Abstract
Purpose
What are the patient characteristics predictive of response to ranibizumab treatment?
Methods
Model-based characterization of best-corrected visual acuity (BCVA) time profiles of patients with neovascular age-related macular degeneration under ranibizumab or sham treatment based on 24-month observations of BCVA in 2419 patients from randomized multicenter phase 3 trials of ranibizumab: ANCHOR, MARINA, PIER, and HARBOR. Goodness-of-fit plots and precision of parameter estimates were used for measure of accuracy.
Results
The model incorporates a long-term effect on disease progression and an additive and more potent short-term effect of ranibizumab. Response to ranibizumab treatment and progression of the disease were found to be a function of seven baseline characteristics (visual acuity, age, leakage size, central retinal lesion thickness, presence or absence of cyst, type of choroidal neovascularization (CNV), and size of pigment epithelium detachment). A composite score of these seven baseline characteristics was derived and used to categorize response to ranibizumab treatment. The ranibizumab treatment arms of two proof-of-concept studies held out from the model development were used to validate the methodology.
Conclusions
A composite score based on seven patient characteristics prior to treatment could be used to discriminate patients with predicted insufficient response to anti–vascular endothelial growth factor treatment.
Translational Relevance
The method could be used to create a virtual ranibizumab treatment arm in clinical trials or to reduce the size of a ranibizumab active control arm.
Keywords: BCVA, anti-VEGF, ranibizumab, meta-marker
Introduction
Neovascular age-related macular degeneration is a leading cause of vision loss.1 It is characterized by the growth of abnormal blood vessels from the choroid into the retina. These vessels are leaky and result in tissue edema, hemorrhage, neovascularization, and, at a later stage, scar formation.2
Vascular endothelial growth factor (VEGF) inhibitors are the current standard of care (SOC) for neovascular age-related macular degeneration (nAMD) as they have proven to be effective in reducing vessel leakiness and choroidal neovascularization, as well as improving vision in patients with nAMD. However, patients do not benefit equally from anti-VEGF treatment and respond with high heterogeneity to these therapies. In the HARBOR study, a phase 3 trial of ranibizumab (Lucentis), for every patient (of the 274 subjects from the 0.5-mg every 4 weeks (q4w) group) who reached “normal” vision (above 84 letters), 13 patients had a suboptimal (below 84 letters) response to treatment, and about half of those patients had a vision below 69 letters (20/40 Snellen) after 2 years of treatment (from our own calculation based on HARBOR results).
In this context of highly variable disease and treatment responses, it is challenging to show that an investigational drug can surpass the SOC or be effective as a second-line therapy when the targeted incremental benefit is not larger than the observed variability. A characterization of patients by their responses to treatment or progressions to disease could reduce the uncertainty in the outcome of a clinical trial and instill confidence in resulting decisions.
Various attempts have been made to characterize and understand the heterogeneity in nAMD disease and response of patients to anti-VEGF therapies. This includes an in silico mechanistic model to test several hypotheses for the mechanism of CNV,3 statistical testing hypotheses to show that fibrovascular pigment epithelium detachment is a risk factor for long-term visual decay in nAMD,4 and general linear models with different correlation structures to quantify the change of visual acuity (VA) over time.5 More elaborated drug-disease models were developed6 to evaluate the impact of an individualized flexible treatment regimen on disease progression, and recently,7 an indirect response model with age and gender as covariates was used to describe the time course of best-corrected visual acuity (BCVA) during treatment with ranibizumab. A predictive model for factors related to anatomic outcome after ranibizumab treatment was developed.8 Also, a linear mixed-effect model9 based on an observational study was used to demonstrate an association between genetic risk factors and response to an anti-VEGF therapy.
VA decay and response to treatment to anti-VEGF therapies in patients with nAMD are likely dependent on the disease status (e.g., leakage, pigment epithelium detachment, retinal thickness). Yet, to our knowledge, there is no nonlinear longitudinal analysis of VA that incorporates in a multivariate manner the relevant pathophysiologic baseline characteristics of these patients from randomized clinical trials.
This article proposes a method that allows the characterization and thereby categorization of patients by their baseline parameters based on their predicted responses to treatment utilizing a model that can predict BCVA at any time point prior to the trial's start.
