Abstract
Plants employ sophisticated mechanisms to control developmental processes and to cope with environmental changes at transcriptional and post-transcriptional levels. MicroRNAs (miRNAs) and long noncoding RNAs (lncRNAs), two classes of endogenous noncoding RNAs, are key regulators of gene expression in plants. Recent studies have identified the interplay between miRNAs and lncRNAs as a novel regulatory layer of gene expression in plants. On one hand, miRNAs target lncRNAs for the production of phased small interfering RNAs (phasiRNAs). On the other hand, lncRNAs serve as origin of miRNAs or regulate the accumulation or activity of miRNAs at transcription and post-transcriptional levels. Theses lncRNA-miRNA interplays are crucial for plant development, physiology and responses to biotic and abiotic stresses. In this review, we summarize recent advances in the biological roles, interaction mechanisms and computational predication methods of the interplay between miRNAs and lncRNAs in plants.
Keywords: Noncoding RNA interactions, Plant gene regulation, Epigenetics, Stress adaptation
1. Introduction
The expression of genes is often spatiotemporally controlled at transcriptional and post-transcriptional levels. Transcription factors and proteins that remodel and modify chromatins play crucial roles in regulating gene transcription [1], [2]. During transcription, pre-mRNAs are subjected to processing such as capping, splicing and adenylation, which provide additional regulations of gene expression. After transcription, the levels and activities of RNAs can be further controlled through RNA modifications, non-coding RNAs (ncRNAs) and various protein factors [3], [4], [5], [6]. In eukaryotes, over 90% RNA transcripts do not encode proteins, which are called ncRNAs [7], [8]. Some of these ncRNAs are basal components of molecular machineries such as ribosome and spliceosome, while others are important riboregulators of gene expression named regulatory ncRNAs [6], [9], [10]. Based on the length, the regulatory ncRNAs are classified into long ncRNAs (lncRNAs, greater than200 nt) and short ncRNAs, including microRNAs (miRNAs), small interfering RNAs (siRNAs) and piwi-interacting RNAs (piRNAs). These regulatory RNAs modulate a variety of biological processes from cell differentiation, organ size and shape determination, to immunity at transcriptional and/or post-transcriptional levels [9], [11], [12], [13]. Interestingly, the emerging evidence has documented the complex interplay between lncRNAs and short ncRNAs on gene regulation in plants and other eukaryotes [14], [15], [16].
To date, thousands of ncRNAs have been identified in plants, such as Arabidopsis [17], rice [18], maize [19], wheat [20], soybean [21], tomato [22], brassica [23], and sorghum [24]. As the two important types of ncRNAs, miRNAs and lncRNAs play critical roles in plant growth, development, biotic and abiotic stress responses such as floret development [25], [26], male sterility [27], [28] , flower time [29], [30], grain yield [31], [32], fruit ripening [33], leaf morphogenesis [34], trichome formation [35], [36], stem elongation [37], [38], cell wall biosynthesis [39], [40], tillering [41], root architecture [42], nodule formation [43], [44] and responses to fungal infection [45], bacterial infection [46], virus infection [47], nematode infection [48], drought [49], cold [50], heat [51], submergence [52], [53], salt [54], [55], light [56] and nutrient stresses [57], [58], [59], [60], [61] (Fig. 1). However, the functional significance and action mechanisms of these regulatory RNAs remain to be deciphered, especially in crops with large and complex genomes. In this mini-review, we will summarize the current advances in the biological functions and action modes of miRNAs and lncRNAs with a focus on their interplays in plants.
Fig. 1.
The biological roles of lncRNAs, miRNAs and their interplay in plant growth and development, biotic and abiotic stress. (a) The representative lncRNAs (green), miRNAs (red) and their interactions (blue) regulate plant growth and development such as floret development [25], [26], male sterility [27], [28], flower time [29], [30], grain yield [31], [32], fruit ripening [33], leaf morphogenesis [34], trichome formation [35], [36], stem elongation [37], [38], cell wall biosynthesis [39], [40], tillering [41], root architecture [42], and nodule formation [43], [44]. (b) The representative lncRNAs (green), miRNAs (red) and their interactions (blue) are involved in biotic and abiotic stress responses such as fungal infection [45], bacterial infection [46], virus infection [47], nematode infection [48], drought [49], cold [50], heat [51], submergence [52], [53], salt [54], [55] , light [56], and nutrient stresses [57], [58], [59], [60], [61]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Biological roles and molecular mechanisms of miRNAs
miRNAs are short (20–24 nucleotides in length) ncRNAs. From interval of 2002 to 2020, 20,388 miRNAs have been annotated in 88 phylogenetically representative plant species [62]. Studies on some miRNAs show that miRNAs regulate almost every biological process of plants from the developmental transition to responses to biotic and abiotic stresses [12], [63], [64], [65], [66] (Fig. 1). We briefly summarize plant miRNA biogenesis, action and related regulatory mechanisms here, since these aspects of miRNAs have been nicely reviewed [67], [68], [69], [70], [71](Fig. 2). In plants, miRNAs mainly inhibit gene expression at the post-transcriptional level through directly targeting mRNA transcripts for cleavage or translational repression [72]. MiRNA biogenesis starts with transcription of primary miRNA transcripts (pri-miRNAs) from miRNA-encoding genes (MIRs) mainly by the DNA-dependent RNA polymerase II (Pol II) [72]. Pri-miRNAs harbor an imperfect stem-loop where the mature miRNAs are embedded. Following transcription, pri-miRNAs are processed into the miRNA/miRNA* duplex in nucleus mainly by the RNase III enzyme, DICER-LIKE1 (DCL1) [73]. Then the miRNA/miRNA* duplex is methylated by HUA ENHANCER 1 (HEN1) to improve its stability and then transported out of nucleus into cytoplasm [74], [75], [76]. MiRNA is incorporated into the ARGONAUTE 1 (AGO1) protein complex to form the miRNA-mediated silencing complex (miRISC) for repressing gene expression [77], [78]. To ensure the efficiency and accuracy of miRNA biogenesis, a plethora of factors such as chromatin modifiers [79], [80], transcriptional factors [81], [82], RNA-associated proteins [83], [84], [85], and protein kinases [86] are employed to regulate the MIR transcription, pri-miRNA processing, RNA stability, and miRNA actions.
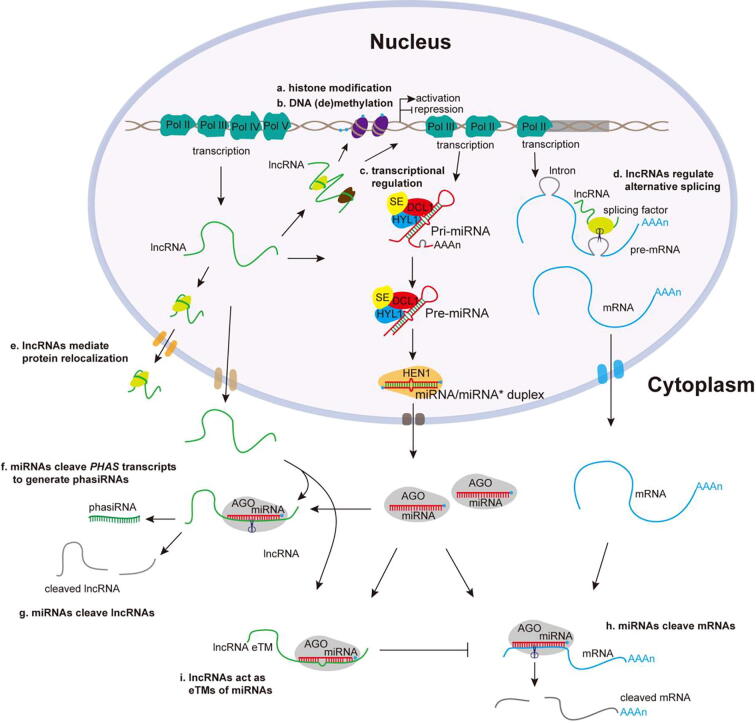
Fig. 2.
