Highlights
-
•
Recurrent, non-resectable ovarian granulosa cell tumor has no standard treatment.
-
•
Estrogen/progesterone receptor signaling regulates granulosa cell tumor growth.
-
•
Granulosa cell tumor responds well to alternating megestrol and tamoxifen therapy.
Keywords: Granulosa cell tumor, Ovary, Megestrol acetate, Tamoxifen, Endocrine therapy
1. Introduction
Sex cord stromal tumors comprise 3–5% of all ovarian malignancies (Scully et al., 1998). Granulosa cell tumors (GCT) constitute 70–90% of the malignant sex cord stromal tumors and are categorized into adult and juvenile subtypes based on different clinical and histopathologic features (Young, 2005). Adult GCTs usually present during the perimenopausal or early postmenopausal years with an estimated incidence of 0.4 to 0.7 per 100,000 (Schumer and Cannistra, 2003). Histologically, GCTs are characterized by grooved or “coffee bean” nuclei also known as Call-Exner bodies, which are circular arrangements of granulosa cells around eosinophilic fluid filled spaces (Young, 2005). GCTs secrete inhibin, which is a useful serum tumor marker during surveillance and treatment of recurrent disease. Inhibin B is the predominant isoform secreted by GCTs (Petraglia et al., 1998). Anti-mullerian hormone (AMH) or CA 125 might be useful in the surveillance of patients with GCT, particularly in the presence of metastatic disease, but are not as established as inhibin B (Petraglia et al., 1998, Geerts et al., 2009, Stine et al., 2013).
GCTs are typically early stage at time of diagnosis with a reported five-year survival greater than 90% for stage I disease (Seagle et al., 2017). The primary treatment is surgical resection while the benefit of adjuvant therapy is limited (Seagle et al., 2017, Mangili et al., 2016). Treatment options for recurrent disease include chemotherapy, radiation and systemic therapies. Combination chemotherapy with carboplatin and paclitaxel has shown response rates of 54% (Brown et al., 2004). Bleomycin, etoposide and cisplatin (BEP) treatment has resulted in response rates of 71%, while single agent taxane has shown responses in 37% of patients (Brown et al., 2005). The addition of bevacizumab to weekly paclitaxel can increase response rates to 44% with however similar six month progression free survival (78% vs. 72%) and median progression free survival (14.9 vs. 14.7 months) (Ray-Coquard et al., 2020). Adjuvant radiotherapy may prolong disease free survival but effects on long term survival are less clear (Wolf et al., 1999).
GCTs are hormone dependent tumors that express estrogen (ER) and progesterone receptors (PR) (van Meurs et al., 2014). Endocrine therapy has therefore emerged as a treatment option for GCT. Selective estrogen receptor antagonists, aromatase inhibitors, gonadotropin releasing hormone agonists and progesterone have been utilized as potential treatments with varying clinical benefit for patients with recurrent GCT (van Meurs et al., 2014). Reported response rates to endocrine therapy range from 18% to 71% (van Meurs et al., 2014, Fishman et al., 1996). We present a case of recurrent GCT that demonstrates an exceptional response to endocrine therapy using an alternating regimen of megestrol acetate and tamoxifen.
2. Case report
A 48 year-old G2P2 female presented with abnormal uterine bleeding and a 12 cm right adnexal mass. She underwent a robotic assisted laparoscopic total hysterectomy with right salpingo-oophorectomy and left salpingectomy. A frozen section diagnosis of the right adnexal mass was not obtained and a staging procedure was not performed. The final pathology showed an adult granulosa cell tumor confined to the right ovary. The patient was subsequently referred to a gynecologic oncologist but declined a surgical staging procedure and adjuvant treatment. She opted for surveillance with serum inhibin levels and office visits every three months. A postoperative PET/CT scan at the time of referral showed no evidence of metabolically active disease.
