Abstract
We developed a method to perform direct ink writing (DIW) three-dimensional (3D) printing of coconut-based products with high oil content by varying compositions of the coconut oil and the coconut cream. The addition of oils is particularly crucial in providing energy, developing neurological functions, and improving the palatability of food. Despite the potential merits of high oil-content foods, there have been limited studies on 3D printing of high oil-content foods. In particular, the effect of oil content on the printability of food inks has not been studied to date. 3D printing of food inks with high oil contents is challenging due to oil separation that leads to unpredictable changes in rheological properties. In this work, we surveyed the behavior of the mixture of the coconut oil and the coconut cream and identified the appropriate conditions for the food inks that show the printability in DIW 3D printing. We initially formulated coconut cream inks added with coconut oil that did not exhibit oil separation, and characterized the rheological properties of such inks. We successfully 3D-printed coconut cream with additional coconut oil and successfully fabricated 3D structures with inks containing 25% water with an additional 10% (w/w) of coconut oil. Texture profile analysis (TPA) suggested that the hardness index and the chewiness index of mesh-shaped 3D-printed coconut cream decreased due to an increase in the water content of the ink. Overall, this study offered an understanding of the stability of the food inks and demonstrated the fabrication of 3D colloidal food with controlled oil content, which can be applied to formulating foods with tunable oil content to cater to individual nutritional needs without compromising the stability of the inks.
Keywords: 3D printing, Direct ink writing, 3D food printing, Rheology, Coconut cream, Coconut oil
1. Introduction
This paper describes a method to perform three-dimensional (3D) printing of food inks with high oil content using a direct ink writing (DIW) 3D printer. We applied this method to 3D print coconut cream with additional coconut oil and successfully fabricated 3D structures with inks containing 25% water with an additional 10% (w/w) coconut oil. While previous works focused on 3D printing of starch-based[1-4] and fiber-based inks[5,6], our work focused on the effect of oil content on the printability of food inks. Oil is often added to food ink to achieve desired texture, flavor, and functions, but the high oil content of the food would alter the property of food inks by reducing the viscosity and causing phase separation[7]. In this work, we studied the property of food ink added with oil to achieve key rheological properties and 3D printability.
3D printing is a method to fabricate 3D models consisting of various materials deposited in a layer-by-layer manner, and it is applied across multiple fields to fabricate metal aerospace parts[8], living organs[9,10], electronic devices[11], and fluidic devices[12,13]. DIW 3D food printing is an emerging field[14,15], which allows customization of nutrients based on individual needs[16], fabrication of aesthetically pleasing food[17], and customization of food texture[4]. Extrusion-based methods, such as hot-melt and cold extrusion, have been widely used in food printing because of their flexibility to dispense liquid-based food materials[18-20]. However, hot-melt extrusion is not always suitable to print temperature-sensitive food because they require an elevated temperature to melt food materials. As such, there are increasing interests in 3D-printed, temperature-sensitive food materials through cold extrusion that relies solely on the rheology of ink[21,22]. Several foods such as chocolate-based ink[22], milk-based ink[23], vegetable-based ink[5], and gelatin[24] have been used to demonstrate 3D food printing. For example, the addition of xanthan gum and k-carrageenan gum has been demonstrated to make mashed potatoes to form self-supporting structures adequate to maintain 3D structures[1]. Printable chocolate inks were achieved by altering the rheological properties of chocolate ink by varying the cocoa powder and chocolate syrup[22]. These demonstrations have highlighted the importance of the rheology of the food ink to achieve extrusion-based 3D printing.
Foods commonly comprise multiple constituents such as carbohydrates, proteins, fats, small molecules, and water. The interactions among different food constituents would affect the rheological properties and stability of inks[25]. The protein in the food is known to be amphiphilic that holds the hydrophobic and hydrophilic aggregates in the food matrix, and acts as an emulsifier that reduces the surface tension between two immiscible materials such as oil and water. The presence of an emulsifier hence permits different constituents to mix[26]. Starch is a polysaccharide carbohydrate consisting of multiple glucose units joined together by glycosidic bonds[27], which can form entanglements networks to control the rheology of the food[28]. The stability of the ink, where no oil separation occurs, is crucial to prevent rancidity, degradation of vitamins, and formation of potentially harmful compounds[29]. Moreover, the printability of ink would be affected due to the change in phase which would alter the rheological properties of the ink. Hence, the control of the interactions among protein, carbohydrate, and oil is important to control the stability of the edible colloidal system[30]. Despite the increasing interest in 3D food printing, there are limited works on the effect of oil content on the printability of food inks to date. 3D printing of food with high oil content is challenging due to the occurrence of oil separation and poor rheological properties. The addition of oils is particularly crucial in providing energy and improving the palatability of food[31]. Moreover, it is important in neurological development especially in infancy and early childhood as it provides medium for absorption of fat-soluble vitamins A, D, E, and K[32].