A drug-disease model for VA was developed on the 24-month patient-level data of phase 3 trials from ranibizumab (MARINA, PIER, ANCHOR, and HARBOR). Data from untreated patients (sham) were included. The model is based on a kinetic pharmacodynamic (K-PD) approach to account for differences in dose and dosing frequency over time and across studies. The time courses of VA in patients with nAMD with and without anti-VEGF treatment were characterized as function of seven baseline characteristics of patients. The model suggests a dual effect of ranibizumab: a short-term effect restoring vision and a long-term effect slowing down the disease.
The capacity of predicting response to ranibizumab for a group of patients based only on their preidentified baseline characteristics was demonstrated using the ranibizumab active-control arms from two proof-of-concept (POC) studies (test data) of faricimab, a bispecific antibody under clinical development. This shows the potential to create a virtual ranibizumab treatment arm in clinical trials with the model.
The concept of a model-based meta-marker (MBMM) is introduced. This is a patient's signature, defined by a composite score of the seven preidentified baseline characteristics that allows categorization of response to ranibizumab.
Materials and Methods
Trial Designs
All studies used here comply with the Declaration of Helsinki, and the trial designs and patient characteristics have been reported in detail previously.10–13
ANCHOR (NCT01256827), MARINA (NCT01256827), and PIER (NCT00090623) were each a three-arm study with a comparator arm and two ranibizumab arms of 0.3 mg and 0.5 mg. ANCHOR was composed of 426 patients’ predominantly classic subfoveal nAMD and compared monthly dosing of ranibizumab against photodynamic therapy (PDT), the previous standard of care for predominantly classic CNV. Patients from the PDT arm were excluded from the analysis. MARINA was composed of 716 patients’ occult or minimally classic subfoveal nAMD and compared monthly dosing of ranibizumab against sham injections in the control arm. PIER was composed of 180 patients with or without classic CNV secondary to AMD and compared monthly dosing of ranibizumab for 3 months and then every 3 months against sham injections in the control arm. Second-year data were excluded due to the reassignment of patients to different treatment arms with nonrandomization.
The HARBOR trial (NCT00891735) was composed of 1097 patients with or without classic CNV subfoveal AMD and compared 0.5 and 2 mg ranibizumab administered monthly or on an as-needed basis.
The mean profiles of BCVA of each arm of these trials are plotted in Supplementary Figure S1.
The ranibizumab 0.5-mg arms of AVENUE (NCT02484690) and STAIRWAY (NCT03038880), 2 phase 2 studies of faricimab, were used for an external validation of the model. AVENUE was a 5-arm study of 36 weeks (273 patients with CNV secondary to AMD) of ranibizumab 0.5 mg (68 patients) compared to different faricimab treatment arms of different doses and dosing regimens. STAIRWAY was a 3-arm study of 52 weeks (76 patients with CNV secondary to AMD) of ranibizumab 0.5 mg (16 patients) compared to faricimab 6 mg with different dosing regimens.
Models
The K-PD Approach
Ranibizumab is injected in the vitreous humor to inhibit VEGF and thereby reduce vessel leakiness that leads to an improvement in VA. To account for ranibizumab concentrations not measured in the vitreous, a K-PD model was used.14 Such an approach consists of creating a pharmacokinetic profile of ranibizumab into a virtual biophase compartment in which the drug concentrations are in equilibrium with the observed effect in VA. To describe the delay between the injection of ranibizumab and the change in VA, a depot compartment representing the vitreous humor was connected to the biophase compartment with a first-order rate KDE, similar to the elimination rate from the virtual compartment.
The model assumed that the virtual infusion rate (IR; drug amount per unit time) drives the dynamics of VA (cf. Equations (1)–(3)).
| (1) |
| (2) |
| (3) |
In the above equations, A1 and A2 are respectively the amount of drug in vitreous humor and in the biophase compartments.
VA of Sham-Treated Patients
VA of untreated patients is likely to deteriorate over time. This was modeled using an exponential decay as defined in Equation (4).
| (4) |
In (4), VA0 is the baseline VA. VAss is VA at steady state of sham treatment so that VA0 − VAss represents the maximum loss in VA. kpr is the rate of deterioration of vision under sham treatment.
