Abstract
The increasing stroke burden in the world strongly suggests that currently implemented primary stroke prevention strategies are not sufficiently effective, and new effective primary prevention strategies with larger effect size are needed. In this article, we reviewed the latest stroke epidemiology literature with the emphasis on the recent Global Burden of Disease 2013 Study stroke burden estimates from 1990 to 2013, highlight the problems with current primary stroke and cardiovascular disease (CVD) prevention strategies, and outline new developments in primary stroke and CVD prevention. We also suggested key priorities for the future, including comprehensive prevention strategies that target people with all levels of CVD risk, implementation of an integrated approach to promote health behaviors and reduce health disparities, capitalizing on information technology to advance prevention approaches and techniques, and incorporation of culturally appropriate education about healthy lifestyle into standard education curricula early in life. Given the already immense and fast increasing burden of stroke and other major noncommunicable diseases (NCDs) which threatens worldwide sustainability, governments could develop and implement an emergency action plan addressing the primary prevention of NCDs.
Keywords: stroke, primary and secondary prevention
Stroke remains a major global health problem1 and its significance is likely to increase in the future due to ongoing demographic changes, including ageing of the population and health transitions observed in developing countries.2,3 The Global Burden of Disease (GBD) Study provides the most comprehensive assessments of the state of health in the world since 1990.4 The most recent assessment of the global, regional and country-specific burden of stroke is the 2013 study (GBD 2013), which provides results for 1990, 2005 and 2013. Data from the GBD 2013 Study showed that while age-standardized rates of stroke mortality have decreased worldwide in the past two decades, there was a significant increase in the absolute number of people affected by stroke worldwide from 1990 to 2013.3 There is currently almost no country in the world where the stroke burden in terms of absolute number of incident and fatal strokes, stroke survivors and disability-adjusted life years (DALYs) has reduced. The increasing stroke burden in the world strongly suggests that currently used high-risk and population-wide primary stroke prevention strategies are not sufficiently effective, and more effective primary prevention strategies with larger effect size are needed.