To bridge the gap, we demonstrated the extrusion of oil-based food ink through simple alteration of rheological properties with different concentrations of water and oil (Supplementary Figure 1 (82.1KB, pdf) ). We first conducted oil separation tests by varying the concentration of water and additional coconut oil to determine the limits of the amount of oil present in the inks; the stability of the ink was crucial to ensure the smooth extrusion of material and the maintenance of the printed structures. Three inks with different water and oil contents were selected to characterize the rheological properties; the viscosity, yield stress, and storage modulus of the ink are important parameters for DIW to determine the printability and structure integrity of the printed models[22,23]. Mesh structures were printed with all three inks to observe the spreading of inks to determine printability. We also performed texture profile analysis (TPA) for the printed mesh structures using inks with varying contents to assess the capability to achieve desired textural properties. Extending the demonstration, we printed various 3D structures with suitable ink that did not exhibit oil separation and spreading of ink. The knowledge we developed here should be useful to fabricate other food structures with high oil content such as sesame paste[33] and peanut butter[34], which should find a broad field of applications in the healthcare and food industries.
2. Materials and methods
2.1. Preparation of coconut cream ink
The base material used was commercially available coconut cream powder (Kara Coconut Cream Powder, PT Pulau Sambu, Riau, Indonesia) that contained coconut extract, hydrolyzed starch, and milk protein. Other food materials used were coconut oil (Benefit Coco, Singapore) and pandan extract (Bake King, Singapore). The samples were first prepared by adding coconut cream powder into deionized water, with 0.2% w/w pandan extract for color and flavor enhancement, at different weight concentrations. Samples were then mixed thoroughly for 5 min at 2000 revolutions per minute (rpm) with a planetary centrifugal mixer (Thinky ARE-250, Thinky Corporation, Tokyo, Japan), at 25°C (room temperature). Finally, coconut oil was added at different weight concentrations (5–30% w/w). The mixture was homogenized again using a planetary centrifugal mixer for 5 min at 2000 rpm.
2.2. Characterization of oil separation
The oil separated from the ink was collected by filtering the ink with a sieve immediately on preparation of the ink. The mass of the collected oil was weighed using a weighing balance. All measurements were conducted in duplicates. The formula of oil separation ratio used was as follows:
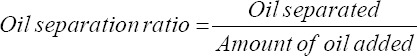
2.3. Rheological characterization
Rheological measurements of coconut cream inks were conducted using an oscillatory rheometer (Discovery Hybrid Rheometer DHR-2, TA Instruments, Delaware, USA) with stainless steel parallel plates with a diameter of 40 mm at a truncation gap of 1000 mm. Shear-thinning tests were conducted by applying a stepwise shear rate ramp of 0.01–100 s−1. Stress sweep measurements were conducted with a logarithmically increasing shear stress at a constant frequency of 1 Hz over the range of 0.1–2000 Pa. Excess food material was removed before the measurements to prevent edge effect. All rheological measurements were conducted at 25 ± 0.1°C in triplicates.
2.4. DIW 3D printing
An extrusion-based DIW printer (SHOTmini 200 Sx, Musashi Engineering, Inc., Tokyo, Japan) was used to perform 3D printing. The printer was placed in an enclosed box to maintain a sterile environment. All 3D models were obtained from a public repository of 3D printable models, Thingiverse, and imported to Slic3r[35] for slicing of the model into layers and generation of G-code. The generated G-code was converted to MuCAD V (Musashi Engineering, Inc., Tokyo, Japan) code through a written Python script and loaded to the DIW printer. All food inks were loaded into a 50-mL luer lock dispensing syringe fitted with 22 G (Birmingham Gauge) nozzle. The standoff distance between the nozzle and substrate was adjusted to the layer thickness, 0.2 mm, with a height feeler gauge. Both printing speed and dispensing pressure were kept constant at 15 mm/s and 0.050 MPa, respectively, throughout the printing process. All printings were conducted at room temperature.
2.5. Texture profile analysis
The texture profile analysis (TPA) was conducted on the 3D printed samples using a 10-kg load texture analyzer (CT3 Texture Analyzer, Brookfield, USA). The printed samples were placed at the center of the fixture base table before the measurements. All TPA measurements were conducted with a probe with a diameter of 38.1 mm at pre-test speed of 2.0 mm/s, test speed of 2.0 mm/s, post-test speed of 2.0 mm/s, trigger force of 5.0 g, and compression strain of 45% to determine the following textural properties: (i) Hardness, (ii) adhesiveness, (iii) cohesiveness, and (iv) chewiness index. All TPA measurements were conducted at 25 ± 0.1°C on duplicate samples.
