Key Points
Question
Is use of long-acting injectable antipsychotics (LAIs) in patients with newly diagnosed schizophrenia associated with decreased mortality?
Findings
This cohort study included 2614 patients with schizophrenia who switched to LAIs, and 2614 propensity-matched patients who continued receiving oral antipsychotics (OAPs) of the same compounds. Patients who switched to LAIs had lower mortality and fewer suicide attempts compared with patients who continued receiving the corresponding OAPs, and patients who switched to LAIs within the first 2 years of OAP initiation exhibited a 47% reduction in the risk of suicide mortality compared with patients who continued receiving OAPs.
Meaning
These findings suggest that LAI use in patients with newly diagnosed schizophrenia should be encouraged because of its association with lower premature mortality.
This cohort study investigates the associations of long-acting injectable antipsychotics with all-cause, natural-cause, and suicide mortality risks in patients with newly diagnosed schizophrenia.
Abstract
Importance
Schizophrenia is generally considered to be among the most severe psychiatric disorders because of the excessive mortality associated with it. Research to find means to reduce this excessive mortality is warranted.
Objective
To investigate associations of long-acting injectable antipsychotics (LAIs) with all-cause, natural-cause, and suicide mortality risks as well as the impacts of early use of LAIs in patients with newly diagnosed schizophrenia.
Design, Setting, and Participants
This cohort study used data from the Taiwan National Health Insurance Research Database to construct a population-based cohort of patients with schizophrenia who received oral antipsychotics (OAPs) from January 1, 2002, to December 31, 2017. Within this cohort, the LAI group was defined as patients who switched to LAIs and were prescribed LAIs at least 4 times within 1 year. The LAI group was propensity matched 1:1 to patients who continued receiving OAPs of the same compounds. All patients were followed up until switching the antipsychotic administration route, death, or the end of the study (December 31, 2018), whichever occurred first. Data analysis was performed from January 2002 to December 2018.
Main Outcomes and Measures
All-cause mortality, natural-cause mortality, suicide mortality, and suicide attempts.
Results
In total, 2614 patients who switched to LAIs (median [interquartile range] {IQR} age, 30 [23-39] years) and 2614 who received OAPs (median [IQR] age, 30 [23-39] years) were included (1333 male patients [51.0%] in each group). During the 16-year follow-up period (median [IQR] follow-up of 14 [10-17] years), patients who switched to LAIs had lower risks of all-cause mortality (adjusted hazard ratio [aHR], 0.66; 95% CI, 0.54-0.81), natural-cause mortality (aHR, 0.63; 95% CI, 0.52-0.76), and suicide attempts (incidence rate ratio, 0.72; 95% CI, 0.55-0.93) compared with patients who received the corresponding OAPs. A 47% lower suicide mortality risk (aHR, 0.53; 95% CI, 0.30-0.92) was observed in patients who switched to LAIs within the first 2 years of OAP initiation.
Conclusions and Relevance
These findings suggest that LAI use in patients with newly diagnosed schizophrenia is associated with decreased all-cause mortality and suicide risk. Furthermore, early treatment with LAIs within the first 2 years of OAP initiation was associated with a decrease in suicide mortality risk. Thus, LAI use in the early stage of treatment should be actively considered for patients with newly diagnosed schizophrenia.
Introduction
Schizophrenia is generally considered to be among the most severe psychiatric disorders because of the excessive mortality associated with it. It has consistently been associated with a life expectancy 10 to 25 years shorter than that of the general population.1,2 In a meta-analysis, Brown et al3 suggested that schizophrenia is associated with a large increase in suicide mortality and a moderately increased risk of natural-cause mortality. Therefore, research on practical methods for reducing these risks is highly warranted.
The lifetime risk of suicide in patients with schizophrenia is approximately 5%.4 The risk is highest during the early stage of the illness or the first episode.5 The suicide rate for patients with schizophrenia spectrum disorders is more than 20 times higher than that for the general population.6 Although suicide is a complex public health challenge, suicide is typically preventable with timely and evidence-based interventions. Risk factors for suicide in this population include being young, male, and highly educated. Illness-related risk factors include prior suicide attempts, depressive symptoms, active positive symptoms, and comorbid substance misuse.4,7 Effective treatment delivery and adherence are vital for suicide prevention in schizophrenia.4,7 Compared with oral antipsychotics (OAPs), long-acting injectable antipsychotics (LAIs) were reported to improve treatment adherence in patients with schizophrenia.8,9 However, studies of LAIs in protecting against suicide are limited; in particular, the association of LAIs with the course of the illness, if administered at an early phase, remains unclear.
The objective of the current study, therefore, was to investigate associations of LAIs and their equivalent OAPs on risks of all-cause, natural-cause, and suicide mortality in patients with newly diagnosed schizophrenia. We also explored whether patients benefited from the early use of LAIs.
