Abstract
Background
A phase 2 trial has suggested that treatment with the melanocortin-4 receptor (MC4R) agonist setmelanotide is associated with a decrease in hunger and weight-related outcomes in participants with Bardet-Biedl syndrome (BBS) and Alström syndrome. Here, we present the study design of an ongoing, randomized, double-blind, placebo-controlled, phase 3 trial to assess the long-term efficacy and safety of setmelanotide for the treatment of obesity and hyperphagia in individuals with BBS or Alström syndrome (ClinicalTrials.gov identifier: NCT03746522).
Methods
It was initially planned that ~30 participants aged ≥6 years with a clinical diagnosis of BBS or Alström syndrome would be enrolled. Participants with obesity as defined by a body mass index ≥30 kg/m2 (in those aged ≥16 years) or a weight >97th percentile (in those aged 6–15 years) are included. Participants are initially randomized in a 1:1 ratio to receive setmelanotide or placebo for 14 weeks (period 1). Following period 1, all participants receive 38 weeks of open-label treatment with setmelanotide (period 2). In each treatment period, setmelanotide is administered at 3 mg once a day following completion of dose escalation. The primary endpoint is the proportion of participants aged ≥12 years achieving a clinically meaningful reduction from baseline (≥10%) in body weight after ~52 weeks (eg, following period 2). Safety and tolerability are assessed by frequency of adverse events.
Conclusions
This pivotal trial is designed to evaluate the efficacy and safety of setmelanotide for the treatment of obesity and hyperphagia in individuals with BBS and Alström syndrome.
Submission category
Study Design, Statistical Design, Study Protocols.
Keywords: Antiobesity drug, Appetite control, Obesity therapy, Phase III study
List of abbreviations
- AE
adverse events
- BBS
Bardet-Biedl syndrome
- CI
confidence interval
- FAS
full analysis set
- MC4R
melanocortin-4 receptor
- QD
once a day
1. Introduction
The hypothalamic melanocortin-4 receptor (MC4R) is activated by neurons in the paraventricular nucleus and is involved in the regulation of metabolism and body weight [1,2]. The MC4R pathway is controlled by 2 opposing types of neurons [2,3]. Inhibition of the MC4R pathway is regulated by release of the inverse agonist agouti-related peptide from neuropeptide Y/agouti-related peptide neurons [3]. Activation of MC4R is dependent on melanocortin-stimulating hormone release by proopiomelanocortin neurons following activation of the leptin receptor [2].
Variants in genes involved in these pathways can result in Bardet-Biedl syndrome (BBS) and Alström syndrome, rare genetic diseases associated with impaired ciliary function and insufficient MC4R signaling [[4], [5], [6], [7]]. BBS is characterized by several major features, including rod-cone dystrophy, polydactyly, learning disabilities, hypogonadism, and renal anomalies [8]. Alström syndrome is characterized by similar features, including cone-rod dystrophy, hearing loss, type 2 diabetes mellitus, dilated cardiomyopathy, and hepatic and renal abnormalities [9]. Additionally, BBS and Alström syndrome are both characterized by early-onset obesity and hyperphagic behaviors likely due at least in part to defects in the MC4R signaling pathway [[10], [11], [12], [13]].
Setmelanotide is a melanocortin agonist that acts as a substitute for α-melanocortin–stimulating hormone at MC4R-activating neurons [14,15]. Treatment with setmelanotide may help overcome many of the effects of genetic deficiencies that occur upstream of MC4R in the pathway. In phase 2 and 3 clinical trials, setmelanotide treatment resulted in reduced body weight and hunger scores in individuals with obesity due to proopiomelanocortin and leptin receptor deficiency [14,15]. In a recent phase 2 trial of 8 individuals with BBS and 4 with Alström syndrome, setmelanotide was associated with a decrease in hunger and weight-related outcomes for up to 12 months [16,17].