The action models of plant lncRNAs, miRNAs and their interplay in diverse biological processes. (a) LncRNAs interact with histone modification complex to regulate histone modification [30], [159]. (b) LncRNAs are involved in DNA (de)methylation to regulate gene transcription [27], [160]. (c) LncRNAs regulate gene transcription by directly binding to proteins required for promoter activity of target genes [101], [102]. (d) LncRNAs interact with alternative splicing factor such as RNA-binding protein to modulate alternative splicing (AS) patterns [97]. (e) LncRNAs mediate protein relocalizaion from nucleus to cytoplasm [161]. (f) miRNAs cleave PHAS transcripts to generate phasiRNAs [28], [33]. (g) LncRNAs are cleaved by miRNAs leading to lncRNA degradation [109]. (h) MiRNAs cleave mRNAs to impact gene transcription [44], [55]. (i) LncRNAs act as eTMs to inhibit miRNA effect on target mRNAs [42], [59].
3. Biological roles and molecular mechanisms of lncRNAs
LncRNAs are a large family of non-coding RNAs with the length of more than 200nt. In plants, similar to mRNAs, Pol II-dependent lncRNA maturation requires capping, splicing and addition of a ploy-A tail, while Pol IV/V-dependent ones do not [87], [88] (Fig. 2). LncRNAs are divided into three groups, long intergenic ncRNAs (no overlapping with protein-coding genes), long intronic ncRNAs (synthesized from intronic region), and natural antisense transcripts (NATs, synthesized from the opposite strand of the associated genes) according to their positions relative to protein-encoding genes in genomes [89]. In the past decades, great efforts have been made toward to the systematic identification and characterization of lncRNAs. For instance, using a reproducibility-based bioinformatics strategy, 6480 lncRNAs were identified from 200 transcriptome data sets in Arabidopsis [17]. Wang et al. identified 37,238 NATs, which are associated with 70% annotated mRNAs, from Arabidopsis [90]. In cotton, 9240 lncRNAs from 21 tissues were identified by integrating multi-strategy RNA-seq data with a pipeline named plant full-length (PULL) [91]. Using the uniform annotation pipeline, 1,246,372 lncRNAs from 80 plant species have been collected according to 13,834 RNA-Seq datasets in Plant Long noncoding RNA Database (PLncDB) V2.0 [92]. Many lncRNAs identified by the prediction tools (see below) have been found to play important roles in plant growth and development. For example, in Arabidopsis, based on the prediction of a pipeline integrating Swiss-Prot database and CPC software [93], [94], the lncRNA MAS, transcribed from MADS AFFECTING FLOWERING4 (MAF4) locus, was found to be induced by cold and required for activating MAF4 transcription by interacting with WDR5A during vernalization [95]. Arabidopsis lncRNA T5120 obtained by the prediction of CPC and CNCI is activated by NLP7, the master nitrate regulatory transcription factor, and promotes plant growth through regulating nitrate assimilation [57].
Although a large number of lncRNAs have been identified, their biological roles and related molecular mechanisms only start to emerge in plants due to the low expression level compared with mRNAs or miRNAs. Studies show that lncRNAs regulate gene expression in both nucleus and cytoplasm via diversified action modes. In the nucleus, lncRNAs modulate gene expression through affecting chromatin remodeling, epigenetic modifications and alternative splicing [96], [97], [98] (Fig. 2). For instance, long intronic ncRNA COLDAIR (COLD ASSISTED INTRONIC NONCODING RNA) controls the transcription of FLOWERING LOCUS C (FLC), which is a key repressor for flowering time [30]. The nuclear-localized COLDAIR is transcribed from a locus within the FLC gene. It physically interacts with a component of polycomb repressive complex 2 (PRC2), and recruit PRC2 to the FLC locus for epigenetic repression during vernalization [30]. Interestingly, the antisense strand of FLC locus encodes another lncRNA named COOLAIR, which interacts with the RNA-binding protein FLOWERING CONTROL LOCUS A (FCA) to represses FLC transcription, accompanied with increased H3K27me3 and decreased H3K36me3 levels in cold condition [99], [100]. The COLDAIR-FLC and COOLAIR-FLC modules represent one of the important roles of lncRNAs in the link of environmental signal and plant development. LincRNA ELF18-INDUCED LONG-NONCODING RNA1 (ELENA1) is induced by the pathogen-associated molecular pattern (PAMP) [101]. ELENA1 directly interacts with the Mediator subunit 19a (MED19a) and promotes its enrichment on PATHOGENESIS-RELATED GENE 1 (PR1) promoter to increase the resistance to bacterial pathogen Pseudomonas ayringae pv. tomato DC3000 [102], [103]. In the cytoplasm, lncRNAs can inhibit protein translation or act as miRNA mimics to inhibit miRNA activity (Fig. 2). MIKKI is a root-specific retrotransposon lncRNA in rice [42]. MIKKI binds and acts as miR171 decoy to inhibit its cleavage on SCARECROW-Like (SCL) mRNAs, leading to the increased cell elongation in root [42].
Despite of their biological importance, lncRNA biogenesis and related regulation mechanisms are still less known. Like the majority of MIR genes, lncRNAs are mostly transcribed by RNA Pol II, but sometimes by Pol III or the plant-specific RNA Pol IV/V [104], [105]. In tomato, 187 lncRNAs are directly targeted by MADS-box transcription factor RIPENING INHIBITOR (RIN), which is a key factor required for tomato fruit ripening [106], suggesting the importance of transcription regulation in lncRNA biogenesis. In addition, cyclin-dependent kinase C (CDKC;2), a component of positive transcription elongation factor b (P-TEFb), is also involved in lncRNA biogenesis [107]. CDKC;2 can promote COOLAIR transcription by enhancing RNA Pol II Ser2 phosphorylation, thereby regulating flowering time in Arabidopsis [107]. Interestingly, the biogenesis of lncRNA and miRNA shares some key components in plants. SERRATE, CBP20 and CBP80, which are key components of miRNA biogenesis, act as regulators of lncRNA biogenesis and intron splicing of some intron-containing lncRNAs [17]. However, DCL1, HYL1 and AGO1 are not required for lincRNA accumulation [17].
4. The interplay between miRNAs and lncRNAs
Recent studies have identified the interplay between miRNAs and lncRNAs. Besides serving as targets or origins of miRNAs, some lncRNAs are able to regulate the biogenesis and function of miRNAs (Fig. 2). The interactions between miRNAs and lncRNAs play important roles in regulating various biological process including development, nutrient abortion, biotic and abiotic stresses [108], [109](Fig. 1).
4.1. LncRNAs are targets of miRNAs to generate phasiRNAs
LncRNA transcripts can be targeted by miRNAs to generate phased small interfering RNAs (phasiRNAs) [92] (Fig. 2). In phasiRNA biogenesis, RNAs including lncRNAs are first typically cleaved by 22 nt miRNAs. Then the RNA-DEPENDENT RNA POLYMERASE6 (RDR6) recruited by AGO1-RISC or AGO7-RISC converts the 3′ fragment into double-stranded RNAs (dsRNAs), which are further processed by a Dicer protein to generate duplexes of phasiRNAs [110], [111], [112]. The resulting phasiRNAs are loaded into AGO proteins and then direct AGOs to find their target transcripts [113]. In plants, ~15 years ago, a subset of lncRNAs that generate a class of phasiRNAs named trans-acting siRNAs (tasiRNAs) were first identified in Arabidopsis [110], [111], [112]. These lncRNAs are targeted by miRNAs including miR173, and miR390, respectively, to produce tasiRNAs [110], [111], [112]. Recently, some lncRNAs from reproductive organ were shown to produce reproductive phasiRNAs [114], [115], [116], [117]. The targets for these phasiRNAs are largely unknown. However, they may regulate reproductive development, given their enrichment in reproductive tissues.