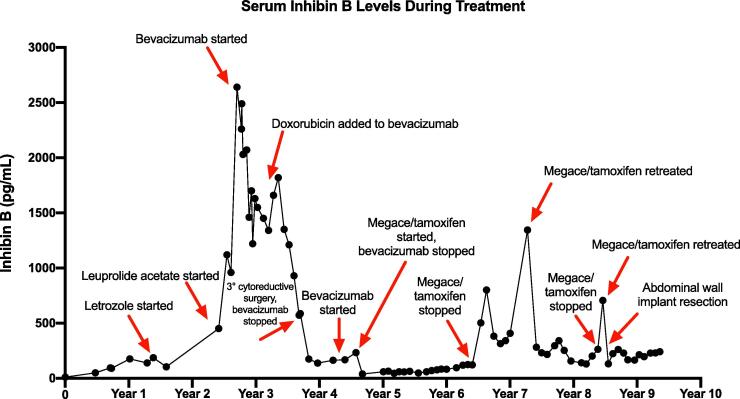
Ten months following the initial surgery, the patient recurred with disease in the pelvis. Serum inhibin level increased to 94 pg/mL. A pelvic ultrasound showed several hypoechoic nodules between the vaginal cuff and bladder, the largest measuring 1.9 cm and was confirmed on pelvic MRI. Fig. 1 shows the serum inhibin B levels during treatment. She underwent a secondary cytoreduction with omentectomy, removal of a left adnexal mass and multiple nodules on the mesentery, peritoneum, vagina, bladder wall, and rectosigmoid colon. Following surgery, she was counseled on adjuvant therapy including chemotherapy, bevacizumab and endocrine therapy. The patient declined chemotherapy due to concerns of side effects and instead chose endocrine therapy. The patient was started on letrozole based on physician’s choice of endocrine therapy. Letrozole resulted in good control of her disease for ten months. Inhibin level increased from 104 to 451 pg/mL. A pelvic ultrasound revealed right adnexal nodules measuring 5.2 × 1.8 cm in aggregate and vaginal cuff nodules measuring 1.9 × 0.5 cm. Treatment was changed to leuprolide acetate which has reported response rates of 40% in recurrent GCT (Fishman et al., 1996). A pelvic MRI three months after initiation of leuprolide acetate revealed however an interval increase of the right adnexal mass to 7.0 × 2.8 × 3.2 cm. Various treatment options including surgical resection were considered at this point. The patient declined surgery and was started on bevacizumab 10 mg/kg every two weeks. This regimen was based on a single reported complete response in a study of eight patients with recurrent GCT treated with a range of bevacizumab dosing intervals (Tao et al., 2009). Bevacizumab treatment resulted in stabilization of the disease over nine months, which was verified by pelvic ultrasounds every two to three months. Upon increase of the right adnexal mass increased to 10.2 × 6.4 × 5.8 cm on pelvic ultrasound, doxorubicin was added to bevacizumab followed by weekly paclitaxel with bevacizumab without clinical response.
Fig. 1.
Serum inhibin B levels during treatment.