In this article, we provide an overview of the most recent literature on stroke epidemiology with the emphasis on main results of the GBD 2013 Study concerning ischaemic stroke (IS) and haemorrhagic stroke (HS) burden in the world in children and adults in 1990 and 2013 by sex, highlight problems with current primary stroke and cardiovascular disease (CVD) prevention strategies, outline several new developments in primary stroke and CVD prevention and suggest key priorities for actions.
THE GLOBAL BURDEN OF STROKE
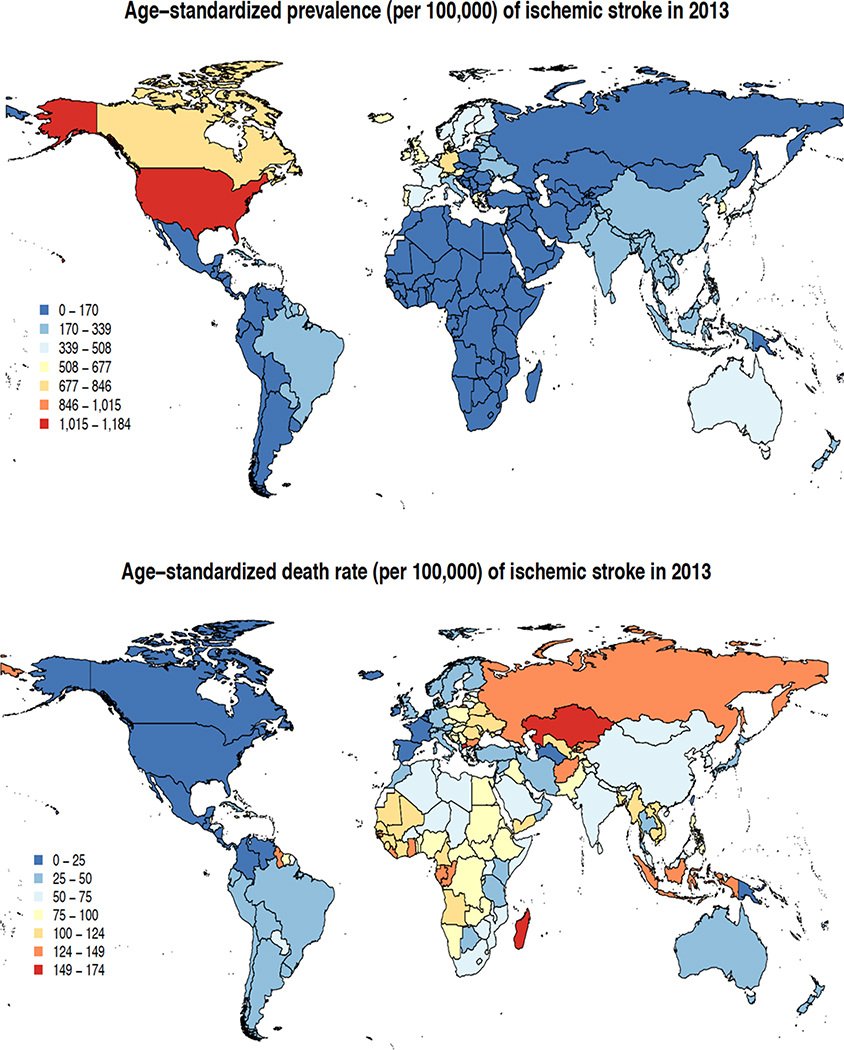
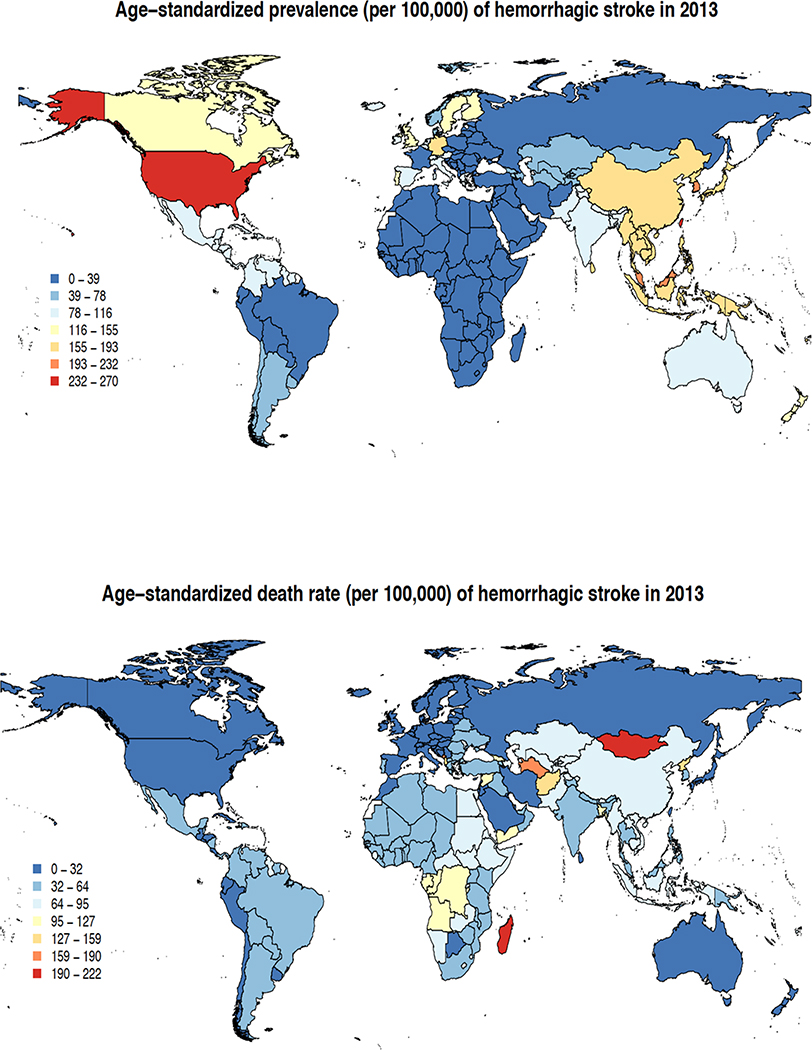
The main results of the GBD 2013 stroke burden estimates were published in a special free-access issue of Neuroepidemiology3,5–11 in October 2015. In 2013, there were almost 25.7 million stroke survivors (71% with IS), 6.5 million deaths from stroke (51% died from IS), 113 million DALYs due to stroke (58% due to IS), and 10.3 million new strokes (67% IS).3 There were large geographical variations in IS and HS age-adjusted incidence, prevalence and mortality rates (Figures 1 and 2). The highest prevalence rate of IS (1,015–1,184/100,000) was observed in developed countries (particularly USA), the lowest (up to 339/100,000) in developing countries. The highest IS mortality rates (124–174/100,000 person-years) were observed in Russia and Kazakhstan, and the lowest (at or below 25/100,000) – in Western Europe, North and Central America, Turkmenistan and Papua New Guinea. Prevalence rates of HS were highest (232–270/100,000) in the USA, and lowest (up to 78/100,000) in Latin America, Africa, Middle East, France, Eastern Europe, Northern part of Asia and Russia. HS mortality rates were highest (159–222/100,000 person-years) in Mongolia and Madagascar, and lowest (up to 32/100,000) in North America, most parts of Western Europe, Russia, Iran, Saudi Arabia, Morocco, Japan, Australia and New Zealand (Figure 2). These geographical variations in stroke burden emphasize the need for regional-specific approach for stroke care planning and prevention.