2.6. Statistical analysis
All experimental data were expressed as mean ± standard deviation with triplicate measurements. Data were subjected to one-way analysis of variance (ANOVA) through Tukey’s test at 5% significance level using statistical software (Minitab, Pennsylvania, USA).
3. Experimental design
3.1. Selection of materials
We selected coconut cream and coconut oil as an example of our demonstration. Coconut cream contains both coconut oil and protein which provide good nutritional value to human health[36]. The proteins present in coconut cream act as an emulsifier that allows the dispersion of oil in the food system. Coconut oil, in contrast, contains primarily saturated fatty acids that is 70% medium-chain fatty acids (MCFA), which are metabolized differently compared to long-chain fatty acids (LCFA) commonly found in human diets such as vegetable oils and dairy fat[37]. Consumption of LCFA would lead to the accumulation of fatty deposits within the artery walls that increase the risk of hypertension and cardiovascular diseases[38]. The metabolism of MCFA is quicker than that of LCFA. The metabolic process converts fats into energy; the reduced deposition of fats in the body tissues decreases the risk of heart diseases[39]. It was also reported that coconut oil possesses antioxidant properties that could boost the immune system as well as prevent and treat infections[40]. Coconut cream and coconut oil also serve as good alternatives to existing vegetable oils such as canola oil. As such, coconut oils and creams are expected to offer potential advantages in healthcare. Despite potential advantages in healthcare, to the best of our knowledge, 3D printing of coconut cream has not been shown in the previous studies.
4. Results and discussion
4.1. Phase separation of coconut cream ink
Initially, we studied the phase separation of the mixture of the coconut cream and the coconut oil. We formulated the coconut cream base with coconut cream powder with different weight concentrations of water, and pandan extract (Figure 1A). A fixed concentration of pandan extract (0.2% w/w, with respect to coconut cream powder) was added to the mixture to color the sample and observe the phase separation of the oil. Then, we added varying weight concentrations of coconut oil (% w/w, with respect to coconut cream base) to the base. The oil separated from the mixture was collected and weighed. As expected, the oil separation ratio increased as the amount of oil separated increased (Figure 1B). Oil separation occurred in inks containing 20% (w/w) water content with additional oil content >5% (w/w), inks containing 25% (w/w) water content with the additional oil content >10% (w/w), and inks containing 33% (w/w) water content with the additional oil content >15% (w/w) (Figure 1B). As the water content increased from 20% to 33% (w/w), the tendency for oil separation to occur decreased. The oil separation ratio increased from 0.10 (10% w/w oil) to 0.80 (30% w/w oil) for inks containing 20% water, 0.01 (12.5% w/w oil) to 0.50 (30% w/w oil) for inks containing 25% water, and 0.01 (20% w/w oil) to 0.35 (30% w/w oil) for inks containing 33% water. As expected, the oil separation became prominent at high concentrations of the oil. With the increase in water content, the amount of dispersed water also increased[41] which allowed the emulsifier to achieve continuous interface between water and oil without phase separation. However, the increased water content decreased the viscosity of the overall ink, which would affect the 3D printability of inks. Overall, we selected three samples (Ink A contains 25% water with 10% (w/w) oil, Ink B contains 25% water with 12.5% (w/w) oil, and Ink C contains 33% water with 10% oil (w/w)) for the characterization of the rheological properties to ensure the 3D printability by cold extrusion.
Figure 1.
An overview of the formulation of coconut cream inks added with coconut oil, and results from oil separation test. (A) Coconut cream base was initially formulated by mixing coconut cream powder with different water concentrations (20, 25, and 33%) and fixed concentration of pandan extract at 0.2%. Next, different weight concentrations of coconut oil (% w/w, with respect to the weight of the coconut cream base) were added to the coconut cream base to observe the occurrence of the oil separation. (B) A plot showing oil separation ratio as a function of oil concentration. (C) A diagram showing inks that exhibited or did not exhibit oil separation at different oil and water content.