4.2. LncRNAs regulate pri-miRNA processing
Natural antisense transcripts (NATs) belong to a class of coding or ncRNAs that are divided into two clades, cis-NATs and trans-NATs, according to their derived region in genome [90]. Cis-NATs are transcribed from the opposite DNA strands at the same genomic locus, while trans-NATs originated from separate genomic loci [118]. Recent studies have revealed the role of cis-NAT in regulating pri-miRNA processing. cis-NAT398b and cis-NAT398c locate on the complementary strands to MIR398b and MIR398c, respectively [119]. Although the RNAs transcribed from these two loci encode proteins, Core-2/I-branching beta-1,6-N-acetylglucosaminyltrasferase and high-affinity nitrate transporter 2.7, they act as lncRNAs in the nucleus to impair the stability and processing of pri-miR398b/c without impacting their transcription [119]. Interestingly, pri-miR398b/c, but not the mature miR398b/c, directly activates NAT398b/c transcription in an unknown mechanism. By this feed-back regulatory loop, plant fine-tunes thermotolerance [119], implying the complexity of miRNA-lncRNA interplay. cis-NATs are widely present in plants and often affect the expression level of the associated sense genes. Bioinformatic analysis has identified 22 cis-NATs that show reverse-complementary to MIR genes in Brassica [119], implying that the NAT-miRNA regulatory mechanism may be widely present.
Besides cis-NATs, some ncRNAs form dsRNA structures similar to that of pri-miRNAs to hijack the DCL1 complex, and thereby inhibit miRNA biogenesis. For instance, the transcripts derived from the short-interspersed elements (SINEs) can form a structure similar to pri-miRNAs, which in turn decoy HYL1 from pri-miRNA processing [120]. Another example is intron lariat RNAs, the byproducts derived from pre-mRNA splicing, which binds the DCL1 complex and prevents pri-miRNA processing as the molecular sponge in Arabidopsis [121]. In addition, lncRNAs may impair microprocessor recognition and processing activity by forming lncRNA-miRNA precursor dimer. Actually, in human cells, some lncRNAs have been found to directly bind to miRNA precursors and block their processing to miRNAs by DICER complex [122], [123]. However, this kind of miRNA processing-related lncRNAs has not been reported in plants to date.
4.3. LncRNAs act as target mimics of miRNAs
Target mimicry is one of the most important mechanisms of miRNA-lncRNA interplay, by which lncRNAs harboring endogenous target mimic (eTM) sites sequester miRNAs by sequence complementarity to inhibit their effects on target mRNA. Target mimicry is also described as miRNA decoy, miRNA sponge or competing endogenous RNA (ceRNA) in animals [124]. Arabidopsis IPS1 (INDUCED BY PHOSPHATE STARVATION 1) is the first identified lncRNA which pairs with miR399 [59]. Both IPS1 and miR399 are induced by Phosphate (Pi) deficiency. In contrast to the cleavage effect of target mRNA by miRNA, miR399-IPS1 pairing contains a bulge which prevents miR399-mediated IPS1 cleavage, and simultaneously cripples miR399-mediated PHO2 degradation [59]. In maize, a novel lncRNA target of miR399, PILNCR1, is also required for low Pi tolerance [125], suggesting the lncRNA-miR399-PHO2 regulatory module may be a widely mechanism in plant response to Pi deficiency.
Moreover, lncRNA39026 binds miR168a and inhibits its function, which in turn improves tomato resistance to Phytophthora infestans [126]. These results suggest that lncRNAs may modulate various biological processes via hijacking miRNAs. Wu et al. developed a computational method and identified 36 and 189 potential eTMs in Arabidopsis and rice, respectively [34]. Since then, additional lncRNAs that potentially decoy miRNAs have been identified in maize [127], cassava[128], tomato [47], [129], [130], and melon [131]. However, the functional significance of these lncRNA-miRNA interactions still needs to be further analyzed.
4.4. LncRNAs inhibit miRNA expression
Some nuclear-localized lncRNAs regulate gene transcription through mediating chromatin modification. They serve as a bridge between transcription factors and chromatin [30], [132], [133]. Recent evidences show that the transcription of MIRs can also be regulated by lncRNAs in plants. Tomato Sl-miR482a functions as a negative regulator in immunity against Phytophthora infestans by repressing the expression of NBS-LRR genes [109]. Interestingly, Sl-lncRNA15492 locates in the reverse strand of Sl-MIR482a and inhibits its transcription [109].
5. Methods to predict the interaction between lncRNAs and miRNAs
Despite the importance of the interplay between lncRNAs and miRNAs, a large portion of lncRNA-miRNA interactions remains to be identified. We summarize the available tools used to identify plant lncRNA-miRNA interactions here.
The prediction of lncRNA-miRNA interactions begins with the identification of miRNAs and lncRNAs. The miRNAs can be identified from the database such as miRBase [134], PmiREN [62], pmiRKB [135] and PMRD [136], while lncRNAs can be obtained from the lncRNA database such as NONCODE [137], lncRNAdb [138], GreeNC [139], PNRD [140], and PlncDB [92]. Once miRNAs and/or lncRNAs are identified, their interactions can be predicted with additional bioinformatic tools, such as TargetFinder [141], TAPIR [142], psRobot [143], spongeScan [144], and PeTMbase [145]. Among these tools, TargetFinder, TAPIR and PmliPred require users to supply miRNA and potential target sequences. After sequence loading, these tools use various methods to find miRNA-target interactions. TargetFinder utilizes a FASTA local sequence alignment program to identify miRNA targets in plants by source code [140]. It should be noted that the FASTA program allows quick identification of targets but cannot find the RNA-miRNA duplexes having a lot of bulges and/or mismatches. Thus, it is less efficient in identifying lncRNA eTMs. In contrast, TAPIR uses both FASTA program and the precise RNAhybrid algorithm to identify miRNA targets [142]. The RNAhybrid algorithm finds the alignment between miRNA and lncRNA sequences that has the minimum free energy, which allows to predict less perfect match targets including lncRNA eTMs [142]. Indeed, using TAPIR, two lncRNA eTMs were identified to act in JA/MeJA biosynthesis in Oolong Tea [146], while 40 lncRNA eTMs of 15 miRNAs were predicted to be involved in early somatic embryogenesis in Dimocarpus longan Lour. [147]. Using python code, PmliPred is specially designed for predicting miRNA-lncRNA interactions in plants based on hybrid model and fuzzy decision [148]. In addition to the tools requiring user-prepared libraries, other ones perform prediction via the database-stored libraries. For instance, SpongeScan predicts miRNA response elements (MREs) within lncRNA eTMs, based on sequence complementary with preloaded miRNA library [144]. By cross-species conservation filter, Tarhunter identifies eTMs in 13 plant species [149]. Another tool called PsRobot discovers small RNAs with stem-loop precursors (e.g. miRNA) and their target transcripts via a Smith-Waterman algorithm up to 26 plant species [143]. A set of lncRNA eTMs identified by PsRobot have been shown to function in responses to tomato yellow leaf curl virus [47]. Based on the predefined scoring schema, PsRNATarget analyzes the complementary match between miRNAs and their target RNAs by evaluating target site accessibility [150]. Using PsRNATarget, Lnc_973 and lnc_253 have been found to serve as eTMs of ghr-miR399 and ghr-156e in cotton, respectively, to regulate salt stress response [151]. Using PsRobot and PsRNATarget, twelve lncRNAs were predicted to function as eTMs involved in Sneb821-induced tomato resistance to M. incognita [48].
Precision and recall rate are two important parameters to evaluate accuracy and sensitivity of the prediction results [152]. A comparation of prediction tools found that Targetfinder has a better efficiency in predicting miRNA targets in Arabidopsis, while PsRNATarget and TAPIR-hybrid perform well in non-Arabidopsis species [152]. The combination of different tools enhances the precision, but may reduce the sensitivity of prediction reducing the numbers of positive predictions [152], [153]. For the precise and sensitive prediction, the algorithm of tools, multiple source of sequence and the co-expression miRNAs need to be taken into consideration [154]. Taken together, with the above tools and database, more lncRNA-miRNA interactions will be identified, which shall provide insight into the cross-talk among ncRNAs in various biological process.