Methods
Data Source
We conducted a population-based cohort study using data from the Taiwan National Health Insurance (NHI) Research Database (NHIRD) and the Taiwan Death Registry (TDR) database. The NHIRD is derived from Taiwan’s single-payer compulsory NHI program, which covers up to 99% of the 23 million people in Taiwan. The NHIRD includes all medical claims data on disease diagnoses, procedures, drug prescriptions, demographic characteristics, and enrollment profiles of all NHI beneficiaries.10 In addition, we linked the NHIRD to the TDR database to ascertain the vital status, date of death, and cause of death of patients. The accuracy of cause-of-death coding in the TDR database was previously validated.11
This study was reviewed and approved by the institutional review board of Taipei Medical University. A waiver of informed consent was granted because the patient information in the national claims data from the NHIRD was deidentified before analysis. All researchers signed an agreement guaranteeing patient confidentiality before using the database. This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.12
Study Population
Base Cohort
Our base cohort included patients with newly diagnosed schizophrenia (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 295; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes F20 and F25) who initiated OAP treatment between January 1, 2002, and December 31, 2017 (170 261 patients). Patients were excluded on the basis of the following criteria: (1) aged less than 16 or greater than 65 years at cohort entry, (2) information regarding sex or age missing, (3) no antipsychotic prescription, and (4) LAI as the first antipsychotic prescription. Consequently, 138 240 patients were enrolled in the base cohort. The base cohort entry date was defined as the date of the first antipsychotic prescription.
Study Cohort
In our study, the LAI group was defined as patients in the base cohort who initiated an OAP prescription and then switched to LAIs during subsequent treatment and were prescribed LAIs at least 4 times within 1 year.13,14 In total, 35 182 patients (25%) switched to LAIs, and 13 826 (10%) were prescribed LAIs at least 4 times within 1 year. For each patient who received LAIs, we selected a propensity-matched reference patient from our base cohort who received an OAP of the same compound and continued receiving OAPs throughout. Using a prevalent new-user design,15 reference patients were selected from OAP initiators who spent the same duration of time in the base cohort as did exposed patients (defined as the period from the first prescription of OAPs to the first prescription of LAI) and continued receiving OAPs when exposed patients switched to LAIs. Accordingly, we randomly selected up to 1 reference patient in each exposure set who had matched OAPs of the same compounds received, age at cohort entry, sex, calendar year of cohort entry, psychiatric comorbidities 1 year before the index date, and psychiatric hospitalization (yes or no) 6 months before the index date with cases, using a time-conditioned propensity score. The index date was defined as the date of the first LAI prescription. All patients were followed up until switching the antipsychotic administration route, death, or the end of the study (December 31, 2018), whichever occurred first. The study flowchart and timeline are presented in eFigure 1 and eFigure 2 in the Supplement.
Antipsychotic Exposure
Information on antipsychotics use was obtained from ambulatory and inpatient prescription claims data using the Anatomical Therapeutic Chemical Classification System code N05A (antipsychotics) but excluding N05AN (lithium).16 The antipsychotics and Anatomical Therapeutic Chemical codes used for the definition are shown in eTable 1 in the Supplement. Patients were stratified into LAI and OAP groups according to the study criteria. LAIs were further categorized into flupentixol, haloperidol, olanzapine, paliperidone, and risperidone to explore the associations of different drugs with the outcomes. To investigate the association of the early use of LAIs, we grouped patients using LAIs into those switching to LAIs within 2 years and those switching after 2 years, according to a literature review.17,18,19,20
Outcomes
The study’s primary outcomes were all-cause mortality (ICD-9-CM codes 001-799 and E800-E999; ICD-10 codes A00-Y98), natural-cause mortality (ICD-9-CM codes 001-799; ICD-10 codes A00-R99), and suicide mortality (ICD-9-CM codes E950-E959; ICD-10 codes X60-X84 and Y87), obtained by linking the NHIRD to the TDR database. Occurrences of all-cause and suicide deaths were determined from January 1, 2002, to December 31, 2018.21 The secondary outcome was suicide attempt occurrence, defined as emergency department (ED) visits and hospitalization with a diagnosis of suicide (ICD-9-CM codes E950-E959; ICD-10 codes X60-X84).22
Covariates
Covariates included age at cohort entry, sex, calendar year of cohort entry, psychiatric comorbidities 1 year before the index date, psychiatric hospitalization (yes or no), Charlson Comorbidity Index (CCI) score23 within 1 year before the index date, history of suicide attempts, and number of psychiatric ED visits within 6 months before the index date. The CCI score is associated with mortality and is a useful tool to measure the comorbid disease status in research including psychiatry.1 ICD-10 and ICD-9-CM diagnosis codes for psychiatric and CCI scores are listed in eTable 2 in the Supplement.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics in the LAI and OAP groups. Potential imbalances among covariates after matching were assessed using standardized mean differences. All-cause, natural-cause, and suicide mortalities per 100 000 person-years were independently estimated for the LAI and OAP groups. Conditional Cox regressions were used to estimate the risk of death.24 The Fine and Gray25 method was adapted to estimate the hazard of natural-cause and suicide deaths, considering competing risks from other causes of death. All Cox models included the CCI score within 1 year before the index date, history of suicide attempts, and number of psychiatric ED visits within 6 months before the index date in the model for adjustment. We used a Kolmogorov-type supremum test based on 1000 simulated residual patterns to test the validity of the proportional hazards assumption.26,27 The Cox model met the proportional hazards assumption (P > .05). Occurrences of suicide attempts were estimated on the basis of a negative binomial regression model by counting events and person-times of follow-ups. Significance was set at 2-tailed P < .05. All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute) and R statistical software version 4.0.0 (R Project for Statistical Computing). Data analysis was performed from January 2002 to December 2018.