4.2. Rheological characterization of coconut cream ink
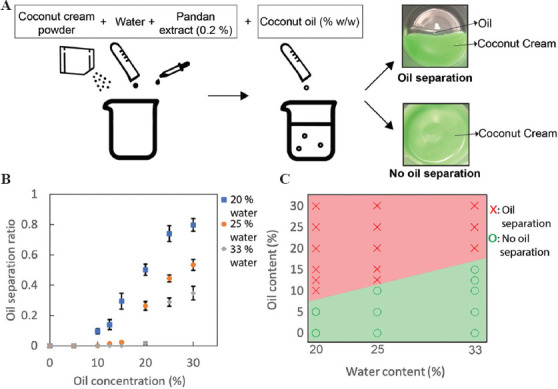
Next, we studied the rheological properties of the inks. Rheological properties such as yield stress and storage modulus (G’) are important to determine the printability of ink[42]. The yield stress of the ink is the minimum shear stress needed to initiate flow in DIW 3D printing. G’ is a measure of mechanical strength at rest condition which determines the structural integrity of the printed material after deposition. The rheological properties were determined using the same method described in previous work[23]. All inks displayed shear thinning property, where the viscosity decreased from an order of 103 to 100 Pa·s with increasing shear rates between 0.01 and 100 s−1 (Figure 2A). Shear-thinning properties are desirable for the extrusion of ink from the nozzle on applied pressure. The increase in water content from 25 to 33% (w/w) resulted in a reduced viscosity due to the weakened colloidal network caused by lower ratios of emulsifier. Similarly, the increase in the oil content from 10 to 12.5% (w/w) led to a decreased viscosity. We also observed mild oil separation in Ink B presumably due to the weakened colloidal network within the ink.
Figure 2.
Rheological characterization and printability of coconut cream ink (Ink A contains 25% water with 10% (w/w) oil, ink B contains 25% water with 12.5% (w/w) oil, and ink C contains 33% water wi 10% (w/w) oil. (A) Viscosity as a function of applied shear rate. (B) Storage moduli (G’) and loss moduli (G’’) as a function of applied oscillatory shear stress. (C) Top and front views of printed cube. (Scale bar: 5 mm).
The previous studies have suggested that the inks with the yield stress of 106–330 Pa and the storage modulus of 1670–49000 Pa were printable using a DIW printer[1,2,22,23,43]. The addition of oil led to a decrease of yield stress from 216 Pa to 160 Pa while the addition of water led to a decrease from 216 Pa to 53 Pa (Table 1). The decrease in yield stress implied that the particle network within the ink was weakened. A sufficiently high value of yield stress allowed to maintain the material in its shape and position after being printed without lateral spreading. The yield stress of Ink C did not meet the yield stress from the previous work[23]; it was hence not deemed printable, which was subsequently experimentally confirmed.
Table 1.
Rheological properties of coconut cream inks. All values were calculated as means (± standard deviations).
| Sample | Yield stress (Pa) | Storage modulus (Pa) |
|---|---|---|
| 25% water with 10% (w/w) oil (Ink A) | 216 ± 35a | 2520 ± 122a |
| 25% water with 12.5% (w/w) oil (Ink B) | 160 ± 44a | 2750 ± 23b |
| 33% water with 10% (w/w) oil (Ink C) | 53 ± 29b | 1320 ± 65c |
a,b,c Means that do not share a superscripted letter are significantly different at p<0.05
The values of G’ were higher than the values of G” in the linear viscoelastic region for all coconut cream inks which indicated that the inks have solid-like behaviors. They allow the deposited inks to self-support themselves (Figure 2B). Ink C exhibited the lower G’ than Inks A and B (Table 1); this difference suggested that the bond strength within the ink matrix was the weakest and would not be able to hold its shape as much as the other two inks. The high values of G’ suggested strong intermolecular bonds within the ink that permit to hold the structures of the printed inks. Both G’ and G” started to deviate from linearity due to the deformation of the bonds within the ink, suggesting the flow of the ink. In this observation, all Inks A, B, and C possessed solid-like behaviors which would allow the printed materials to retain their shapes.
4.3. 3D printability of coconut cream ink
A printable ink should display shear thinning behavior and no spreading of ink that allows the printed material to retain its shape. We printed mesh structures (20 mm × 20 mm × 20 mm) with Inks A, B, and C to verify the ability of the inks to self-sustain the printed structures with fidelity (Figure 2C). In this design of the 3D model (i.e., mesh grid), the spreading of inks results in the reduction in the space surrounded by the printed ink. Based on the rheological characterization, Ink C exhibited the lowest yield stress and storage modulus; Ink C exhibited more lateral spreading than Inks A and B. In crucial constant, no lateral spreading of ink was observed for structures printed with Inks A and B; the printed structures were well maintained. Despite the good printability, we observed the phase separation of the oil from the structures printed in Ink B over time, which was also previously characterized (Figure 1). While the current study focused on characterizing the rheological properties of different coconut inks for their printability, other parameters such as dispensing pressure, nozzle velocity, and nozzle diameter would affect the dimension of the printed inks, which is essential to achieve print fidelity[44]. Overall, we identified that Ink A was the promising candidate to perform cold extrusion to create complex 3D structures.