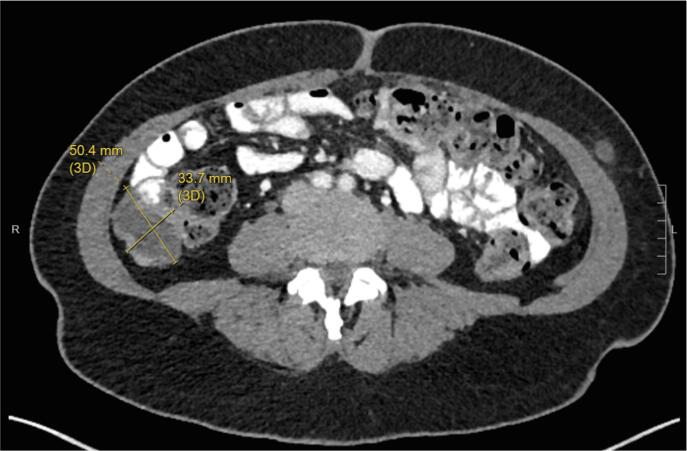
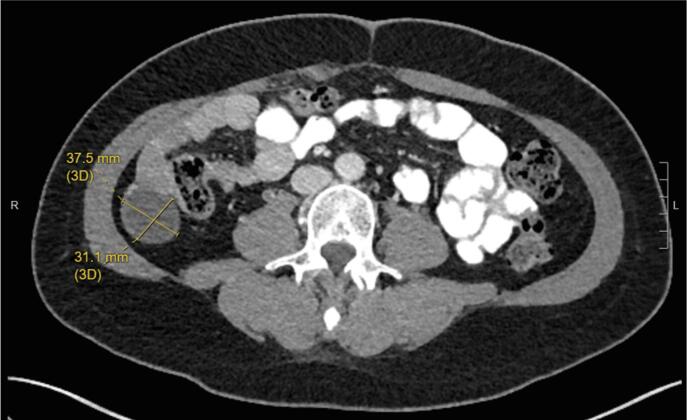
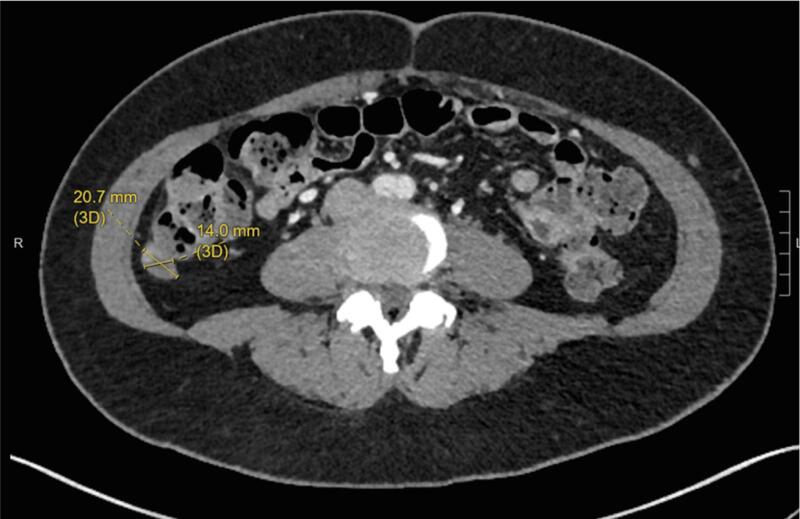
The patient subsequently underwent a tertiary cytoreduction with removal of an 11 cm pelvic mass followed by pelvic radiation and vaginal brachytherapy. Six months later, bevacizumab 15 mg/kg every three weeks was started for progressive disease with multiple peritoneal metastases and lymphadenopathy. Bevacizumab treatment resulted in a mixed response on MRI with decreased nodularity along the vaginal cuff but increased size of multiple peritoneal soft tissue implants, including a nodule lateral to the cecum, which increased from 3.5 × 4.3 cm to 3.4 × 5.0 cm. In addition, inhibin level increased from 167 to 233 pg/ml. Based on positive ER (2–3+, 80%) and PR (3+, 90%) expression in tumor tissue obtained at the time of tertiary cytoreduction, treatment was changed to megestrol acetate 40 mg b.i.d. for two weeks alternating with tamoxifen 20 mg q.d. for two weeks. Using this endocrine therapy, inhibin level decreased from 233 to 39 pg/ml. The patient’s disease related symptoms including abdominal pain resolved. CT imaging also showed decreased bulk of tumor lateral to the cecum over the course of treatment (Fig. 2A, Fig. 2B, Fig. 2C).
Fig. 2A.
Prior to megace/tamoxifen treatment. Peritoneal implant 5.0 × 3.4 cm.
Fig. 2B.
Month 15 of megace/tamoxifen treatment. Peritoneal implant 3.8 × 3.1 cm.
Fig. 2C.
Month 26 of megace/tamoxifen treatment. Peritoneal implant 2.1 × 1.4 cm.