6. Future perspectives
MiRNAs and lncRNAs play essential roles in regulating various biological processes. The interplay between miRNAs and lncRNAs not only provides additional layers of gene express, but also contributes to the complexity of biological systems. Technologies based on eTMs such as target MIMICs and short tandem target MIMICs have also been developed to study gene function and to improve agricultural traits such as grain yield and quality, resistance to environmental stresses [59], [155], [156], [157], [158]. However, studies on the interplay between miRNAs and lncRNAs are still in the infant stage. Identification of the potential miRNA-lncRNA interaction in various plant species and in various physiological and developmental conditions is still a huge task. Moreover, among identified miRNA-lncRNA interactions, only a few have been analyzed in terms of biological significance. The detailed functional mechanisms for these interactions are still unclear. It will also be interesting to know if these miRNA-lncRNA interactions, related functions and mechanisms are conserved among different plant species. In addition, how various interplays between miRNAs and lncRNAs themselves are modulated at physiological and/or spatiotemporal levels and integrated into gene regulatory network are still largely unknown. Despite of these challenges, studies on the interplay between miRNAs and lncRNAs will be a rich source for exciting new discoveries, lead to a better understanding of gene regulation network and provide intellectual basis for improving important agricultural traits.
CRediT authorship contribution statement
Xiangxiang Meng: Writing - original draft, Writing - review & editing, Visualization. Aixia Li: Writing - original draft, Writing - review & editing. Bin Yu: Conceptualization, Supervision, Writing - original draft, Writing - review & editing. Shengjun Li: Conceptualization, Supervision, Writing - original draft, Writing - review & editing, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by National Natural Science Foundation of China (31872816 and 32070621 to S.L.), NIH (GM127414 to B.Y.), NSF (MCB1818082 to B.Y.), China Postdoctoral Science Foundation (2020 M672156 to X.M.), and The Fundamental Research Funds of Shandong University (61200079614090 to A.L.). We regret that many original articles cannot be included owing to space limitation.
Contributor Information
Bin Yu, Email: byu3@unl.edu.
Shengjun Li, Email: li_sj@qibebt.ac.cn.
References
- 1.Deng X., Song X.W., Wei L.Y., Liu C.Y., Cao X.F. Epigenetic regulation and epigenomic landscape in rice. Natl Sci Rev. 2016;3:309–327. [Google Scholar]
- 2.Schvartzman J.M., Thompson C.B., Finley L.W.S. Metabolic regulation of chromatin modifications and gene expression. J Cell Biol. 2018;217:2247–2259. doi: 10.1083/jcb.201803061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Zhao B.S., Roundtree I.A., Lu Z.K., Han D.L., Ma H.H. N-6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frye M., Jaffrey S.R., Pan T., Rechavi G., Suzuki T. RNA modifications: what have we learned and where are we headed? Nat Rev Genet. 2016;17:365–372. doi: 10.1038/nrg.2016.47. [DOI] [PubMed] [Google Scholar]
- 5.Laloum T., Martin G., Duque P. Alternative Splicing Control of Abiotic Stress Responses. Trends Plant Sci. 2018;23:140–150. doi: 10.1016/j.tplants.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y., Zhang Y., Chen X., Chen Y. Plant Noncoding RNAs: Hidden Players in Development and Stress Responses. Annu Rev Cell Dev Biol. 2019;35:407–431. doi: 10.1146/annurev-cellbio-100818-125218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chekanova J.A., Gregory B.D., Reverdatto S.V., Chen H., Kumar R., Hooker T. Genome-wide high-resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell. 2007;131:1340–1353. doi: 10.1016/j.cell.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 8.Xue Y., Chen R., Qu L., Cao X. Noncoding RNA: from dark matter to bright star. Sci China Life Sci. 2020;63:463–468. doi: 10.1007/s11427-020-1676-5. [DOI] [PubMed] [Google Scholar]
- 9.Cech T.R., Steitz J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Ariel F., Romero-Barrios N., Jegu T., Benhamed M., Crespi M. Battles and hijacks: noncoding transcription in plants. Trends Plant Sci. 2015;20:362–371. doi: 10.1016/j.tplants.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Malmuthuge N., Guan L.L. Noncoding RNAs: Regulatory Molecules of Host-Microbiome Crosstalk. Trends Microbiol. 2021 doi: 10.1016/j.tim.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Li S., Castillo-Gonzalez C., Yu B., Zhang X. The functions of plant small RNAs in development and in stress responses. Plant J. 2017;90:654–670. doi: 10.1111/tpj.13444. [DOI] [PubMed] [Google Scholar]
- 13.Dahariya S., Paddibhatla I., Kumar S., Raghuwanshi S., Pallepati A., Gutti R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol Immunol. 2019;112:82–92. doi: 10.1016/j.molimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Cajal S.R.Y., Segura M.F. A Proposal for Understanding Cell-Specific Signaling Pathways. Frontiers in Genetics; Interplay Between ncRNAs and Cellular Communication: 2019. Hummer S; p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C., Wang Z., Zhang J., Zhao X., Xu P., Liu X. Crosstalk of mRNA, miRNA, lncRNA, and circRNA and Their Regulatory Pattern in Pulmonary Fibrosis. Mol Ther Nucleic Acids. 2019;18:204–218. doi: 10.1016/j.omtn.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai Y.H., Dai X.Z., Harrison A.P., Chen M. RNA regulatory networks in animals and plants: a long noncoding RNA perspective. Briefings in Functional Genomics. 2015;14:91–101. doi: 10.1093/bfgp/elu017. [DOI] [PubMed] [Google Scholar]
- 17.Liu J., Jung C., Xu J., Wang H., Deng S., Bernad L. Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell. 2012;24:4333–4345. doi: 10.1105/tpc.112.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan J., Li J., Yang Y., Tan C., Zhu Y., Hu L. Stress-responsive regulation of long non-coding RNA polyadenylation in Oryza sativa. Plant J. 2018;93:814–827. doi: 10.1111/tpj.13804. [DOI] [PubMed] [Google Scholar]
- 19.Li D.D., Qiao H.L., Qiu W.J., Xu X., Liu T.M., Jiang Q.L. Identification and functional characterization of intermediate-size non-coding RNAs in maize. BMC Genomics. 2018;19. doi: 10.1186/s12864-018-5103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cagirici H.B., Alptekin B., Budak H. RNA Sequencing and Co-expressed Long Non-coding RNA in Modern and Wild Wheats. Sci Rep. 2017;7:10670. doi: 10.1038/s41598-017-11170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golicz A.A., Singh M.B., Bhalla P.L. The Long Intergenic Noncoding RNA (LincRNA) Landscape of the Soybean Genome. Plant Physiol. 2018;176:2133–2147. doi: 10.1104/pp.17.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Gao L., Li J., Zhu B., Zhu H., Luo Y. Analysis of long-non-coding RNAs associated with ethylene in tomato. Gene. 2018;674:151–160. doi: 10.1016/j.gene.2018.06.089. [DOI] [PubMed] [Google Scholar]
- 23.Wei W., Li G., Jiang X., Wang Y., Ma Z., Niu Z. Small RNA and degradome profiling involved in seed development and oil synthesis of Brassica napus. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0204998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X., Zheng H.X., Li J.L., Liu L.N., Zhang X.S., Sui N. Comparative Transcriptome Analysis Reveals New lncRNAs Responding to Salt Stress in Sweet Sorghum. Front Bioeng Biotechnol. 2020;8. doi: 10.3389/fbioe.2020.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J., Yan B., Qu Y., Qin F., Yang Y., Hao X. Zm401, a short-open reading-frame mRNA or noncoding RNA, is essential for tapetum and microspore development and can regulate the floret formation in maize. J Cell Biochem. 2008;105:136–146. doi: 10.1002/jcb.21807. [DOI] [PubMed] [Google Scholar]