VA of Ranibizumab-Treated Patients
Two different modes of action of ranibizumab were investigated: a short-term effect by increasing VA additively and a long-term effect by reducing the rate of deterioration of VA. Such a long-term effect alone cannot describe the improvement observed with ranibizumab treatment. Therefore, a model with a short-term effect alone (model 1 described by Equation (5)) was compared to a model that combines both short-term and long-term effects (model 2 in Equation (6)).
| (5) |
| (6) |
In Equations (5) and (6):
-
•
Emax is the maximum short-term effect on VA.
-
•
E50 is the drug amount per unit time corresponding to half of the maximal short-term effect.
-
•
Ed50 is the drug amount per unit time corresponding to half of the maximal long-term effect.
The residual variability was described by an additive normally distributed error model with variance σ2. The baseline VA0 was modeled as normally distributed around the observed baseline VA (BVA) with the same variance as that of the residual error. Hence,
| (7) |
N(0, 1) is the normal distribution with mean 0 and variance 1. To account for ceiling and floor effects, the maximum short-term effect Emax as well as VAss were assumed to be a function of baseline VA0. More specifically:
| (8) |
| (9) |
Emax 0, VAss0 , and p were estimated. Between-subject variability in structural model parameters was estimated using a lognormal distribution for the random effects.
Goodness-of-fit diagnostics and structural adequacy were used to select between model 1 and model 2.
Covariate Analysis
The influence of age, sex, and baseline disease-related covariates (cf. Table 1) from fundus fluorescence angiography and optical coherence tomography (OCT) were tested on all model parameters. OCT parameters were available for the HARBOR study only. Therefore, while the data from all ranibizumab studies were used to select the structurally most adequate model, the covariate analysis was limited to HARBOR. Moreover, as there were no sham-treated patients in HARBOR, the disease-related parameters kpr, VAss0 were fixed to their population values estimated when data from all studies were used without including the covariates.
Table 1.
Patient Baseline Characteristics From HARBOR (n = 1097)
| Variable | Definition | Categories/Units | Type of Measurement | Median [min, max]/Proportion |
|---|---|---|---|---|
| AGE | Age | Years | Demographic | 80 [50, 98] |
| SEX | Gender | 0 = male, 1 = female | Demographic | 41% male |
| 59% female | ||||
| BFED | Fibrovascular PED | 0 = absence,a 1 = presence | FFA | 1% Missinga |
| 20% absence | ||||
| 79% presence | ||||
| BFEDA | Fibrovascular PED area | FFA | ||
| BSED | Serous PED | 0 = absence, 1 = presence | FFA | 98% absence |
| 2% presence | ||||
| BSEDA | Serous PED area | Continuous | FFA | 3.04 [0.23, 13.73] |
| TCNV | Type of CNV | 1 = occult, 2 = minimally classic, 3 = predominantly classic | FFA | 38% occult, 46% minimally classic, 16% predominantly classic |
| BCNV | CNV size | Continuous | FFA | 2.76 [0.05, 13.73] |
| BLEA | Leakage size | Continuous | FFA | 3.04 [0.23, 13.73] |
| BLES | Lesion size | Continuous | FFA | 2.82 [0.08, 13.73] |
| BSFTA | Subretinal fibrosis tissue area | Continuous | FFA | 0 [0, 1.46] |
| BTVOL | Total volume | Continuous | OCT | 10.7 [4.1, 18] |
| BCFT | Central foveal thickness | Continuous | OCT | 307.5 [69, 997.5] |
| BCSUBT | Central subfield thickness | Continuous | OCT | 349 [1, 913] |
| BCYST | Cyst | 0 = absence, 1 = presence | OCT | 0.2% missing |
| 39.1% absence | ||||
| 60.7% presence | ||||
| BIRF | Intraretinal fluid | 0 = absence, 1 = presence | OCT | 0.1% missing |
| 0.4% absence | ||||
| 99.5% presence | ||||
| BEDT | Pigment epithelium detachment thickness | Continuous | OCT | 141.5 [0, 1126.5] |
| BCNVT | CNV thickness | Continuous | OCT | 163 [0, 616] |
| BLT | Lesion thickness | Continuous | OCT | 579 [293.5, 1475.5] |
| BCRLTb | Central retinal lesion thickness | Continuous | OCT | 408 [140.5, 1089.5] |
| BCRT | Central retinal thickness | Continuous | OCT | 307.5 [69, 997.5] |
| BCVOL | Cube volume | Continuous | OCT | 10.7 [4.1, 18] |
| BSRFT | Sub retinal fluid thickness | Continuous | OCT | 148.5 [31.5, 704.5] |
FFA, fundus fluorescence angiography; PED, pigment epithelium detachment.