BBS is a rare genetic disease of obesity, with a global prevalence ranging from 1 in 3700 to 1 in 160,000 individuals, and with higher prevalence rates being observed in isolated populations [18]. Clinical trial design for rare disease has novel challenges additional to those associated with trial design for common diseases [19]. Although a randomized controlled trial design is considered the gold standard for evaluating therapeutic efficacy, the large number of participants required is often not feasible to recruit for rare diseases such as BBS [[20], [21], [22]]. Recruiting individuals with rare diseases can be challenging because of a lack of disease awareness, diagnostic challenges, and the overall rarity of the disease [19]. Insufficient available research on the natural history of the disease can make determining relevant outcome measures, efficacy endpoints, and follow-up duration challenging [19,20]. In addition, interindividual heterogeneity and the smaller study size of rare disease trials can complicate the study analysis. These factors highlight the importance of clear and well-designed trials tailored for rare diseases.
Here, we present the study design of an ongoing, randomized, double-blind, placebo-controlled, phase 3 trial with an open-label extension to assess the long-term efficacy and safety of setmelanotide for the treatment of obesity and hyperphagia in individuals with BBS or Alström syndrome (ClinicalTrials.gov identifier: NCT03746522).
2. Methods
2.1. Study design and participants
This is a randomized, placebo-controlled, double-blind, phase 3 trial with an open-label extension evaluating the efficacy and safety of setmelanotide in individuals with BBS or Alström syndrome. The trial is being conducted at ~10 centers worldwide. Participants aged ≥6 years with a clinical diagnosis of BBS (according to Beales criteria) [8] or Alström syndrome (according to Marshall criteria) [9] are eligible (Table 1). All participants with Alström syndrome must have a genetically confirmed diagnosis at the time of enrollment. Although genetic testing is common in individuals with BBS, diagnosis of BBS is based on meeting clinical criteria [8]. A small proportion of participants with BBS (≤10%) may be enrolled in the study without prior genetic testing to assess whether previous genetic testing changes the patient population in a manner that might affect responsiveness to setmelanotide. It was initially planned that the trial would enroll ~30 participants, including at least 20 with BBS and 6 with Alström syndrome, with a maximum of 6 participants aged <12 years being initially enrolled, including ~4 with BBS and 2 with Alström syndrome. Recruitment of the primary cohort was completed in 2019, with 38 participants (32 with BBS and 6 with Alström syndrome) being enrolled.
Table 1.
Diagnostic criteria for BBS and Alström syndrome.
| Requirement | Primary/major features | Secondary/minor features | |
|---|---|---|---|
| BBS [8] | 4 primary features or 3 primary and 2 secondary features |
|
|
| Alström syndrome [9] (in those aged 6 to ≤14 years) | 2 major criteria or 1 major and 3 minor criteria |
|
|
| Alström syndrome [9] (in those aged ≥15 years) | 2 major and 2 minor criteria or 1 major and 4 minor criteria |
|
BBS, Bardet-Biedl syndrome; CHF, congestive heart failure; DCM, dilated cardiomyopathy; ERG, electroretinography; T2DM, type 2 diabetes mellitus.
If old enough for testing: cone dystrophy by ERG.
In males.
In females.
Participants with obesity as defined by a body mass index ≥30 kg/m2 (in those aged ≥16 years) or weight >97th percentile (in those aged 6–15 years) are included. Those receiving an intensive diet or exercise regimen with or without the use of weight loss agents that has resulted in >2% weight loss within the last 2 months are excluded. Additionally, those using any medication approved to treat obesity within 3 months of randomization (eg, orlistat, lorcaserin, phentermine-topiramate, naltrexone-bupropion) are also excluded. Additional key inclusion and exclusion criteria are shown in Table 2.
Table 2.
Key inclusion and exclusion criteria.
| Inclusion criteria | |
| |
| |
| Exclusion criteria |
|
| |
| |
| |
| |
|
BBS, Bardet-Biedl syndrome; BMI, body mass index; HbA1c, glycated hemoglobin.
It was planned that a maximum of 6 participants under the age of 12 years would be initially enrolled into the pivotal cohort.
This trial is being conducted in accordance with the International Council on Harmonisation for Good Clinical Practice, Declaration of Helsinki, and appropriate regulatory requirements. The trial is being conducted only at sites in which institutional review board approval has been obtained. Written informed consent from participants or guardians prior to participation is required.