Results
Patient Characteristics
The database extracted for the current analysis comprised 5228 patients with newly diagnosed schizophrenia (median [interquartile range] {IQR} age, 30 [23-39] years) who met the inclusion criteria (eFigure 1 in the Supplement). Further demographic and clinical characteristics of these patients are detailed in Table 1. Among them, 2614 patients who switched to LAIs (median [IQR] age, 30 [23-39] years) were compared with 2614 propensity-matched OAP (mean [IQR] age, 30 [23-39] years) patients in our study (1333 male patients [51.0%] in each group). The mean (SD) interval from the first LAI prescription to the fourth LAI prescription was 109 (69.3) days. Compared with the OAP group, the LAI group was more likely to have a history of suicide attempts and psychiatric ED visits 6 months before the index date, which implied that the LAI group consisted of patients with clinically more-severe disease (Table 1). The covariates mentioned were included in the matching and were evenly distributed between the 2 groups, except for the CCI score, history of suicide attempts, and number of psychiatric ED visits. Thus, these 3 covariates were not included in the matching.
Table 1. Baseline Characteristics of Patients Who Switched to LAIs and Their Matched Controls Who Continued Receiving OAPs of the Same Compounds.
| Characteristic | Patients, No. (%) (N = 5228) | SDa | P value | |
|---|---|---|---|---|
| LAIs (n = 2614) | OAPs (n = 2614) | |||
| Demographic characteristics | ||||
| Age at first antipsychotics, median (IQR), y | 30 (23-39) | 30 (23-39) | 0.00 | NA |
| Age group, y | ||||
| 16-35 | 1696 (64.9) | 1691 (64.7) | 0.01 | NA |
| 36-65 | 918 (35.1) | 923 (35.3) | 0.01 | |
| Sex | ||||
| Male | 1333 (51.0) | 1333 (51.0) | 0.00 | NA |
| Female | 1281 (49.0) | 1281 (49.0) | 0.00 | |
| Duration from first antipsychotic to index date, median (IQR), mo | 57 (20-105) | 57 (20-105) | 0.00 | NA |
| Type of antipsychotic | ||||
| Haloperidol | 455 (17.4) | 455 (17.4) | 0.00 | NA |
| Flupentixol | 619 (23.7) | 619 (23.7) | 0.00 | |
| Olanzapine | 152 (5.8) | 152 (5.8) | 0.00 | |
| Risperidone | 987 (37.8) | 987 (37.8) | 0.00 | |
| Paliperidone | 401 (15.3) | 401 (15.3) | 0.00 | |
| Disease severity measured 1 y before the index date | ||||
| Psychiatric comorbidities | ||||
| Depression | 477 (17.1) | 477 (17.1) | 0.00 | NA |
| Anxiety disorder | 520 (19.9) | 520 (19.9) | 0.00 | |
| Bipolar disorder | 493 (18.9) | 493 (18.9) | 0.00 | |
| Substance use disorder | 139 (5.3) | 139 (5.3) | 0.00 | |
| Charlson Comorbidity Index score | ||||
| 0 | 2435 (93.2) | 2434 (93.1) | .47b | |
| 1 | 123 (4.7) | 117 (4.5) | ||
| 2 | 39 (1.5) | 36 (1.4) | ||
| ≥3 | 17 (0.7) | 27 (1.0) | ||
| Disease severity measured 6 mo before the index date | ||||
| Psychiatric hospitalization | ||||
| No | 2076 (79.4) | 2076 (79.4) | 0.00 | NA |
| Yes | 538 (20.6) | 538 (20.6) | 0.00 | |
| History of suicide attempts | ||||
| No | 2556 (97.8) | 2584 (98.9) | <.001b | |
| Yes | 58 (2.2) | 30 (1.2) | ||
| Psychiatric emergency department visits, No. | ||||
| 0 | 2039 (78.0) | 2235 (85.5) | <.001b | |
| 1 | 388 (14.8) | 247 (9.5) | ||
| ≥2 | 187 (7.2) | 132 (5.0) | ||
Abbreviations: IQR, interquartile range; LAI, long-acting injectable antipsychotic; NA, not applicable; OAP, oral antipsychotic.
SD = |P1 − P2|/[P1(1 − P1) + P2(1 − P2)/2]0.5. They are the same for all categorical variables with 2 levels. Charlson Comorbidity Index score, history of suicide attempts, and number of psychiatric emergency department visits were not included in matching.
The P value was generated using a χ2 test.
