDP-43 (Stallings et al., 2010; Wegorzewska et al., 2009; Wils et al., 2010; Xu et al., 2010). However, these data should be interpreted with caution because manipulation of TDP-43 in mice appears to be challenging. In fact, it has been reiterated that TDP-43 toxicity in mice is dose-dependent, and that a few folds increase of WT or mutant TDP-43 leads to a range of toxicity from mild neuronal defects to severe lifespan reduction (Barmada et al., 2014; Da Cruz and Cleveland, 2011; Janssens et al., 2013; Wegorzewska and Baloh, 2011). Most robust mouse models either produce TDP-43 CTFs after cleavage or express CTFs transgenes on their own (Table 3). A knock-in mouse model expressing human mutant TDP-43 was recently created, which exhibits the full spectrum of ALS-like pathology (Huang et al., 2020). In these mice, the presence of TDP-43 truncation is associated with disease onset which is followed by progressive neurodegeneration, motor defects, and death. This physiologically relevant model shows high levels of TDP-43 in the spinal cord but not in brain. Accordingly, the levels of TDP-35 are also elevated in the spinal cord, but in an age-dependent manner, exerting great analogy to ALS. Previous knock-in models failed to exhibit such robust phenotypes probably due to dose-dependency of the mutant transgenes (Ebstein et al., 2019; White et al., 2018).
Table 3.
Animal models expressing truncated TDP-43
| CTF transgene | Model organism | Phenotypes and key characteristics | Reference |
|---|---|---|---|
| TDP-25 | Rat (Rattus) | Forelimb impairment | (Dayton et al., 2013) |
| TDP-25 | Mouse (Mus musculus) | Reduced motor function, impaired autophagy and proteasomal function | (Caccamo et al., 2015) |
| hTDP-25 | Fruit fly (Drosophila melanogaster) | Reduced longevity and locomotor function when expressed in motor neurons | (Gregory et al., 2012) |
| TDP-43CTF (~25 kDa) | “ | Reduced longevity and induced Caspase-3 activation when expressed in motor neurons | (Voigt et al., 2010) |
| Flag-TDP-35 | “ | Pupal lethality when expressed in the eye | (Crippa et al., 2016) |
| Flag-TDP-25 | “ | Mild disorganization of retinal structures when expressed in the eye | (Crippa et al., 2016) |
| TDP-25-YFP | Worm (Caenorhabditis elegans) | Protein aggregation and locomotor defects | (Zhang et al., 2011) |
TDP-43 regulates its own expression (Budini and Buratti, 2011). This autoregulation can be impaired under certain pathological conditions, inducing abnormal levels of endogenous TDP-43 or even higher levels of transgenic TDP-43 protein in model organisms. In the case of mutant transgenic TDP-43, its stability may increase (Ling et al., 2010), resulting in higher TDP-43 protein levels. To avoid uncontrolled overexpression of the transgene, a knock in TDP-43Q331K mouse model was created using CRISPR-Cas9 technology (White et al., 2018). This pathophysiologically improved mouse model showed cognitive impairment and interneuronal dysfunction, yet no motor defects in spite of vast arrays of abnormal splicing. Another study reported that knock-in TDP-43M337V mice display mild neuronal defects but high dysregulation of TDP-43-linked splicing mechanisms (Watanabe et al., 2020). Here, the authors suggested that the short life span of mice (~2–2.5 years) may be insufficient to observe a significant gain-of-function phenotype via splicing dysregulations in the mutant mice, consistent with earlier reports (Ebstein et al., 2019; Fratta et al., 2018). In contrast, as shown in Table 3, TDP-25 mice and rat models show reduced motor function (Caccamo et al., 2015; Dayton et al., 2013). Truncation of RRM domains in this case is expected to result in dysfunctional splicing (Figure 2). However, the mechanism of splicing dysfunction and its role in neurodegeneration remain to be elucidated.
In addition to ALS models, TDP-43 CTFs were also expressed in a conditional mouse model of FTLD (Walker et al., 2015). In this work, cytosolic accumulation of truncated TDP-43 resulted in phosphorylation of endogenous full-length TDP-43 and hippocampal neurodegeneration. These phenotypes were alleviated when CTFs expression was silenced in old mice, supporting the pathogenic role of TDP-43 CTFs. On the other side, amyloid beta (Aβ) expression has been reported to trigger TDP-43 pathology with significant TDP-43 truncation in AD mouse models (Herman et al., 2011), but TDP-43 depletion has also been linked to neurodegeneration in AD mice (LaClair et al., 2016). These observations highlight an important cross talk between Aβ and TDP-43-mediated pathologies. Truncated TDP-43 was also observed in rats that survived from acute ischemic strokes (Kanazawa et al., 2011; Thammisetty et al., 2018) and mislocalization, phosphorylation and cleavage of TDP-43 into TDP-25 and TDP-35 were evident in mouse and rat models of traumatic brain injury (Huang et al., 2017; Tan et al., 2018; Wang et al., 2015a). These studies suggest that TDP-43 insults could be determined, at least in part, through mechanisms involving production of CTFs, even beyond the ALS-FTD spectrum.
Invertebrate organisms have also contributed to understand TDP-43 function and pathology. For instance, Drosophila null models of TBPH, the fly ortholog of human TDP-43, display motor dysfunction along with defective neuromuscular junctions in larvae (Diaper et al., 2013), which supports the fundamental role of TDP-43 in vivo. In contrast, expression of the human TDP-43M337V mutant in flies leads to dose-dependent toxicity (Ritson et al., 2010), the severity of which correlates with higher accumulation of TDP-35 and TDP-25. Transgenic worm models of C. elegans expressing several ALS-linked mutants of TDP-43 (G290A, A315T and M337V) exhibit phosphorylation, ubiquitination and truncation of the protein along with motor dysfunction (Liachko et al., 2010). To better understand how different CTFs behave in vivo, Drosophila models that independently express TDP-25 and TDP-35 at similar expression levels were created (Crippa et al., 2016). In this case, TDP-25 showed mild toxicity, while TDP-35 induced lethality compared to flies expressing full-length TDP-43. We anticipate that conditional expression of TDP-35 in adult flies will help to define its precise pathological role.