2.2. Procedures and assessments
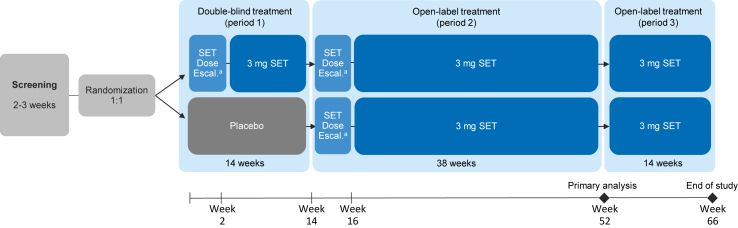
The trial has 3 treatment periods (Fig. 1). Participants are initially randomized in a 1:1 ratio to receive setmelanotide or placebo for 14 weeks (period 1). Participants who are ≥16 years of age and randomized to setmelanotide receive a subcutaneous injection of setmelanotide 2 mg once a day (QD) during a 2-week dose escalation, which increases to 3 mg at the beginning of week 3. Participants who are <16 years of age and randomized to setmelanotide initially receive a subcutaneous injection of setmelanotide 1 mg QD for the first week, which increases to 2 mg for the second week and to 3 mg at the beginning of the third week. Similarly, matching placebo is administered via a subcutaneous injection in those randomized to placebo.
Fig. 1.
Schematic for the design of the overall study. Escal., escalation; QD, once a day; SET, setmelanotide. aDuring dose escalation, participants who are ≥16 years of age receive setmelanotide 2 mg QD for 2 weeks, which increases to 3 mg at the beginning of week 3; participants who are <16 years of age receive setmelanotide 1 mg QD for the first week, 2 mg for the second week, and 3 mg at the beginning of week 3.
Following period 1, all participants receive 38 weeks of open-label treatment with setmelanotide (period 2). To maintain the blind, an upward dose escalation to a fixed dose of 3 mg of setmelanotide is performed during the first 2 weeks of period 2. This dose escalation follows the same procedure as that for period 1 and is performed independently of the initial treatment received. The primary analysis is performed following period 2. Following period 2, participants continue to receive open-label setmelanotide for another 14 weeks (period 3).
Participants are assessed via site visits during period 1 at the beginning of weeks 1, 3, 7, and 11; during period 2 at the beginning of weeks 15, 17, 23, 29, 35, 41, 47, and 53; and during period 3 at the beginning of week 60 and at the end of the study. At each visit, the study drug is dispensed; body weight, height, vital signs, and concomitant medications are reviewed. Adverse events (AEs) are monitored throughout the study. Laboratory parameters are recorded at each visit during period 1 and 2 and at the end of study. Additional body composition measurements, including body fat, are recorded at screening and at the beginning of week 53.
All participants who are able to self-assess their hunger complete a daily hunger questionnaire in the morning prior to a morning meal and dosing. For participants ≥12 years of age, the assessments use a Likert-type scale, where 0 = not hungry at all and 10 = hungriest possible, to generate a hunger score. Participants are asked to provide scores for morning hunger, most hunger (ie, hunger at their hungriest point in the past 24 h), and average hunger over the past day. For participants between 6 and < 12 years of age, morning hunger is assessed using a pictorial (smiley face) version with scores ranging from 0 to 4, with 0 = not hungry at all and 4 = hungriest possible.
During weeks 2 and 15, AEs are assessed, and concomitant medications are reviewed via a telephone call. Changes in depression and suicidality are assessed and monitored using the Columbia Suicide Severity Rating Scale and the Patient Health Questionnaire-9 over the entire course of the trial.