“Absence” = characteristic not present while “missing”= measurement not available.
Additional information on how some of the OCT parameters were defined or measured is provided in the supplementary information file.
The distributions of these baseline characteristics were similar across the different treatment arms of HARBOR.
The likelihood ratio test was used to identify the significant covariates for each parameter of the model. A full model was then developed using the forward inclusion–backward exclusion procedure for all significant covariates. The model development and qualification were guided by the objective function (OFV), which is proportional to the log-likelihood (lower OFV means higher likelihood of describing the data), the goodness-of-fit plots, and the precision of parameter estimates. Note that power analyses or testing hypotheses were not used in the article.
Model Validation
An external validation of the model was performed by predicting typical trends of VA time course of ranibizumab treatment arms from AVENUE and STAIRWAY using only the baseline characteristics of patients. The predictive performance of the model was assessed: observed patients who achieved 20/40 (at least 69 letters) vision at month 9 in AVENUE were compared to model predictions to create a receiver operating characteristic curve (ROC).
Software
Model parameter estimation and model evaluation were done with NONMEM software (Icon Development Solutions, Ellicott City, Maryland, USA) (version 7, level 4, double precision).15 Analysis data sets were created using SAS System for Windows version 9.4 (SAS Institute, Cary, NC, USA). Graphical analyses were performed with S-PLUS version 8 (Statistical Sciences, Seattle, WA, USA).
Results
Evaluation of Dual Effects of Ranibizumab
Short-term effect alone (model 1) and the combination of short-term and long-term effects (model 2) of ranibizumab were investigated. The addition of the long-term effect on top of the short-term effect led to a drop in OFV of 300 points for only 1 additional parameter (Ed50).
There was additional evidence that the dual effects of ranibizumab were needed to adequately describe the VA time course. Indeed, it was expected that, within the same randomized trial, the predicted sham time course of the ranibizumab treatment arms (assuming they had received sham instead of ranibizumab) would be similar to that of the sham comparator. Therefore, the predicted median time course of BCVA under sham injections between the treatment arms of MARINA should be similar (this holds true for PIER too). This could be demonstrated only when the dual effects of ranibizumab were taken into account in the model, as shown in Supplementary Figure S2.
Thus, model 2 was retained as the structurally more adequate model and was subsequently used to test the influence of patient characteristics on treatment response and deterioration of vision.
Covariates Analysis
The covariates analysis was limited to HARBOR. The disease parameters were fixed to their estimated population values in Supplementary Table S1. The influence of the baseline characteristics listed in Table 1 was tested on each of the parameters of model 2.
The final model defined by Equations (6) to (9) includes the covariate models defined in Table 2, which also provides the parameter estimates.
Table 2.
Final Model Parameter Estimates
| Parameter | Covariate | Estimate | RSE | ω22 BSV (RSE) |
|---|---|---|---|---|
| kpr0 | TCNV = 3 or BCYST = 1 | 0.00036 | — | 3.18 (46%) |
| TCNV < 3 and BCYST = 0 | 0.00019 | <1% | ||
| θ9 | Size of BCRLT | 0.063 | <1% | |
| θ2 | 20.1 | — | — | |
| θ7 | VA0 | 2.41 | <1% | |
| θ3 | 0.00318 | <1% | 1.07 (2%) | |
| θ11 | Dose | –0.432 | <1% | |
| θ13 | AGE | 1.14 | <1% | |
| Emax0 | TCNV > 1 and BCYST = 0 | 25.2 | <1% | 0.159 (1%) |
| TCNV = 1 or BCYST = 1 | 21 | <1% | ||
| θ7 | VA0 | 2.41 | <1% | |
| θ9 | Size of BCRLT | 0.063 | <1% | |
| θ13 | AGE | 1.14 | <1% | |
| θ5 | 0.981 | <1% | 2.49 (<1%) | |
| θ8 | BEDT | 0.0771 | <1% | |
| Ed50 (µg/day) | ||||
| θ6 | 9.4 | — | — | |
| Residual error (letter) | ||||
| σ | 4.41 | <1% | — | |
BSV, between-subject variability; RSE, relative standard error.