Figure 1.
Age-standardised prevalence and mortality of ischaemic stroke per 100,000 person-years in various regions in 2013
Figure 2.
Age-standardised prevalence and mortality of haemorrhagic stroke per 100,000 person-years in various regions in 2013
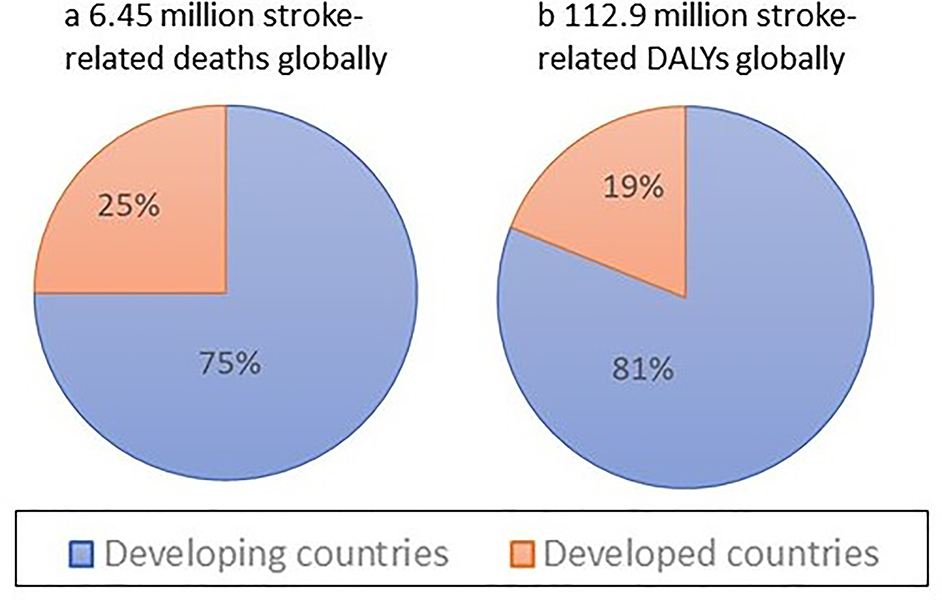
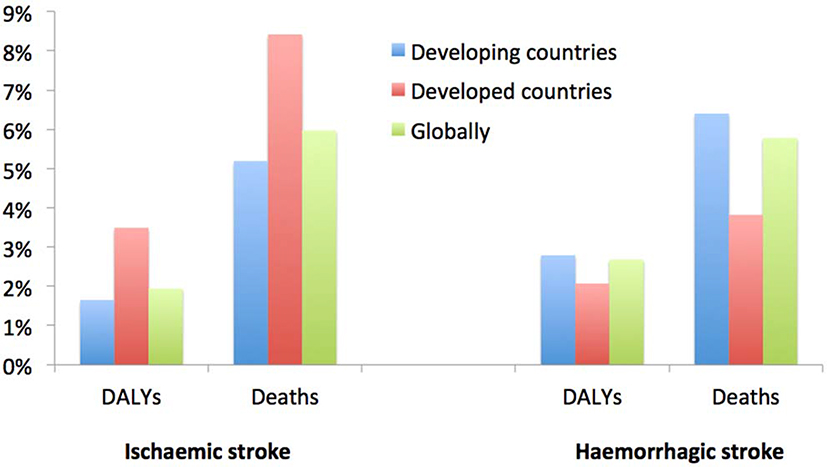
Globally, over the last few decades stroke burden in terms of absolute number of people affected by and died from stroke has increased dramatically1,3 largely due to ageing of the population and population growth in most regions,2 and there were no countries in the world where this burden was declining.12 Over the 1990–2013 period, there was a significant increase in the absolute number of disability-adjusted life-years (DALYs) due to IS, and of deaths from IS and HS, survivors and incident events for both IS and HS (Box 1) in both men and women.1,3,9,10,13 The bulk of the burden of stroke continued to reside in developing countries, where it comprised 75.2% of the global deaths from stroke and 81.0% of the global stroke-related DALYs (Figure 3). Globally, the proportional contribution of stroke-related DALYs and deaths due to stroke compared to all diseases increased by about 20–25% from 1990 (3.5% [95% uncertainty intervals (UI) 3.1 to 4.0] and 9.66% [95% UI 8.5 to 10.7], respectively) to 2013 (4.6% [95% UI 4.0 to 5.3] and 11.8% [95% UI 10.5 to 13.3], respectively). However, there was a diverging trend in developed and developing countries. For example, there was a significant increase in DALYs and deaths in developing countries, and no measurable change in the proportional contribution of DALYs and deaths