2.3. Randomization and blinding
On the first day of period 1, participants who remain eligible are assigned a unique randomization number indicating the initial treatment assignment based on a code that is generated prior to the start of the study. Randomization is stratified by age group (≥12 vs < 12 years of age) and disease (BBS vs Alström syndrome). The study remains blinded to participants, caregivers, and assessors through the end of period 2. Placebo and setmelanotide are identical in appearance and supplied in identical packaging. At each visit during period 1, the study pharmacist selects the correct treatment using a package code and provides the blinded study medication to the participant or caregiver; following period 1, the study pharmacist provides open-label setmelanotide. Unblinding only occurs in the event of a medical emergency where the identity of the study drug may be necessary to appropriately treat the participant. If a participant is unblinded, the reason and timing of unblinding is documented. Of note, it may be difficult to fully maintain treatment blinding as tanning and hyperpigmentation can be very noticeable in patients receiving setmelanotide treatment.
2.4. Study endpoints
The primary endpoint is the proportion of participants aged ≥12 years achieving a reduction from baseline (≥10%) in body weight after ~52 weeks of treatment with setmelanotide (ie, following period 2). For participants randomized to the setmelanotide group, baseline is defined as the last available measurement prior to the randomization. For participants randomized to the placebo group, baseline is defined as the last available measurement prior to the first dose of open-label setmelanotide treatment.
Key secondary efficacy endpoints include percent change in body weight and hunger scores after ~52 weeks of treatment with setmelanotide and proportion of participants with ≥25% improvement in hunger score after ~52 weeks of treatment with setmelanotide in participants aged ≥12 years. A complete list of primary and secondary efficacy endpoints is included in Table 3.
Table 3.
Study efficacy endpoints.
| Primary efficacy endpoint | Timing | Study population |
|---|---|---|
| Proportion of participants who achieve ≥10% reduction from baseline in body weight | ~52 weeks of treatmenta | FAS among those who are ≥12 years of age at baseline |
| Key secondary endpoints | ||
| Mean percent change from baseline in body weight | ~52 weeks of treatmenta | FAS among those who are ≥12 years of age at baseline |
| Mean percent change in weekly average of daily hunger score | ~52 weeks of treatmenta | FAS among those who are ≥12 years of age at baseline |
| Proportion of participants who achieve ≥25% reduction in weekly average of daily hunger score | ~52 weeks of treatmenta | FAS among those who are ≥12 years of age at baseline |
| Other secondary endpoints | ||
| Mean percent change from baseline in body weight compared with placebo | Week 14 | PCS among those who are ≥12 years of age at baseline |
| Mean percent change in weekly average of daily hunger score compared with placebo | Week 14 | PCS among those who are ≥12 years of age at baseline |
FAS, full analysis set; PCS, 14-week placebo-controlled analysis set.
Treatment with setmelanotide. For participants randomized to the setmelanotide group, baseline is defined as the last available measurement prior to the randomization; for participants randomized to the placebo group, baseline is defined as the last available measurement prior to the first dose of open-label setmelanotide treatment.
Exploratory endpoints include various body composition/metabolic outcomes, safety/tolerability, and changes in depression or suicidality as assessed by the Columbia Suicide Severity Rating Scale and Patient Health Questionnaire-9. Safety and tolerability are assessed by frequency of AEs. All AEs are graded using the National Cancer Institute Common Terminology Criteria for Adverse Events.
2.5. Statistical analyses
Efficacy is assessed primarily in the full analysis set (FAS; Table 3), which includes all participants who received at least 1 dose of study drug and have baseline data. The primary analysis is conducted in the FAS among those who are ≥12 years of age at baseline. Safety is assessed in the safety analysis set, which comprises participants who received at least 1 dose of study drug or placebo. Efficacy analyses are performed according to randomization, and safety analyses are performed according to treatment received. No interim analyses are planned.
The primary statistical hypothesis is that the proportion of participants treated with setmelanotide for ~52 weeks who achieve ≥10% reduction from baseline in body weight is greater than a historical control rate of 10% in the FAS among those who are ≥12 years of age at baseline. Data from the Clinical Registry Investigating Bardet-Biedl Syndrome (ClinicalTrials.gov identifier: NCT02329210) was used to provide input for the sample size/power calculations. A sample size of 7 participants provides ~91% power at 1-sided alpha of 0.025 to yield a statistically significant difference, assuming a 66% response rate in participants treated with setmelanotide. This suggests that powering the study for the primary endpoint requires <10 participants; the size of the trial also considers the rarity of BBS and Alström syndrome and a desire to better understand the effect of setmelanotide in these individuals. Thus, ~30 participants (including 6 participants with Alström syndrome) are planned to be enrolled in the study; this number is suitable for a single pivotal trial to support the indications in BBS and Alström syndrome and to provide robust information for both the between-group analysis in period 1 and the comparison to the historical control rate in period 2.
