Abstract
Background
CDK4/6 inhibitors have become the standard for first-line treatment of metastatic luminal breast cancer based on consistent data from several phase 3 trials demonstrating clinically meaningful improvement of progression-free as well as overall survival. In addition, they are about to become a part of adjuvant treatment for patients with high-risk luminal disease based on positive results from the first randomized phase 3 trial on abemaciclib. Nevertheless, the majority of patients with advanced or metastatic luminal breast cancer and prospectively a relevant proportion of patients treated in the adjuvant setting will eventually develop resistance to this endocrine based combination within 12–36 months, depending on the line of treatment.
Conclusion
Potential subsequent therapies include PI3K inhibitors, mTOR inhibitors, endocrine monotherapy, PARP inhibitors, and chemotherapy. However, these therapies have mainly been developed in the pre-CDK4/6 inhibitor era and little is known about potential cross-resistance. The concept of continuing CDK4/6 inhibition beyond progression is supported by some preclinical data, but to date there is very limited clinical evidence to support this strategy. Therefore, treatment of metastatic luminal breast cancer after progression on CDK4/6 inhibitors remains a challenge.
Key Messages
Here we review current evidence from pro- and retrospective studies and give an outlook on future developments with respect to novel therapeutic agents, including oral SERD and AKT inhibitors, which have the potential to change the therapeutic landscape in the future. Furthermore, clinical treatment algorithms and current research will also be discussed.
Keywords: PI3K inhibitors, mTOR inhibitors, Oral selective estrogen receptor degraders, AKT inhibitors
Introduction
Metastatic breast cancer (MBC) accounts for more than 600,000 deaths per year worldwide and it is the leading cause of cancer mortality among women [1]. Classification of breast cancer subtypes, initially based on gene expression profiling [2, 3], routinely relies on the expression of estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor 2 (HER-2), as well as the proliferation index Ki67, assessed by immunohistochemistry. In combination with a limited number of targetable mutations, this allows for individualized therapeutic strategies [4]. Up to 70% of MBC patients have luminal breast cancer, defined by expression of ER and HER-2 negativity [5], with a median overall survival (OS) as long as 57 months [6]. Estrogen deprivation, including selective ER modulators, such as tamoxifen [7], aromatase inhibitors (AI) like letrozole [8], anastrozole [9], and exemestane [10], and selective ER degraders (SERD) like fulvestrant [11], has long been the hallmark of treatment, albeit with a limited clinical efficacy. In endocrine-naive metastatic luminal breast cancer (MLBC) patients receiving anastrozole or fulvestrant as first-line treatment, the median progression-free survival (PFS) was 13.8 and 16.6 months [12], respectively. The PFS is considerably shorter, i.e., only 6.5 months, in endocrine-pretreated patients receiving fulvestrant as second-line therapy [11]. In BOLERO-2, addition of the mTORC1 inhibitor everolimus to exemestane in patients resistant to a nonsteroidal AI demonstrated a significant PFS benefit of 6.5 months [13] but fell short of demonstrating a statistically significant OS benefit. Subsequently, dual mTORC1/2 inhibitors like vistusertib have been developed to circumvent possible negative feedback loops that might result in resistance to mTORC1 inhibition. However, vistusertib did not lead to improved efficacy when directly compared to everolimus [14].
With the introduction of the CDK4/6 inhibitors (CDK4/6i) palbociclib, ribociclib, and abemaciclib added to the backbone of endocrine therapy, response and survival rates substantially improved in both first- and second-line treatments. All 3 CDK4/6i have consistently increased PFS in several phase 3 clinical trials [15, 16, 17, 18, 19, 20]. For example, abemaciclib added to a nonsteroidal AI as first-line treatment for postmenopausal women led to a PFS improvement of 13.4 months in the MONARCH-3 study [19]. In addition, ribociclib and abemaciclib have provided a statistically significant OS benefit in the first-line setting as well as in the second-line setting within MONALEESA-3, MONALEESA-7, and MONARCH 2 [20, 21, 22, 23].
These excellent results have rendered CDK4/6i combined with endocrine treatment as the new standard of care (SOC) for both endocrine-naive and endocrine-pretreated MLBC patients. In the clinical routine, AI are the preferred endocrine backbone among de novo MLBC patients or among patients with disease recurrence >12 months upon completion of adjuvant endocrine treatment. In a direct comparison of letrozole and fulvestrant as the endocrine backbone for palbociclib as first-line therapy in the PARSIFAL study, fulvestrant did not demonstrate improved efficacy or noninferiority compared to letrozole [24]. Fulvestrant might be preferred as the endocrine combination partner in patients with disease progression on AI or with recurrence <12 months after completion of adjuvant endocrine treatment [4]. However, fulvestrant also functions as the endocrine partner for potential subsequent therapies after CDK4/6i, as it is approved in combination with alpelisib in PIK3CA-mutant patients. Therefore, strategic considerations about further treatment lines will also influence the choice of the best endocrine combination partner for CDK4/6i in an individual patient. In any case, selection of the most appropriate CDK4/6i should consider the toxicity profile of each compound (gastrointestinal toxicity for abemaciclib and hepatotoxicity and QTcF prolongation for ribociclib) with regard to the patient's comorbidities. Under special circumstances, selected patients with a low tumor burden or multiple limiting comorbidities and difficult access to their treating physicians may also be considered for endocrine monotherapy [4]. On the other hand, chemotherapy is still the SOC in patients presenting with a visceral crisis [4]. Today, the vast majority of MLBC patients receive CDK4/6i as first-line treatment and the time to subsequent chemotherapy has been substantially increased, thus reducing systemic toxicities and the improving quality of life [15].
Principal Mechanisms of Resistance to CDK4/6i
Despite this significant clinical progress, the majority of patients will show tumor progression within up to 36 months of CDK4/6i treatment [15, 16, 18, 20, 23]. Preclinical models suggest multiple resistance mechanisms against CDK4/6i in MLBC involving either hyperactivation of the CDK4/6 G1 checkpoint kinase, thus mandating the development of more potent CDK4/6i, or bypassing of the CDK4/6 G1 checkpoint kinase through CCNE1/CDK2 leading to retinoblastoma protein (Rb) phosphorylation or through loss of target by acquired loss-of-function RB1 mutations [25]. In addition, ineffective ER inhibition by de novo ERα (ESR1) driver mutations [26], or due to constitutive activation of the PI3K/AKT/mTOR signal cascade through extensive cross-talk between the ER and HER-2, IGF-1R, or FGFR1 pathways [27, 28], has been recognized as being of clinical importance. Under treatment with CDK4/6i, acquired ERS1 mutations, detected in up to 30% of patients, primarily reflect a resistance to endocrine therapies [26, 29], whereas de novo RB1 mutations, found in up to 10% of CDK4/6i-pretreated patients, indicate CDK4/6i resistance [25, 26, 30]. Increased CCNE1 mRNA levels, documented among half of the PALOMA-3 patients, were shown to correlate with a short median PFS of 7.6 months and, conversely, low CCNE1 mRNA levels correlated with an increased PFS of 14.1 months under palbociclib [31], suggesting CCNE1 expression as a potential predictive biomarker for CDK4/6 inhibition. This supports the need for development of dual CDK2- and CDK4/6 inhibitors, such as PF-06873600, currently being tested in a phase 1/2 trial (NCT03519178; Table 1). Interestingly, neither CCND1 nor CDK4 or CDK6 amplification could be associated with PFS in the PALOMA-3 trial [31], although resistance mechanisms through CDK6 overexpression via the FAT1/Hippo pathway have been described [32, 33]. In addition, acquired resistance mechanisms specific to certain CDK4/6i have also been postulated; abemaciclib has been demonstrated to maintain in vitro anti-tumor activity in Rb-deficient, palbociclib/ribociclib-resistant cell lines [34] since it can effectively inhibit further CDK complexes, such as CDK7/Cyclin H1 and CDK9/Cyclin T1 [35, 36]. This preclinical finding supports the rationale for continuing CDK4/6 inhibition beyond progression, e.g., with an alternative CDK4/6i.
Table 1.