The patient continued on megestrol acetate and tamoxifen treatment for 22 months before inhibin levels started to rise again. A biopsy obtained from a paracolic lymph node showed recurrent granulosa cell tumor with positive ER (3+, 70%) and PR (3+, 70%) expression, FOXL2 C134W mutation, stable microsatellite status, and low tumor mutation burden. The patient was restarted on alternating megestrol acetate and tamoxifen, which was followed by another rapid decrease of serum inhibin from 1345 to 282 pg/mL. She continued on this treatment regimen for 14 months with control of her disease burden. Treatment was paused again for two months due to rising inhibin levels, but restarted for a third time due to a lack of other treatment options. In addition, she underwent excision of a GCT tumor implant in the anterior abdominal wall. Four years after her first and twelve months after her third treatment cycle of megestrol acetate and tamoxifen, the disease remains under control based on stable inhibin levels and imaging studies. The patient denies any symptoms possibly related to the disease or treatment.
3. Discussion
Recurrent, non-resectable adult granulosa cell tumor of the ovary remains a clinical challenge without standard treatment. An ongoing Gynecologic Oncology Group (GOG) trial is comparing carboplatin and paclitaxel with BEP for the treatment of ovarian sex cord stromal tumors and will provide additional efficacy data on chemotherapy in recurrent GCT (NCT01042522). Adjuvant radiotherapy may prolong disease free survival (Wolf et al., 1999). Anti-angiogenic therapy using bevacizumab has demonstrated some clinical activity with stabilization of disease in up to 78% of relapsed cases (Brown et al., 2014). Immunotherapy using the PD-1 inhibitor pembrolizumab has recently shown a potential clinical benefit in adult GCT with disease control of over 12 months (How et al., 2021).
The biological rationale for the use of endocrine therapy for GCT is the high level of ER and PR expression. In addition, FOXL2 mutations are found in over 90% of GCTs, resulting in upregulation of aromatase expression and subsequent increased biosynthesis of estradiol and estrone from androgens (Shah et al., 2009). Data on the clinical efficacy of aromatase inhibitors in recurrent GCT are limited to fewer than 20 case reports, such as three patients with a reported complete response sustained over 12 months (Korach et al., 2009).
The mechanisms of anti-tumor efficacy in GCT using endocrine treatment include progestin-mediated apoptosis, a negative feedback inhibition of gonadotropins and subsequent decreased estrogen levels, or a combination of both. The ER and PR expression status of GCT might be a predictive biomarker for response to hormone treatment, and possibly guide the selection of specific endocrine treatments. However, about two-thirds of adult GCT express ER and almost all are PR positive, and data on the predictive value for response to endocrine therapy are scant (Farinola et al., 2007). In a systematic review evaluating the effectiveness of various endocrine treatments in advanced GCT, patients with ER negative and PR positive tumors had the highest overall response rate of 66.7% versus 33.3% in patients with both ER and PR positive tumors (van Meurs et al., 2014). Hormone receptor status information was only available for 7 out of 31 patients in this study and more data are needed to identify a predictive biomarker profile based on ER and PR status.
Our case study illustrates the great potential of endocrine therapy for the treatment of recurrent GCT. There are several notable aspects to this case. First, an alternating regimen of megestrol acetate and tamoxifen showed a greater clinical benefit compared to the patient’s prior therapies, which included an aromatase inhibitor and a gonadotropin-releasing hormone agonist. Meurs and colleagues described 31 adult GCT patients with an overall response rate of 71.0% to endocrine therapy, including 18 partial responses and 8 complete responses (van Meurs et al., 2014). One patient was treated with an alternating regimen of megestrol acetate and tamoxifen with a complete response at 22 months. In contrast to our case, this patient was ER negative and PR positive (Hardy et al., 2005). Second, our patient’s treatment was interrupted twice due to rising inhibin levels and presumed resistance to endocrine therapy. However, re-initiation of the same hormonal regimen after both treatment interruptions showed significant clinical efficacy, suggesting a re-sensitization during the treatment break. While the mechanisms underlying the development of resistance to endocrine treatment are unclear, it is possible that the continuous treatment with megestrol acetate and tamoxifen modified the ER and PR pathways. Megestrol treatment might have induced a down-regulation of PR expression and signaling, and thus rendered tumor cells resistant to the pro-apoptotic effects of progesterone. The treatment interruption might have reconstituted an ER/PR expression profile that re-sensitized the tumor cells to endocrine treatment.
