Abstract
This study describes oral temperatures in a large sample of nonsurgical inpatients without infections to inform evaluation of fever in the hospital.
Oral temperature is measured frequently in hospital care and elevated temperatures may indicate infection. Studies describe temperature of healthy ambulatory patients, but analogous data for noninfected inpatients are lacking.1,2,3 To inform evaluation of fever in the hospital, we describe oral temperatures in a large sample of nonsurgical inpatients without infections.
Methods
Cleveland Clinic’s institutional review board approved this study with a waiver of informed consent. We collected electronic health record data from nonsurgical patients hospitalized within the Cleveland Clinic Health System in 2017-2018. Patients with evidence of malignancy, infection, or immunological dysfunction, identified through diagnosis codes, medications (eg, antibiotics), or laboratory tests, were excluded. Data are from 18 hospitals ranging from large academic centers to community hospitals.
Oral temperatures measured during the first week of hospitalization were included. We excluded implausible values (<82 °F or >110 °F) and temperatures measured less than 6 hours following administration of acetaminophen or nonsteroidal anti-inflammatory drugs.
We describe temperature among this sample with means and 99% ranges,1 and we measured within-patient variability for patients with 20 or more measurements. A mixed linear model with patient-level clustering (to account for repeated measures) was used to estimate associations between temperature and other variables, including age, sex, race/ethnicity, body mass index (calculated as weight in kilograms divided by height in meters squared), smoking status, pregnancy status, time of day, and common diagnoses (heart failure, shock, myocardial infarction, kidney disease, cerebrovascular accident, pulmonary embolism, liver disease, gastrointestinal bleeding, poisoning, pancreatitis, prediabetes, diabetes, hypertension, chronic obstructive pulmonary disease, hypothyroidism, hyperthyroidism, and deep venous thrombosis). The medical record contains self-identified race/ethnicity, which was included because of associations in prior work.1 Standard definitions identified kidney disease.4 Analyses were conducted using R version 4.0.0 (R Foundation for Statistical Computing).
Results
Of 45 989 patients who met inclusion criteria, 3367 (7%) were excluded for missing demographic or temperature data, leaving 42 622 patients with 705 910 temperature measurements. Further excluded temperatures were those that were nonorally measured (100 612 [14%]), outside the first week of hospitalization (48 411 [7%]), implausible (31 [0.004%]), or within 6 hours following administration of nonsteroidal anti-inflammatory drugs (15 745 [2%]) or acetaminophen (45 225 [6%]), leaving 495 886 (70%) for analysis. Patients contributed a median of 9 temperatures (interquartile range, 5-16) over a median of 2 days (interquartile range, 1-4). Among the patients, the mean age was 61 (SD, 19) years, 50% were female, 68% were White, 25% were Black, and the mean body mass index was 30 (SD, 8).
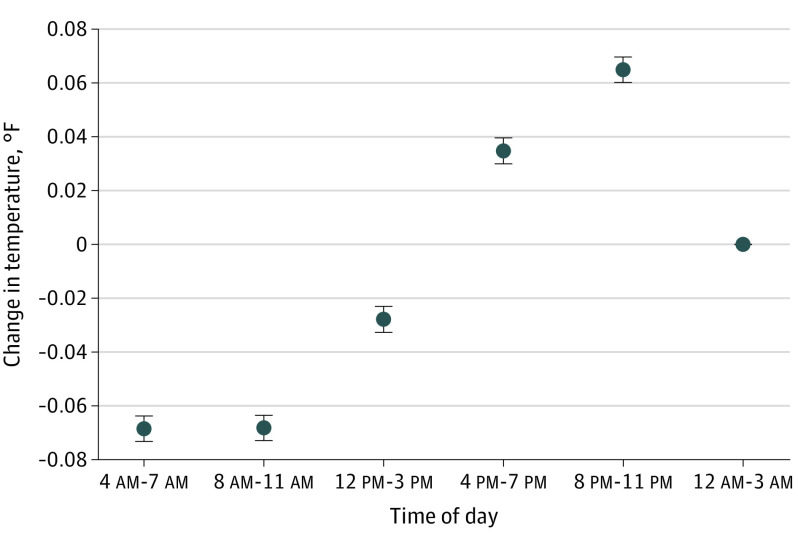
Patients’ mean temperature was 98.04 °F (99% range, 95.80 °F-99.90 °F). Of 6832 patients with 20 or more temperature measurements, the mean per-patient SD was 0.46 °F. Temperatures were lower among patients with older age (−0.025 °F per decade) and body mass index (−0.02 °F per 10-point increase) and were higher in female (+0.070 °F), Black (+0.074 °F), and pregnant (+0.045 °F) patients (Table). Temperatures varied among patients with different diagnoses. Diurnal variation demonstrated late evening peaks and morning troughs (Figure).