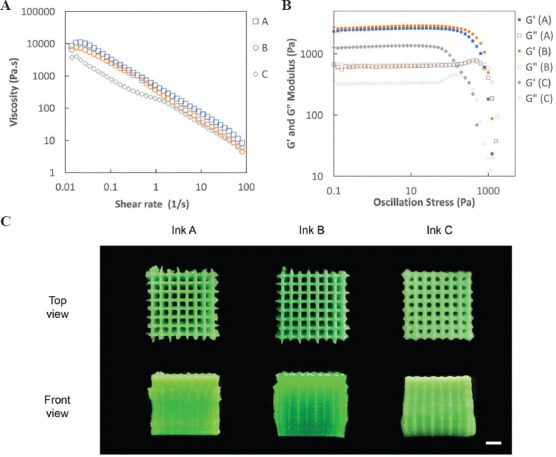
Finally, we demonstrated the fabrication of various 3D structures with Ink A using the DIW printer. All printed structures are shown (Figure 3). The deposited inks exhibited structural integrity, and all printed structures were self-supporting. In this demonstration, we also printed a humanoid structure with overhang features and the deposited material was able to maintain the structure without any support. Thus, it is important to ensure that the yield stress and storage modulus are sufficiently high to allow the ink to self-support themselves on deposition. These observations confirmed the printability of Ink A that was suitable to create 3D food structures.
Figure 3.
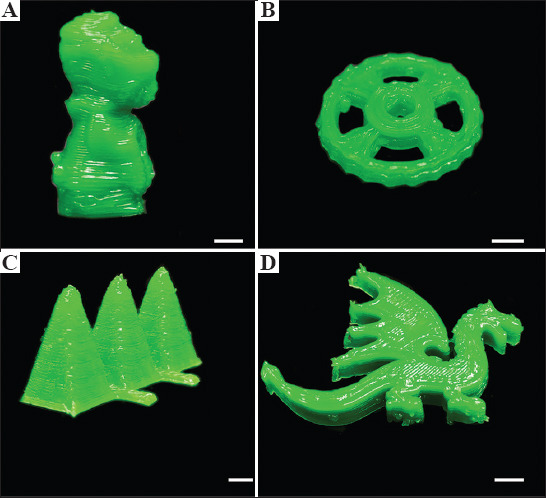
DIW 3D printed models with coconut cream ink A. (A) humanoid, (B) wheel, (C) pyramids, and (D) dragon (All scale bars: 5 mm)
4.4. Texture profile analysis
We characterized the textural properties of the inks. We printed mesh structures with the dimensions of 20 mm × 20 mm × 20 mm with Inks A, B, and C and performed a double compression test to mimic the biting behavior of humans. The hardness decreased from 0.60 N (Ink A) to 0.35 N (Ink C) and the chewiness also decreased from 0.33 (Ink A) to 0.14 (Ink C), which correlated to the increase in water content (Table 2). As the oil content increased, there was no significant change in hardness and chewiness between Ink A and B. There were no significant changes in adhesiveness and cohesiveness of the inks in response to the addition of neither oil nor water for the range of parameters we investigated.
Table 2.
Texture profile analysis of coconut cream inks. All values were calculated as means (± standard deviations).
| Sample | Hardness (N) | Adhesiveness (mJ) | Cohesiveness | Chewiness Index |
|---|---|---|---|---|
| 25% water with 10% (w/w) oil (Ink A) | 0.60 ± 0.02a | 2.95 ± 0.35a | 0.48 ± 0.02a | 0.33 ± 0.01a |
| 25% water with 12.5% (w/w) oil (Ink B) | 0.51 ± 0.08ab | 2.30 ± 0a | 0.46 ± 0.03a | 0.23 ± 0.06ab |
| 33% water with 10% (w/w) oil (Ink C) | 0.35 ± 0.02b | 2.25 ± 0.21a | 0.42 ± 0.06a | 0.14 ± 0.04b |
a,b,cMeans that do not share a superscripted letter are significantly different at p<0.05
TPA suggested that hardness and chewiness could be varied by adding water. However, the rheological properties of the inks would be simultaneously compromised, which affected the printability of the inks. For example, the hardness of structures printed with Ink C was lower than that of Ink A but the rheological properties of Ink C were not adequate for 3D printing, which caused the inks to spread on deposition (Figure 2C). The previous studies reported that textural properties could be controlled by varying geometrical and process parameters such as infill density and nozzle diameter[45]. Overall, the desired textural properties should be achieved by altering material properties as well as designing the structures of the printed material, which are under investigation.
5. Conclusions
This paper discussed the 3D printing of coconut cream added with coconut oil using a DIW 3D printer. 3D-printable coconut cream inks were formulated with additional coconut oil without causing oil separation, and 3D structures were fabricated at room temperature. We conducted oil separation tests to determine the limits of the amount of oil that could be added into the inks at different water concentrations because the stability of the ink was crucial to ensure smooth extrusion of material. The oil separation ratio increased as the concentration of the oil increased. Rheological characterization of the selected formulation of the coconut inks was performed to determine the yield stress and storage modulus of the inks. The textural properties of the inks were characterized to determine the hardness, adhesiveness, cohesiveness, and chewiness indexes. Coconut cream inks of 25% water content with 10% (w/w) added coconut oil did not exhibit oil separation and were suitable for DIW 3D printing with a yield stress of 216 Pa and storage modulus of 2520 Pa. Using the ink, we fabricated various 3D structures, and all printed structures were able to maintain its shape.