All-Cause, Natural-Cause, and Suicide Mortality
During the follow-up period (median [IQR], 14 [10-17] years), 235 patients in the LAI group died and 287 patients in the OAP group died. All-cause mortality rates were 66 and 90 deaths per 100 000 person-years in the LAI and OAP groups, respectively (Table 2). The multivariate Cox model revealed that the LAI group had a 34% lower risk for all-cause mortality (adjusted hazard ratio [aHR], 0.66; 95% CI, 0.54-0.81) and a 37% lower risk of natural-cause mortality (aHR, 0.63; 95% CI, 0.52-0.76) compared with the OAP group after adjusting for the covariates. The median (IQR) follow-up time of suicide was 8 (5-11) years. The LAI group exhibited lower suicide mortality than the OAP group (13 vs 17 deaths per 100 000 person-years), but the risk estimate was not significant (aHR, 0.80; 95% CI, 0.58-1.11).
Table 2. All-Cause, Natural, and Suicide Mortality Risks in 5228 Patients With Schizophrenia Who Switched to LAIs Compared With Their OAP Using Counterparts.
| Mortality | Deaths, No. | Person-y, No. | Mortality rate, deaths/100 000 person-y (95% CI) | Cox regression model | Fine-Gray model | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | aHR (95% CI)a | HR (95% CI) | aHR (95% CI)a | ||||
| All cause | |||||||
| LAIs | 235 | 35 084 | 66 (58-75) | 0.67 (0.55-0.81) | 0.66 (0.54-0.81) | NA | NA |
| OAPs | 287 | 31 643 | 90 (80-101) | 1 [Reference] | 1 [Reference] | NA | NA |
| Natural cause | |||||||
| LAIs | 153 | 35 084 | 43 (36-50) | 0.62 (0.49-0.78) | 0.62 (0.48-0.81) | 0.63 (0.54-0.75) | 0.63 (0.52-0.76) |
| OAPs | 194 | 31 643 | 61 (52-70) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Suicide | |||||||
| LAIs | 48 | 35 084 | 13 (9-17) | 0.91 (0.59-1.40) | 0.87 (0.55-1.38) | 0.87 (0.65-1.15) | 0.80 (0.58-1.11) |
| OAPs | 55 | 31 643 | 17 (13-22) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
Abbreviations: aHR, adjusted hazard ratio; LAI, long-acting injectable; NA, not applicable; OAP, oral antipsychotic.
The aHR was derived from the Cox hazard model adjusted for the Charlson Comorbidity Index score within 1 year before the index date, suicide attempts, and the number of psychiatric emergency department visits within 6 months before the index date. The Fine and Gray method considered competing risks from other causes of death.
Suicide Attempts
During the follow-up period, patients who switched to LAIs had fewer hospital visits for suicide attempts than their matched OAP controls (3.5 vs 4.2 visits) (Table 3). The negative binomial regression results indicated that patients who switched to LAIs exhibited a 28% lower risk of suicide attempts than patients who received only OAPs (incidence rate ratio, 0.72; 95% CI, 0.55-0.93).
Table 3. Risk of Suicide Attempts in the LAI and OAP Groups.
| Group | Patients, No. | Suicide attempts, mean (SD), No. | IRR (95% CI)a | P value |
|---|---|---|---|---|
| LAI | 264 | 3.5 (6.5) | 0.72 (0.55-0.93) | .01 |
| OAP | 263 | 4.2 (8.19) | 1 [Reference] |
Abbreviations: IRR, incidence rate ratio; LAI, long-acting injectable antipsychotic; OAP, oral antipsychotic.
The IRR was estimated using a negative binomial regression model adjusted for the Charlson Comorbidity Index score within 1 year before the index date, suicide attempts, and the number of psychiatric emergency department visits within 6 months before the index date.
Benefit of Early Use of LAIs
We examined the risk of mortality associated with the early use of LAIs (ie, within 2 years) compared with the matched OAP group (Table 4). In our population, 742 patients switched to LAIs within the first 2 years of OAP initiation, and 1872 switched to LAIs more than 2 years after OAP initiation. Patients who switched to LAIs within the first 2 years of OAP initiation had a 47% decreased risk in suicide mortality compared with those who continued receiving OAPs (aHR, 0.53; 95% CI, 0.30-0.92). The benefit of LAIs for protecting against suicide mortality was not observed in patients who switched to LAIs more than 2 years after OAP initiation (aHR, 1.03; 95% CI, 0.70-1.52). Regarding all-cause mortality, patients who switched to LAIs within 2 years had lower all-cause mortality than the OAP group (114 vs 129 deaths per 100 000 person-years), but the risk estimate did not reach statistical significance (aHR, 0.93; 95% CI, 0.67-1.30).