TDP-43 proteinopathies have diverse symptoms ranging from motor defects in ALS to cognitive impairments in FTLD and AD. The animal models discussed above provide insights into how TDP-43 fragments may correlate with neuropathological and disease-linked behavioral changes. However, understanding the relevance of TDP-43 fragments in phenotypic manifestation has been challenging. As shown in Table 3, several models of truncated TDP-43 show motor defects along with neurodegeneration. This is controversial because, unlike many studies, some reports do not show TDP-43 fragments in animal models (Wegorzewska and Baloh, 2011). Varied range of phenotypes in different models may be attributed to the use of different expression systems, distinct mutations and even different methods of detection. Species-specific mechanisms may also influence the outcome as significantly higher CTFs were detected in primates compared to mice using the same mutant TDP-43M337V transgene (Yin et al., 2019). Also, the splicing function of TDP-43 may affect phenotypes differently in animal models. For instance, a mediator of motor neuron growth, STMN2, is not regulated by TDP-43 in mice (Klim et al., 2019; Melamed et al., 2019). A similar argument can be made for TDP-43-mediated repression of non-conserved cryptic exons (Ling et al., 2015). On the other side, elimination of the aggregation prone C-terminal domain in TDP-ΔC mice results in mild age-related motor dysfunction (Nishino et al., 2019), suggesting that CTF aggregation may not be required for age-dependent neurodegeneration in mice. Hence, the extent to which truncated forms of TDP-43 contribute to disease pathogenesis is still unclear.
Approaches to prevent TDP-43 truncation and potential toxicity
Given the potential toxic effects of TDP-43 CTFs, various approaches were undertaken to block the generation of TDP-43 fragments as well as to promote the degradation of these toxic species (Table 4). For instance, Lin et al. found that over-expression of heat shock factor 1 (HSF1) reduces TDP-35 fragments after proteasomal inhibition. The authors also showed that HSF1 overexpression reduces the levels of cleaved (activated) Caspase 3, highlighting the potential role of HSF1 in decreasing caspase-dependent TDP-43 truncation (Lin et al., 2016). A separate report also indicated that the Caspase 4 inhibitor LEVD-fmk can reduce TDP-43 truncation (Yin et al., 2019). Since many caspases and other enzymes can also cleave TDP-43, these studies suggest that multiple approaches aimed at blocking enzymes responsible for cleaving TDP-43 may contribute to prevent TDP-43 fragmentation. Additionally, TDP-43 and its CTFs are degraded through proteasomal and autophagy pathways (Wang et al., 2010), both of which are impaired in ALS and FTD (Ling et al., 2013). Neurons are sensitive to TDP-43 levels and autophagy induction can mitigate neurodegeneration through preventing cell death and clearing excess TDP-43 (Barmada et al., 2014). Recently, Cicardi et al. provided evidence that induction of the proteasome or autophagy can promote degradation of TDP-43, TDP-35 and TDP-25 in motor neurons and in myoblasts (Cicardi et al., 2018). Since the ratio of BAG1/BAG3 determines the route of degradation for ubiquitinated proteins (Gamerdinger et al., 2009), the authors used BAG1 overexpression to degrade TDP-43 and its truncated forms through proteasome, while achieved their autophagic degradation through BAG3 overexpression. Interestingly, an interacting partner of BAG3, HSPB8 also reduces truncated TDP-43 (TDP-25 and TDP-35) mediated toxicity in flies and mammalian neuronal cells (Crippa et al., 2016). Further, an efficient degradation of truncated TDP-43 forms was achieved via autophagy by Rapamycin administration, a well-known autophagy inducer. Rapamycin treatment significantly contributes to clearing of both TDP-25 and TDP-35 (Wang et al., 2012b), while Raloxifene, a drug with estrogenic properties, reduces TDP-25-mediated toxicity through enhancing autophagy and reducing apoptosis in cultured cells (Zhou et al., 2018). Valproate is another drug reported to ameliorate TDP-25 toxicity through regulation of ER stress and autophagy (Wang et al., 2015b). In addition to the clearance mechanisms via autophagy induction, the herbal product Berberine can reverse accumulation of insoluble TDP-25 aggregates in neuronal cell cultures by deregulating mTOR/p70S6K pathway (Chang et al., 2016). Several studies have relied on the mTOR pathway as an effective mean of inducing autophagy, but it is considered to be less efficient in neurons (Tsvetkov et al., 2010). Hence, it should be of interest to test novel small molecules to achieve autophagy induction beyond the mTOR pathway while avoiding neurotoxicity. Despite the overall progress in this area (Table 4), we are still far from any robust treatment for TDP-43 proteinopathies. More comprehensive and integrative studies are required to understand the pathobiology of TDP-43 fragmentation and to identify effective therapeutic targets. Integrating the mechanisms that generate TDP-43 fragments through alternative splicing may also contribute to that end.
Table 4.
Therapeutic approaches to ameliorate toxicity of TDP-43 CTFs
| Approach | Category | Mechanism of action | Reference |
|---|---|---|---|
| Raloxifene | Small molecule | Limits TDP-25 toxicity through autophagy | (Zhou et al., 2018) |
| Valproate | Small molecule | Attenuates TDP-25 toxicity mediated by ER stress and autophagy | (Wang et al., 2015b) |
| HSF1 overexpression | Transcription factor | Reduces TDP-35 levels, even after proteasomal inhibition | (Lin et al., 2016) |
| Berberine | Plant product | Reverses TDP-25 accumulation | (Chang et al., 2016) |
| Rapamycin | Small molecule | Clears TDP-25 and TDP-35 through autophagy | (Wang et al., 2012b) |
| LEVD-fmk | Small molecule | Inhibits Caspase 4 and reduces TDP-43 truncation | (Yin et al., 2019) |
| HSPB8 overexpression | Chaperone | Reduces TDP-25 levels in motor neurons as well as in myocytes through autophagy | (Cicardi et al., 2018) (Crippa et al., 2016) |
| KPT-335 | Small molecule | Suppresses CTF-linked toxicity through inhibition of the Xpo1/Crm1 nuclear export pathway | (Chou et al., 2018) |
Concluding remarks
The presence of TDP-43 fragments in a broad spectrum of neurodegenerative diseases has been extensively documented. Still, key questions regarding their potential neurotoxic role remain unanswered. Cumulative evidence suggests that TDP-43 CTFs may mediate toxicity through a toxic “gain-of-function” due to their deleterious effects in the cytosol, and also to a “loss-of-function” in the nucleus due to sequestration of WT TDP-43 into cytoplasmic inclusions. TDP-43 is an essential RNA-binding protein that regulates the processing of thousands of target RNAs and, therefore, its loss-of-function effect is not surprising. Considering the pivotal role of TDP-43 CTFs in generating toxic protein aggregates, it is tempting to speculate that these species may serve as key players in the development and/or progression of TDP-43 proteinopathies. These toxic aggregates could facilitate the entrapment of functional WT TDP-43, resulting in a severe “domino effect” with multiple adverse impacts on cellular homeostasis. Thus, it is of paramount importance to continue studying the role of TDP-43 CTFs in the pathogenesis of many age-related neurodegenerative disorders including ALS, FTLD and AD. In this regard, the development of antibodies that could recognize specific CTFs in human tissues as well as tools for reliable detection of TDP-43 amyloids will accelerate progress in the field.