Overview of current clinical phase 1–3 trials in MLBC
| Trial | Phase | Indication | Regimen | Status | Conclusions/remarks | Reference |
|---|---|---|---|---|---|---|
| NCT03519178 | 1/2 | Pretreated MLBC | PF-06873600 alone and in combination with endocrine treatment | Recruiting | Evaluates the safety of dual CDK2 and CDK4/6i PF-06873600 alone and in combination with endocrine treatment among CDK4/6i-pretreated patients | − |
| NCT02632045 (MAINTAIN) | 2 | Pretreated MLBC | Ribociclib/fulvestrant vs. placebo/fulvestrant | Recruiting | Evaluates the efficacy of ribociclib/fulvestrant after progression on CDK4/6i | − |
| NCT02738866 | 2 | Pretreated MLBC | Palbociclib/fulvestrant | Recruiting | Evaluates the efficacy of palbociclib/fulvestrant after progression on palbociclib/AI | − |
| NCT02778685 | 2 | MLBC | Pembrolizumab/palbociclib/letrozole | Recruiting | Evaluates the efficacy of pembrolizumab/palbociclib/letrozole among treatment-naive patients | − |
| NCT03147287 (PACE) | 2 | Pretreated MLBC | Avelumab/palbociclib/fulvestrant vs. palbociclib/fulvestrant vs. fulvestrant | Recruiting | Evaluates 3 regimens after progression: − on CDK4/6i in the metastatic setting − <12 months after completion of CDK4/6i in adjuvant setting |
|
| NCT03294694 | 1 | Pretreated MLBC (cohort B) | PDR001 (spartalizumab)/ribociclib/fulvestrant | Recruiting | Evaluates the safety of anti-PD-1 spartalizumab in combination with ribociclib/fulvestrant among CDK4/6i pretreated patients (cohort B) | − |
| NCT03280563 (MORPHEUS HR+BC) | 1/2 | Pretreated MLBC | Atezolizumab/entinostat vs. atezolizumab/fulvestrant vs. atezolizumab/abemaciclib/fulvestrant vs. atezolizumab/ipatasertib vs. atezolizumab/ipatasertib/fulvestrant vs. atezolizumab/bevacizumab/endocrine treatment | Recruiting | Evaluates the ORR in multiple regimens among CDK4/6i-pretreated patients | − |
| NCT02732119 (TRINITY-1) | 1/2 | Pretreated MLBC | Ribociclib/everolimus/exemestane | Completed | Evaluated the efficacy of ribociclib/everolimus/exemestane after progression on CDK4/6i | 50 |
| NCT03099174 | 1 | Untreated MLBC (cohort D) Pretreated MLBC (cohorts D and F) | Xentuzumab/abemaciclib/fulvestrant | Recruiting | Evaluates the safety and efficacy of anti-IGF-1/2 xentuzumab combined with abemaciclib/fulvestrant in: − de novo MLBC after progression on AI (cohort D) − untreated patients <12 months of AI completion in the adjuvant setting (cohort D) − AI progression when recurrence occurs >12 months of AI completion in the adjuvant setting (cohort D) − progression on CDK4/6i (cohort F) |
51 |
| NCT02684032 | 1 | Untreated MLBC (cohort A) Pretreated MLBC (cohorts B, C, and D) | Gedatolisib/palbociclib/letrozole vs. gedatolisib/palbociclib/fulvestrant vs. fulvestrant | Active, not recruiting | Evaluated the safety and MTD of the dual mTOR/PI3K inhibitor gedatolisib in multiple combinations in treatment-naive patients (cohort A), CDK4/6i-naive patients (cohort B), and CDK4/6i-pretreated patients (cohorts C/D) | 52 |
| NCT03238196 | 1 | Pretreated MLBC | Erdafitinib/palbociclib/fulvestrant | Recruiting | Evaluates the safety and tolerability of the FGFR inhibitor erdafitinib in combination with palbociclib/fulvestrant in CDK4/6i-pretreated patients with FGFR-amplified MLBC | − |
| NCT04504331 | 1 | Pretreated MLBC (cohorts 1 and 2) | Infigratinib/tamoxifen (cohort 1), infigratinib/palbociclib/fulvestrant (cohort 2) | Recruiting (cohort 1), planned (cohort 2) | Evaluates the safety and tolerability of the FGFR inhibitor infigratinib in multiple combinations in FGFR-altered MLBC | − |
| NCT03584009 (VERONICA) | 2 | Pretreated MLBC | Venetoclax/fulvestrant vs. fulvestrant | Completed | Evaluated the efficacy of fulvestrant with or without the BCL-2 inhibitor venetoclax after progression on CDK4/6i | − |
| NCT03900884 (PALVEN) | 1 | Pretreated BCL-2 positive MLBC | Venetoclax/palbociclib/letrozole | Recruiting | Evaluates the safety and tolerability of the BCL-2 inhibitor venetoclax in combination with palbociclib/letrozole in pretreated, CDK4/6i-naive patients | − |
| NCT02860000 | 2 | Pretreated MLBC | Alisertib/fulvestrant vs. alisertib | Completed | Evaluated the efficacy of the AURKA inhibitor alisertib with or without fulvestrant in endocrine-refractory MLBC | − |
| NCT01872260 | 1/2 | Untreated MLBC (arms 1, 2, 3, and 4) | Ribociclib/letrozole vs. alpelisib/letrozole vs. ribociclib/alpelisib/letrozole | Completed | Evaluated the safety, tolerability, and preliminary clinical antitumor activity of the 3 combinations in treatment-naive patients | − |
| NCT03006172 | 1 | Untreated PIK3CA-mutant MLBC (arms B and E) Pretreated PIK3CA-mutant MLBC (arms C and D) | arm B: GDC-0077 (inavolisib)/palbociclib/letrozole, arm E: inavolisib/palbociclib/fulvestrant, arm C: inavolisib/letrozole, arm D: GDC-0077/fulvestrant | Recruiting | Evaluated the safety, tolerability, and preliminary clinical anti-tumor activity of the PI3K inhibitor inavolisib in combination with palbociclib/letrozole or fulvestrant in CDK4/6i-naive patients (arms B and E) or in combination with letrozole or fulvestrant in CDK4/6i-pretreated patients (arms C and D) | − |
| NCT04191499 (IN AVO 120; WO41554) | 2/3 | Untreated PIK3CA-mutant MLBC | Inavolisib/palbociclib/fulvestrant vs. palbociclib/fulvestrant | Recruiting | Evaluates the efficacy and safety of inavolisib in combination with palbociclib/fulvestrant vs. palbociclib/fulvestrant in PIK3CA-mutant treatment-naive MLBC with progression after <12 months since completion of adjuvant endocrine therapy | − |
| NCT03939897 | 1/2 | Pretreated MLBC | Phase 1: copanlisib/abemaciclib/fulvestrant, phase 2: copanlisib/abemaciclib/fulvestrant vs. abemaciclib/fulvestrant | Suspended | Evaluates the efficacy and safety of the PI3Kδ inhibitor copanlisib in combination with abemaciclib/fulvestrant in CDK4/6i- and PI3K inhibitor-pretreated patients (phase 1) and in CDK4/6i- and PI3K inhibitor-nonpretreated patients (phase 2) | − |
| NCT03778931 (EMERALD) | 3 | Pretreated MLBC | RAD1901 (elacestrant) vs. SOC (AI or fulvestrant) | Active, not recruiting | Compared PFS between oral SERD elacestrant and SOC in CDK4/6i-pretreated patients | − |
| NCT02569801 (HydranGea) | 2 | Pretreated MLBC | GDC-0810 (brilanestrant) vs. fulvestrant | Withheld | Evaluated the efficacy, safety, and tolerability of the oral SERD brilanestrant vs. fulvestrant in ΑI-refractory MLBC | − |
| NCT02734615 | 1 | Pretreated MLBC | LSZ102 vs. LSZ102/ribociclib vs. LSZ102/alpelisib | Completed | Evaluated the safety and tolerability of the oral SERD LSZ102 alone or in combination with alpelisib or ribociclib in fulvestrant- and/or CDK4/6i-pretreated MLBC | 119 |
| NCT03284957 (AMEERA-1) | 1/2 | Pretreated MLBC | SAR 439859 (amcenestrant) alone vs. in combination with palbociclib, midazolam or alpelisib | Recruiting | Evaluates the safety, tolerability, and preliminary clinical antitumor activity of the oral SERD amcenestrant in multiple combinations in CDK4/6i-pretreated MLBC | 116 |
| NCT04059484 (AMEERA-3) | 2 | Pretreated MLBC | Amcenestrant vs. SOC endocrine monotherapy | Recruiting | Compares PFS between amcenestrant and SOC endocrine monotherapy in CDK4/6i-pretreated MLBC | − |
| NCT04478266 (AMEERA-5) | 3 | Untreated MLBC | Amcenestrant/palbociclib vs. letrozole/palbociclib | Recruiting | Compares PFS between amcenestrant/palbociclib and letrozole/palbociclib in treatment-naive MLBC | − |
| NCT04576455 | 2 | Pretreated MLBC | GDC-9545 (giredestrant) vs. SOC endocrine monotherapy | Recruiting | Compares PFS between the oral SERD giredestrant and the SOC endocrine monotherapy in CDK4/6i-pretreated MLBC | |
| NCT04546009 (BO41843) | 3 | Untreated MLBC | Giredestrant/palbociclib vs. letrozole/palbociclib | Recruiting | Compares PFS between giredestrant/palbociclib and letrozole/palbociclib in treatment-naive MLBC | − |
| NCT04188548 (EMBER) | 1 | Pretreated MLBC | LY3484356/abemaciclib/AIs (part A) vs. LY3484356 vs. LY3484356/alpelisib vs. LY3484356/everolimus (part B) | Recruiting | Evaluates the safety and tolerability of the oral SERD LY3484356 combination with abemaciclib/AI in CDK4/6i-naive patients (part A), or alone, with alpelisib or everolimus in CDK4/6i-pretreated patients (part B) | − |
| NCT04305496 (CAPItello-291) | 3 | Pretreated MLBC | Capivasertib/fulvestrant vs. placebo/fulvestrant | Recruiting | Compares PFS between AKT inhibitor capivasertib in combination with fulvestrant vs. fulvestrant in CDK4/6i-pretreated MLBC | − |
| NCT03959891 (TAKTIC) | 1 | Pretreated MLBC | Ipatasertib/fulvestrant vs. ipatasertib/letrozole vs. ipatasertib/palbociclib/fulvestrant | Recruiting | Evaluates the safety and tolerability of the AKT inhibitor ipatasertib in CDK4/6i-pretreated, PI3K inhibitor-naive MLBC | − |
| NCT04060862 (IPA Tunity 150) | 3 | Untreated MLBC | Ipatasertib/palbociclib/fulvestrant | Recruiting | Compares PFS in ipatasertib in combination with palbociclib/fulvestrant vs. palbociclib/fulvestrant in untreated patients | − |
| NCT 04256941 (INTERACT) | 2 | Untreated MLBC | Palbociclib or ribociclib or abemaciclib with AI or fulvestrant | Recruiting | Assesses PFS under multiple regimens in untreated MLBC patients with ESR1 activating mutations | − |
| NCT04534283 | 2 | Tumors harboring pathogenic alterations in BRAF, RAF1, MEK1/2, ERK1/2, and NF1 | LY3214996/abemaciclib | Recruiting | Assesses the clinical efficacy of the ERK1/2 inhibitor LY3214996 in combination with abemaciclib | − |
CDK4/6i beyond Progression
In PALOMA-3, four percent of the patients in the palbociclib arm received CDK4/6i beyond progression [37]. Real-world data support this observation. In an analysis of 525 patients who received further systemic therapies after CDK4/6i progression, 12.3% of those having CDK4/6i in combination with an AI as first-line therapy were treated with a CDK4/6i in the subsequent line [38]. To some extent, the concept of “treatment beyond progression” with CDK4/6i has therefore entered routine clinical practice despite the fact that there is only very limited clinical evidence to support this strategy so far.