Overall, the fixed-effect parameters and the between-subject variabilities were estimated with a good precision. The goodness-of-fit plots and visual predictive check (VPC) in Supplementary Figures S3 and S4 respectively show the adequacy of the model to describe the data and to reproduce the median profile and the associated between-patient variability with a slight underprediction of observed 95th percentiles.
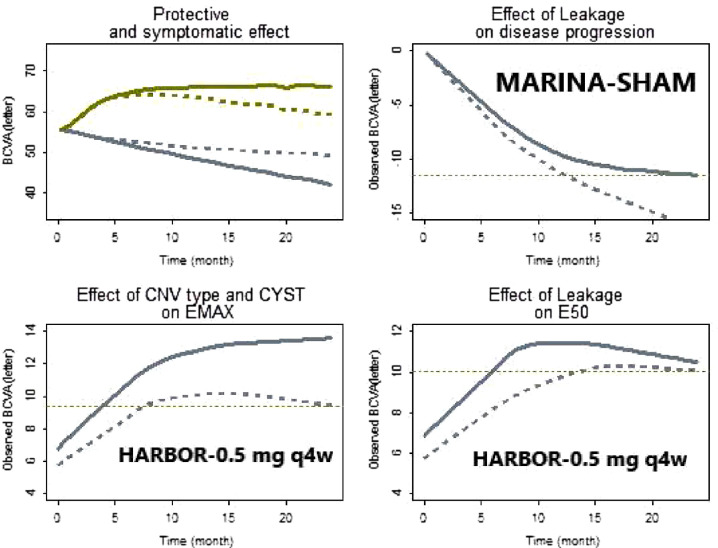
Figure 1 (top left) shows for a typical patient (baseline characteristics set to their median value) the predicted progression, long-term effect alone, short effect alone, and the combination of both effects of ranibizumab 0.5 mg q4w. The E50 of a typical patient was estimated to be ∼10-fold smaller than Ed50. This indicates that ranibizumab is more potent for a short-term restorative effect on VA than for slowing the progression of the disease. More specifically, the 0.5-mg monthly ranibizumab is ∼18 * E50 and therefore corresponds to ∼95% of the maximum short-term effect and only ∼65% of the maximum long-term effect.
Figure 1.
Effect of baseline characteristics on model parameters. Top left: Profiles of a typical patient. Olive line predicted time profile of BCVA change from baseline of the 0.5-mg q4w (protective + symptomatic effects). Gray line represents the predicted visual decay for untreated patients. Dotted gray line is the predicted visual decay or protective effect (not observed) of treated patients. Dotted olive line is the predicted symptomatic effect (not observed) of treated patients. Top right: Gray line observed mean profile for patients with low (≤ median) leakage size. Gray dotted line is the observed mean profile for patients with high (≥ median) leakage size. The latter group shows a faster decay of visual acuity. Bottom left: Gray line observed mean profile of not occult patients without cyst. Gray dotted line is the observed mean profile for the remaining patients. Bottom right: Gray line observed mean profile for patients with low (≤ median) leakage size. Gray dotted line is the observed mean profile for patients with high (≥ median) leakage size.
Plausibility of Baseline Characteristics
Identified baseline characteristics seem plausible from a pathophysiologic context. Additional evidence based on observed data is provided below.
-
–
Influence on kpr: kpr increases with the size of leakage. In Figure 1 (top right), untreated patients with small size of leakage (< median) lost ∼12 letters after 2 years while those with larger size (≥ median) of leakage show a similar decline in just 1 year.
-
–
Influence on E50: E50 increases with the size of leakage or of pigment epithelium detachment thickness. For the same dose, higher E50 translates into longer treatment duration to achieve similar efficacy to lower E50. In Figure 1 (bottom right), patients with small leakage size (< median) have reached 10-letter improvement at ∼month 5 while patients with large leakage size (≥ median) have shown similar improvement only at month 15.
-
–
Influence on Emax: the maximum symptomatic effect increases with the size of central retinal lesion thickness and decreases when the age of patients increases (observed but not shown here). No occult CNV patients without a cyst have a higher Emax showing a difference > ∼5 letters (cf. Fig. 1, bottom left).