On the other side, it is critical to clarify the controversial role of TDP-43 fragments in disease pathogenesis. Earlier studies demonstrated that TDP-43 CTFs seem to be pathogenic triggers as they appear before the development of symptoms in transgenic mice (Wegorzewska et al., 2009). However, other reports show little or no TDP-43 fragments, questioning their pathological significance. These discrepancies could be due to differences in experimental models and procedures, detection methods, species-specific mechanisms and perhaps differences in cell vulnerability. In addition, the recent discovery of neurotoxic TDP-43 NTFs created by alternative splicing (sTDP43) adds another layer of complexity. These alternative splicing variants accumulate in the cytosol due to a unique NES domain and seem to aggregate before nuclear depletion and cytosolic sequestration of full-length TDP-43, suggesting an early involvement in disease pathogenesis. However, these isoforms have not been studied yet in animal models and the extent and prevalence of sTDP43 pathology in ALS and related disorders are largely unknown. A more integrative framework will be needed to fully address these major questions in the field. Thus, it is still unclear if TDP-43 fragments are triggers, byproducts, or late-stage contributors of pathogenesis.
Acknowledgments
We thank all Rincon-Limas lab members for valuable discussions. This work was supported by National Institutes of Health grant R01AG059871 to D.E.R.-L.
Authors contributions
D.C. and D.E.R.-L. conceived the original idea and designed the outlines of the study. D.C. performed literature review, wrote the first draft, and prepared the figures. D.C., A.M.P., and D.E.R.-L. revised the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2021.102459.
Supplemental information
References
- Afroz T., Hock E.-M., Ernst P., Foglieni C., Jambeau M., Gilhespy L.A.B., Laferriere F., Maniecka Z., Plückthun A., Mittl P. Functional and dynamic polymerization of the ALS-linked protein TDP-43 antagonizes its pathologic aggregation. Nat. Commun. 2017;8:45. doi: 10.1038/s41467-017-00062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves S., Marais T., Biferi M.-G., Furling D., Marinello M., Hachimi K.E., Cartier N., Ruberg M., Stevanin G., Brice A. Lentiviral vector-mediated overexpression of mutant ataxin-7 recapitulates SCA7 pathology and promotes accumulation of the FUS/TLS and MBNL1 RNA-binding proteins. Mol. Neurodegener. 2016;11:58. doi: 10.1186/s13024-016-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Archbold H.C., Jackson K.L., Arora A., Weskamp K., Tank E.M.-H., Li X., Miguez R., Dayton R.D., Tamir S., Klein R.L. TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Sci. Rep. 2018;8:4606. doi: 10.1038/s41598-018-22858-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmada S.J., Serio A., Arjun A., Bilican B., Daub A., Ando D.M., Tsvetkov A., Pleiss M., Li X., Peisach D. Autophagy induction enhances TDP43 turnover and survival in neuronal ALS models. Nat. Chem. Biol. 2014;10:677–685. doi: 10.1038/nchembio.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berning B.A., Walker A.K. The pathobiology of TDP-43 C-terminal fragments in ALS and FTLD. Front. Neurosci. 2019;13:335. doi: 10.3389/fnins.2019.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budini M., Buratti E. TDP-43 autoregulation: implications for disease. J. Mol. Neurosci. 2011;45:473–479. doi: 10.1007/s12031-011-9573-8. [DOI] [PubMed] [Google Scholar]
- Buratti E. Functional significance of TDP-43 mutations in disease. Adv. Genet. 2015;91:1–53. doi: 10.1016/bs.adgen.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Buratti E., Baralle F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J. Biol. Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- Buratti E., Dörk T., Zuccato E., Pagani F., Romano M., Baralle F.E. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A., Shaw D.M., Guarino F., Messina A., Walker A.W., Oddo S. Reduced protein turnover mediates functional deficits in transgenic mice expressing the 25 kDa C-terminal fragment of TDP-43. Hum. Mol. Genet. 2015;24:4625–4635. doi: 10.1093/hmg/ddv193. [DOI] [PubMed] [Google Scholar]
- Cairns N.J., Neumann M., Bigio E.H., Holm I.E., Troost D., Hatanpaa K.J., Foong C., White C.L., Schneider J.A., Kretzschmar H.A. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am. J. Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitini C., Conti S., Perni M., Guidi F., Cascella R., De Poli A., Penco A., Relini A., Cecchi C., Chiti F. TDP-43 inclusion bodies formed in bacteria are structurally amorphous, non-amyloid and inherently toxic to neuroblastoma cells. PLoS One. 2014;9:e86720. doi: 10.1371/journal.pone.0086720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-F., Lee Y.-C., Lee K.-H., Lin H.-C., Chen C.-L., Shen C.-K., Huang C.-C. Therapeutic effect of berberine on TDP-43-related pathogenesis in FTLD and ALS. J. Biomed. Sci. 2016;23:72. doi: 10.1186/s12929-016-0290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che M.-X., Jiang L.-L., Li H.-Y., Jiang Y.-J., Hu H.-Y. TDP-35 sequesters TDP-43 into cytoplasmic inclusions through binding with RNA. FEBS Lett. 2015;589:1920–1928. doi: 10.1016/j.febslet.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Chen P.-C., Wu D., Hu C.-J., Chen H.-Y., Hsieh Y.-C., Huang C.-C. Exosomal TAR DNA-binding protein-43 and neurofilaments in plasma of amyotrophic lateral sclerosis patients: a longitudinal follow-up study. J. Neurol. Sci. 2020;418:117070. doi: 10.1016/j.jns.2020.117070. [DOI] [PubMed] [Google Scholar]
- Chhangani D., Mishra A. Protein quality control system in neurodegeneration: a healing company hard to beat but failure is fatal. Mol. Neurobiol. 2013;48:141–156. doi: 10.1007/s12035-013-8411-0. [DOI] [PubMed] [Google Scholar]