A multicenter analysis of 58 MLBC patients receiving abemaciclib as monotherapy or in combination with endocrine treatment after progression on palbociclib suggested some clinical benefit based on a median PFS of 5.8 months and a treatment duration of 6 months or longer in one third of the patients [39]. Two further retrospective analyses of small patient cohorts receiving abemaciclib as a monotherapy [40] or in combination with endocrine therapy [41] in the same clinical setting seem to confirm a limited clinical activity of abemaciclib beyond CDK4/6i progression, with a median PFS up to 7.0 months. Continuation of palbociclib beyond progression, while switching the endocrine therapy, was evaluated in a small, retrospective, single-institution study [42]. The estimated median PFS for the entire duration while on CDK4/6i was 23.5 months (95% CI 12.8–27.8), of which 11.8 months (95% CI 5.34–13.13) was the median PFS beyond the first progression. The median OS from first-line CDK4/6i treatment was 45.4 months [42]. A retrospective analysis from MSKCC evaluated 135 patients receiving more than 2 lines of CDK4/6i. Patients who discontinued their first CDK4/6i due to progression had a relatively short duration of the second CDK4/6i therapy, with a median time to subsequent therapy of 19.9–22.2 weeks. The data also provide evidence of radiologically demonstrated responses in some of the patients [43]. In current consensus guidelines, no routine use of CDK4/6i beyond progression in MLBC is recommended [4]. Nevertheless, participation in clinical trials is strongly recommended. This question is currently addressed by phase 2 trials, like MAINTAIN and NCT02738866, investigating the benefit of ribociclib and palbociclib added to fulvestrant as a second-line treatment after progression under upfront CDK4/6i (Table 1).
Investigational CDK4/6i Combinations after CDK4/6i Progression
Preclinical models suggest an interaction of checkpoint inhibitors with CDK4/6i through inhibition of proteasome-mediated PD-L1 degradation via SPOP, a cullin 3 E3 ubiquitin ligase adaptor protein [44], as well as through direct stimulation of PD-1-expressing T cells by CDK4/6i, resulting in an enhanced in vitro intratumoral T-cell infiltration [45]. The first promising results have come from a phase 1/2 open-label single-arm study investigating palbociclib and letrozole in combination with pembrolizumab in CDK4/6i-pretreated patients [46]. Further checkpoint inhibitors like spartalizumab (PDR001), avelumab, or atezolizumab in combination with CDK4/6i and endocrine therapy are currently being investigated in several clinical trials (NCT03294694, PACE, and MORPHEUS HR + BC; Table 1).
The synergistic activity between CDK4/6i and PI3K or mTOR inhibitors observed in vitro appears to be mediated by p21-mediated cell quiescence [47], cell senescence [48], or even augmentation of tumor-infiltrating cytotoxic NK cells, CD4+ and CD8+ T cells, and suppression of Tregs [49]. First clinical data from a prospective trial (NCT02871791) evaluating palbociclib in combination with exemestane and everolimus in CKD4/6i-pretreated MLBC patients revealed substantial systemic toxicities including high-grade mucositis and neutropenia among 15.6 and 71.9% of the patients, respectively. Poor overall response rates (ORR) and a median PFS of 3.8 months precluded further investigation of this combination [50]. In contrast, the phase 1/2 TRINITY-1 trial, evaluating ribociclib in combination with exemestane/everolimus in the same clinical setting, revealed a manageable toxicity profile and demonstrated a clinical benefit with a 1-year PFS of 33% [51]. Alternative strategies of concurrent CDK4/6 and PI3K/AKT/mTOR pathway blockade beyond CDK4/6i progression include the monoclonal antibody xentuzumab, which binds IGF-1 and IGF-2 [52] and thus suppresses IGF-1R and IR-A signaling upstream of the mTOR/PI3K pathway, or dual mTOR/PI3K inhibitors like gedatolisib [53], and are currently being investigated in several phase 1 trials (Table 1).
Preclinical data also demonstrate an association of FGFR1 amplification, detectable among 15% of MLBC patients with endocrine resistance [54]; treatment of FGFR-amplified breast cancer cells with FGF-2 strongly induces CCND1 expression and may thus lead to an escape from CDK4/6 inhibition [55]. Among patients in the MONALEESA-2 study, FGFR1 amplification detected in ctDNA was associated with a shorter PFS under ribociclib [56]. Initial studies failed to demonstrate a significant activity of the FGFR1–3 inhibitors lucitanib [57] and dovitinib [58] in combination with fulvestrant in endocrine-resistant patients. Clinical development is now focusing on novel FGFR inhibitors, such as erdafitinib, which is currently being investigated in combination with palbociclib and fulvestrant in CDK4/6i-pretreated patients (Table 1). Preclinical evidence also suggests interactions between the CDK4/6/RB1 axis and AURKA [59], PLK1 [60], and BCL2 [61] inhibitors. Currently, the BLC-2 inhibitor venetoclax and the AURKA inhibitor alisertib are being investigated in clinical trials (VERONICA, PALVEN, and NCT02860000), promising further advances in the treatment of MLBC beyond CDK4/6i progression.