The primary endpoint is assessed using an exact binomial test at a 1-sided significance level of 0.025, which was chosen on the basis of the small sample size. A 2-sided 95% confidence interval (CI) is calculated using the exact Clopper-Pearson method. The statistical criterion for rejection of the null hypothesis corresponds to the 2-sided 95% CI for setmelanotide of the response rate excluding 10% (ie, the lower bound of the 95% CI > 0.10). Because of the rarity of the disease indication and small sample size, no multiplicity adjustments are being performed for key secondary endpoints. The P-values and the corresponding CIs are being provided. All AEs and discontinuations due to AEs are being summarized descriptively with frequencies and percentages.
For participants randomized to the setmelanotide group, baseline for statistical analyses following ~52 weeks of treatment is defined as the last available measurement prior to the randomization. For participants randomized to the placebo group, baseline is defined as the last available measurement prior to the first dose of open-label setmelanotide treatment. For participants randomized to the placebo arm with <52 weeks of setmelanotide treatment by the timing of the primary analysis at the end of period 2, multiple imputation is used to impute measurements after ~52 weeks of treatment for the primary analysis.
3. Discussion
Hyperphagia can be an overwhelming burden to both the individual and the family, severely affecting health, quality of life, and finances. Environmental controls, such as supervising children around food, securing food sources, reducing energy intake, and adhering to meal schedules, are essential management strategies for weight regulation [23]. However, these controls do not address the persistent hyperphagia [24], which can negatively affect quality of life and social dynamics.
Individuals with BBS and Alström syndrome can experience hyperphagia leading to obesity, and no treatments have been commercially approved to manage these symptoms in these individuals. This is the first pivotal phase 3 clinical trial to investigate the safety and efficacy of an MC4R agonist for the treatment of obesity and hyperphagia in individuals with BBS or Alström syndrome. Because of the rarity of BBS and Alström syndrome, a traditional trial design is not being applied, and the primary endpoint does not directly compare the placebo and treatment arms. However, an initial placebo-controlled period is included in the trial design, which allows some secondary endpoints to be assessed as a comparison between treatment and placebo.
This trial includes a 14-week randomized, double-blind placebo period allowing for evaluation of any placebo effect associated with setmelanotide, which is often difficult to achieve in rare disease clinical trials [19], Additionally, all participants receive open-label setmelanotide treatment following the randomized placebo period, which can alleviate ethical concerns of placebo delivery in rare diseases as well as ease reluctance for enrollment [20].
BBS is known to be associated with variants in >20 genes, with variants in BBS1, BBS2, and BBS10 representing approximately half of the total BBS cases [25]. There are known genotype-phenotype relationships in patients with BBS [25], and response to setmelanotide could be dependent on genotype. Additional analyses from this trial could also assess response to setmelanotide by genetic background.
This study is designed to evaluate the efficacy and safety of setmelanotide for the treatment of obesity and hyperphagia in individuals with BBS or Alström syndrome. This study aims to build upon the results of a phase 2 study that suggested setmelanotide may reduce hunger and body weight in individuals with BBS and Alström syndrome. If successful, the results from this pivotal trial may support the use of setmelanotide for treatment of obesity and hyperphagia in individuals with BBS or Alström syndrome.
Role of the funding source
The sponsor of the study (Rhythm Pharmaceuticals, Inc) designed the trial with assistance from academic investigators. The sponsor aided in data collection, data analysis, data interpretation, and writing of the report. All authors had final responsibility for the decision to submit for publication.
Data statement
Because this article presents a clinical study design, no data are currently available to share.
Funding source
This study was supported by Rhythm Pharmaceuticals, Inc.