Virtual Ranibizumab Treatment Arm
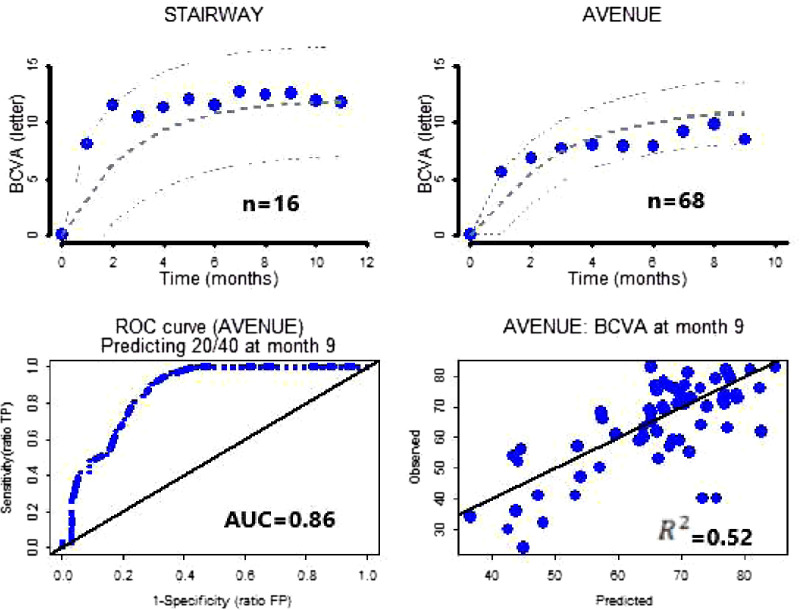
The ranibizumab active control arms of AVENUE and STAIRWAY were used for external validations of the model and of the concept of virtual ranibizumab treatment arm. The individual BCVA time course of patients with nAMD under ranibizumab treatment was predicted using only the seven preidentified baseline characteristics with no other information (similar to what would have been done prior to start of the trials). As shown in Figure 2 (top), the model predictions of ranibizumab mean profiles in both trials were in excellent agreement with the observed data. The predictive performance of the model was tested on the ranibizumab arm of AVENUE by comparing patients who achieved 20/40 (≥69 letters) vision at month 9 with their predictions from baseline. This resulted in an area under the curve (AUC) of 0.86 for the ROC curve and R2 of 0.52 (Fig. 2, bottom). The AUC would reduce to 0.80 if 20/40 had been substituted for 20/50.
Figure 2.
Predictions of ranibizumab treatment arms with baseline data only. Top left: Blue dots are observed mean BCVA over time of ranibizumab q4w from STAIRWAY. Gray dotted lines are predicted mean over time and 90% confidence interval (CI). Top right: Blue dots are observed mean BCVA over time of ranibizumab q4w from AVENUE. Gray dotted lines are predicted mean over time and 90% CI. Bottom left: Blue dotted line is the ROC curve for the ranibizumab treatment arm from AVENUE for patients who have achieved 20/40 vision at month 9. Bottom right: Blue dots are predicted versus observed at month 9 of the ranibizumab treatment arm from AVENUE.
MBMM of Response to Ranibizumab Treatment
The MBMM is defined for any patient as the expected change from baseline of BCVA at 2 years on ranibizumab treatment at a given dose and dosing frequency. This can be likened to a patient's signature based on the model equations and population parameters and is uniquely defined by the seven preidentified baseline characteristics.
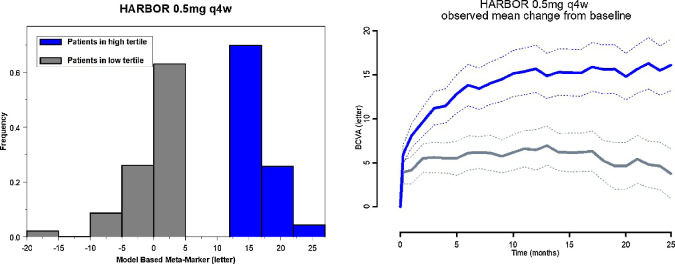
Figure 3 (left) shows the distribution of the MBMM for the low and high tertiles of the 0.5-mg q4w treatment group from HARBOR. Figure 3 (right) shows the corresponding mean change from baseline of BCVA for each group. The group of patients with high MBMM score shows a significantly higher response to treatment. Hence, the MBMM allows a categorization of patients by predicting how well they would respond to ranibizumab treatment.