PI3K Inhibitors
The latest advance in the treatment of MLBC is the FDA and EMA approval of the first α-selective PI3K inhibitor alpelisib in 2019/2020 for postmenopausal MLBC patients harboring PIK3CA mutations. PIK3CA encodes the isoform p110α, i.e., the catalytic subunit of class IA PI3K [27]. Somatic, heterozygous gain-of-function PIK3CA mutations lead to a constitutive activation of PI3K and are detectable in up to 47% of patients with MLBC [62, 63, 64]. More than 80% of PIK3CA mutations occur within the helical domain (exon 9, mostly PIK3CAE542K and PIK3CAE545K) and the kinase domain (exon 20, mostly PIK3CAH1047R) [62, 64]. PIK3CA mutations are early events in breast cancer pathogenesis [65, 66]. PIK3CA mutations were detectable among a third of the patients in the PALOMA-3 study and in most cases they persisted throughout the study treatment, with up to 6% of the subjects developing de novo PIK3CA mutations under treatment in both study arms [26]. PIK3CA mutations did not impact the efficacy of CDK4/6i [15]. Similarly, in the BOLERO-2 study, i.e., the registrational trial for everolimus, no association between PIK3CA mutations and response to everolimus/exemestane among MLBC patients could be demonstrated [67]. In the SAFIR02 trial, PIK3CA mutations conferred resistance to chemotherapy and were associated with a poorer median OS [68]. Clinical development of first-generation pan-PI3K inhibitors like pictilisib [69], buparlisib [70, 71], and the α-selective inhibitor taselisib [72] was halted due to significant systemic toxicities, which outweighed the agents' therapeutic benefit. In contrast, in the phase 3 SOLAR-1 trial, the α-selective alpelisib demonstrated significant activity with a manageable toxicity profile, mainly consisting of metabolic, dermatologic, and gastrointestinal toxicities [73]. This international, randomized, double-blind study investigated fulvestrant in combination with alpelisib in postmenopausal patients. Enrollment preferentially focused on patients with a secondary endocrine resistance with progression >6 months upon first-line palliative AI treatment in de novo MLBC or recurrence after ≥24 months of adjuvant endocrine therapy or relapse <12 months after the completion of adjuvant AI therapy. Only 13–14% of the PIK3CA-mutant patients and 22–27% of the PIK3CA wild-type patients had a primary endocrine resistance and only 6% of the patients were pretreated with CDK4/6i, whereas patients with prior palliative chemotherapy, fulvestrant, or mTOR inhibitors were not eligible. Subjects with a visceral crisis, inflammatory breast cancer, a fasting plasma glucose level ≥140 mg/dL and/or glycosylated hemoglobin (HbA1c) ≥6.4% were excluded. Mutational PIK3CA analysis was predominantly performed on archived tumor tissue (>90% of patients) and focused on hotspot exon 7, 9, and 20 mutations. Median PFS was the primary endpoint in the PIK3CA-mutant cohort. Herein, the median PFS was 11.0 months for alpelisib versus 5.7 months for the control arm. In the PIK3CA-mutant cohort, the 12-months PFS was 46.3% for alpelisib versus 28.4% in the PIK3CA wild-type cohort. In the PIK3CA-mutant cohort, alpelisib improved the ORR to 26.6%, with the majority of patients showing a partial remission. The clinical benefit rate at 24 weeks was also increased to 61.5% with the addition of alpelisib. OS, the key secondary endpoint, was prolonged by 7.9 months in the PIK3CA-mutant cohort; however, this did not reach statistical significance (HR = 0.86; 95% CI 0.64–1.15; p = 0.15) [74]. Moreover, 74 and 62% of the patients had dose interruptions and dose adjustments of alpelisib, respectively, whereas a fourth of the patients permanently discontinued alpelisib due to intolerable side effects [73]. These primarily consisted of high-grade hyperglycemia, commonly developing within the first weeks of treatment, as well as diarrhea and rash, all manageable with well-defined therapeutic algorithms [75, 76, 77]. Antidiabetic management of alpelisib-induced hyperglycemia is of special importance; whereas metformin and gliflozins are the preferable agents [77], administration of insulin should be avoided since it has been demonstrated to suppress PI3K inhibition by reactivating the PI3K/mTOR cascade [77].
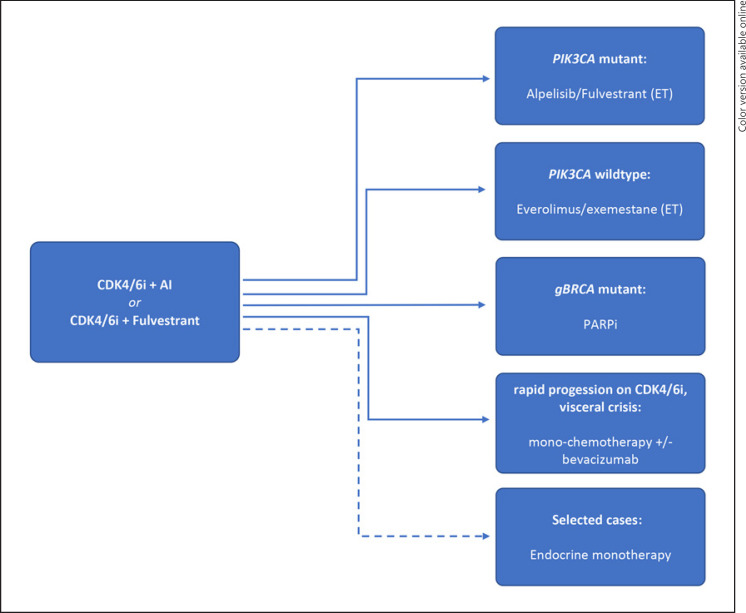
Since only 6% of SOLAR-1 patients were pretreated with CDK4/6i, clarification of the role of PI3Kα inhibition after CDK4/6i therapy is of importance. First data supporting the efficacy of PIK3CA inhibition after CKD4/6 inhibition has been provided by the phase II BYLIEVE trial, testing alpelisib within multiple cohorts and in combination with letrozole or fulvestrant for PIK3CA-mutant MLBC patients after CDK4/6i progression (cohort A, 121 patients) [78]. Preliminary results for this cohort have demonstrated a 6-month PFS of 50.4% for the combination of alpelisib and fulvestrant [79] and a median PFS of 7.3 months, superior to an extrapolated PFS of 3.6 months based on real-world data of patients after progression on CDK4/6i [80]. This supports alpelisib as second-line treatment after CDK4/6 inhibition in PIK3CA-mutant MLBC patients [81] (Fig. 1). Although alpelisib has received FDA approval for PIK3CA-mutant MLBC patients upon progression on or after an endocrine-based regimen, the EMA approval is limited for progression upon an endocrine monotherapy. This complicates the use of alpelisib in patients with de novo PIK3CA-mutant MLBC progressing on first-line CDK4/6i and encourages their recruitment into running clinical trials. Combined inhibition of PI3Kα and the CDK4/6/RB1 axis [82], with the aim of preventing or delaying the development of resistance, has been investigated for the combination of alpelisib, ribociclib, and letrozole in a phase 1 trial (NCT01872260) and is currently being evaluated in the phase 2/3 INAVO120 study for inavolisib (GDC-0077), in combination with palbociclib and fulvestrant (Table 1).
Fig. 1.
Proposed treatment algorithms of MLBC beyond CDK4/6i progression. ET, endocrine therapy.
mTOR Inhibitors
The combination of everolimus and exemestane has long been approved by the FDA and the EMA for patients progressing on or after a nonsteroidal AI. In addition, everolimus in combination with tamoxifen or fulvestrant has been demonstrated to be superior to endocrine therapy alone in randomized trials (TAMRAD and PrE0102), based on significant improvement of the PFS [83, 84]. However, none of these studies has shown a significant OS benefit. In the registrational BOLERO-2 trial everolimus significantly prolonged the investigator-assessed PFS, i.e., the primary endpoint, from 3.2 to 7.8 months (HR = 0.45; 95% CI 0.38–0.54; p < 0.0001) [85] and numerically prolonged OS by 4.4 months, but without reaching statistical significance (HR = 0.89; 95% CI 0.73–1.10; p = 0.14) [86]. In the PALOMA-3 trial, 16% of the patients in the palbociclib arm received everolimus/exemestane as a first subsequent treatment, with a median PFS of 4.3 months [37]. A small (n = 33) single-institution retrospective analysis in patients treated with everolimus and exemestane demonstrated comparable PFS and OS, regardless of prior CDK4/6i exposure [87]. Two further retrospective studies indicated a median PFS between 4.2 and 5.7 months for everolimus-based therapies in CDK4/6i-pretreated patients [88, 89]. Even though data from the pivotal BOLERO-2 trial also stem from the pre-CDK4/6i era, treatment with everolimus/exemestane remains a relevant second-line option [4], especially for patients without activating PIK3CA mutations (Fig. 1). Ongoing research, like in the EMBER study (Table 1), is currently evaluating oral SERD in combination with everolimus upon CDK4/6i progression. This will further elucidate the role of mTOR inhibitors after CDK4/6i.
Endocrine Monotherapy or Cytostatic Treatment
In the PALOMA-3 study, a third of the palbociclib-pretreated patients were subsequently treated with chemotherapy and a further quarter of the patients were treated with endocrine monotherapy upon CDK4/6i progression [37]. Palbociclib-pretreated patients who received chemotherapy as their immediate subsequent line showed a median duration of therapy of 5.6 (4.3–6.1) months. The duration of the subsequent chemotherapy was identical among patients in the placebo arm (5.6 months; 95% CI 3.7–6.9). Patients who were selected for chemotherapy as their first subsequent therapy had a shorter median PFS on study treatment compared to the overall study population, demonstrating a selection bias towards a more rapidly progressing and presumably more endocrine- and CDK4/6i-resistant disease. Patients receiving an endocrine therapy as their immediate subsequent line of therapy, in contrast, had a longer median PFS on study treatment compared to the overall study population, supporting this observation. The duration of endocrine therapy as the first subsequent line was 4.0 (3.2–5.7) vs. 6.2 (4.8–8.3) months in the palbociclib and placebo arm, respectively. In an efficacy analysis of subsequent therapies of patients treated in the multicenter phase 2 TREnd trial, the time to treatment failure (TTF) of the subsequent therapy was similar for both chemotherapeutic and endocrine treatments upon CDK4/6i progression and ranged from 3.7 to 4.2 months [90]. Within 2 retrospective single-center analyses of patients pretreated with palbociclib in a second or later line, the TTF ranged from 4.1 to 4.7 months for subsequent single-agent chemotherapy [91, 92], in line with real-world data [93], whereas TTF data regarding endocrine treatment upon CDK4/6i progression were less consistent [91, 92]. In a population-based observational study with a total of 525 patients receiving treatment after CDK4/6i progression, subsequent chemotherapy was more common among younger patients, with a rapid progression and non-AI backbone under CDK4/6i, whereas elderly patients, with bone-only disease or no prior cytostatic treatment, were more likely to receive subsequent CDK4/6i beyond progression [38]. Thus, age, metastatic site, clinical course of the disease, and prior endocrine treatment influence the selection of subsequent therapy after progression on CDK4/6i [38, 94].