- 26.Anwar N., Ohta M., Yazawa T., Sato Y., Li C., Tagiri A. miR172 downregulates the translation of cleistogamy 1 in barley. Ann Bot. 2018;122:251–265. doi: 10.1093/aob/mcy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding J., Lu Q., Ouyang Y., Mao H., Zhang P., Yao J. A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acad Sci U S A. 2012;109:2654–2659. doi: 10.1073/pnas.1121374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Y., Yang J., Mathioni S.M., Yu J., Shen J., Yang X. PMS1T, producing phased small-interfering RNAs, regulates photoperiod-sensitive male sterility in rice. Proc Natl Acad Sci U S A. 2016;113:15144–15149. doi: 10.1073/pnas.1619159114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivaraj S.M., Jain A., Singh A. Highly preserved roles of Brassica MIR172 in polyploid Brassicas: ectopic expression of variants of Brassica MIR172 accelerates floral transition. Mol Genet Genomics. 2018;293:1121–1138. doi: 10.1007/s00438-018-1444-3. [DOI] [PubMed] [Google Scholar]
- 30.Heo J.B., Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331:76–79. doi: 10.1126/science.1197349. [DOI] [PubMed] [Google Scholar]
- 31.Jiao Y., Wang Y., Xue D., Wang J., Yan M., Liu G. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet. 2010;42:541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Luo X., Sun F., Hu J., Zha X., Su W. Overexpressing lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat Commun. 2018;9:3516. doi: 10.1038/s41467-018-05829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G., Chen D., Zhang T., Duan A., Zhang J., He C. Transcriptomic and functional analyses unveil the role of long non-coding RNAs in anthocyanin biosynthesis during sea buckthorn fruit ripening. DNA Res. 2018;25(5):465–476. doi: 10.1093/dnares/dsy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H.J., Wang Z.M., Wang M., Wang X.J. Widespread long noncoding RNAs as endogenous target mimics for microRNAs in plants. Plant Physiol. 2013;161:1875–1884. doi: 10.1104/pp.113.215962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao X., Wang J., Zhu S., Xie Q., Wang L., Yu H. Transcriptomic and functional analyses uncover the regulatory role of lncRNA000170 in tomato multicellular trichome formation. Plant J. 2020;104:18–29. doi: 10.1111/tpj.14902. [DOI] [PubMed] [Google Scholar]
- 36.Yu N., Cai W.J., Wang S., Shan C.M., Wang L.J., Chen X.Y. Temporal control of trichome distribution by microRNA156-targeted SPL genes in Arabidopsis thaliana. Plant Cell. 2010;22:2322–2335. doi: 10.1105/tpc.109.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Wang Y, Zhao J, Huang J, Shi Y, Deng D. Unveiling gibberellin-responsive coding and long noncoding RNAs in maize. Plant Mol Biol 2018 b;98:427-38. [DOI] [PubMed]
- 38.Patil V., McDermott H.I., McAllister T., Cummins M., Silva J.C., Mollison E. APETALA2 control of barley internode elongation. Development. 2019;146. doi: 10.1242/dev.170373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Held M.A., Penning B., Brandt A.S., Kessans S.A., Yong W., Scofield S.R. Small-interfering RNAs from natural antisense transcripts derived from a cellulose synthase gene modulate cell wall biosynthesis in barley. Proc Natl Acad Sci U S A. 2008;105:20534–20539. doi: 10.1073/pnas.0809408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X., Wang C., Xiang N., Li X., Yang S., Du J. Activation of secondary cell wall biosynthesis by miR319-targeted TCP4 transcription factor. Plant Biotechnol J. 2017;15:1284–1294. doi: 10.1111/pbi.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gou J., Fu C., Liu S., Tang C., Debnath S., Flanagan A. The miR156-SPL4 module predominantly regulates aerial axillary bud formation and controls shoot architecture. New Phytol. 2017;216:829–840. doi: 10.1111/nph.14758. [DOI] [PubMed] [Google Scholar]
- 42.Cho J., Paszkowski J. Regulation of rice root development by a retrotransposon acting as a microRNA sponge. Elife. 2017;6. doi: 10.7554/eLife.30038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H., Li Y., Zhang K., Li M., Fu S., Tian Y. miR169c-NFYA-C-ENOD40 modulates nitrogen inhibitory effects in soybean nodulation. New Phytol. 2020 doi: 10.1111/nph.17115. [DOI] [PubMed] [Google Scholar]
- 44.Tsikou D., Yan Z., Holt D.B., Abel N.B., Reid D.E., Madsen L.H. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science. 2018;362:233–236. doi: 10.1126/science.aat6907. [DOI] [PubMed] [Google Scholar]
- 45.Jiang N., Cui J., Shi Y., Yang G., Zhou X., Hou X. Tomato lncRNA23468 functions as a competing endogenous RNA to modulate NBS-LRR genes by decoying miR482b in the tomato-Phytophthora infestans interaction. Hortic Res. 2019;6:28. doi: 10.1038/s41438-018-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Y., Zhou Y.F., Feng Y.Z., He H., Lian J.P., Yang Y.W. Transcriptional landscape of pathogen-responsive lncRNAs in rice unveils the role of ALEX1 in jasmonate pathway and disease resistance. Plant Biotechnol J. 2020;18:679–690. doi: 10.1111/pbi.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J., Yu W., Yang Y., Li X., Chen T., Liu T. Genome-wide analysis of tomato long non-coding RNAs and identification as endogenous target mimic for microRNA in response to TYLCV infection. Sci Rep. 2015;5:16946. doi: 10.1038/srep16946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang F., Zhao D., Fan H., Zhu X., Wang Y., Liu X. Functional Analysis of Long Non-Coding RNAs Reveal Their Novel Roles in Biocontrol of Bacteria-Induced Tomato Resistance to Meloidogyne incognita. Int J Mol Sci. 2020;21(3) doi: 10.3390/ijms21030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S., Yu X., Lei N., Cheng Z., Zhao P., He Y. Genome-wide identification and functional prediction of cold and/or drought-responsive lncRNAs in cassava. Sci Rep. 2017;7:45981. doi: 10.1038/srep45981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu Q., Guo F., Xu Q., Cang J. LncRNA improves cold resistance of winter wheat by interacting with miR398. Funct Plant Biol. 2020;47:544–557. doi: 10.1071/FP19267. [DOI] [PubMed] [Google Scholar]
- 51.Wang A., Hu J., Gao C., Chen G., Wang B., Lin C. Genome-wide analysis of long non-coding RNAs unveils the regulatory roles in the heat tolerance of Chinese cabbage (Brassica rapa ssp.chinensis) Sci Rep. 2019;9:5002. doi: 10.1038/s41598-019-41428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu F., Tan Z., Fang T., Tang K., Liang K., Qiu F. A Comprehensive Transcriptomics Analysis Reveals Long Non-Coding RNA to be Involved in the Key Metabolic Pathway in Response to Waterlogging Stress in Maize. Genes (Basel) 2020;11. doi: 10.3390/genes11030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo F., Han N., Xie Y., Fang K., Yang Y., Zhu M. The miR393a/target module regulates seed germination and seedling establishment under submergence in rice (Oryza sativa L.) Plant Cell Environ. 2016;39:2288–2302. doi: 10.1111/pce.12781. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X., Dong J., Deng F., Wang W., Cheng Y., Song L. The long non-coding RNA lncRNA973 is involved in cotton response to salt stress. BMC Plant Biol. 2019;19:459. doi: 10.1186/s12870-019-2088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan S., Zhao J., Li Z., Hu Q., Yuan N., Zhou M. MicroRNA396-mediated alteration in plant development and salinity stress response in creeping bentgrass. Hortic Res. 2019;6:48. doi: 10.1038/s41438-019-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun Z., Huang K., Han Z., Wang P., Fang Y. Genome-wide identification of Arabidopsis long noncoding RNAs in response to the blue light. Sci Rep. 2020;10:6229. doi: 10.1038/s41598-020-63187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu F., Xu Y., Chang K., Li S., Liu Z., Qi S. The long noncoding RNA T5120 regulates nitrate response and assimilation in Arabidopsis. New Phytol. 2019;224:117–131. doi: 10.1111/nph.16038. [DOI] [PubMed] [Google Scholar]