The alternating regimen of megestrol acetate and tamoxifen has not been studied systematically in GCT. A phase II GOG trial in recurrent or advanced endometrial cancer using a similar regimen showed a 27% overall response rate including 21.4% with a complete response (Fiorica et al., 2004). Response rates were higher for grade 1 histology and extra-pelvic measurable disease (38% and 31%, respectively), and in women less than 60 years of age (44% vs. 20%). In contrast to our case, patients on this trial were chemo- and endocrine therapy naïve. In addition, megestrol acetate was dosed at 80 mg p.o. b.i.d for three weeks followed by tamoxifen citrate 20 mg p.o. b.i.d for three weeks. These significantly higher doses might have contributed to the notable weight gain, gastrointestinal side effects, and development of pulmonary embolism in four patients on the trial.
We report a case of exceptional response to endocrine therapy in a recurrent and heavily pretreated adult granulosa cell tumor using moderate doses of alternating megestrol acetate and tamoxifen. The patient continues on this regimen with control of her disease and a good quality of life for now over four years. Given our experience and the published data using endocrine therapies in hormone responsive gynecologic cancer, it would be interesting to study the efficacy of alternating megestrol acetate and tamoxifen in GCT in a clinical trial. The infrequent diagnosis, slow tumor growth and lack of standard treatment for recurrent GCTs present challenges for a clinical trial. Any clinical trial will need to include a biomarker analysis to study response predictors including the ER and PR pathways.
Written informed consent was obtained from the patient for publication of this case report.
CRediT authorship contribution statement
Ashley S. Moon: Writing - original draft, Writing - review & editing, Visualization. Oliver Dorigo: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or other conflicts of interest.
References
- Scully RE, Young RH, Clement PB, Pathology (U.S.) AFI of, Pathology UA for R and E in. Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament. Armed Forces Institute of Pathology; 1998.
- Young R.H. Sex cord-stromal tumors of the ovary and testis: their similarities and differences with consideration of selected problems. Mod. Pathol. 2005;18:S81–S98. doi: 10.1038/modpathol.3800311. [DOI] [PubMed] [Google Scholar]
- Schumer S.T., Cannistra S.A. Granulosa Cell Tumor of the Ovary. J. Clin. Oncol. 2003;21:1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Petraglia F., Luisi S., Pautier P., Sabourin J.-C., Rey R., Lhomme C. Inhibin B Is the Major Form of Inhibin/Activin Family Secreted by Granulosa Cell Tumors. J. Clin. Endocrinol. Metab. 1998;83:1029–1032. doi: 10.1210/jcem.83.3.4800. [DOI] [PubMed] [Google Scholar]
- Geerts I., Vergote I., Neven P., Billen J. The role of inhibins B and antimüllerian hormone for diagnosis and follow-up of granulosa cell tumors. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2009;19:847–855. doi: 10.1111/IGC.0b013e3181a702d1. [DOI] [PubMed] [Google Scholar]
- Stine J.E., Suri A., Gehrig P.A., Chiu M., Erickson B.K., Huh W.K. Pre-operative imaging with CA125 is a poor predictor for granulosa cell tumors. Gynecol. Oncol. 2013;131:59–62. doi: 10.1016/j.ygyno.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Seagle B.-L.L., Ann P., Butler S., Shahabi S. Ovarian granulosa cell tumor: A National Cancer Database study. Gynecol. Oncol. 2017;146:285–291. doi: 10.1016/j.ygyno.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Mangili G., Ottolina J., Cormio G., Loizzi V., De Iaco P., Pellegrini D.A. Adjuvant chemotherapy does not improve disease-free survival in FIGO stage IC ovarian granulosa cell tumors: The MITO-9 study. Gynecol. Oncol. 2016;143:276–280. doi: 10.1016/j.ygyno.2016.08.316. [DOI] [PubMed] [Google Scholar]