PARP Inhibitors
More than half of the patients in the phase III OlympiAD trial, evaluating olaparib versus single-agent chemotherapy (capecitabine, vinorelbine, or eribulin) in patients with germline BRCA1/2 (gBRCA 1/2) mutations had hormone receptor-positive, HER-negative disease. For this cohort, the ORR was 65.4%, compared to 36.4% in the control arm, demonstrating a significant activity of olaparib in MLBC [95]. A second randomized phase 3 PARP inhibitor (PARPi) trial with a very similar study design, i.e., the EMBRACA trial, investigating talazoparib in the same clinical setting yielded comparable results. In the overall population, talazoparib significantly improved PFS from 5.6 to 8.6 months (HR = 0.54; 95% CI 0.41–0.71; p < 0.001). The treatment effects in EMBRACA were identical for hormone receptor-positive and hormone receptor-negative patients, with an HR for PFS of 0.47 (95% CI 0.32–0.71) and an ORR of 63.2% in hormone receptor-positive disease [96]. Even though these data also stem from the pre-CDK4/6i era, due to a sustained PFS benefit of both PARPis over physicians' choice chemotherapy, olaparib and talazoparib remain relevant treatment options for taxane- and anthracycline-pretreated, endocrine-resistant patients with gBRCA 1/2 mutations beyond CDK4/6i progression [94].
Novel Oral SERDS beyond CDK4/6i Progression
Under selective pressure of endocrine AI treatment, up to 30% of patients develop ESR1D538 or ESR1Y537S/N/C mutations [97]. In CDK4/6i trials, the prevalence of ESR1 mutations varies from 4% among first-line patients in the MONALEESA-2 study [17] to 25.3% among pretreated patients in the PALOMA-3 study [26]. The prevalence of ESR1 mutations in the BOLERO-2 study was 28.8% [98], and it was even higher among heavily pretreated patients in the FERGI and SOFeA trials (i.e., 37 and 39%, respectively) [99]. The most common mutations, i.e., ESR1Y537S and ESR1D538G, cluster in the ligand-binding domain of ER, leading to ligand-independent receptor activity [100]. Since AI only reduce levels of the ligand but cannot inhibit constitutively activated mutant ER, ESR1 mutations are predictive of resistance to AI [101, 102]. ESR1 mutations have no predictive value as biomarkers for CDK4/6i, but rather they function as factors of endocrine resistance [29]. A retrospective analysis of the SoFEA study suggests that fulvestrant might be superior to exemestane in patients with ESR1 mutations [103]. However, the numbers in this analysis are too small to come to a conclusion. The recently published plasmaMATCH study investigated an extended 500-mg dose of fulvestrant in patients with activating ESR1 mutations [104]. That study cohort did not meet its predefined response rate, but it demonstrated an objective response in 8% of the patients, suggesting at least some activity for SERD in this setting. More promising results are expected from novel oral SERD, able to overcome endocrine resistance owed to ESR1 mutations.
RAD1901 (elacestrant), demonstrating antitumor activity both against ESR1Y537S and ESR1D538G mutations, is the first oral SERD being tested against fulvestrant, anastrozole, letrozole, or exemestane in a phase 3 trial (EMERALD) recruiting CDK4/6i-pretreated patients with or without ESR1 mutations [105] (Table 1). In contrast, the clinical development of brilanestrant has recently been halted. SAR 439859 (amcenestrant) has also shown clinical activity irrespectively of the ESR1 mutational status among heavily pretreated MLBC patients in the AMEERA-1 trial [106] and it is currently being evaluated in the phase 2 AMEERA-3 trial versus physicians' choice among CDK4/6i-pretreated MLBC patients, as well as in the phase 3 AMEERA-5 trial in combination with palbociclib versus palbociclib/letrozole among CDK4/6i-naive MLBC patients. Another oral SERD, i.e., GDC-9545 (giredestrant), with a 10-fold higher potency than fulvestrant, showing promising results in a phase Ib trial [107] is similarly being tested in a phase 2 trial (NCT04576455) versus SOC endocrine therapy in CDK4/6i-pretreated MLBC patients and in the phase 3 BO41843 study as a first-line treatment in combination with palbociclib versus palbociclib/letrozole alone for CDK4/6i-naive MLBC patients (Table 1). LSZ 102 was the first oral SERD evaluated in escalating doses in combination with alpelisib among fulvestrant- and/or CDK4/6i-pretreated MLBC patients in a phase Ib trial [108, 109] with good tolerability and sustained antitumor activity. The phase I EMBER trial is evaluating yet another SERD, i.e., LY3484356, in combination with alpelisib or everolimus in CDK4/6i-pretreated patients. Data from these trials will hopefully provide additional information regarding the incorporation of oral SERD into the current endocrine treatment landscape and for patients who progress on a CDK4/6i.
AKT Inhibitors beyond CDK4/6i Progression
The serine/threonine kinases AKT 1, AKT 2, and AKT 3, downstream effectors of the PI3K/AKT/mTOR pathway, mediate cell proliferation and resistance to apoptosis and can be activated by many upstream receptor tyrosine kinases [110]. AKT activation is mediated by phosphorylation at at least 2 sites (i.e., pT308 from PDK1 and pS473 from mTORC2) [111], promoting breast cancer cell survival as well as resistance to endocrine and cytostatic treatments [112, 113, 114]. Multiple negative feedback loops have been described within the PI3K/AKT/mTOR signal pathway, including PTEN, IRS-1, FOXO, and PHLPP1 [111], which may in fact explain the limited efficacy of agents such as everolimus [114]. In addition, different levels of negative feedback loop activity during acute or chronic PI3K/AKT/mTOR pathway inhibition may indeed have an effect on dosing schedules for AKT inhibitors [111]. Whereas immunohistochemical loss of PTEN expression or AKT1–3 amplification is frequent in breast cancer, somatic gain-of-function AKT1E17K mutation is found only in 1–8% of breast cancer patients [115] and results in a constitutive activation of this pathway. AKT1E17K activity can be successfully suppressed by both allosteric AKT inhibitors, impeding AKT localization to the plasma membrane, and catalytic, ATP-competitive AKT inhibitors in breast cancer models [116]. The latter encompasses oral AKT inhibitors like GDC-0068 (ipatasertib) and AZD5363 (capivasertib). Capivasertib synergizes with fulvestrant in breast cancer models irrespectively of endocrine sensitivity and has been shown to overcome endocrine resistance by reducing PTEN levels [117]. The randomized phase 2 trial FAKTION evaluated the addition of capivasertib to fulvestrant in CDK4/6i-naive, AI-resistant MLBC patients after a maximum of 3 lines of endocrine treatment and after 1 palliative chemotherapy line [118]. The addition of capivasertib to fulvestrant leads to an improved PFS by 5.5 months, irrespectively of the PIK3CA or PTEN mutational status. The ORR and the clinical benefit rate were both 41% in favor of capivasertib. The toxicity of capivasertib was similar to that reported for the α-selective PI3K inhibitor alpelisib and included rash, diarrhea, hyperglycemia, nausea/vomiting, and stomatitis. In addition, hypertension and a single case of atypical pulmonary infection were reported. Capivasertib is currently being evaluated in the phase 3 trial CAPItello-291 in combination with fulvestrant as a second-line treatment among CDK4/6i-pretreated MLBC patients. In parallel, the phase 3 IPATunity150 trial is investigating ipatasertib in combination with SOC palbociclib/ fulvestrant among CDK4/6i-untreated MLBC patients either with relapse during adjuvant AI therapy or with progression after <12 months of first-line palliative AI therapy (Table 1). An additional phase 1 trial (TAKTIC) is also evaluating ipatasertib in multiple combination regimens among CDK4/6i-pretreated, PI3Ki-naive MLBC patients. Since the addition of ipatasertib to palliative taxane chemotherapy failed to improve PFS in PIK3CA/AKT1/PTEN-altered MLBC as well as TNBC in the phase 3 IPATunity130 trial [119], clinical development of AKT inhibitors is expected to focus on increasing the efficacy of and overcoming the resistance to endocrine therapy.