The nutrients of coconut cream are considered to be advantageous to human health. The antioxidant properties of coconut cream boost the immune system that could prevent and treat infections. Coconut oil serves as an alternative source of oil that reduces the risk of cardiovascular disease. This method offered a simple route to control the rheological properties and stability of the food inks and fabricate 3D colloidal food with personalized oil content. Our study demonstrated 3D printing of high-oil-content foods, which offers potential applications in the personalization of foods tailored for individual nutritional needs and preferences through 3D food printing.
Acknowledgments
C.P.L. acknowledged the financial support from the President’s Graduate Fellowship awarded by Ministry of Education (MOE), Singapore. The authors thank International Design Centre (IDC) at Singapore University of Technology and Design (SUTD) for the project support (IDG11700103) and SUTD Growth Plan, Healthcare Sector Thrust 3-3 3D Food Printing (SGPHCRS1907).
Conflict of interest
There are no conflicts to declare.
Author contributions
C.P.L., J.Y.H., and M.H. planned the experiments. C.P.L. and J.Y.H. carried out the experiments. M.H. supervised the experiments. C.P.L. and M.H. wrote the paper.
References
- 1.Liu Z, Zhang M, Yang CH. Dual Extrusion 3D Printing of Mashed Potatoes/Strawberry Juice Gel. LWT. 2018;96:589–96. https://doi.org/10.1016/j.lwt.2018.06.014. [Google Scholar]
- 2.Liu Z, Zhang M, Bhandari B, et al. Impact of Rheological Properties of Mashed Potatoes on 3D Printing. J Food Eng. 2018;220:76–82. https://doi.org/10.1016/j.jfoodeng.2017.04.017. [Google Scholar]
- 3.Liu Z, Zhang M, Bhandari B. Effect of Gums on the Rheological, Microstructural and Extrusion Printing Characteristics of Mashed Potatoes. Int J Biol Macromol. 2018;117:1179–87. doi: 10.1016/j.ijbiomac.2018.06.048. https://doi.org/10.1016/j.ijbiomac.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Bhandari B, Prakash S, et al. Creation of Internal Structure of Mashed Potato Construct by 3D Printing and its Textural Properties. Food Res Int. 2018;111:534–43. doi: 10.1016/j.foodres.2018.05.075. https://doi.org/10.1016/j.foodres.2018.05.075. [DOI] [PubMed] [Google Scholar]
- 5.Pant A, Lee AY, Karyappa R, et al. 3D Food Printing of Fresh Vegetables Using Food Hydrocolloids for Dysphagic Patients. Food Hydrocoll. 2021;114:106546. https://doi.org/10.1016/j.foodhyd.2020.106546. [Google Scholar]
- 6.Kim HW, Lee JH, Park SM, et al. Effect of Hydrocolloids on Rheological Properties and Printability of Vegetable Inks for 3D Food Printing. J Food Sci. 2018;83:2923–32. doi: 10.1111/1750-3841.14391. https://doi.org/10.1111/1750-3841.14391. [DOI] [PubMed] [Google Scholar]
- 7.Zheng LM, Yang J, Zhang C, et al. Effect of Oil Content and Emulsifier Type on the Properties and Antioxidant Activity of Sea Buckthorn Oil-in-Water Emulsions. J Food Qual. 2020;2020:8. https://doi.org/10.1155/2020/1540925. [Google Scholar]
- 8.Shapiro AA, Borgonia JP, Chen QN, et al. Additive Manufacturing for Aerospace Flight Applications. J Spacecr Rockets. 2016;53:952–9. [Google Scholar]
- 9.Wang X, Ao Q, Tian X, et al. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers. 2017;9:401. doi: 10.3390/polym9090401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noor N, Shapira A, Edri R, et al. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv Sci. 2019;6:1900344. doi: 10.1002/advs.201900344. https://doi.org/10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin SR, Farzad R, Tamayol A, et al. A Bioactive Carbon Nanotube-Based Ink for Printing 2D and 3D Flexible Electronics. Adv Mater. 2016;28:3280–89. doi: 10.1002/adma.201506420. https://doi.org/10.1002/adma.201506420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ching T, Li Y, Karyappa R, et al. Fabrication of Integrated Microfluidic Devices by Direct Ink Writing (DIW) 3D Printing. Sens Actuators B Chem. 2019;297:126609. https://doi.org/10.1016/j.snb.2019.05.086. [Google Scholar]