Table 4. Risk of Suicide Mortality in 5228 Patients With Schizophrenia Who Switched to LAIs Within 2 Years of OAP Initiation or More Than 2 Years After OAP Initiation Compared with Their Corresponding Counterparts.
| Group | Deaths, No. | Person-y, No. | Mortality rate, deaths/100 000 person-y (95% CI) | Cox regression model | Fine-Gray model | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | aHR (95% CI)a | HR (95% CI) | aHR (95% CI)a | ||||
| Switched to LAIs ≤2 y after initiating OAPsb | |||||||
| All-cause mortality | |||||||
| LAIs | 94 | 8233 | 114 (93-139) | 0.89 (0.65-1.22) | 0.93 (0.67-1.30) | NA | NA |
| OAPs | 92 | 7109 | 129 (105-158) | 1 [Reference] | 1 [Reference] | NA | NA |
| Natural-cause mortality | |||||||
| LAIs | 67 | 8233 | 81 (63-102) | 1.04 (0.70-1.56) | 1.25 (0.80-1.95) | 1.06 (0.81-1.39) | 1.22 (0.88-1.69) |
| OAPs | 57 | 7109 | 80 (61-103) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Suicide | |||||||
| LAIs | 12 | 8233 | 14 (7-24) | 0.61 (0.28-1.29) | 0.59 (0.27-1.29) | 0.55 (0.33-0.92) | 0.53 (0.30-0.92) |
| OAPs | 22 | 7109 | 30 (19-45) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Switched to LAIs >2 y after initiating OAPsc | |||||||
| All-cause mortality | |||||||
| LAIs | 141 | 26 851 | 52 (44-61) | 0.58 (0.46-0.74) | 0.54 (0.41-0.70) | NA | NA |
| OAPs | 195 | 24 534 | 79 (68-90) | 1 [Reference] | 1 [Reference] | NA | NA |
| Natural-cause mortality | |||||||
| LAIs | 86 | 26 851 | 32 (25-39) | 0.47 (0.35-0.64) | 0.41 (0.29-0.60) | 0.49 (0.39-0.61) | 0.42 (0.31-0.55) |
| OAPs | 137 | 24 534 | 55 (46-65) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Suicide | |||||||
| LAIs | 36 | 26 851 | 13 (9-18) | 1.11 (0.66-1.89) | 1.10 (0.61-1.97) | 1.1 (0.78-1.56) | 1.03 (0.70-1.52) |
| OAPs | 33 | 24 534 | 13 (9-18) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
Abbreviations: aHR, adjusted hazard ratio; LAI, long-acting injectable antipsychotic; NA, not applicable; OAP, oral antipsychotic.
The aHR was derived using the Cox hazard model adjusted for the Charlson Comorbidity Index score within 1 year before the index date, suicide attempts, and the number of psychiatric emergency department visits within 6 months before the index date. The Fine and Gray method considered competing risks from other causes of death.
This group includes 1484 patients, 742 using LAIs and 742 using OAPs.
This group includes 3744 patients, 1872 using LAIs and 1872 using OAPs.
Individual Antipsychotics
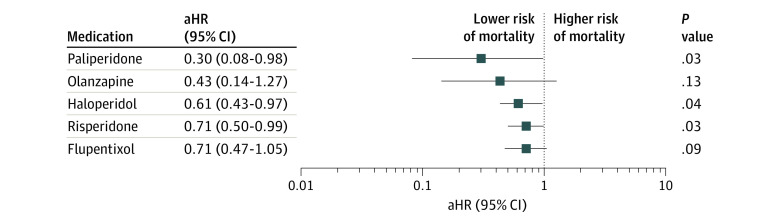
The effects of individual antipsychotics on mortality were also examined. The results indicated that the LAI forms of paliperidone (aHR, 0.30; 95% CI, 0.08-0.98), haloperidol (aHR, 0.61; 95% CI, 0.43-0.97), and risperidone (aHR, 0.71; 95% CI, 0.50-0.99) were associated with lower risks of all-cause mortality than were their corresponding OAPs (Figure).
Figure. Adjusted Hazard Ratios (aHRs) Estimating the Risk of All-Cause Mortality Associated With Individual Long-Acting Injectable Antipsychotics Compared With Their Corresponding Oral Antipsychotics.
Discussion
In this study, the use of LAIs was associated with significantly lower risks of all- and natural-cause mortality and suicide attempts than was the use of OAPs after adjusting for covariates. With regard to the benefits of early use of LAIs, patients who switched to LAIs within the first 2 years of OAP initiation had a 47% decrease in the suicide mortality risk compared with those who continued receiving OAPs.
Here, the LAI group had a 34% lower all-cause and a 37% lower natural-cause mortality risk than did the OAP group. These results are in line with those of previous large observational cohort studies and meta-analyses of randomized clinical trials, in which receiving LAIs was associated with lower mortality than was receiving OAPs.9,28,29 Brown et al30 suggested that some of the excess mortality of schizophrenia could be lessened by reducing patient exposure to environmental risk factors and improving disease, mood disturbance, and psychosis management. Because LAIs were associated with lower risks of rehospitalization and antipsychotic treatment discontinuation than were OAPs,9,14,29 better outcomes in all-cause and natural-cause mortality might be attributable to improved medication adherence and less disease fluctuation and relapse.