- 58.Vidal E.A., Araus V., Lu C., Parry G., Green P.J., Coruzzi G.M. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2010;107:4477–4482. doi: 10.1073/pnas.0909571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franco-Zorrilla J.M., Valli A., Todesco M., Mateos I., Puga M.I., Rubio-Somoza I. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- 60.Kawashima C.G., Matthewman C.A., Huang S., Lee B.R., Yoshimoto N., Koprivova A. Interplay of SLIM1 and miR395 in the regulation of sulfate assimilation in Arabidopsis. Plant J. 2011;66:863–876. doi: 10.1111/j.1365-313X.2011.04547.x. [DOI] [PubMed] [Google Scholar]
- 61.Sun Z., Shu L., Zhang W., Wang Z. Cca-miR398 increases copper sulfate stress sensitivity via the regulation of CSD mRNA transcription levels in transgenic Arabidopsis thaliana. PeerJ. 2020;8 doi: 10.7717/peerj.9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Z., Kuang Z., Wang Y., Zhao Y., Tao Y., Cheng C. PmiREN: a comprehensive encyclopedia of plant miRNAs. Nucleic Acids Res. 2020;48:D1114–D1121. doi: 10.1093/nar/gkz894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D'Ario M., Griffiths-Jones S., Kim M. Small RNAs: Big Impact on Plant Development. Trends Plant Sci. 2017;22:1056–1068. doi: 10.1016/j.tplants.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Huang C.Y., Wang H., Hu P., Hamby R., Jin H. Small RNAs - Big Players in Plant-Microbe Interactions. Cell Host Microbe. 2019;26:173–182. doi: 10.1016/j.chom.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 66.Song X.W., Li Y., Cao X.F., Qi Y.J. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu Rev Plant Biol. 2019;70(70):489–525. doi: 10.1146/annurev-arplant-050718-100334. [DOI] [PubMed] [Google Scholar]
- 67.Wang J., Mei J., Ren G. Plant microRNAs: Biogenesis, Homeostasis, and Degradation. Front Plant Sci. 2019;10:360. doi: 10.3389/fpls.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Y., Jia T., Chen X. The 'how' and 'where' of plant microRNAs. New Phytol. 2017;216:1002–1017. doi: 10.1111/nph.14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Achkar N.P., Cambiagno D.A., Manavella P.A. miRNA Biogenesis: A Dynamic Pathway. Trends Plant Sci. 2016;21:1034–1044. doi: 10.1016/j.tplants.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Manavella P.A., Yang S.W., Palatnik J. Keep calm and carry on: miRNA biogenesis under stress. Plant J. 2019;99:832–843. doi: 10.1111/tpj.14369. [DOI] [PubMed] [Google Scholar]
- 71.Jodder J. Regulation of pri-MIRNA processing: mechanistic insights into the miRNA homeostasis in plant. Plant Cell Rep. 2021 doi: 10.1007/s00299-020-02660-7. [DOI] [PubMed] [Google Scholar]
- 72.Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- 73.Kurihara Y., Watanabe Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. PNAS. 2004;101:12753–12758. doi: 10.1073/pnas.0403115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu B., Yang Z.Y., Li J.J., Minakhina S., Yang M.C., Padgett R.W. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park M.Y., Wu G., Gonzalez-Sulser A., Vaucheret H., Poethig R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci U S A. 2005;102:3691–3696. doi: 10.1073/pnas.0405570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang B., You C., Zhang Y., Zeng L., Hu J., Zhao M. Linking key steps of microRNA biogenesis by TREX-2 and the nuclear pore complex in Arabidopsis. Nat Plants. 2020;6:957–969. doi: 10.1038/s41477-020-0726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bologna N.G., Iselin R., Abriata L.A., Sarazin A., Pumplin N., Jay F. Nucleo-cytosolic Shuttling of ARGONAUTE1 Prompts a Revised Model of the Plant MicroRNA Pathway. Mol Cell. 2018;69(709–19) doi: 10.1016/j.molcel.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Cui Y., Fang X., Qi Y. TRANSPORTIN1 Promotes the Association of MicroRNA with ARGONAUTE1 in Arabidopsis. Plant Cell. 2016;28:2576–2585. doi: 10.1105/tpc.16.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z., Ma Z., Castillo-Gonzalez C., Sun D., Li Y., Yu B. SWI2/SNF2 ATPase CHR2 remodels pri-miRNAs via Serrate to impede miRNA production. Nature. 2018;557:516–521. doi: 10.1038/s41586-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 80.Choi K., Kim J., Muller S.Y., Oh M., Underwood C., Henderson I. Regulation of MicroRNA-Mediated Developmental Changes by the SWR1 Chromatin Remodeling Complex. Plant Physiol. 2016;171:1128–1143. doi: 10.1104/pp.16.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Z., Guo T., Liu Y., Liu Q., Fang Y. The Roles of Arabidopsis CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laubinger S. MicroRNA transcription and processing: Elongator caught in the act. Nat Plants. 2015;1:15076. doi: 10.1038/nplants.2015.76. [DOI] [PubMed] [Google Scholar]
- 83.Zhang S., Liu Y., Yu B. PRL1, an RNA-binding protein, positively regulates the accumulation of miRNAs and siRNAs in Arabidopsis. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li S., Xu R., Li A., Liu K., Gu L., Li M. SMA1, a homolog of the splicing factor Prp28, has a multifaceted role in miRNA biogenesis in Arabidopsis. Nucleic Acids Res. 2018;46:9148–9159. doi: 10.1093/nar/gky591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li S., Li M., Liu K., Zhang H., Zhang S., Zhang C. MAC5, an RNA-binding protein, protects pri-miRNAs from SERRATE-dependent exoribonuclease activities. Proc Natl Acad Sci U S A. 2020;117:23982–23990. doi: 10.1073/pnas.2008283117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su C., Li Z., Cheng J., Li L., Zhong S., Liu L. The Protein Phosphatase 4 and SMEK1 Complex Dephosphorylates HYL1 to Promote miRNA Biogenesis by Antagonizing the MAPK Cascade in Arabidopsis. Dev Cell. 2017;41(527–39) doi: 10.1016/j.devcel.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 88.Wierzbicki A.T. The role of long non-coding RNA in transcriptional gene silencing. Curr Opin Plant Biol. 2012;15:517–522. doi: 10.1016/j.pbi.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Chekanova J.A. Long non-coding RNAs and their functions in plants. Curr Opin Plant Biol. 2015;27:207–216. doi: 10.1016/j.pbi.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Wang H., Chung P.J., Liu J., Jang I.C., Kean M.J., Xu J. Genome-wide identification of long noncoding natural antisense transcripts and their responses to light in Arabidopsis. Genome Res. 2014;24:444–453. doi: 10.1101/gr.165555.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng X., Chen Y., Zhou Y., Shi K., Hu X., Li D. Full-length annotation with multi-strategy RNA-seq uncovers transcriptional regulation of lncRNAs in cotton1. Plant Physiol. 2020 doi: 10.1093/plphys/kiaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin J., Lu P., Xu Y., Li Z., Yu S., Liu J. PLncDB V2.0: a comprehensive encyclopedia of plant long noncoding RNAs. Nucleic Acids Res. 2021;49:D1489–D1495. doi: 10.1093/nar/gkaa910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Apweiler R. Activities at the Universal Protein Resource (UniProt) (vol 42, pg D198, 2014). Nucleic Acids Research 2014;42:7486-. [DOI] [PMC free article] [PubMed]