Table. Patient Characteristics Associated With Oral Temperature in a Multivariable Model.
| Characteristics | No. (%) | Mean change in temperature (95% CI), °Fa |
|---|---|---|
| Demographic | ||
| Age (per decade) | −0.025 (−0.027 to −0.023) | |
| Sex | ||
| Female | 21 284 (50) | 0.070 (0.063 to 0.077) |
| Male | 21 338 (50) | 0 [Reference] |
| Race/ethnicityb | ||
| White | 29 144 (68) | 0 [Reference] |
| Black | 10 637 (25) | 0.074 (0.066 to 0.082) |
| Other | 2841 (7) | 0.017 (0.003 to 0.031) |
| Body mass index, mean (SD)c | 30 (8) | −0.002 (−0.002 to −0.001) |
| Pregnant | 1541 (4) | 0.045 (0.025 to 0.065) |
| Smoker | 9572 (23) | 0.006 (−0.002 to 0.015) |
| Diagnosisd | ||
| Acute heart failure | 4080 (10) | −0.154 (−0.165 to −0.143) |
| Shock | 161 (0.4) | −0.082 (−0.135 to −0.028) |
| Myocardial infarction | 1856 (4) | −0.026 (−0.042 to −0.009) |
| Kidney disease | 916 (2) | −0.023 (−0.046 to −0.001) |
| Cerebrovascular accident | 2388 (6) | 0.041 (0.027 to 0.055) |
| Poisoning | 321 (0.8) | 0.056 (0.016 to 0.096) |
| Pulmonary embolism | 859 (2) | 0.079 (0.055 to 0.102) |
| Liver disease | 594 (1) | 0.095 (0.067 to 0.123) |
| Gastrointestinal bleeding | 1905 (5) | 0.100 (0.084 to 0.116) |
| Acute pancreatitis | 960 (2) | 0.167 (0.144 to 0.190) |
Demographics, diagnoses, and time of day were controlled for in the model, which was constructed using individual temperature measurements rather than patients, and accounted for clustering within patient.
A patient self-identified field in the medical record indicated race/ethnicity, which was used because of associations found in prior work. The reference group for race is non-Hispanic White.
Calculated as weight in kilograms divided by height in meters squared. The mean change in temperature is per 1 kg/m2.
Other diagnoses variables were associated with absolute temperature changes of 0.025 °F or less, including prediabetes (0.019 °F), diabetes (0.012 °F), hypertension (−0.009 °F), chronic obstructive pulmonary disease (−0.014 °F), hypothyroidism (−0.025 °F), hyperthyroidism (not significant), and deep vein thrombosis (not significant).
Figure. Diurnal Variation in Oral Temperature of Noninfected Hospitalized Patients.
The graph shows mean change in temperature with 95% CI error bars for the time-of-day variable in the mixed linear regression model on oral temperature, which includes the covariables listed in the Table.
Discussion
In this study of medical inpatients without malignancy, infection, or immunological dysfunction, the range of 95.8 °F to 99.9 °F encompassed 99% of almost half a million temperature measurements and could serve as a normal range against which to compare infected patients. The top of this range is half a degree lower than the accepted 100.4 °F, which is based on axillary temperatures taken in the 1860s.5 Temperatures have been decreasing for 100 years, and 99.9 °F has been found to be the top of the 99% range among outpatients in previous work as well as in this study.1,2 The bottom of the range was slightly warmer in this study of inpatients than in a study of outpatients (95.5 °F).1 Demographics, comorbidities, and time of day were associated with small temperature differences, which should not be considered during clinical interpretation of temperature. Within-patient variability exceeded temperature differences associated with most patient attributes. Compared with outpatients, whose temperatures peak at 4 pm,1 inpatient temperatures peaked later, perhaps reflecting disordered sleep.6
This study is limited by the use of electronic health records, which may contain some inaccurate data. Furthermore, the study included only oral temperature measurement. In light of these findings, however, it may be appropriate to reassess whether fever begins at 100.4 °F.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Obermeyer Z, Samra JK, Mullainathan S. Individual differences in normal body temperature: longitudinal big data analysis of patient records. BMJ. 2017;359:j5468. doi: 10.1136/bmj.j5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sund-Levander M, Forsberg C, Wahren LK. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci. 2002;16(2):122-128. doi: 10.1046/j.1471-6712.2002.00069.x [DOI] [PubMed] [Google Scholar]
- 3.Protsiv M, Ley C, Lankester J, Hastie T, Parsonnet J. Decreasing human body temperature in the United States since the industrial revolution. Elife. 2020;9:e49555. doi: 10.7554/eLife.49555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Section 2: AKI definition. Kidney Int Suppl. 2012;2(1):19-36. doi: 10.1038/kisup.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6°F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268(12):1578-1580. doi: 10.1001/jama.1992.03490120092034 [DOI] [PubMed] [Google Scholar]
- 6.Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. doi: 10.12788/jhm.3091 [DOI] [PubMed] [Google Scholar]