- Chiang C.-H., Grauffel C., Wu L.-S., Kuo P.-H., Doudeva L.G., Lim C., Shen C.-K.J., Yuan H.S. Structural analysis of disease-related TDP-43 D169G mutation: linking enhanced stability and caspase cleavage efficiency to protein accumulation. Sci. Rep. 2016;6:21581. doi: 10.1038/srep21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.-C., Zhang Y., Umoh M.E., Vaughan S.W., Lorenzini I., Liu F., Sayegh M., Donlin-Asp P.G., Chen Y.H., Duong D.M. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat. Neurosci. 2018;21:228–239. doi: 10.1038/s41593-017-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicardi M.E., Cristofani R., Rusmini P., Meroni M., Ferrari V., Vezzoli G., Tedesco B., Piccolella M., Messi E., Galbiati M. Tdp-25 routing to autophagy and proteasome ameliorates its aggregation in amyotrophic lateral sclerosis target cells. Sci. Rep. 2018;8:12390. doi: 10.1038/s41598-018-29658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa V., Cicardi M.E., Ramesh N., Seguin S.J., Ganassi M., Bigi I., Diacci C., Zelotti E., Baratashvili M., Gregory J.M. The chaperone HSPB8 reduces the accumulation of truncated TDP-43 species in cells and protects against TDP-43-mediated toxicity. Hum. Mol. Genet. 2016;25:3908–3924. doi: 10.1093/hmg/ddw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cruz S., Cleveland D.W. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr. Opin. Neurobiol. 2011;21:904–919. doi: 10.1016/j.conb.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammer E.B., Fallini C., Gozal Y.M., Duong D.M., Rossoll W., Xu P., Lah J.J., Levey A.I., Peng J., Bassell G.J., Seyfried N.T. Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination. PLoS One. 2012;7:e38658. doi: 10.1371/journal.pone.0038658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson Y., Kelley T., Mackenzie I.R.A., Pickering-Brown S., Du Plessis D., Neary D., Snowden J.S., Mann D.M.A. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- Dayton R.D., Gitcho M.A., Orchard E.A., Wilson J.D., Wang D.B., Cain C.D., Johnson J.A., Zhang Y.-J., Petrucelli L., Mathis J.M. Selective forelimb impairment in rats expressing a pathological TDP-43 25 kDa C-terminal fragment to mimic amyotrophic lateral sclerosis. Mol. Ther. 2013;21:1324–1334. doi: 10.1038/mt.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco G., Lomartire A., Mandili G., Lupino E., Buccinnà B., Ramondetti C., Moglia C., Novelli F., Piccinini M., Mostert M. Reduced cellular Ca(2+) availability enhances TDP-43 cleavage by apoptotic caspases. Biochim. Biophys. Acta. 2014;1843:725–734. doi: 10.1016/j.bbamcr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Diaper D.C., Adachi Y., Sutcliffe B., Humphrey D.M., Elliott C.J.H., Stepto A., Ludlow Z.N., Vanden Broeck L., Callaerts P., Dermaut B. Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Hum. Mol. Genet. 2013;22:1539–1557. doi: 10.1093/hmg/ddt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein S.Y., Yagudayeva I., Shneider N.A. Mutant TDP-43 causes early-stage dose-dependent motor neuron degeneration in a TARDBP knockin mouse model of ALS. Cell Rep. 2019;26:364–373.e4. doi: 10.1016/j.celrep.2018.12.045. [DOI] [PubMed] [Google Scholar]
- Feneberg E., Charles P.D., Finelli M.J., Scott C., Kessler B.M., Fischer R., Ansorge O., Gray E., Talbot K., Turner M.R. Detection and quantification of novel C-terminal TDP-43 fragments in ALS-TDP. Brain Pathol. 2020 doi: 10.1111/bpa.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R., Kapogiannis D., Huey E.D., Momeni P. FTD and ALS: a tale of two diseases. Curr. Alzheimer Res. 2011;8:273–294. doi: 10.2174/156720511795563700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratta P., Sivakumar P., Humphrey J., Lo K., Ricketts T., Oliveira H., Brito-Armas J.M., Kalmar B., Ule A., Yu Y. Mice with endogenous TDP-43 mutations exhibit gain of splicing function and characteristics of amyotrophic lateral sclerosis. EMBO J. 2018;37 doi: 10.15252/embj.201798684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y., Kaneko K., Nukina N. Molecular properties of TAR DNA binding protein-43 fragments are dependent upon its cleavage site. Biochim. Biophys. Acta. 2011;1812:1577–1583. doi: 10.1016/j.bbadis.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Gamerdinger M., Hajieva P., Kaya A.M., Wolfrum U., Hartl F.U., Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28:889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gámez-Valero A., Beyer K. Alternative splicing of alpha- and beta-Synuclein genes plays differential roles in Synucleinopathies. Genes (Basel) 2018;9:63. doi: 10.3390/genes9020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C.W., Lee I.C., Sundaram J.R., George S.E., Yusoff P., Brush M.H., Sze N.S.K., Shenolikar S. Chronic oxidative stress promotes GADD34-mediated phosphorylation of the TAR DNA-binding protein TDP-43, a modification linked to neurodegeneration. J. Biol. Chem. 2017;293:163–176. doi: 10.1074/jbc.M117.814111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J.M., Barros T.P., Meehan S., Dobson C.M., Luheshi L.M. The aggregation and neurotoxicity of TDP-43 and its ALS-associated 25 kDa fragment are differentially affected by molecular chaperones in Drosophila. PLoS One. 2012;7:e31899. doi: 10.1371/journal.pone.0031899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Muñoz M.J., Castillo-Carranza D.L., Krishnamurthy S., Paulucci-Holthauzen A.A., Sengupta U., Lasagna-Reeves C.A., Ahmad Y., Jackson G.R., Kayed R. Amyloid-β oligomers as a template for secondary amyloidosis in Alzheimer’s disease. Neurobiol. Dis. 2014;71:14–23. doi: 10.1016/j.nbd.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Hart M.P., Gitler A.D. ALS-associated ataxin 2 polyQ expansions enhance stress-induced caspase 3 activation and increase TDP-43 pathological modifications. J. Neurosci. 2012;32:9133–9142. doi: 10.1523/JNEUROSCI.0996-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergesheimer R.C., Chami A.A., de Assis D.R., Vourc’h P., Andres C.R., Corcia P., Lanznaster D., Blasco H. The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: a resolution in sight? Brain. 2019;142:1176–1194. doi: 10.1093/brain/awz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.M., Khandelwal P.J., Rebeck G.W., Moussa C.E.-H. Wild type TDP-43 induces neuro-inflammation and alters APP metabolism in lentiviral gene transfer models. Exp. Neurol. 2012;235:297–305. doi: 10.1016/j.expneurol.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.M., Khandelwal P.J., Stanczyk B.B., Rebeck G.W., Moussa C.E.-H. β-Amyloid triggers ALS-associated TDP-43 pathology in AD models. Brain Res. 2011;1386:191–199. doi: 10.1016/j.brainres.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz J.H., Gozal Y.M., Duong D.M., Dammer E.B., Gearing M., Ye K., Lah J.J., Peng J., Levey A.I., Seyfried N.T. Asparaginyl endopeptidase cleaves TDP-43 in brain. Proteomics. 