Conclusion and Perspectives
With CDK4/6i becoming the preferred standard in first-line therapy and even moving into the adjuvant setting, treatment of MLBC beyond CDK4/6i progression remains challenging. CDK4/6i treatment beyond progression cannot be recommended and should be further evaluated within clinical trials [4]. The combination of alpelisib with fulvestrant constitutes a preferred second-line treatment option for PIK3CA-mutant patients after progression on endocrine monotherapy. The requirement for progression on endocrine monotherapy according to the EMA label implies some strategic considerations for its use in de novo PIK3CA-mutant MLBC patients who received CDK4/6i in the first line. As an alternative to first-line CDK4/6i therapy, patients with a rapid relapse on or after adjuvant endocrine therapy should be strongly considered for enrollment into one of the current phase 3 trials investigating the upfront concurrent use of CDK4/6i and PI3K inhibitors (e.g., NCT04191499). For PIK3CA wild-type MLBC patients as well as PIK3CA-mutant patients with no access to alpelisib, or concomitant diseases precluding its use, the combination of everolimus/exemestane as a second-line treatment beyond CDK4/6i progression remains a valid option [4]. For younger patients with aggressive disease and a rapid clinical progression or a visceral crisis, cytostatic monotherapies (potentially in combination with bevacizumab) should probably be prioritized, whereas elderly patients in selected cases, e.g., with slowly progressing disease and a limited disease burden, may be considered as candidates for endocrine monotherapies [38, 94]. In taxane- and anthracycline- pretreated patients harboring gBRCA1/2 mutations, PARPis like olaparib and talazoparib constitute a preferred treatment option after progression on CDK4/6i [94].
Current phase 1–3 clinical trials will hopefully reshape the treatment landscape of MLBC. Oral SERDS and AKT inhibitors are being developed beyond CDK4/6i in phase 2–3 trials. Novel PI3K inhbitors, such as inavolisib, but also BCL-2 inhibitor venetoclax and the AURKA inhibitor alisertib, have already reached clinical phase 2 trials with CDK4/6i-pretreated patients, whereas FGFR inhibitors and PD-1/ PD-L1 antibodies are still in early clinical development in this setting. These new advances are expected to pave the way for the development of further biomarkers in MLBC. Regarding the expected shift of CDK4/6i toward adjuvant treatment among high-risk early breast cancer patients within the next years [120], accelerated incorporation of novel therapeutic agents into the treatment of MLBC is mandated, allowing for the development of highly-efficient, individualized antitumor therapies.
Conflict of Interest Statement
F.M. has received honoraria from Roche, Amgen, Astra Zeneca, Eisai, Celgene, Novartis, Pfizer, Genomic Health, Myriad, Seagen, and Lilly and acted as a consultant for Roche, Astra Zeneca, and Novartis. A.M. has received travel sponsorship from Pfizer, Eisai, and Celgene, as well as honoraria from Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this paper apart from those disclosed.
Funding Sources
This review was not funded.
Author Contributions
All of the authors contributed significantly to the literature review and the writing of this work and approved the final version.
verified
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wesolowski R, Ramaswamy B. Gene expression profiling: changing face of breast cancer classification and management. Gene Expr. 2011;15((3)):105–15. doi: 10.3727/105221611x13176664479241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015 Nov;24(Suppl 2):S26–35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020 Dec;31((12)):1623–49. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013 Oct;141((3)):507–14. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 6.Caswell-Jin JL, Plevritis SK, Tian L, Cadham CJ, Xu C, Stout NK, et al. Change in survival in metastatic breast cancer with treatment advances: Meta-Analysis and systematic review. JNCI Cancer Spectr. 2018 Nov;2((4)):pky062. doi: 10.1093/jncics/pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971 Jun;25((2)):270–5. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombernowsky P, Smith I, Falkson G, Leonard R, Panasci L, Bellmunt J, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol. 1998 Feb;16((2)):453–61. doi: 10.1200/JCO.1998.16.2.453. [DOI] [PubMed] [Google Scholar]
- 9.Buzdar AU, Jonat W, Howell A, Jones SE, Blomqvist CP, Vogel CL, et al. Arimidex Study Group Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials. Cancer. 1998 Sep;83((6)):1142–52. [PubMed] [Google Scholar]
- 10.Kaufmann M, Bajetta E, Dirix LY, Fein LE, Jones SE, Zilembo N, et al. The Exemestane Study Group Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. J Clin Oncol. 2000 Apr;18((7)):1399–411. doi: 10.1200/JCO.2000.18.7.1399. [DOI] [PubMed] [Google Scholar]
- 11.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010 Oct;28((30)):4594–600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 12.Robertson JF, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016 Dec;388((10063)):2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012 Feb;366((6)):520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid P, Zaiss M, Harper-Wynne C, Ferreira M, Dubey S, Chan S, et al. Fulvestrant plus vistusertib vs fulvestrant plus everolimus vs fulvestrant alone for women with hormone receptor–positive metastatic breast cancer. The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019 Aug;5((11)):1556–63. doi: 10.1001/jamaoncol.2019.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 Apr;17((4)):425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 16.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016 Nov;375((18)):1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 17.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018 Jul;29((7)):1541–7. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 18.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017 Nov;35((32)):3638–46. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 19.Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019 Jan;5((1)):5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Abstract 7184 − Overall survival (OS) results of the phase III MONALEESA-3 trial of postmenopausal patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor 2-negative (HER2−) advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB) Ann Oncol. 2019;30((suppl_5)):v851–v934. [Google Scholar]
- 21.Hurvitz SA, Im SA, Lu YS, Colleoni M, Franke FA, Bardia A, et al. Phase III MONALEESA-7 trial of premenopausal patients with HR+/HER2− advanced breast cancer (ABC) treated with endocrine therapy ± ribociclib Overall survival (OS) results. J Clin Oncol. 2019 Jun;37((suppl 18)) [Google Scholar]
- 22.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019 Jul;381((4)):307–16. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 23.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy − MONARCH 2, a randomized clinical trial. JAMA Oncol. 2019 Sep;6((1)):116–24. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llombart-Cussac A, Pérez-García JM, Bellet M, Dalenc F, Gil MJ, Borrego MR, et al. PARSIFAL: A randomized, multicenter, open-label, phase II trial to evaluate palbociclib in combination with fulvestrant or letrozole in endocrine-sensitive patients with estrogen receptor (ER)[+]/HER2[-] metastatic breast cancer. J Clin Oncol. 2020 May;38((15_suppl suppl_15)):1007–1007. [Google Scholar]
- 25.Chandarlapaty S, Razavi P. Cyclin E mRNA: assessing cyclin-dependent kinase (CDK) activation state to elucidate breast cancer resistance to CDK4/6 inhibitors. J Clin Oncol. 2019 May;37((14)):1148–50. doi: 10.1200/JCO.19.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018 Nov;8((11)):1390–403. doi: 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol. 2019 Dec;30(suppl_10):x3–11. doi: 10.1093/annonc/mdz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res. 2014 Jan;16((1)):201. doi: 10.1186/bcr3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Leary B, Hrebien S, Morden JP, Beaney M, Fribbens C, Huang X, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018 Mar;9((1)):896. doi: 10.1038/s41467-018-03215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razavi P, dos Anjos CH, Brown DN, Qing L, Ping C, Herbert J, et al. Molecular profiling of ER+ metastatic breast cancers to reveal association of genomic alterations with acquired resistance to CDK4/6 inhibitors. J Clin Oncol. 2019 May;37((15_suppl suppl_15)):1009–1009. [Google Scholar]
- 31.Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2019 May;37((14)):1169–78. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell. 2018 Dec;34((6)):893–905.e8. doi: 10.1016/j.ccell.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, Yang X. Targeting the Hippo pathway for breast cancer therapy. Cancers (Basel) 2018 Nov;10((11)):422. doi: 10.3390/cancers10110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro-Yepes J, Chen X, Bui T, Kettner NM, Junt KK, Keyomarsi K, Abstract PD2-05: Differential mechanisms of acquired resistance to abemaciclib versus palbociclib reveal novel therapeutic strategies for CDK 4/6 therapy-resistant breast cancer SABCS 2019. AACR. Cancer Res. 2020;80((suppl_4)) Abstract PD2-05. [Google Scholar]
- 35.Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014 Oct;32((5)):825–37. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell. 2018 Jul;34((1)):9–20. doi: 10.1016/j.ccell.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018 Nov;379((20)):1926–36. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 38.Princic N, Aizer A, Tang DH, Smith DM, Johnson W, Bardia A. Predictors of systemic therapy sequences following a CDK 4/6 inhibitor-based regimen in post-menopausal women with hormone receptor positive, HEGFR-2 negative metastatic breast cancer. Curr Med Res Opin. 2019 Jan;35((1)):73–80. doi: 10.1080/03007995.2018.1519500. [DOI] [PubMed] [Google Scholar]