- Brown J., Shvartsman H.S., Deavers M.T., Burke T.W., Munsell M.F., Gershenson D.M. The activity of taxanes in the treatment of sex cord-stromal ovarian tumors. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:3517–3523. doi: 10.1200/JCO.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Brown J., Shvartsman H.S., Deavers M.T., Ramondetta L.M., Burke T.W., Munsell M.F. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord-stromal ovarian tumors. Gynecol. Oncol. 2005;97:489–496. doi: 10.1016/j.ygyno.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Ray-Coquard I., Harter P., Lorusso D., Dalban C., Vergote I., Fujiwara K. Effect of Weekly Paclitaxel With or Without Bevacizumab on Progression-Free Rate Among Patients With Relapsed Ovarian Sex Cord-Stromal Tumors: The ALIENOR/ENGOT-ov7 Randomized Clinical Trial. JAMA Oncol. 2020;6:1923–1930. doi: 10.1001/jamaoncol.2020.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J.K., Mullen J., Eifel P.J., Burke T.W., Levenback C., Gershenson D.M. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecol. Oncol. 1999;73:35–41. doi: 10.1006/gyno.1998.5287. [DOI] [PubMed] [Google Scholar]
- van Meurs H.S., van Lonkhuijzen L.R.C.W., Limpens J., van der Velden J., Buist M.R. Hormone therapy in ovarian granulosa cell tumors: A systematic review. Gynecol. Oncol. 2014;134:196–205. doi: 10.1016/j.ygyno.2014.03.573. [DOI] [PubMed] [Google Scholar]
- Fishman A., Kudelka A.P., Tresukosol D., Edwards C.L., Freedman R.S., Kaplan A.L. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J. Reprod. Med. 1996;41:393–396. [PubMed] [Google Scholar]
- Tao X., Sood A.K., Deavers M.T., Schmeler K.M., Nick A.M., Coleman R.L. Anti-angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecol. Oncol. 2009;114:431–436. doi: 10.1016/j.ygyno.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Brady W.E., Schink J., Van Le L., Leitao M., Yamada S.D. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the Gynecologic Oncology Group. Cancer. 2014;120:344–351. doi: 10.1002/cncr.28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How J.A., Jazaeri A., Westin S.N., Sood A.K., Ramondetta L.M., Xu M. The clinical efficacy and safety of single-agent pembrolizumab in patients with recurrent granulosa cell tumors of the ovary: a case series from a phase II basket trial. Invest. New Drugs. 2021 doi: 10.1007/s10637-020-01043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S.P., Köbel M., Senz J., Morin R.D., Clarke B.A., Wiegand K.C. Mutation of FOXL2 in Granulosa-Cell Tumors of the Ovary. N. Engl. J. Med. 2009;360:2719–2729. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- Korach J., Perri T., Beiner M., Davidzon T., Fridman E., Ben-Baruch G. Promising effect of aromatase inhibitors on recurrent granulosa cell tumors. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2009;19:830–833. doi: 10.1111/IGC.0b013e3181a261d7. [DOI] [PubMed] [Google Scholar]
- Farinola M.A., Gown A.M., Judson K., Ronnett B.M., Barry T.S., Movahedi-Lankarani S. Estrogen Receptor α and Progesterone Receptor Expression in Ovarian Adult Granulosa Cell Tumors and Sertoli-Leydig Cell Tumors. Int. J. Gynecol. Pathol. 2007;26:375–382. doi: 10.1097/pgp.0b013e31805c0d99. [DOI] [PubMed] [Google Scholar]
- Hardy R.D., Bell J.G., Nicely C.J., Reid G.C. Hormonal treatment of a recurrent granulosa cell tumor of the ovary: case report and review of the literature. Gynecol. Oncol. 2005;96:865–869. doi: 10.1016/j.ygyno.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Fiorica J.V., Brunetto V.L., Hanjani P., Lentz S.S., Mannel R., Andersen W. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2004;92:10–14. doi: 10.1016/j.ygyno.2003.11.008. [DOI] [PubMed] [Google Scholar]