- 13.Goh WH, Hashimoto M. Fabrication of 3D Microfluidic Channels and In-Channel Features Using 3D Printed, Water-Soluble Sacrificial Mold. Macromol Mater Eng. 2018;303:1700484. https://doi.org/10.1002/mame.201700484. [Google Scholar]
- 14.Voon SL, An J, Wong G, et al. 3D Food Printing:A Categorised Review of Inks and their Development. Virtual Phys Prototyp. 2019;14:203–18. [Google Scholar]
- 15.Tan C, Chua CK, Li L, et al. Enhancing 3D Printability of Pureed Food by Addition of Hydrocolloids. 3rd International Conference on Progress in Additive Manufacturing. 2018 [Google Scholar]
- 16.Derossi A, Caporizzi R, Azzollini D, et al. Application of 3D Printing for Customized Food. A Case on the Development of a Fruit-based Snack for Children. J Food Eng. 2018;220:65–75. https://doi.org/10.1016/j.jfoodeng.2017.05.015. [Google Scholar]
- 17.Dankar I, Haddarah A, Omar FE, et al. 3D Printing Technology:The New Era for Food Customization and Elaboration. Trends Food Sci Technol. 2018;75:231–42. https://doi.org/10.1016/j.tifs.2018.03.018. [Google Scholar]
- 18.Lille M, Nurmela A, Nordlund E, et al. Applicability of Protein and Fiber-rich Food Materials in Extrusion-based 3D Printing. J Food Eng. 2018;220:20–7. https://doi.org/10.1016/j.jfoodeng.2017.04.034. [Google Scholar]
- 19.Wang L, Zhang M, Bhandari B, et al. Investigation on Fish Surimi Gel as Promising Food Material for 3D Printing. J Food Eng. 2018;220:101–8. https://doi.org/10.1016/j.jfoodeng.2017.02.029. [Google Scholar]
- 20.Tan C, Toh WY, Wong G, et al. Extrusion-based 3D Food Printing Materials and Machines. Int J Bioprinting. 2018;4:2. doi: 10.18063/IJB.v4i2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gholamipour-Shirazi A, Nortonand IT, Mills T. Designing Hydrocolloid Based Food-ink Formulations for Extrusion 3D Printing. Food Hydrocoll. 2019;95:161–7. https://doi.org/10.1016/j.foodhyd.2019.04.011. [Google Scholar]
- 22.Karyappa R, Hashimoto M. Chocolate-based Ink Three-dimensional Printing (Ci3DP) Sci Rep. 2019;9:14178. doi: 10.1038/s41598-019-50583-5. https://doi.org/10.1038/s41598-019-50583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CP, Karyappa R, Hashimoto M. 3D Printing of Milk-based Product. RSC Adv. 2020;10:29821–8. doi: 10.1039/d0ra05035k. https://doi.org/10.1039/d0ra05035k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan JJ, Lee CP, Hashimoto M. Preheating of Gelatin Improves its Printability with Transglutaminase in Direct Ink Writing 3D Printing. Int J Bioprint. 2020;6:296. doi: 10.18063/ijb.v6i4.296. https://doi.org/10.18063/ijb.v6i4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao MA. Springer; Boston, MA: 2007. Rheological Behavior of Processed Fluid and Semisolid Foods; pp. 223–337. https://doi.org/10.1007/978-0-387-70930-7_5. [Google Scholar]
- 26.Wilde P, Mackie A, Husband F, et al. Proteins and Emulsifiers at Liquid Interfaces. Adv Colloid Interface Sci. 2004;108–109:63–71. doi: 10.1016/j.cis.2003.10.011. https://doi.org/10.1016/j.cis.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Buléon PC, Planchot V, Ball SG. Starch Granules:Structure and Biosynthesis. Int J Biol Macromol. 1998;23:85–112. doi: 10.1016/s0141-8130(98)00040-3. https://doi.org/10.1016/s0141-8130(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 28.Cuvelier BL. Concentration Regimes in Xanthan Gum Solutions Deduced from Flow and Viscoelastic Properties. Carbohydr Polym. 1986;6:321–333. https://doi.org/10.1016/0144-↩(86)90023-8. [Google Scholar]
- 29.Decker EA, Chen B, Panya A, et al. Woodhead Publishing; Cambridge: 2010. Understanding Antioxidant Mechanisms in Preventing Oxidation in Foods. In:Oxidation in Foods and Beverages and Antioxidant Applications; pp. 225–248. https://doi.org/10.1533/9780857090447.2.225. [Google Scholar]