Figure 3.
Distribution of the MBMM (low and high tertiles) from the 0.5-mg q4w and corresponding observed mean profiles of BCVA. Left: Blue bars are distribution of high-tertile MBMM. Gray bars are distribution of low-tertile MBMM. Right: Blue bold and dotted lines are respectively BCVA mean and 90% CI of the mean of patients in the high tertile of MBMM. Gray bold and dotted lines are respectively BCVA mean and 90% CI of the mean of patients in the low tertile of MBMM.
Discussion
BCVA time course in patients with nAMD on ranibizumab or sham treatment was characterized using a drug-disease model that postulated a dual effect of ranibizumab: a long-term effect that slows down visual decay and a short-term effect that improves VA. The model indicates that ranibizumab is more potent for restoring vision than slowing down progression. The 0.5 mg q4w of ranibizumab reaches a near-maximal short-term effect but only ∼65% of the maximum long-term effect. That is, an increase of ranibizumab dose would not translate into a higher short-term effect but may further slow down the disease and thus increases durability of the effect. This dual effect with different potencies may explain partly why in real-world data, beyond the limited number of injections received by patients, there is decay of efficacy for anti-VEGF in long-term treated patients as there is room for the disease to progress while no further (short-term) improvement can be achieved, suggesting an efficacy ceiling for anti-VEGF. This is probably why several therapies currently under clinical development target simultaneously VEGF and a second pathophysiologic mechanism.16 Thus, the question of the optimal therapy for every patient: patients with insufficient response to anti-VEGF treatment may, for instance, be switched to long-acting therapies such as the ranibizumab Port Delivery System16 or to a dual-targeted drug such as faricimab, while for other patients, the treatment burden may be lowered with an appropriate personal treatment interval. It is therefore critical to identify which patient needs what, when, and how much. There will not be a unique set of predictors. Indeed, many patient characteristics have been associated with anti-VEGF treatment response, but their predictive powers remained to be proven. With the disease heterogeneity and high variability in treatment response, it is likely that no single patient characteristic can predict alone with high accuracy the response to anti-VEGF therapies. For instance, in 1 study,6 this variability in response to anti-VEGF therapies has been associated with dose and/or dosing frequency, while others17,18 postulated that the disease status at entry times into clinical trials is an important factor explaining the high variability observed. In that respect, some findings,8,9 based on small sample sizes of observational studies, are interesting since composite scores of patient characteristics were shown to be predictive of response to ranibizumab treatment. This is line with the findings of the present article. Indeed, the drug-disease model incorporates seven baseline characteristics that are not only plausible in the pathophysiologic context but also supported by the observed data as demonstrated above. The model suggests that the rate of VA decay increases with the size of leakage or of central retinal thickness. Progression of patients without cysts and not predominantly classic CNV is ∼1/2 slower than that of the others. Leakage size also has an influence on ranibizumab potency on short-term effect so that patients with larger size (≥ median) of leakage require longer treatment duration than those patients with smaller size (< median) of leakage. Short-term effect was higher in the no-occult CNV patients with no cyst and with a large size of central retinal lesion thickness. Overall, older patients with cysts and high leakage would progress faster, require longer treatment duration, and show poorer short-term effect. In HARBOR 0.5 mg q4w, the BCVA mean change from baseline of that group of patients (∼50) did not exceed five letters after 2 years of treatment. These patients may be appropriate candidates for different therapy.
Two POC studies held out from the model development were used for further validation. The outcomes of their ranibizumab treatment arms using only the seven preidentified patient characteristics could be predicted with good accuracy. The capacity of predicting response to ranibizumab for a group of patients based only on their baseline characteristics suggests that the model could be used to create a virtual ranibizumab treatment arm in clinical trials or to reduce the size of a ranibizumab active control arm.
The MBMM was introduced as a patient's signature uniquely defined for a given dose and dosing regimen by a composite score of preidentified seven baseline characteristics, allowing a categorization of responses to ranibizumab treatment and prediction of patients with insufficient response. It could be used for patient enrichment in clinical trials.