- 94.Kong L., Zhang Y., Ye Z.Q., Liu X.Q., Zhao S.Q., Wei L. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007;35:W345–W349. doi: 10.1093/nar/gkm391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao X., Li J., Lian B., Gu H., Li Y., Qi Y. Global identification of Arabidopsis lncRNAs reveals the regulation of MAF4 by a natural antisense RNA. Nat Commun. 2018;9:5056. doi: 10.1038/s41467-018-07500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heo J.B., Lee Y.S., Sung S. Epigenetic regulation by long noncoding RNAs in plants. Chromosome Res. 2013;21:685–693. doi: 10.1007/s10577-013-9392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bardou F., Ariel F., Simpson C.G., Romero-Barrios N., Laporte P., Balzergue S. Long noncoding RNA modulates alternative splicing regulators in Arabidopsis. Dev Cell. 2014;30:166–176. doi: 10.1016/j.devcel.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 98.Long Y.C., Wang X.Y., Youmans D.T., Cech T.R. How do lncRNAs regulate transcription? Science. Advances. 2017;3. doi: 10.1126/sciadv.aao2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Csorba T., Questa J.I., Sun Q.W., Dean C. Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. PNAS. 2014;111:16160–16165. doi: 10.1073/pnas.1419030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian Y., Zheng H., Zhang F., Wang S., Ji X., Xu C. PRC2 recruitment and H3K27me3 deposition at FLC require FCA binding of COOLAIR. Sci Adv. 2019;5:eaau7246. doi: 10.1126/sciadv.aau7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seo J.S., Sun H.X., Park B.S., Huang C.H., Yeh S.D., Jung C. ELF18-INDUCED LONG-NONCODING RNA Associates with Mediator to Enhance Expression of Innate Immune Response Genes in Arabidopsis. Plant Cell. 2017;29:1024–1038. doi: 10.1105/tpc.16.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seo J.S., Diloknawarit P., Park B.S., Chua N.H. ELF18-INDUCED LONG NONCODING RNA 1 evicts fibrillarin from mediator subunit to enhance PATHOGENESIS-RELATED GENE 1 (PR1) expression. New Phytol. 2019;221:2067–2079. doi: 10.1111/nph.15530. [DOI] [PubMed] [Google Scholar]
- 103.Mach J. The Long-Noncoding RNA ELENA1 Functions in Plant Immunity. Plant Cell. 2017;29:916. doi: 10.1105/tpc.17.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wierzbicki A.T., Haag J.R., Pikaard C.S. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell. 2008;135:635–648. doi: 10.1016/j.cell.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li L., Eichten S.R., Shimizu R., Petsch K., Yeh C.T., Wu W. Genome-wide discovery and characterization of maize long non-coding RNAs. Genome Biol. 2014;15:R40. doi: 10.1186/gb-2014-15-2-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu T., Tzeng D.T.W., Li R., Chen J., Zhong S., Fu D. Genome-wide identification of long non-coding RNA targets of the tomato MADS box transcription factor RIN and function analysis. Ann Bot. 2019;123:469–482. doi: 10.1093/aob/mcy178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z.W., Wu Z., Raitskin O., Sun Q., Dean C. Antisense-mediated FLC transcriptional repression requires the P-TEFb transcription elongation factor. Proc Natl Acad Sci U S A. 2014;111:7468–7473. doi: 10.1073/pnas.1406635111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou X., Cui J., Meng J., Luan Y. Interactions and links among the noncoding RNAs in plants under stresses. Theor Appl Genet. 2020;133:3235–3248. doi: 10.1007/s00122-020-03690-1. [DOI] [PubMed] [Google Scholar]
- 109.Jiang N., Cui J., Hou X., Yang G., Xiao Y., Han L. Sl-lncRNA15492 interacts with Sl-miR482a and affects Solanum lycopersicum immunity against Phytophthora infestans. Plant J. 2020;103:1561–1574. doi: 10.1111/tpj.14847. [DOI] [PubMed] [Google Scholar]
- 110.Axtell M.J., Jan C., Rajagopalan R., Bartel D.P. A two-hit trigger for siRNA biogenesis in plants. Cell. 2006;127:565–577. doi: 10.1016/j.cell.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 111.Howell M.D., Fahlgren N., Chapman E.J., Cumbie J.S., Sullivan C.M., Givan S.A. Genome-wide analysis of the RNA-DEPENDENT RNA POLYMERASE6/DICER-LIKE4 pathway in Arabidopsis reveals dependency on miRNA- and tasiRNA-directed targeting. Plant Cell. 2007;19:926–942. doi: 10.1105/tpc.107.050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoshikawa M., Peragine A., Park M.Y., Poethig R.S. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 2005;19:2164–2175. doi: 10.1101/gad.1352605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y.L., Teng C., Xia R., Meyers B.C. PhasiRNAs in Plants: Their Biogenesis, Genic Sources, and Roles in Stress Responses, Development, and Reproduction. Plant Cell. 2020;32:3059–3080. doi: 10.1105/tpc.20.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson C., Kasprzewska A., Tennessen K., Fernandes J., Nan G.L., Walbot V. Clusters and superclusters of phased small RNAs in the developing inflorescence of rice. Genome Res. 2009;19:1429–1440. doi: 10.1101/gr.089854.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhai J.X., Zhang H., Arikit S., Huang K., Nan G.L., Walbot V. Spatiotemporally dynamic, cell-type-dependent premeiotic and meiotic phasiRNAs in maize anthers. PNAS. 2015;112:3146–3151. doi: 10.1073/pnas.1418918112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fei Q.L., Yang L., Liang W.Q., Zhang D.B., Meyers B.C. Dynamic changes of small RNAs in rice spikelet development reveal specialized reproductive phasiRNA pathways. J Exp Bot. 2016;67:6037–6049. doi: 10.1093/jxb/erw361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xia R., Chen C.J., Pokhrel S., Ma W.Q., Huang K., Patel P. 24-nt reproductive phasiRNAs are broadly present in angiosperms. Nature. Communications. 2019;10. doi: 10.1038/s41467-019-08543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rinn J.L., Chang H.Y. Genome Regulation by Long Noncoding RNAs. Annu Rev Biochem. 2012;81(81):145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Y., Li X., Yang J., He Y. Natural antisense transcripts of MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. Nat Commun. 2020;11:5351. doi: 10.1038/s41467-020-19186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pouch-Pelissier M.N., Pelissier T., Elmayan T., Vaucheret H., Boko D., Jantsch M.F. SINE RNA induces severe developmental defects in Arabidopsis thaliana and interacts with HYL1 (DRB1), a key member of the DCL1 complex. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Z., Wang S., Cheng J., Su C., Zhong S., Liu Q. Intron Lariat RNA Inhibits MicroRNA Biogenesis by Sequestering the Dicing Complex in Arabidopsis. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tian T., Lv X., Pan G., Lu Y., Chen W., He W. Long Noncoding RNA MPRL Promotes Mitochondrial Fission and Cisplatin Chemosensitivity via Disruption of Pre-miRNA Processing. Clin Cancer Res. 2019;25:3673–3688. doi: 10.1158/1078-0432.CCR-18-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Y., Song Y., Wang Z., Zhang Z., Lu M., Wang Y. Long Non-coding RNA LINC01787 Drives Breast Cancer Progression via Disrupting miR-125b Generation. Front Oncol. 2019;9:1140. doi: 10.3389/fonc.2019.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du Q., Wang K., Zou C., Xu C., Li W.X. The PILNCR1-miR399 Regulatory Module Is Important for Low Phosphate Tolerance in Maize. Plant Physiol. 2018;177:1743–1753. doi: 10.1104/pp.18.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hou X.X., Cui J., Liu W.W., Jiang N., Zhou X.X., Qi H.Y. LncRNA39026 Enhances Tomato Resistance to Phytophthora infestans by Decoying miR168a and Inducing PR Gene Expression. Phytopathology. 2020;110:873–880. doi: 10.1094/PHYTO-12-19-0445-R. [DOI] [PubMed] [Google Scholar]
- 127.Fan C.Y., Hao Z.Q., Yan J.H., Li G.L. Genome-wide identification and functional analysis of lincRNAs acting as miRNA targets or decoys in maize. BMC Genomics. 2015;16. doi: 10.1186/s12864-015-2024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li SX, Yu X, Lei N, Cheng ZH, Zhao PJ, He YK, et al. Genome-wide identification and functional prediction of cold and/or drought-responsive lncRNAs in cassava (vol 7, 45981, 2017). Scientific Reports 2017;7c [DOI] [PMC free article] [PubMed]