- 30.Dickinson E. Interfacial Structure and Stability of Food Emulsions as Affected Byprotein-Polysaccharide Interactions. Soft Matter. 2008;4:932–42. doi: 10.1039/b718319d. https://doi.org/10.1039/b718319d. [DOI] [PubMed] [Google Scholar]
- 31.Calder PC. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J Parenter Enteral Nutr. 2015;39:18S–32. doi: 10.1177/0148607115595980. https://doi.org/10.1177/014↟115595980. [DOI] [PubMed] [Google Scholar]
- 32.Milner JA, Allison RG. The Role of Dietary Fat in Child Nutrition and Development:Summary of an ASNS Workshop. J Nutr. 1999;129:2094–105. doi: 10.1093/jn/129.11.2094. https://doi.org/10.1093/jn/129.11.2094. [DOI] [PubMed] [Google Scholar]
- 33.Guneser O, Zorba M. Effect of Emulsifiers on Oil Separation Problem and Quality Characteristics of Tahin Helva during Storage. J Food Sci Technol. 2014;51:1085–93. doi: 10.1007/s13197-011-0594-7. https://doi.org/10.1007/s13197-011-0594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gills LA, Resurreccion AV. Sensory and Physical Properties of Peanut Butter Treated with Palm Oil and Hydrogenated Vegetable Oil to Prevent Oil Separation. J Food Sci. 2000;65:173–80. https://doi.org/10.1111/j.1365-2621.2000.tb15975.x. [Google Scholar]
- 35. [Last accessed 2020 Jan 17];Slic3r Open Source 3D Printing Toolbox. Available from: http://www.slic3r.org .
- 36.Patil U, Benjakul S. Coconut Milk and Coconut Oil:Their Manufacture Associated with Protein Functionality. J Food Sci. 2018;83:2019–27. doi: 10.1111/1750-3841.14223. https://doi.org/10.1111/1750-3841.14223. [DOI] [PubMed] [Google Scholar]
- 37.Gu Y, Luchsinger JA, Stern Y, et al. Mediterranean Diet, Inflammatory and Metabolic Biomarkers, and Risk of Alzheimer's Disease. J Alzheimers Dis. 2010;22:483–92. doi: 10.3233/JAD-2010-100897. https://doi.org/10.3233/jad-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji MK, Takeuchi H, Nakamura M, et al. Dietary Medium-chain Triacylglycerols Suppress Accumulation of Body Fat in a Double-blind, Controlled Trial in Healthy Men and Women. J Nutr. 2001;131:2853–9. doi: 10.1093/jn/131.11.2853. https://doi.org/10.1093/jn/131.11.2853. [DOI] [PubMed] [Google Scholar]
- 39.Tholstrup CE, Jauhiainen M, Petersen M, et al. Effects of Medium-chain Fatty Acids and Oleic Acid on Blood Lipids, Lipoproteins, Glucose, Insulin, and Lipid Transfer Protein Activities. Am J Clin Nutr. 2004;79:564–9. doi: 10.1093/ajcn/79.4.564. https://doi.org/10.1093/ajcn/79.4.564. [DOI] [PubMed] [Google Scholar]
- 40.Marina AM, Man YB, Nazimah SA, et al. Antioxidant Capacity and Phenolic Acids of Virgin Coconut Oil. Int J Food Sci Nutr. 2009;60:114–23. doi: 10.1080/09637480802549127. https://doi.org/10.1080/09637480802549127. [DOI] [PubMed] [Google Scholar]
- 41.El-Din MR, El-Hamouly SH, Mohamed HM, et al. Water-in-diesel Fuel Nanoemulsions:Preparation, Stability and Physical Properties. Egypt J Pet. 2013;22:517–30. https://doi.org/10.1016/j.ejpe.2013.11.006. [Google Scholar]
- 42.Godoi FC, Prakash S, Bhandari BR. 3d Printing Technologies Applied for Food Design:Status and Prospects. J Food Eng. 2016;179:44–54. https://doi.org/10.1016/j.jfoodeng.2016.01.025. [Google Scholar]
- 43.Yang F, Zhang M, Bhandari B, et al. Investigation on Lemon Juice Gel as Food Material for 3D Printing and Optimization of Printing Parameters. LWT. 2018;87:67–76. https://doi.org/10.1016/j.lwt.2017.08.054. [Google Scholar]
- 44.Karyappa R, Ohno A, Hashimoto M. Immersion Precipitation 3D Printing (ip3DP) Mater Horizons. 2019;6:1834–44. https://doi.org/10.1039/c9mh00730j. [Google Scholar]
- 45.Huang MS, Zhang M, Bhandari B. Assessing the 3D Printing Precision and Texture Properties of Brown Rice Induced by Infill Levels and Printing Variables. Food Bioproc Tech. 2019;12:1185–96. https://doi.org/10.1007/s11947-019-02287-x. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