With regard to associations of individual antipsychotics with mortality, the LAI forms of paliperidone, haloperidol, and risperidone were associated with lower risks of all-cause mortality compared with their corresponding OAPs. Notably, LAI flupentixol and LAI olanzapine were not associated with significant benefits for all-cause mortality. These findings are consistent with those of Taipale et al.28 However, studies further assessing the efficacy of individual antipsychotics against mortality are needed.
The lifetime risk of suicide in patients with schizophrenia is approximately 5%,4 and suicide is another leading contributor to excessive premature mortality in this population.6,30 The risk is highest among patients during the early stage of their illness or their first episode.5 Most suicide attempts occur within 2 years after the first episode of psychosis,31,32 and suicide-related mortality is higher among individuals recently diagnosed as having schizophrenia (ie, ≤5 years from diagnosis).33 The higher risk of suicide compared with later stages of schizophrenia might be explained by the critical period hypothesis, which states that the primary clinical and psychosocial deterioration associated with schizophrenia occurs within the first 5 years, the critical period.34,35 Within this period, biological, psychological, and psychosocial influences are developing and have maximum plasticity to adequate interventions. However, relapse is also common in this period because of low levels of insight and nonadherence to medication.36 Although suicide is a complex and multifactorial phenomenon, suicides are typically preventable with timely, evidence-based interventions. Hence, active early intervention and even the early application of LAIs to reduce risks of relapse, suicide attempts, and suicide mortality in the early stage have been proposed.36,37,38,39
Our findings suggest that LAI users had a lower suicide attempt risk and that patients who switched to LAIs within 2 years of OAPs treatment had decreased suicide mortality compared with those who continued receiving OAPs. These results are consistent with a previous study38 that revealed that the use of second-generation LAIs for recent-onset schizophrenia reduced suicidal ideation compared with chronic schizophrenia. However, we did not observe a decrease in suicide mortality among patients who switched to LAIs more than 2 years after OAP treatment. This might have been due to the neurological processes underlying schizophrenia having been ongoing for many years before the first episode.40 Therefore, with later LAI use, their protective effects might not be sufficient to compensate for the cognitive and psychosocial deterioration that occurs in the first few years of the illness.
Clinically, most psychiatrists use LAIs with a conservative attitiude,41,42 and the reasons for this attitude are generally not well supported by current scientific evidence.41 This attitude was also observed in our study; only 10% of our base cohort was prescribed LAIs at least 4 times within 1 year. Heres et al43 investigated factors associated with negative psychiatrist attitudes toward LAI use and identified 3 main ones: limited availability of different types of second-generation antipsychotic depot drugs, the frequent rejection of the depot by patients, and patient skepticism based on the lack of experiencing a relapse. Several other factors also influence psychiatrist practices, such as patient or psychiatrist perceptions, drug costs and insurance coverage, difficulty of adjusting LAI dosages in response to adverse effects, and the conservative position regarding LAI use in treating early-stage schizophrenia in the majority of guidelines.14,44,45
In our findings, the LAI group had significantly lower risks of all-cause and natural-cause mortality, and those who switched to LAIs in the early stage (≤2 years) had a 47% decreased risk in suicide mortality as well. Given that inadequate medication adherence is common among patients with newly diagnosed schizophrenia, more active consideration of LAIs in this stage for better long-term outcomes should be encouraged, particularly for those who have already exhibited poor adherence attitudes.39,37,45,46,47,48 Additional studies are also needed to clarify long-term adverse effects specific to LAIs use and identify specific psychotherapeutic and psychosocial interventions that may offer other benefits to patients in the early stage.
Strengths and Limitations
This study’s major strength is its population-based design that used a prevalent new-user design to examine long-term mortality, for up to 16 years, as our primary outcome in patients with newly diagnosed schizophrenia. This design enabled head-to-head comparisons between LAIs and their corresponding OAPs by enrolling patients and considering the disease duration, age, sex, and psychiatric comorbidities at the baseline.
Several limitations should be addressed. First, all observational studies have residual confounding. However, we made our best effort to minimize this potential bias by stringently selecting our matched cohort using the duration of OAPs treatment, year of first antipsychotic treatment, and psychotic comorbidities at the baseline. Second, lifestyle factors, socioeconomic status, social support, and the personal preferences of psychiatrists were not included in the study because of the NHIRD’s limitations. Third, the CCI score, history of suicide attempts, and number of psychiatric ED visits were included in the Cox hazard model but not in the matching. Fourth, an immortal time bias may exist in cohort studies. In this study, the mean (SD) duration from the first LAI prescription to the fourth LAI prescription was 109 (69.3) days, and no participants died during this period. Thus, the likelihood of immortal time bias in our study was deemed to be low.