2012;12:2455–2463. doi: 10.1002/pmic.201200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-Y., Lee Y.-C., Li P.-C., Liliang P.-C., Lu K., Wang K.-W., Chang L.-C., Shiu L.-Y., Chen M.-F., Sun Y.-T. TDP-43 proteolysis is associated with astrocyte reactivity after traumatic brain injury in rodents. J. Neuroimmunol. 2017;313:61–68. doi: 10.1016/j.jneuroim.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Huang S.-L., Wu L.-S., Lee M., Chang C.-W., Cheng W.-C., Fang Y.-S., Chen Y.-R., Cheng P.-L., Shen C.-K.J. A robust TDP-43 knock-in mouse model of ALS. Acta Neuropathol. Commun. 2020;8:3. doi: 10.1186/s40478-020-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz L.M., Kwong L.K., Xu Y., Truax A.C., Uryu K., Neumann M., Clark C.M., Elman L.B., Miller B.L., Grossman M. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am. J. Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi P., Kim G., Shahidehpour R.K., Bolbolan K., Gefen T., Bigio E.H., Mesulam M.-M., Geula C. Distribution of TDP-43 pathology in hippocampal synaptic relays suggests transsynaptic propagation in frontotemporal lobar degeneration. J. Neuropathol. Exp. Neurol. 2020;79:585–591. doi: 10.1093/jnen/nlaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens J., Wils H., Kleinberger G., Joris G., Cuijt I., Ceuterick-de Groote C., Van Broeckhoven C., Kumar-Singh S. Overexpression of ALS-associated p.M337V human TDP-43 in mice worsens disease features compared to wild-type human TDP-43 mice. Mol. Neurobiol. 2013;48:22–35. doi: 10.1007/s12035-013-8427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.-L., Xue W., Hong J.-Y., Zhang J.-T., Li M.-J., Yu S.-N., He J.-H., Hu H.-Y. The N-terminal dimerization is required for TDP-43 splicing activity. Sci. Rep. 2017;7:6196. doi: 10.1038/s41598-017-06263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Murray M.E., Whitwell J.L., Tosakulwong N., Weigand S.D., Petrucelli L., Liesinger A.M., Petersen R.C., Parisi J.E., Dickson D.W. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 2016;131:571–585. doi: 10.1007/s00401-016-1537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani F., Obi T., Shishido T., Akatsu H., Murayama S., Saito Y., Yoshida M., Hasegawa M. Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains. Sci. Rep. 2016;6:23281. doi: 10.1038/srep23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa M., Kakita A., Igarashi H., Takahashi T., Kawamura K., Takahashi H., Nakada T., Nishizawa M., Shimohata T. Biochemical and histopathological alterations in TAR DNA-binding protein-43 after acute ischemic stroke in rats. J. Neurochem. 2011;116:957–965. doi: 10.1111/j.1471-4159.2010.06860.x. [DOI] [PubMed] [Google Scholar]
- Kitamura A., Nakayama Y., Shibasaki A., Taki A., Yuno S., Takeda K., Yahara M., Tanabe N., Kinjo M. Interaction of RNA with a C-terminal fragment of the amyotrophic lateral sclerosis-associated TDP43 reduces cytotoxicity. Sci. Rep. 2016;6:19230. doi: 10.1038/srep19230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klim J.R., Williams L.A., Limone F., Guerra San Juan I., Davis-Dusenbery B.N., Mordes D.A., Burberry A., Steinbaugh M.J., Gamage K.K., Kirchner R. ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat. Neurosci. 2019;22:167–179. doi: 10.1038/s41593-018-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoulina P., Rohn T.T. Caspase-cleaved transactivation response DNA-binding protein 43 in Parkinson’s disease and dementia with Lewy bodies. Neurodegener. Dis. 2010;7:243–250. doi: 10.1159/000287952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo P.-H., Doudeva L.G., Wang Y.-T., Shen C.-K.J., Yuan H.S. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic Acids Res. 2009;37:1799–1808. doi: 10.1093/nar/gkp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaClair K.D., Donde A., Ling J.P., Jeong Y.H., Chhabra R., Martin L.J., Wong P.C. Depletion of TDP-43 decreases fibril and plaque β-amyloid and exacerbates neurodegeneration in an Alzheimer’s mouse model. Acta Neuropathol. 2016;132:859–873. doi: 10.1007/s00401-016-1637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A.E., Riherd Methner and D.N., Ferreira A. Neuronal degeneration, synaptic defects, and behavioral abnormalities in tau₄₅₋₂₃₀ transgenic mice. Neuroscience. 2014;275:322–339. doi: 10.1016/j.neuroscience.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.-Y., Yeh P.-A., Chiu H.-C., Tang C.-Y., Tu B.P. Hyperphosphorylation as a defense mechanism to reduce TDP-43 aggregation. PLoS One. 2011;6:e23075. doi: 10.1371/journal.pone.0023075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yokoshi M., Okada H., Kawahara Y. The cleavage pattern of TDP-43 determines its rate of clearance and cytotoxicity. Nat. Commun. 2015;6:6183. doi: 10.1038/ncomms7183. [DOI] [PubMed] [Google Scholar]
- Li W., Reeb A.N., Lin B., Subramanian P., Fey E.E., Knoverek C.R., French R.L., Bigio E.H., Ayala Y.M. Heat shock-induced phosphorylation of TAR DNA-binding protein 43 (TDP-43) by MAPK/ERK kinase regulates TDP-43 function. J. Biol. Chem. 2017;292:5089–5100. doi: 10.1074/jbc.M116.753913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liachko N.F., Guthrie C.R., Kraemer B.C. Phosphorylation promotes neurotoxicity in a Caenorhabditis elegans model of TDP-43 proteinopathy. J. Neurosci. 2010;30:16208–16219. doi: 10.1523/JNEUROSCI.2911-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liachko N.F., McMillan P.J., Guthrie C.R., Bird T.D., Leverenz J.B., Kraemer B.C. CDC7 inhibition blocks pathological TDP-43 phosphorylation and neurodegeneration: CDC7 and TDP-43 Pathology. Ann. Neurol. 2013;74:39–52. doi: 10.1002/ana.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.-Y., Folorunso O., Taglialatela G., Pierce A. Overexpression of heat shock factor 1 maintains TAR DNA binding protein 43 solubility via induction of inducible heat shock protein 70 in cultured cells. J. Neurosci. Res. 2016;94:671–682. doi: 10.1002/jnr.23725. [DOI] [PubMed] [Google Scholar]