- 39.Wander SA, Zangardi M, Niemierko A, Kambadakone A, Kim LS, Xi J, et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients (pts) with hormone receptor-positive (HR+)/ HER2-metastatic breast cancer (MBC) J Clin Oncol. 2019 May;37((15_suppl suppl_15)):1057–1057. [Google Scholar]
- 40.Tamragouri K, Cobleigh MA, Rao RD. Abemaciclib with or without fulvestrant for the treatment of hormone receptor-positive and HER2-negative metastatic breast cancer with disease progression following prior treatment with palbociclib. J Clin Oncol. 2019 May;37((15_suppl suppl_15)):1053–1053. [Google Scholar]
- 41.Mariotti V, Khong HT, Soliman HH, Costa RL, Fisher S, Boulware D. Efficacy of abemaciclib (abema) after palbociclib (palbo) in patients (pts) with metastatic breast cancer (MBC) J Clin Oncol. 2019 May;37((suppl 15)):e.12521. [Google Scholar]
- 42.Eziokwu AS, Varella L, Kruse ML, Jia X, Moore HCF, Budd GT, et al. Real-world evidence evaluating continuation of CDK4/6 inhibitors beyond first progression in hormone receptor-positive (HR+) metastatic breast cancer. J Clin Oncol. 2019 May;37((suppl 15)):e12538. [Google Scholar]
- 43.dos Anjos CH, Razavi P, Herbert J, Colon J, Gill K, Modi S, et al. A large retrospective analysis of CDK 4/6 inhibitor retreatment in ER+ metastatic breast cancer (MBC) J Clin Oncol. 2019 May;37((suppl 15)):1053. [Google Scholar]
- 44.Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018 Jan;553((7686)):91–5. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018 Feb;8((2)):216–33. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yost SE, Lee JS, Egelston C, Frankel PH, Ruel C, Padam S, et al. Abstract P3-11-04: A phase II study of pembrolizumab, letrozole and palbociclib in patients with metastatic estrogen receptor positive breast cancer. SABCS 2019. AACR. Cancer Res. 2020;80((suppl_4)) Abstract P3-11-04. [Google Scholar]
- 47.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010 Jun;2((6)):344–52. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida A, Lee EK, Diehl JA. Induction of therapeutic senescence in Vemurafenib-resistant melanoma by extended inhibition of CDK4/6. Cancer Res. 2016 May;15(76(10)):2990–3002. doi: 10.1158/0008-5472.CAN-15-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teo ZL, Versaci S, Dushyanthen S, Caramia F, Savas P, Mintoff CP, et al. Combined CDK4/6 and PI3Kalpha inhibition is synergistic and immunogenic in triple-negative breast cancer. Cancer Res. 2017 Nov;77((22)):6340–52. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 50.Barroso-Sousa R, Li T, Trippa L, Rees R, Andrews C, Ferreira AR, Abstract P5-11-04: A phase I/IIb study of palbociclib (PALBO) plus everolimus (EVE) and exemestane (EXE) in hormone-receptor positive (HR+)/HER2- metastatic breast cancer (MBC) after progression on a CDK4/6 inhibitor (CDK4/6i): Results of the phase II study. SABCS 2019 AACR. Cancer Res. 2020;80((suppl_4)) Abstract P5-11-04. [Google Scholar]
- 51.Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak AB, Yardley DA, et al. Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2- advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITY-1): Efficacy, safety, and biomarker results. J Clin Oncol. 2019 May;37((15_suppl suppl_15)):1016–1016. [Google Scholar]
- 52.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials—early lessons. J Mammary Gland Biol Neoplasia. 2008 Dec;13((4)):471–83. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forero-Torres A, Han H, Dees EC, Wesolowski R, Bardia A, Kabos P, et al. Phase Ib study of gedatolisib in combination with palbociclib and endocrine therapy (ET) in women with estrogen receptor (ER) positive (+) metastatic breast cancer (MBC) (B2151009) J Clin Oncol. 2018 May;36((15_suppl suppl_15)):1040–1040. [Google Scholar]
- 54.Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010 Mar;70((5)):2085–94. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Formisano L, Lu Y, Jansen VM, Bauer JA, Hanker A, Ericsson PG, et al. Abstract GS6-05: Gain-of-function kinase library screen identifies FGFR1 amplification as a mechanism of resistance to antiestrogens and CDK4/6 inhibitors in ER+ breast cancer. SABCS 2017. AACR. Cancer Res. 2018;78((suppl_4)) Abstract GS6-05. [Google Scholar]
- 56.Formisano L, Lu Y, Servetto A, Hanker AB, Jansen VM, Bauer JA, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019 Mar;10((1)):1373. doi: 10.1038/s41467-019-09068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campone M, Bachelot T, Penault-Llorca F, Pallis A, Agrapart V, Pierrat MJ, et al. A phase Ib dose allocation study of oral administration of lucitanib given in combination with fulvestrant in patients with estrogen receptor-positive and FGFR1-amplified or non-amplified metastatic breast cancer. Cancer Chemother Pharmacol. 2019 Apr;83((4)):743–53. doi: 10.1007/s00280-018-03765-3. [DOI] [PubMed] [Google Scholar]
- 58.Musolino A, Campone M, Neven P, Denduluri N, Barrios CH, Cortes J, et al. Phase II, randomized, placebo-controlled study of dovitinib in combination with fulvestrant in postmenopausal patients with HR+, HER2- breast cancer that had progressed during or after prior endocrine therapy. Breast Cancer Res. 2017 Feb;19((1)):18. doi: 10.1186/s13058-017-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong X, Du J, Parsons SH, Merzoug FF, Webster Y, Iversen PW, et al. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 2018 Oct;••• doi: 10.1158/2159-8290.CD-18-0469. [DOI] [PubMed] [Google Scholar]
- 60.Montaudon E, Nikitorowicz-Buniak J, Sourd L, Morisset L, El Botty R, Huguet L, et al. PLK1 inhibition exhibits strong anti-tumoral activity in CCND1-driven breast cancer metastases with acquired palbociclib resistance. Nat Commun. 2020 Aug;11((1)):4053. doi: 10.1038/s41467-020-17697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whittle JR, Vaillant F, Surgenor E, Policheni AN, Giner G, Capaldo BD, et al. Dual targeting of CDK4/6 and BCL2 pathways augments tumor response in estrogen receptor-positive breast cancer. Clin Cancer Res. 2020 Aug;26((15)):4120–34. doi: 10.1158/1078-0432.CCR-19-1872. [DOI] [PubMed] [Google Scholar]
- 62.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004 Nov;64((21)):7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 63.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr;304((5670)):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 64.Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004 Aug;3((8)):772–5. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 65.Miron A, Varadi M, Carrasco D, Li H, Luongo L, Kim HJ, et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 2010 Jul;70((14)):5674–8. doi: 10.1158/0008-5472.CAN-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dunlap J, Le C, Shukla A, Patterson J, Presnell A, Heinrich MC, et al. Phosphatidylinositol-3-kinase and AKT1 mutations occur early in breast carcinoma. Breast Cancer Res Treat. 2010 Apr;120((2)):409–18. doi: 10.1007/s10549-009-0406-1. [DOI] [PubMed] [Google Scholar]
- 67.Moynahan ME, Chen D, He W, Sung P, Samoila A, You D, et al. Correlation between PIK3CA mutations in cell-free DNA and everolimus efficacy in HR+, HER2- advanced breast cancer: results from BOLERO-2. Br J Cancer. 2017 Mar;116((6)):726–30. doi: 10.1038/bjc.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N, et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol. 2020 Mar;31((3)):377–86. doi: 10.1016/j.annonc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016 Jun;17((6)):811–21. doi: 10.1016/S1470-2045(16)00106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo in postmenopausal, hormone receptor-positive, HER-2 negative, advanced breast cancer (BELLE-2): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 Jul;18((7)):904–16. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Leo A, Johnston S, Lee KS, Ciruelos E, Lønning PE, Janni W, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018 Jan;19((1)):87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 72.Baselga J, Dent SF, Cortés J, Im Y-H, Diéras V, Harbeck N, et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J Clin Oncol. 2018;36((suppl_18)):1006–1006. [Google Scholar]
- 73.André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. SOLAR-1 Study Group Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019 May;380((20)):1929–40. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 74.André F, Ciruelos E, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2020 Nov; doi: 10.1016/j.annonc.2020.11.011. S0923-7534(20):43166-7. [DOI] [PubMed] [Google Scholar]
- 75.Busaidy NL, Farooki A, Dowlati A, Perentesis JP, Dancey JE, Doyle LA, et al. Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol. 2012 Aug;30((23)):2919–28. doi: 10.1200/JCO.2011.39.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benson AB, 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, Jr, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004 Jul;22((14)):2918–26. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 77.Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018 Aug;560((7719)):499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rugo HS, Bianchi GV, Chia SK, Turner NC, Juric D, Jacot W, et al. BYLieve: A phase II study of alpelisib (ALP) with fulvestrant (FUL) or letrozole (LET) for treatment of PIK3CA mutant, hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (aBC) progressing on/after cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) therapy. J Clin Oncol. 2018 Jun;36((15_suppl suppl_15)):TPS1107–1107. [Google Scholar]
- 79.Rugo HS, Lerebours F, Ciruelos E, Drullinsky P, Borrego MR, Neven P, et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDKi) + aromatase inhibitor (AI): BYLieve study results. J Clin Oncol. 2020 May;38((15_suppl suppl_15)):1006–1006. [Google Scholar]