Conclusions
Our findings suggest that the use of LAIs in patients with newly diagnosed schizophrenia was associated with significantly reduced risks of all-cause mortality and suicide attempts. Furthermore, early treatment with LAIs (within the first 2 years) was also associated with decreased suicide mortality risks. Thus, more active consideration of LAIs in the early stage of treatment should be encouraged.
eFigure 1. Flowchart Showing the Study Cohorts
eFigure 2. Study Timeline
eTable 1. ATC Codes Used to Identify Antipsychotics: Long-Acting Injectable Antipsychotics (LAIs) and Oral Antipsychotics (OAPs)
eTable 2. ICD-10 and ICD-9 CM Codes for Psychiatric and Charlson Comorbidities
References
- 1.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425-448. doi: 10.1146/annurev-clinpsy-032813-153657 [DOI] [PubMed] [Google Scholar]
- 2.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83-88. doi: 10.1097/YCO.0b013e32835035ca [DOI] [PubMed] [Google Scholar]
- 3.Brown S. Excess mortality of schizophrenia: a meta-analysis. Br J Psychiatry. 1997;171(6):502-508. doi: 10.1192/bjp.171.6.502 [DOI] [PubMed] [Google Scholar]
- 4.Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4)(suppl):81-90. doi: 10.1177/1359786810385490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordentoft M, Madsen T, Fedyszyn I. Suicidal behavior and mortality in first-episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392. doi: 10.1097/NMD.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 6.Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388. doi: 10.1016/j.schres.2020.04.025 [DOI] [PubMed] [Google Scholar]
- 7.Hawton K, Sutton L, Haw C, Sinclair J, Deeks JJ. Schizophrenia and suicide: systematic review of risk factors. Br J Psychiatry. 2005;187(1):9-20. doi: 10.1192/bjp.187.1.9 [DOI] [PubMed] [Google Scholar]
- 8.Keating D, McWilliams S, Schneider I, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open. 2017;7(1):e013881. doi: 10.1136/bmjopen-2016-013881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686-693. doi: 10.1001/jamapsychiatry.2017.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med. 2008;148(4):258-267. doi: 10.7326/0003-4819-148-4-200802190-00004 [DOI] [PubMed] [Google Scholar]
- 11.Lu T-H, Lee M-C, Chou M-C. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol. 2000;29(2):336-343. doi: 10.1093/ije/29.2.336 [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 13.Ritchie CW, Harrigan S, Mastwyk M, Macfarlane S, Cheesman N, Ames D. Predictors of adherence to atypical antipsychotics (risperidone or olanzapine) in older patients with schizophrenia: an open study of 3(1/2) years duration. Int J Geriatr Psychiatry. 2010;25(4):411-418. doi: 10.1002/gps.2354 [DOI] [PubMed] [Google Scholar]
- 14.Fang SC, Liao DL, Huang CY, Hsu CC, Cheng SL, Shao YJ. The effectiveness of long-acting injectable antipsychotics versus oral antipsychotics in the maintenance treatment of outpatients with chronic schizophrenia. Hum Psychopharmacol. 2020;35(3):e2729. doi: 10.1002/hup.2729 [DOI] [PubMed] [Google Scholar]
- 15.Suissa S, Moodie EE, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26(4):459-468. doi: 10.1002/pds.4107 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Collaborating Centre for Drug Statistics . Methodology. Published 2014. Accessed October 31, 2020. https://www.whocc.no/atc_ddd_index/
- 17.Napryeyenko O, Burba B, Martinez G, et al. Risperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measures. J Clin Psychopharmacol. 2010;30(2):200-202. doi: 10.1097/JCP.0b013e3181d438e2 [DOI] [PubMed] [Google Scholar]
- 18.Eaton WW, Thara R, Federman B, Melton B, Liang KY. Structure and course of positive and negative symptoms in schizophrenia. Arch Gen Psychiatry. 1995;52(2):127-134. doi: 10.1001/archpsyc.1995.03950140045005 [DOI] [PubMed] [Google Scholar]
- 19.Parellada E, Velligan DI, Emsley R, Kissling W. Long-acting injectable antipsychotics in first-episode schizophrenia. Schizophr Res Treatment. 2012;2012:318535. doi: 10.1155/2012/318535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventriglio A, Gentile A, Bonfitto I, et al. Suicide in the early stage of schizophrenia. Front Psychiatry. 2016;7:116. doi: 10.3389/fpsyt.2016.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative effectiveness of antipsychotics for risk of attempted or completed suicide among persons with schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi: 10.1093/schbul/sbaa031.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin H-T, Lai C-H, Perng H-J, et al. Insomnia as an independent predictor of suicide attempts: a nationwide population-based retrospective cohort study. BMC Psychiatry. 2018;18(1):117. doi: 10.1186/s12888-018-1702-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson Comorbidity Index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288-1294. doi: 10.1016/j.jclinepi.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 24.Fox J. Cox proportional-hazards regression for survival data: appendix to an R and S-PLUS companion to applied regression. Published 2002. Accessed April 1, 2021. https://socialsciences.mcmaster.ca/jfox/Books/Companion-1E/appendix-cox-regression.pdf