- Ling J.P., Pletnikova O., Troncoso J.C., Wong P.C. Science. 2015;7:650–655. doi: 10.1126/science.aab0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S.-C., Albuquerque C.P., Han J.S., Lagier-Tourenne C., Tokunaga S., Zhou H., Cleveland D.W. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc. Natl. Acad. Sci. U S A. 2010;107:13318–13323. doi: 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S.-C., Polymenidou M., Cleveland D.W. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscic R.M., Grinberg L.T., Zidar J., Gitcho M.A., Cairns N.J. ALS and FTLD: two faces of TDP-43 proteinopathy. Eur. J. Neurol. 2008;15:772–780. doi: 10.1111/j.1468-1331.2008.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Duan W., Guo Y., Li Z., Han H., Zhang S., Yuan P., Li C. A new cellular model of pathological TDP-43: the neurotoxicity of stably expressed CTF25 of TDP-43 depends on the proteasome. Neuroscience. 2014;281:88–98. doi: 10.1016/j.neuroscience.2014.09.043. [DOI] [PubMed] [Google Scholar]
- Lutz C. Mouse models of ALS: past, present and future. Brain Res. 2018;1693:1–10. doi: 10.1016/j.brainres.2018.03.024. [DOI] [PubMed] [Google Scholar]
- McDonald K.K., Aulas A., Destroismaisons L., Pickles S., Beleac E., Camu W., Rouleau G.A., Vande Velde C. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum. Mol. Genet. 2011;20:1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- Melamed Z., López-Erauskin J., Baughn M.W., Zhang O., Drenner K., Sun Y., Freyermuth F., McMahon M.A., Beccari M.S., Artates Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat. Neurosci. 2019;22:180–190. doi: 10.1038/s41593-018-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mompeán M., Chakrabartty A., Buratti E., Laurents D.V. Electrostatic repulsion governs TDP-43 C-terminal domain aggregation. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mompeán M., Romano V., Pantoja-Uceda D., Stuani C., Baralle F.E., Buratti E., Laurents D.V. Point mutations in the N-terminal domain of transactive response DNA-binding protein 43 kDa (TDP-43) compromise its stability, dimerization, and functions. J. Biol. Chem. 2017;292:11992–12006. doi: 10.1074/jbc.M117.775965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Christopher M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nishino K., Watanabe S., Shijie J., Murata Y., Oiwa K., Komine O., Endo F., Tsuiji H., Abe M., Sakimura K. Mice deficient in the C-terminal domain of TAR DNA-binding protein 43 develop age-dependent motor dysfunction associated with impaired Notch1−Akt signaling pathway. Acta Neuropathol. Commun. 2019;7:118. doi: 10.1186/s40478-019-0776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T., Kametani F., Arai T., Akiyama H., Hasegawa M. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum. Mol. Genet. 2009;18:3353–3364. doi: 10.1093/hmg/ddp275. [DOI] [PubMed] [Google Scholar]
- Nonaka T., Masuda-Suzukake M., Arai T., Hasegawa Y., Akatsu H., Obi T., Yoshida M., Murayama S., Mann D.M.A., Akiyama H. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Ou S.H., Wu F., Harrich D., García-Martínez L.F., Gaynor R.B. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J. Virol. 1995;69:3584–3596. doi: 10.1128/JVI.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesiridis G.S., Tripathy K., Tanik S., Trojanowski J.Q., Lee V.M.-Y. A “two-hit” hypothesis for inclusion formation by carboxyl-terminal fragments of TDP-43 protein linked to RNA depletion and impaired microtubule-dependent transport. J. Biol. Chem. 2011;286:18845–18855. doi: 10.1074/jbc.M111.231118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T., Rothstein J.D. Rodent models of amyotrophic lateral sclerosis. Curr. Protoc. Pharmacol. 2015;69:21. doi: 10.1002/0471141755.ph0567s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinarbasi E.S., Cağatay T., Fung H.Y.J., Li Y.C., Chook Y.M., Thomas P.J. Active nuclear import and passive nuclear export are the primary determinants of TDP-43 localization. Sci. Rep. 2018;8:7083. doi: 10.1038/s41598-018-25008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A., Bharathi V., Sivalingam V., Girdhar A., Patel B.K. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2019;12:25. doi: 10.3389/fnmol.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J.P., Corbett N.J., Kellett K.A.B., Hooper N.M. Tau proteolysis in the pathogenesis of tauopathies: neurotoxic fragments and novel biomarkers. J. Alzheimers Dis. 2018;63:13–33. doi: 10.3233/JAD-170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti A., Gumina V., Lenzi P., Bossolasco P., Fulceri F., Volpe C., Bardelli D., Pregnolato F., Maraschi A., Fornai F. Chronic stress induces formation of stress granules and pathological TDP-43 aggregates in human ALS fibroblasts and iPSC-motoneurons. Neurobiol. Dis. 2020;145:105051. doi: 10.1016/j.nbd.2020.105051. [DOI] [PubMed] [Google Scholar]
- Riku Y. Reappraisal of the anatomical spreading and propagation hypothesis about TDP-43 aggregation in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Neuropathology. 2020:12644. doi: 10.1111/neup.12644. [DOI] [PubMed] [Google Scholar]
- Ritson G.P., Custer S.K., Freibaum B.D., Guinto J.B., Geffel D., Moore J., Tang W., Winton M.J., Neumann M., Trojanowski J.Q. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J. Neurosci. 2010;30:7729–7739. doi: 10.1523/JNEUROSCI.5894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn T.T. Caspase-cleaved TAR DNA-binding protein-43 is a major pathological finding in Alzheimer’s disease. Brain Res. 2008;1228:189–198. doi: 10.1016/j.brainres.2008.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn T.T., Kokoulina P. Caspase-cleaved TAR DNA-binding protein-43 in Pick’s disease. Int. J. Physiol. Pathophysiol. Pharmacol. 2009;1:25–32. [PMC free article] [PubMed] [Google Scholar]
- Sasaguri H., Chew J., Xu Y.-F., Gendron T.F., Garrett A., Lee C.W., Jansen-West K., Bauer P.O., Perkerson E.A., Tong J. The extreme N-terminus of TDP-43 mediates the cytoplasmic aggregation of TDP-43 and associated toxicity in vivo. Brain Res. 2016;1647:57–64. doi: 10.1016/j.brainres.2016.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton C.F., Cenik C., Kucukural A., Dammer E.B., Cenik B., Han Y., Dewey C.M., Roth F.P., Herz J., Peng J. Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J. Biol. Chem. 2011;286:1204–1215. doi: 10.1074/jbc.M110.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy J., El Mammeri N., Dutour A., Berbon M., Saad A., Lends A., Morvan E., Grélard A., Lecomte S., Kauffmann B. Structural dissection of amyloid aggregates of TDP-43 and its C-terminal fragments TDP-35 and TDP-16. FEBS J. 2019;287:2449–2467. doi: 10.1111/febs.15159. [DOI] [PubMed] [Google Scholar]