- 80.Turner S, Chia SK, Kanakamedala H, Hsu WC, Park J, Chandiwana D, et al. Real-world effectiveness of alpelisib (ALP) + fulvestrant (FUL) compared with standard treatment among patients (Pts) with hormone-receptor positive (HR+) human epidermal growth factor receptor-2 negative (HER2e) PIK3CA-mutated (Mut) advanced breast cancer (ABC) Ann Oncol. 2020;31((S4)):S366. [Google Scholar]
- 81.AGO [Internet]., Taufkirchen Leitlinien/Empfehlungen − Empfehlungen gynäkologische Onkologie Kommission Mamma [accessed 2021 Jan 6] Available from: https://www.ago-online.de/leitlinien-empfehlungen/leitlinien-empfehlungen/kommission-mamma. [Google Scholar]
- 82.Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014 Jul;26((1)):136–49. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012 Aug;30((22)):2718–24. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 84.Kornblum N, Zhao F, Manola J, Klein P, Ramaswamy B, Brufsky A, et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: results of PrE0102. J Clin Oncol. 2018 Jun;36((16)):1556–63. doi: 10.1200/JCO.2017.76.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yardley DA, Noguchi S, Pritchard KI, Burris HA, 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013 Oct;30((10)):870–84. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol. 2014 Dec;25((12)):2357–62. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cook M, Al Rabadi L, Mitri ZI. Everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer patients previously treated with CDK4/6 inhibitor-based therapies. J Clin Oncol. 2019 May;37((15_suppl suppl_15)):1058–1058. [Google Scholar]
- 88.Dhakal A, Antony Thomas R, Levine EG, Brufsky A, Takabe K, Hanna MG, et al. Outcome of Everolimus-Based Therapy in Hormone-Receptor-Positive Metastatic Breast Cancer Patients After Progression on Palbociclib. Breast Cancer (Auckl) 2020 Jul;14:1178223420944864. doi: 10.1177/1178223420944864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nichetti F, Marra A, Giorgi CA, Randon G, Scagnoli S, De Angelis C, et al. Efficacy of everolimus plus exemestane in CDK 4/6 inhibitors-pretreated or naïve HR-positive/HER2-negative breast cancer patients: A secondary analysis of the EVERMET study. Ann Oncol. 2020;31(S4):S382. [Google Scholar]
- 90.Rossi L, Biagioni C, McCartney A, Migliaccio I, Curigliano G, Sanna G, et al. Clinical outcomes after palbociclib with or without endocrine therapy in postmenopausal women with hormone receptor positive and HER2-negative metastatic breast cancer enrolled in the TREnd trial. Breast Cancer Res. 2019 May;21((1)):71. doi: 10.1186/s13058-019-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giridhar KV, Choong GM, Leon-Ferre RA, O'Sullivan CC, Ruddy KJ, Haddad TC, et al. Clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: A retrospective single-institution study [abstract]. SABCS 2018 AACR. Cancer Res. 2019;79((suppl_4)) doi: 10.1007/s10549-022-06713-1. Abstract P6-18-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, et al. Retrospective analysis of treatment patterns and the effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Canc Netw. 2019 Feb;17((2)):141–7. doi: 10.6004/jnccn.2018.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sirvén MB, Fernández-Ortega A, Stradella A, Morilla I, Falo C, Vázquez S, et al. Real-world efficacy and safety of eribulin in advanced and pretreated HER2-negative breast cancer in a Spanish comprehensive cancer center. BMC Pharmacol Toxicol. 2019 Nov;20((1)):68. doi: 10.1186/s40360-019-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sammons S, Shastry M, Dent S, Anders C, Hamilton E. Practical treatment strategies and future directions after progression while receiving CDK4/6 inhibition and endocrine therapy in advanced HR+/HER2- breast cancer. Clin Breast Cancer. 2020 Feb;20((1)):1–11. doi: 10.1016/j.clbc.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017 Aug;377((6)):523–33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 96.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;37988:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clatot F, Perdrix A, Augusto L, Beaussire L, Delacour J, Calbrix C, et al. Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget. 2016 Nov;7((46)):74448–59. doi: 10.18632/oncotarget.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016 Oct;2((10)):1310–5. doi: 10.1001/jamaoncol.2016.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Santo I, McCartney A, Migliaccio I, Di Leo A, Malorni L. The emerging role of ESR1 mutations in luminal breast cancer as a prognostic and predictive biomarker of response to endocrine therapy. Cancers (Basel) 2019 Nov;11((12)):1894. doi: 10.3390/cancers11121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013 Dec;45((12)):1439–45. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang K, Hong R, Xu F, Xia W, Kaping L, Qin G, et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res. 2018 Aug;10:2573–80. doi: 10.2147/CMAR.S173193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toy W, Weir H, Razavi P, Lawson M, Goeppert AU, Mazzola AM, et al. Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Cancer Discov. 2017 Mar;7((3)):277–87. doi: 10.1158/2159-8290.CD-15-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016 Sep;34((25)):2961–8. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 104.Turner NC, Kingston B, Kilburn LS, Kernaghan S, Wardley AM, Macpherson IR, et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020 Oct;21((10)):1296–308. doi: 10.1016/S1470-2045(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bardia A, Aftimos P, Bihani T, Anderson-Villaluz AT, Jung J, Conlan MG, et al. EMERALD: phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019 Oct;15((28)):3209–18. doi: 10.2217/fon-2019-0370. [DOI] [PubMed] [Google Scholar]
- 106.Campone M, Bardia A, Ulaner GA, Chandarlapaty S, Gosselin A, Doroumian S, et al. Phase I/II study of SAR439859, an oral selective estrogen receptor degrader (SERD), in estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC) J Clin Oncol. 2020 May;38((15_suppl suppl_15)):1070–1070. [Google Scholar]
- 107.Lim E, Jhaveri KL, Perez-Fidalgo JA, Bellet M, Boni V, Perez Garcia JM, et al. A phase Ib study to evaluate the oral selective estrogen receptor degrader GDC-9545 alone or combined with palbociclib in metastatic ER-positive HER2-negative breast cancer. J Clin Oncol. 2020 May;38((15_suppl suppl_15)):1023–1023. [Google Scholar]
- 108.Jhaveri K, Juric D, Cresta S, Yap YS, Duhoux FP, Terret C, et al. Abstract PD7-09: A phase 1/1b study of LSZ102, an oral selective estrogen receptor degrader (SERD), in combination with ribociclib in patients with estrogen receptor-positive (ER+) advanced breast cancer (ABC) who had progressed after endocrine therapy (ET) SABCS 2019. AACR. Cancer Res. 2020;80((suppl_4)) Abstract PD7-09. [Google Scholar]
- 109.Jhaveri K, Juric D, Yap YS, Cresta S, Layman R, Duhoux F, et al. LBA1 Interim results of a phase I/Ib study of LSZ102, an oral selective estrogen receptor degrader (SERD), in combination with ribociclib (RIB) or alpelisib (ALP) in patients with ER+ breast cancer (BC) who had progressed after endocrine therapy (ET) Ann Oncol. 2020 May;31(suppl_2):S62. [Google Scholar]
- 110.Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol Ther. 2017 Apr;172:101–15. doi: 10.1016/j.pharmthera.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frogne T, Jepsen JS, Larsen SS, Fog CK, Brockdorff BL, Lykkesfeldt AE. Antiestrogen-resistant human breast cancer cells require activated protein kinase B/Akt for growth. Endocr Relat Cancer. 2005 Sep;12((3)):599–614. doi: 10.1677/erc.1.00946. [DOI] [PubMed] [Google Scholar]
- 112.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Patents Anticancer Drug Discov. 2010 Jan;5((1)):29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 113.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005 Oct;207((2)):139–46. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- 114.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006 Feb;66((3)):1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y, Huang L, Dong Y, Tao C, Zhang R, Shao H, et al. Effect of AKT1 (p. E17K) Hotspot Mutation on Malignant Tumorigenesis and Prognosis. Front Cell Dev Biol. 2020 Oct;8((8)):573599. doi: 10.3389/fcell.2020.573599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davies BR, Guan N, Logie A, Crafter C, Hanson L, Jacobs V, et al. Tumors with AKT1E17K mutations are rational targets for single agent or combination therapy with AKT inhibitors. Mol Cancer Ther. 2015 Nov;14((11)):2441–51. doi: 10.1158/1535-7163.MCT-15-0230. [DOI] [PubMed] [Google Scholar]
- 117.Fu X, Creighton CJ, Biswal NC, Kumar V, Shea M, Herrera S, et al. Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-targeting mammalian target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase. Breast Cancer Res. 2014 Sep;16((5)):430. doi: 10.1186/s13058-014-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020 Mar;21((3)):345–57. doi: 10.1016/S1470-2045(19)30817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Turner N, Dent R, O'Shaughnessy J, Kim SB, Isakoff S, Barrios CH, et al. Ipatasertib (IPAT) + paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered hormone receptor-positive (HR+) HER2-negative advanced breast cancer (aBC): Primary results from Cohort B of the IPATunity130 randomised phase III trial. Ann Oncol. 2020 Sep;31((suppl:4)):S354–S355. doi: 10.1007/s10549-021-06450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnston SR, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. monarchE Committee Members and Investigators Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020 Dec;38((34)):3987–98. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]