Declaration of competing interest
RMH is a consultant for Rhythm Pharmaceuticals, Inc and Trinity Life Sciences and receives grant funding from the Bardet-Biedl Syndrome Foundation.
GG, GY, and MWS are employed by and may own stock in Rhythm Pharmaceuticals, Inc.
JCH has received grant support for clinical investigations from the Memphis Research Consortium, Le Bonheur Children's Foundation Research Institute, and Rhythm Pharmaceuticals, Inc.
JAY receives grant support for clinical investigations from the NICHD, NIH, Soleno Therapeutics Inc, and Rhythm Pharmaceuticals, Inc.
Acknowledgments
Editorial assistance was provided under the direction of the authors by Scott Houck, PhD, CMPP, and David Boffa, ELS, MedThink SciCom, and funded by Rhythm Pharmaceuticals, Inc.
References
- 1.Yazdi F.T., Clee S.M., Meyre D. Obesity genetics in mouse and human: back and forth, and back again. PeerJ. 2015;3:e856. doi: 10.7717/peerj.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huvenne H., Dubern B., Clement K., Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obesity Facts. 2016;9(3):158–173. doi: 10.1159/000445061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015;48(4):229–233. doi: 10.5483/BMBRep.2015.48.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo S., Guo D.F., Bugge K., Morgan D.A., Rahmouni K., Sheffield V.C. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 2009;18(7):1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davenport J.R., Watts A.J., Roper V.C., Croyle M.J., van Groen T., Wyss J.M., Nagy T.R., Kesterson R.A., Yoder B.K. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 2007;17(18):1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hearn T., Spalluto C., Phillips V.J., Renforth G.L., Copin N., Hanley N.A., Wilson D.I. Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes. 2005;54(5):1581–1587. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- 7.Li G., Vega R., Nelms K., Gekakis N., Goodnow C., McNamara P., Wu H., Hong N.A., Glynne R. A role for Alstrom syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS Genet. 2007;3(1):e8. doi: 10.1371/journal.pgen.0030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beales P.L., Elcioglu N., Woolf A.S., Parker D., Flinter F.A. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J. Med. Genet. 1999;36(6):437–446. [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall J.D., Beck S., Maffei P., Naggert J.K. Alstrom syndrome. Eur. J. Hum. Genet. 2007;15(12):1193–1202. doi: 10.1038/sj.ejhg.5201933. [DOI] [PubMed] [Google Scholar]
- 10.Feuillan P.P., Ng D., Han J.C., Sapp J.C., Wetsch K., Spaulding E., Zheng Y.C., Caruso R.C., Brooks B.P., Johnston J.J., Yanovski J.A., Biesecker L.G. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J. Clin. Endocrinol. Metab. 2011;96(3):E528–E535. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherafat-Kazemzadeh R., Ivey L., Kahn S.R., Sapp J.C., Hicks M.D., Kim R.C., Krause A.J., Shomaker L.B., Biesecker L.G., Han J.C., Yanovski J.A. Hyperphagia among patients with Bardet-Biedl syndrome. Pediatr. Obes. 2013;8(5):e64–e67. doi: 10.1111/j.2047-6310.2013.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J.C., Reyes-Capo D.P., Liu C.Y., Reynolds J.C., Turkbey E., Turkbey I.B., Bryant J., Marshall J.D., Naggert J.K., Gahl W.A., Yanovski J.A., Gunay-Aygun M. Comprehensive endocrine-metabolic evaluation of patients with Alström syndrome compared with BMI-matched controls. J. Clin. Endocrinol. Metab. 2018;103(7):2707–2719. doi: 10.1210/jc.2018-00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks M.D., Marshall J.D., Maffei P., Hanish A.E., Hunter L.A., Brady S.M., Sedaka N.M., Sherafat Kazemzadeh R., Tsao J.W., Milan G., Naggert J., Yanovski J.A., Han J.C. 8 November 2012. Hyperphagia, Leptin, and Brain-Derived Neurotrophic Factor in Subjects with Alström Syndrome and BMI-Z Matched Controls (Poster 2702T), Poster Presented at the Annual Scientific Meeting of the American Society for Human Genetics. San Francisco, CA. [Google Scholar]