- Sorrentino Z.A., Giasson B.I. The emerging role of α-synuclein truncation in aggregation and disease. J. Biol. Chem. 2020;295:10224–10244. doi: 10.1074/jbc.REV120.011743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings N.R., Puttaparthi K., Luther C.M., Burns D.K., Elliott J.L. Progressive motor weakness in transgenic mice expressing human TDP-43. Neurobiol. Dis. 2010;40:404–414. doi: 10.1016/j.nbd.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Lee K., Matsuoka M. TDP-43-induced death is associated with altered regulation of BIM and Bcl-xL and attenuated by caspase-mediated TDP-43 cleavage. J. Biol. Chem. 2011;286:13171–13183. doi: 10.1074/jbc.M110.197483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Coon E.A., Osmand A.P., Kirby P.A., Martin W., Wieler M., Shiga A., Shirasaki H., Tada M., Makifuchi T. Coexistence of Huntington’s disease and amyotrophic lateral sclerosis: a clinicopathologic study. Acta Neuropathol. 2012;124:749–760. doi: 10.1007/s00401-012-1005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.-F., Yamada M., Toyoshima Y., Yokoseki A., Miki Y., Hoshi Y., Kaneko H., Ikeuchi T., Onodera O., Kakita A. Selective occurrence of TDP-43-immunoreactive inclusions in the lower motor neurons in Machado–Joseph disease. Acta Neuropathol. 2009;118:553–560. doi: 10.1007/s00401-009-0552-x. [DOI] [PubMed] [Google Scholar]
- Tan X.L., Sun M., Brady R.D., Liu S., Llanos R., Cheung S., Wright D.K., Casillas-Espinosa P.M., Sashindranath M., O’Brien T.J. Transactive response DNA-binding protein 43 abnormalities after traumatic brain injury. J. Neurotrauma. 2018;36 doi: 10.1089/neu.2017.5491. [DOI] [PubMed] [Google Scholar]
- Thammisetty S.S., Pedragosa J., Weng Y.C., Calon F., Planas A., Kriz J. Age-related deregulation of TDP-43 after stroke enhances NF-κB-mediated inflammation and neuronal damage. J. Neuroinflammation. 2018;15:312. doi: 10.1186/s12974-018-1350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey J.R., Curk T., Rogelj B., Briese M., Cereda M., Kayikci M., König J., Hortobágyi T., Nishimura A.L., Župunski V. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomé S.O., Vandenberghe R., Ospitalieri S., Van Schoor E., Tousseyn T., Otto M., von Arnim C.A.F., Thal D.R. Distinct molecular patterns of TDP-43 pathology in Alzheimer’s disease: relationship with clinical phenotypes. Acta Neuropathol. Commun. 2020;8:61. doi: 10.1186/s40478-020-00934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima Y., Tanaka H., Shimohata M., Kimura K., Morita T., Kakita A., Takahashi H. Spinocerebellar ataxia type 2 (SCA2) is associated with TDP-43 pathology. Acta Neuropathol. 2011;122:375–378. doi: 10.1007/s00401-011-0862-7. [DOI] [PubMed] [Google Scholar]
- Tsai K.-J., Yang C.-H., Fang Y.-H., Cho K.-H., Chien W.-L., Wang W.-T., Wu T.-W., Lin C.-P., Fu W.-M., Shen C.-K.J. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J. Exp. Med. 2010;207:1661–1673. doi: 10.1084/jem.20092164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi P.S., Choi K.-J., Leonard P.G., Sizovs A., Moosa M.M., MacKenzie K.R., Ferreon J.C., Ferreon A.C.M. The N-terminal domain of ALS-linked TDP-43 assembles without misfolding. Angew. Chem. Int. Ed. 2017;56:12590–12593. doi: 10.1002/anie.201706769. [DOI] [PubMed] [Google Scholar]
- Tsvetkov A.S., Miller J., Arrasate M., Wong J.S., Pleiss M.A., Finkbeiner S. A small-molecule scaffold induces autophagy in primary neurons and protects against toxicity in a Huntington disease model. Proc. Natl. Acad. Sci. U S A. 2010;107:16982–16987. doi: 10.1073/pnas.1004498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K., Nakashima-Yasuda H., Forman M.S., Kwong L.K., Clark C.M., Grossman M., Miller B.L., Kretzschmar H.A., Lee V.M.-Y., Trojanowski J.Q., Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in alzheimer disease and corticobasal degeneration but not in other tauopathies. J. Neuropathol. Exp. Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vintilescu C.R., Afreen S., Rubino A.E., Ferreira A. The neurotoxic TAU45-230 fragment accumulates in upper and lower motor neurons in amyotrophic lateral sclerosis subjects. Mol. Med. 2016;22:477–486. doi: 10.2119/molmed.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt A., Herholz D., Fiesel F.C., Kaur K., Müller D., Karsten P., Weber S.S., Kahle P.J., Marquardt T., Schulz J.B. TDP-43-mediated neuron loss in vivo requires RNA-binding activity. PLoS One. 2010;5:e12247. doi: 10.1371/journal.pone.0012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.K., Tripathy K., Restrepo C.R., Ge G., Xu Y., Kwong L.K., Trojanowski J.Q., Lee V.M.-Y. An insoluble frontotemporal lobar degeneration-associated TDP-43 C-terminal fragment causes neurodegeneration and hippocampus pathology in transgenic mice. Hum. Mol. Genet. 2015;24:7241–7254. doi: 10.1093/hmg/ddv424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-K., Lee Y.-C., Huang C.-Y., Liliang P.-C., Lu K., Chen H.-J., Li Y.-C., Tsai K.-J. Traumatic brain injury causes frontotemporal dementia and TDP-43 proteolysis. Neuroscience. 2015;300:94–103. doi: 10.1016/j.neuroscience.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Wang I.-F., Chang H.-Y., Hou S.-C., Liou G.-G., Way T.-D., Shen C.-K.J. The self-interaction of native TDP-43 C terminus inhibits its degradation and contributes to early proteinopathies. Nat. Commun. 2012;3:766. doi: 10.1038/ncomms1766. [DOI] [PubMed] [Google Scholar]
- Wang I.-F., Guo B.-S., Liu Y.-C., Wu C.-C., Yang C.-H., Tsai K.-J., Shen C.-K.J. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc. Natl. Acad. Sci. U S A. 2012;109:15024–15029. doi: 10.1073/pnas.1206362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fan H., Ying Z., Li B., Wang H., Wang G. Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neurosci. Lett. 2010;469:112–116. doi: 10.1016/j.neulet.2009.11.055. [DOI] [PubMed] [Google Scholar]