from stroke in developed countries. While the proportional contribution of IS-related DALYs and deaths to DALYs and deaths from all causes was greatest in developed countries, for HS these proportions were greatest in developing countries (Figure 4). While the increase in the incidence of HS in developing countries may be related to the high rate of undetected and/or poorly controlled arterial hypertension,14–17 stroke incidence increase18 or even better detection of stroke in those countries, the increase in the prevalence of IS and HS in developed countries could be related to the improvements in acute stroke care, or more effective secondary prevention and greater identification of minor stroke cases (including wider use of advanced neuroimaging),19 which is highly dependent on universal access to primary care.20,21
Box 1.
Total number of DALYs, deaths, incident and prevalent cases of ischaemic and haemorrhagic stroke in the world in 1990 and 2013 (modified from Feigin et al.3)
| 1990 (in millions) | 2013 (in millions) | % Increase | ||
|---|---|---|---|---|
| Ischaemic stroke | DALYs | 34.2 (29.6 – 38.3) | 47.4 (40.5 – 52.2) | 38.6% |
| Deaths | 2.2 (1.9 – 2.4) | 3.3 (2.8 – 3.6) | 50% | |
| Incidence | 4.3 (4.1 – 4.5) | 6.9 (6.5 – 7.4) | 60.5% | |
| Prevalence | 10.0 (9.6 – 10.5) | 18.3 (17.8 – 18.9) | 83% | |
| Haemorrhagic stroke | DALYs | 56.0 (49.9 – 62.2) | 65.5 (59.5 – 74.7) | 17% |
| Deaths | 2.4 (2.1 – 2.7) | 3.2 (2.9 – 3.7) | 33.3% | |
| Incidence | 1.9 (1.8 – 2.0) | 3.4 (3.2 – 3.5) | 78.9% | |
| Prevalence | 7.4 (7.1 – 7.6) | 89.7% |
Figure 3:
Stroke-related deaths and DALYs by country development status.
Figure 4.