- 14.Clement K., Biebermann H., Farooqi I.S., Van der Ploeg L., Wolters B., Poitou C., Puder L., Fiedorek F., Gottesdiener K., Kleinau G., Heyder N., Scheerer P., Blume-Peytavi U., Jahnke I., Sharma S., Mokrosinski J., Wiegand S., Muller A., Weiss K., Mai K., Spranger J., Gruters A., Blankenstein O., Krude H., Kuhnen P. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018;24(5):551–555. doi: 10.1038/s41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- 15.Kühnen P., Clement K., Wiegand S., Blankenstein O., Gottesdiener K., Martini L.L., Mai K., Blume-Peytavi U., Gruters A., Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 2016;375(3):240–246. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 16.Haws R., Stewart M., Han J.C. vol. 10. Banff; Alberta, Canada: February 2019. (Clinical Study Experience of the MC4R Agonist Setmelanotide in the Treatment of Rare Genetic Disorders of Obesity: Results from Bardet-Biedl Syndrome and Alström Syndrome Cohorts in a Phase 2 Open-Label Study, Poster Presented at the Keystone Symposia on Molecular and Cellular Biology 2019). [Google Scholar]
- 17.Haws R., Brady S., Davis E., Fletty K., Yuan G., Gordon G., Stewart M., Yanovski J. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes. Metabol. 2020 doi: 10.1111/dom.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro-Sánchez S., Álvarez-Satta M., Valverde D. Bardet-Biedl syndrome: a rare genetic disease. J. Pediatr. Genet. 2013;2(2):77–83. doi: 10.3233/PGE-13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rath A., Salamon V., Peixoto S., Hivert V., Laville M., Segrestin B., Neugebauer E.A.M., Eikermann M., Bertele V., Garattini S., Wetterslev J., Banzi R., Jakobsen J.C., Djurisic S., Kubiak C., Demotes-Mainard J., Gluud C. A systematic literature review of evidence-based clinical practice for rare diseases: what are the perceived and real barriers for improving the evidence and how can they be overcome? Trials. 2017;18(1):556. doi: 10.1186/s13063-017-2287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augustine E.F., Adams H.R., Mink J.W. Clinical trials in rare disease: challenges and opportunities. J. Child Neurol. 2013;28(9):1142–1150. doi: 10.1177/0883073813495959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day S., Jonker A.H., Lau L.P.L., Hilgers R.D., Irony I., Larsson K., Roes K.C., Stallard N. Recommendations for the design of small population clinical trials. Orphanet J. Rare Dis. 2018;13(1):195. doi: 10.1186/s13023-018-0931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sriram P. Beyond placebo: alternative options to the randomized control trial design in rare disease studies. Clin. Trial Pract. Open J. 2020;1(1):42–45. [Google Scholar]
- 23.Dykens E.M., Miller J., Angulo M., Roof E., Reidy M., Hatoum H.T., Willey R., Bolton G., Korner P. Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome. JCI Insight. 2018;3(12) doi: 10.1172/jci.insight.98333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heymsfield S.B., Avena N.M., Baier L., Brantley P., Bray G.A., Burnett L.C., Butler M.G., Driscoll D.J., Egli D., Elmquist J., Forster J.L., Goldstone A.P., Gourash L.M., Greenway F.L., Han J.C., Kane J.G., Leibel R.L., Loos R.J., Scheimann A.O., Roth C.L., Seeley R.J., Sheffield V., Tauber M., Vaisse C., Wang L., Waterland R.A., Wevrick R., Yanovski J.A., Zinn A.R. Hyperphagia: current concepts and future directions proceedings of the 2nd international conference on hyperphagia. Obesity. 2014;22(Suppl 1):S1–s17. doi: 10.1002/oby.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsyth Rl R.L., Gunay-Aygun M. Bardet-Biedl syndrome overview. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., editors. GeneReviews®. University of Washington, Seattle; 2020. NBK1363. [Google Scholar]