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 26.Austin PC. Statistical power to detect violation of the proportional hazards assumption when using the Cox regression model. J Stat Comput Simul. 2018;88(3):533-552. doi: 10.1080/00949655.2017.1397151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gharibvand L, Fernandez G. Advanced statistical and graphical features of SAS® PHREG. Presented at SAS Global Forum 2008. Accessed April 1, 2021. https://support.sas.com/resources/papers/proceedings/pdfs/sgf2008/375-2008.pdf
- 28.Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280. doi: 10.1016/j.schres.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 29.Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609. doi: 10.1176/appi.ajp.2011.10081224 [DOI] [PubMed] [Google Scholar]
- 30.Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217. doi: 10.1192/bjp.177.3.212 [DOI] [PubMed] [Google Scholar]
- 31.Dutta R, Murray RM, Hotopf M, Allardyce J, Jones PB, Boydell J. Reassessing the long-term risk of suicide after a first episode of psychosis. Arch Gen Psychiatry. 2010;67(12):1230-1237. doi: 10.1001/archgenpsychiatry.2010.157 [DOI] [PubMed] [Google Scholar]
- 32.Jakhar K, Beniwal RP, Bhatia T, Deshpande SN. Self-harm and suicide attempts in schizophrenia. Asian J Psychiatr. 2017;30:102-106. doi: 10.1016/j.ajp.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleischhacker WW, Kane JM, Geier J, et al. Completed and attempted suicides among 18,154 subjects with schizophrenia included in a large simple trial. J Clin Psychiatry. 2014;75(3):e184-e190. doi: 10.4088/JCP.13m08563 [DOI] [PubMed] [Google Scholar]
- 34.Birchwood M, Todd P, Jackson C. Early intervention in psychosis: the critical period hypothesis. Br J Psychiatry Suppl. 1998;172(33):53-59. doi: 10.1192/S0007125000297663 [DOI] [PubMed] [Google Scholar]
- 35.Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884-897. doi: 10.1016/S0006-3223(01)01303-8 [DOI] [PubMed] [Google Scholar]
- 36.Přikryl R, Přikrylová Kučerová H, Vrzalová M, Cešková E. Role of long-acting injectable second-generation antipsychotics in the treatment of first-episode schizophrenia: a clinical perspective. Schizophr Res Treatment. 2012;2012:764769. doi: 10.1155/2012/764769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224. doi: 10.1001/jamapsychiatry.2020.2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corigliano V, Comparelli A, Mancinelli I, et al. Long-acting injectable second-generation antipsychotics improve negative symptoms and suicidal ideation in recent diagnosed schizophrenia patients: a 1-year follow-up pilot study. Schizophr Res Treatment. 2018;2018:4834135. doi: 10.1155/2018/4834135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan SKW, Chan SWY, Pang HH, et al. Association of an early intervention service for psychosis with suicide rate among patients with first-episode schizophrenia-spectrum disorders. JAMA Psychiatry. 2018;75(5):458-464. doi: 10.1001/jamapsychiatry.2018.0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry. 2015;20(1):84-97. doi: 10.1038/mp.2014.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heres S, Hamann J, Kissling W, Leucht S. Attitudes of psychiatrists toward antipsychotic depot medication. J Clin Psychiatry. 2006;67(12):1948-1953. doi: 10.4088/JCP.v67n1216 [DOI] [PubMed] [Google Scholar]
- 42.Jaeger M, Rossler W. Attitudes towards long-acting depot antipsychotics: a survey of patients, relatives and psychiatrists. Psychiatry Res. 2010;175(1-2):58-62. doi: 10.1016/j.psychres.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 43.Heres S, Reichhart T, Hamann J, Mendel R, Leucht S, Kissling W. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301. doi: 10.1016/j.eurpsy.2009.12.020 [DOI] [PubMed] [Google Scholar]
- 44.Parellada E, Bioque M. Barriers to the use of long-acting injectable antipsychotics in the management of schizophrenia. CNS Drugs. 2016;30(8):689-701. doi: 10.1007/s40263-016-0350-7 [DOI] [PubMed] [Google Scholar]
- 45.Kim B, Lee SH, Yang YK, Park JI, Chung YC. Long-acting injectable antipsychotics for first-episode schizophrenia: the pros and cons. Schizophr Res Treatment. 2012;2012:560836. doi: 10.1155/2012/560836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horvitz-Lennon M, Predmore Z, Orr P, et al. Simulated long-term outcomes of early use of long-acting injectable antipsychotics in early schizophrenia. Early Interv Psychiatry. 2019;13(6):1357-1365. doi: 10.1111/eip.12770 [DOI] [PubMed] [Google Scholar]
- 47.Emsley R, Medori R, Koen L, Oosthuizen PP, Niehaus DJ, Rabinowitz J. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213. doi: 10.1097/JCP.0b013e318167269d [DOI] [PubMed] [Google Scholar]
- 48.Tiihonen J, Walhbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224. doi: 10.1136/bmj.38881.382755.2F [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart Showing the Study Cohorts
eFigure 2. Study Timeline
eTable 1. ATC Codes Used to Identify Antipsychotics: Long-Acting Injectable Antipsychotics (LAIs) and Oral Antipsychotics (OAPs)
eTable 2. ICD-10 and ICD-9 CM Codes for Psychiatric and Charlson Comorbidities