Supplementary Material
Acknowledgments
Disclosure: C. Diack, Roche (E, F); D. Schwab, Roche (E, F); V. Cosson, Roche (E, F); V. Buchheit, Roche (E, F); N. Mazer, Roche (E, F); N. Frey, Roche (E, F)
References
- 1. Bourne RR, Steven GA, White RA, et al.. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013; 1: e339–e349. [DOI] [PubMed] [Google Scholar]
- 2. Van Lookeren Campagne M, LeCouter J,Yaspan BL,. Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014; 232: 151–164. [DOI] [PubMed] [Google Scholar]
- 3. Shirinifard A, James AG, Maciej S, et al.. Adhesion failures determine the pattern of choroidal neovascularization in the eye: a computer simulation study. PLoS Comput Biol. 2012; 8: e1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoerster R, Philipp SM, Sitnilska V, et al.. Fibrovascular pigment epithelial detachment is a risk factor for long-term visual decay in neovascular age-related macular degeneration. Retina. 2014; 34(9): 1767–1773. [DOI] [PubMed] [Google Scholar]
- 5. Burzykowski T, Buyse M.. The correlation structure of longitudinal measurements of vision in patients with macular degeneration. Pharma Stat. 2011; 10: 115–121. [DOI] [PubMed] [Google Scholar]
- 6. Holz FG, Korobelnik JF, Lanzetta Pet al.. The effects of a flexible visual acuity–driven ranibizumab treatment regimen in age-related macular degeneration: outcomes of a drug and disease. Invest Ophthalmol Vis Sci. 2010; 51(1): 405–412. [DOI] [PubMed] [Google Scholar]
- 7. Mulyukov Z, Weber S, Pigeolet E, et al.. Neovascular age-related macular degeneration: a visual acuity model of natural disease progression and ranibizumab treatment effect. CPT Pharmacometrics Syst Pharmacol. 2018; 7(10): 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez-Buendia L, Delgado-Tirado S, Sanabria MR, Fernandez I, Coco RM.. Predictive models of long-term anatomic outcome in age-related macular degeneration treated with as-needed ranibizumab. BMC Ophthalmol. 2017; 17(1): 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah AR, Williams S, Baumal CR, Rosner B, Duker JS, Seddon JM.. Predictors of response to intravitreal anti–vascular endothelial growth factor treatment of age-related macular degeneration. Am J Ophthalmol. 2016; 163: 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown DM, Kaiser PK, Michels M, et al.. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355(14): 1432–1444. [DOI] [PubMed] [Google Scholar]
- 11. Rosenfeld PJ, Brown DM, Heier JS, et al.. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355(14): 1419–1431. [DOI] [PubMed] [Google Scholar]
- 12. Regillo CD, Brown DM, Abraham P, et al.. Randomized double-masked sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J. Ophthalmol. 2008; 145: 239–248. [DOI] [PubMed] [Google Scholar]
- 13. Allen CH, Busbee BG, Regillo CD, et al.. Twenty-four-month efficacy and safety of 0.5mg or 2mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014; 121(11): 2181–2192. [DOI] [PubMed] [Google Scholar]
- 14. Jacqmin P, Snoeck E, Van Schaick EA, et al.. Modelling response time profiles in the absence of drug concentrations: definition and performance evaluation of the K-PD model. J Pharmacokinet Pharamacodyn. 2007; 34(1): 57–85. [DOI] [PubMed] [Google Scholar]
- 15. Beal S, Sheiner L, eds. NONMEM Users Guides, NONMEM Projects Group. San Francisco: University of California; 1998. [Google Scholar]
- 16. Adamis AP, Brittain CJ, Dandekar A, Hopkins JJ.. Building on the success of anti-vascular endothelial growth factor therapy: a vision for the next decade. Eye. 2020; 34: 1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah AR, Del Priore LV.. Natural history of predominantly classic, minimally classic, and occult subgroups in exudative age-related macular degeneration. Ophthalmology. 2009; 116(10): 1901–1907. [DOI] [PubMed] [Google Scholar]
- 18. Liu T, Shah AR, Del Priore LV.. Progression of lesion size in untreated eyes with exudative age-related macular degeneration: a meta-analysis using Lineweaver-Burk plots. JAMA Ophthalmol. 2013; 131(3): 335–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.