- Wang X., Ma M., Teng J., Che X., Zhang W., Feng S., Zhou S., Zhang Y., Wu E., Ding X. Valproate attenuates 25-kDa C-terminal fragment of TDP-43-induced neuronal toxicity via suppressing endoplasmic reticulum stress and activating autophagy. Int. J. Biol. Sci. 2015;11:752–761. doi: 10.7150/ijbs.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-T.T., Kuo P.-H.H., Chiang C.-H.H., Liang J.-R.R., Chen Y.-R.R., Wang S., Shen J.C., Yuan H.S. The truncated C-terminal RNA recognition motif of TDP-43 protein plays a key role in forming proteinaceous aggregates. J. Biol. Chem. 2013;288:9049–9057. doi: 10.1074/jbc.M112.438564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Oiwa K., Murata Y., Komine O., Sobue A., Endo F., Takahashi E., Yamanaka K. ALS-linked TDP-43M337V knock-in mice exhibit splicing deregulation without neurodegeneration. Mol. Brain. 2020;13:8. doi: 10.1186/s13041-020-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegorzewska I., Baloh R.H. TDP-43-Based animal models of neurodegeneration: new insights into ALS pathology and pathophysiology. Neurodegener Dis. 2011;8:262–274. doi: 10.1159/000321547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegorzewska I., Bell S., Cairns N.J., Miller T.M., Baloh R.H. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. U S A. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp K., Tank E.M., Miguez R., McBride J.P., Gómez N.B., White M., Lin Z., Gonzalez C.M., Serio A., Sreedharan J. Shortened TDP43 isoforms upregulated by neuronal hyperactivity drive TDP43 pathology in ALS. J. Clin. Invest. 2020;130:1139–1155. doi: 10.1172/JCI130988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.A., Kim E., Duffy A., Adalbert R., Phillips B.U., Peters O.M., Stephenson J., Yang S., Massenzio F., Lin Z. TDP-43 gains function due to perturbed autoregulation in a Tardbp knock-in mouse model of ALS-FTD. Nat. Neurosci. 2018;21:552–563. doi: 10.1038/s41593-018-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wils H., Kleinberger G., Janssens J., Pereson S., Joris G., Cuijt I., Smits V., Ceuterick-de Groote C., Van Broeckhoven C., Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. U S A. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.C., Dugger B.N., Dickson D.W., Wang D.-S. TDP-43 in aging and Alzheimer’s disease - a review. Int. J. Clin. Exp. Pathol. 2011;4:147–155. [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Sanelli T., Chiang H., Sun Y., Chakrabartty A., Keith J., Rogaeva E., Zinman L., Robertson J. Low molecular weight species of TDP-43 generated by abnormal splicing form inclusions in amyotrophic lateral sclerosis and result in motor neuron death. Acta Neuropathol. 2015;130:49–61. doi: 10.1007/s00401-015-1412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Sanelli T., Dib S., Sheps D., Findlater J., Bilbao J., Keith J., Zinman L., Rogaeva E., Robertson J. RNA targets of TDP-43 identified by UV-CLIP are deregulated in ALS. Mol. Cell Neurosci. 2011;47:167–180. doi: 10.1016/j.mcn.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Xu Y.-F., Gendron T.F., Zhang Y.-J., Lin W.-L., D’Alton S., Sheng H., Casey M.C., Tong J., Knight J., Yu X. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J. Neurosci. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.-S. Does a loss of TDP-43 function cause neurodegeneration? Mol. Neurodegener. 2012;7:27. doi: 10.1186/1750-1326-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Hideyama T., Hachiga K., Teramoto S., Takano J., Iwata N., Saido T.C., Kwak S. A role for calpain-dependent cleavage of TDP-43 in amyotrophic lateral sclerosis pathology. Nat. Commun. 2012;3:1307. doi: 10.1038/ncomms2303. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Teramoto S., Kwak S. Phosphorylated TDP-43 becomes resistant to cleavage by calpain: a regulatory role for phosphorylation in TDP-43 pathology of ALS/FTLD. Neurosci. Res. 2016;107:63–69. doi: 10.1016/j.neures.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Yang C., Wang H., Qiao T., Yang B., Aliaga L., Qiu L., Tan W., Salameh J., McKenna-Yasek D.M., Smith T. Partial loss of TDP-43 function causes phenotypes of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U S A. 2014;111:E1121–E1129. doi: 10.1073/pnas.1322641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Lin F., Robertson C.S., Wang K.K.W. Dual vulnerability of TDP-43 to calpain and caspase-3 proteolysis after neurotoxic conditions and traumatic brain injury. J. Cereb. Blood Flow Metab. 2014;34:1444–1452. doi: 10.1038/jcbfm.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P., Guo X., Yang W., Yan S., Yang S., Zhao T., Sun Q., Liu Y., Li S., Li X.-J. Caspase-4 mediates cytoplasmic accumulation of TDP-43 in the primate brains. Acta Neuropathol. 2019;137:919–937. doi: 10.1007/s00401-019-01979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tan C.-F., Mori F., Tanji K., Kakita A., Takahashi H., Wakabayashi K. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;115:115–122. doi: 10.1007/s00401-007-0285-7. [DOI] [PubMed] [Google Scholar]
- Zhang T., Mullane P.C., Periz G., Wang J. TDP-43 neurotoxicity and protein aggregation modulated by heat shock factor and insulin/IGF-1 signaling. Hum. Mol. Genet. 2011;20:1952–1965. doi: 10.1093/hmg/ddr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-J., Caulfield T., Xu Y.-F., Gendron T.F., Hubbard J., Stetler C., Sasaguri H., Whitelaw E.C., Cai S., Lee W.C., Petrucelli L. The dual functions of the extreme N-terminus of TDP-43 in regulating its biological activity and inclusion formation. Hum. Mol. Genet. 2013;22:3112–3122. doi: 10.1093/hmg/ddt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-J., Xu Y., Dickey C.A., Buratti E., Baralle F., Bailey R., Pickering-Brown S., Dickson D., Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J. Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-J.J., Xu Y.-F.F., Cook C., Gendron T.F., Roettges P., Link C.D., Lin W.-L.L., Tong J., Castanedes-Casey M., Ash P. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc. Natl. Acad. Sci. U S A. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Beers D.R., Bell S., Wang J., Wen S., Baloh R.H., Appel S.H. TDP-43 activates microglia through NF-κB and NLRP3 inflammasome. Exp. Neurol. 2015;273:24–35. doi: 10.1016/j.expneurol.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Zhou F., Dong H., Liu Y., Yan L., Sun C., Hao P., Liu Y., Zhai J., Liu Y. Raloxifene, a promising estrogen replacement, limits TDP-25 cell death by enhancing autophagy and suppressing apoptosis. Brain Res. Bull. 2018;140:281–290. doi: 10.1016/j.brainresbull.2018.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.