- 129.Yang Y.W., Liu T.L., Shen D.Y., Wang J.Y., Ling X.T., Hu Z.Z. Tomato yellow leaf curl virus intergenic siRNAs target a host long noncoding RNA to modulate disease symptoms. PLoS Pathog. 2019;15. doi: 10.1371/journal.ppat.1007534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cui J., Jiang N., Hou X., Wu S., Zhang Q., Meng J. Genome-Wide Identification of lncRNAs and Analysis of ceRNA Networks During Tomato Resistance to Phytophthora infestans. Phytopathology. 2020;110:456–464. doi: 10.1094/PHYTO-04-19-0137-R. [DOI] [PubMed] [Google Scholar]
- 131.Gao C., Sun J.L., Dong Y.M., Wang C.Q., Xiao S.H., Mo L.F. Comparative transcriptome analysis uncovers regulatory roles of long non-coding RNAs involved in resistance to powdery mildew in melon. BMC Genomics. 2020;21. doi: 10.1186/s12864-020-6546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dykes I.M., Emanueli C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genomics Proteomics Bioinformatics. 2017;15:177–186. doi: 10.1016/j.gpb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y., Tao Y., Liao Q. Long noncoding RNA: a crosslink in biological regulatory network. Brief Bioinform. 2018;19:930–945. doi: 10.1093/bib/bbx042. [DOI] [PubMed] [Google Scholar]
- 134.Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meng Y., Gou L., Chen D., Mao C., Jin Y., Wu P. PmiRKB: a plant microRNA knowledge base. Nucleic Acids Res. 2011;39:D181–D187. doi: 10.1093/nar/gkq721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Z., Yu J., Li D., Zhang Z., Liu F., Zhou X. PMRD: plant microRNA database. Nucleic Acids Res. 2010;38:D806–D813. doi: 10.1093/nar/gkp818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fang S., Zhang L., Guo J., Niu Y., Wu Y., Li H. NONCODEV5: a comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 2018;46:D308–D314. doi: 10.1093/nar/gkx1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quek X.C., Thomson D.W., Maag J.L., Bartonicek N., Signal B., Clark M.B. lncRNAdb v2.0: expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res. 2015;43:D168–D173. doi: 10.1093/nar/gku988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paytuví Gallart A., Hermoso Pulido A., Martínez Anzar, de Lagrán I., Sanseverino W., Aiese Cigliano R. GREENC: a Wiki-based database of plant lncRNAs. Nucleic Acids Res. 2016;44:D1161–D1166. doi: 10.1093/nar/gkv1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yi X., Zhang Z., Ling Y., Xu W., Su Z. PNRD: a plant non-coding RNA database. Nucleic Acids Res. 2015;43:D982–D989. doi: 10.1093/nar/gku1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fahlgren N., Howell M.D., Kasschau K.D., Chapman E.J., Sullivan C.M., Cumbie J.S. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS ONE. 2007;2 doi: 10.1371/journal.pone.0000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bonnet E., He Y., Billiau K., Van de Peer Y. TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics. 2010;26:1566–1568. doi: 10.1093/bioinformatics/btq233. [DOI] [PubMed] [Google Scholar]
- 143.Wu H.J., Ma Y.K., Chen T., Wang M., Wang X.J. PsRobot: a web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res. 2012;40:W22–W28. doi: 10.1093/nar/gks554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Furió-Tarí P., Tarazona S., Gabaldón T., Enright A.J., Conesa A. spongeScan: A web for detecting microRNA binding elements in lncRNA sequences. Nucleic Acids Res. 2016;44:W176–W180. doi: 10.1093/nar/gkw443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Karakülah G., Yücebilgili Kurtoğlu K., Unver T. PeTMbase: A Database of Plant Endogenous Target Mimics (eTMs) PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0167698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhu C., Zhang S., Fu H., Zhou C., Chen L., Li X. Transcriptome and Phytochemical Analyses Provide New Insights Into Long Non-Coding RNAs Modulating Characteristic Secondary Metabolites of Oolong Tea (Camellia sinensis) in Solar-Withering. Front Plant Sci. 2019;10:1638. doi: 10.3389/fpls.2019.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen Y., Li X., Su L., Chen X., Zhang S., Xu X. Genome-wide identification and characterization of long non-coding RNAs involved in the early somatic embryogenesis in Dimocarpus longan Lour. BMC Genomics. 2018;19:805. doi: 10.1186/s12864-018-5158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kang Q., Meng J., Cui J., Luan Y., Chen M. PmliPred: a method based on hybrid model and fuzzy decision for plant miRNA-lncRNA interaction prediction. Bioinformatics. 2020;36:2986–2992. doi: 10.1093/bioinformatics/btaa074. [DOI] [PubMed] [Google Scholar]
- 149.Ma X., Liu C., Gu L., Mo B., Cao X., Chen X. TarHunter, a tool for predicting conserved microRNA targets and target mimics in plants. Bioinformatics. 2018;34:1574–1576. doi: 10.1093/bioinformatics/btx797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dai X., Zhuang Z., Zhao P.X. psRNATarget: a plant small RNA target analysis server (2017 release) Nucleic Acids Res. 2018;46:W49–W54. doi: 10.1093/nar/gky316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Deng F., Zhang X., Wang W., Yuan R., Shen F. Identification of Gossypium hirsutum long non-coding RNAs (lncRNAs) under salt stress. BMC Plant Biol. 2018;18:23. doi: 10.1186/s12870-018-1238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Srivastava P.K., Moturu T.R., Pandey P., Baldwin I.T., Pandey S.P. A comparison of performance of plant miRNA target prediction tools and the characterization of features for genome-wide target prediction. BMC Genomics. 2014;15:348. doi: 10.1186/1471-2164-15-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oliveira A.C., Bovolenta L.A., Nachtigall P.G., Herkenhoff M.E., Lemke N., Pinhal D. Combining Results from Distinct MicroRNA Target Prediction Tools Enhances the Performance of Analyses. Front Genet. 2017;8:59. doi: 10.3389/fgene.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ritchie W., Flamant S., Rasko J.E. Predicting microRNA targets and functions: traps for the unwary. Nat Methods. 2009;6:397–398. doi: 10.1038/nmeth0609-397. [DOI] [PubMed] [Google Scholar]
- 155.Todesco M., Rubio-Somoza I., Paz-Ares J., Weigel D. A collection of target mimics for comprehensive analysis of microRNA function in Arabidopsis thaliana. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yan J., Gu Y., Jia X., Kang W., Pan S., Tang X. Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell. 2012;24:415–427. doi: 10.1105/tpc.111.094144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang H., Zhang J., Yan J., Gou F., Mao Y., Tang G. Short tandem target mimic rice lines uncover functions of miRNAs in regulating important agronomic traits. Proc Natl Acad Sci U S A. 2017;114:5277–5282. doi: 10.1073/pnas.1703752114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peng T., Qiao M., Liu H., Teotia S., Zhang Z., Zhao Y. A Resource for Inactivation of MicroRNAs Using Short Tandem Target Mimic Technology in Model and Crop Plants. Mol Plant. 2018;11:1400–1417. doi: 10.1016/j.molp.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 159.Kim D.H., Xi Y., Sung S. Modular function of long noncoding RNA, COLDAIR, in the vernalization response. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ariel F., Jegu T., Latrasse D., Romero-Barrios N., Christ A., Benhamed M. Noncoding transcription by alternative RNA polymerases dynamically regulates an auxin-driven chromatin loop. Mol Cell. 2014;55:383–396. doi: 10.1016/j.molcel.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 161.Campalans A., Kondorosi A., Crespi M. Enod40, a short open reading frame-containing mRNA, induces cytoplasmic localization of a nuclear RNA binding protein in Medicago truncatula. Plant Cell. 2004;16:1047–1059. doi: 10.1105/tpc.019406. [DOI] [PMC free article] [PubMed] [Google Scholar]