Proportional (%) contribution of ischaemic and haemorrhagic strokes burden (with 95% uncertainty intervals [UI]) to all health conditions by country development status in 2013 (modified from Feigin et al.)3
Of additional concern is a significant increase in the number of younger adults (aged <65 years) affected by stroke.1,22,23 In 2013 there were 97,800 [95% UI 90,600 to 106,000] prevalent cases of childhood IS and 67,621 [95% UI 62,900 to 72,200] prevalent cases of childhood HS, reflecting an increase of approximately 35% in the absolute numbers of prevalent childhood strokes since 1990.8 Between 1990 and 2013 there were significant increases in the global prevalence rates of childhood IS, as well as significant decreases in the global death rate and DALYs rate of all strokes in 0 to 19 year olds. While prevalence rates for childhood IS and HS decreased significantly in developed countries, in developing countries, a decline was seen only in HS, with no change in IS prevalence rates. In 2013, the prevalence rate of both IS and HS in children was significantly higher in developed countries than developing countries. However, both death and DALY rates for all strokes in children were significantly lower in developed countries than in developing countries in 2013. Also, between 1990 and 2013 there were significant increases in prevalent cases, total deaths, and DALYs due to HS and IS in younger adults (20–64 years).9 If these trends continue, the burden of stroke will increase even faster24 and the UN Global Targets25 to reduce premature mortality from non-communicable diseases (NCDs), including stroke, by 25% by 2025 will not be met. Moreover, for stroke in particular, mortality reduction should not be the primary health goal12 because about three quarter of stroke survivors remain disabled and reduced mortality will inevitably lead to a greater number of people disabled from stroke. Focus on acute phase treatment and rehabilitation alone will not significantly reduce the burden of stroke.26 There is an urgent need to address these disparities in stroke burden in children and young adults (taking into account some specific causes for childhood stroke)27 with both global and country level initiatives targeting prevention early in life as well as improved access to acute and chronic stroke care.8
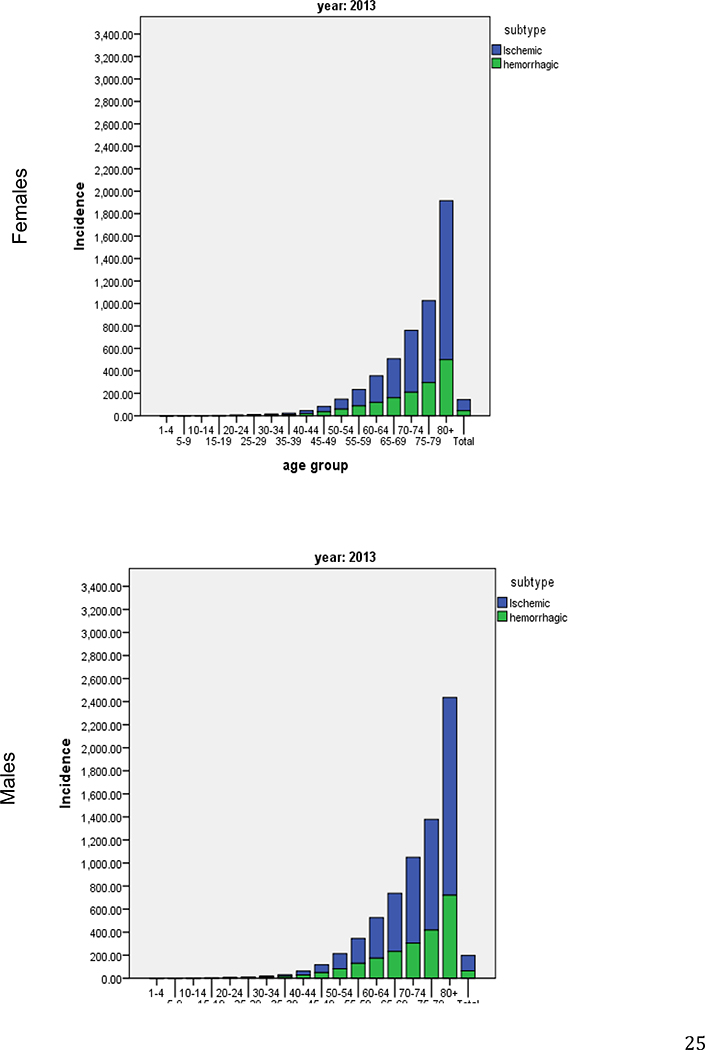
The GBD 2013 Study also demonstrated important sex differences in IS and HS burden in the world.10 In 2013, men had a higher incidence of IS then women while significant sex differences in the incidence of HS were not observed (Figure 5). The total DALYs were similar for men and women for both stroke subtypes in 2013. Both IS and HS DALYs showed an increasing trend for both men and women since 1990 which was statistically significant only for IS among men. These findings show a clear need for additional research into factors that potentially contribute to the causes of sex differences in risk and outcomes. In addition, there is adequate evidence of differences in stroke incidence and burden between the sexes to support the need for well-designed stroke intervention trials, which are equally powered for men and women to examine effectiveness of primary care, risk factor management strategies, and hospital services.10
Figure 5.
Incidence per 100,000 of ischemic and haemorrhagic stroke in females and males by 5-year age bands in 2013 (modified from S Barker-Collo et al.)10
Stroke is currently the third largest contributor to DALYs after ischemic heart disease and lower respiratory infection globally in developing countries, and it is third largest contributor to DALYs in developed countries (after ischemic heart disease and low back and neck pain).28 This emphasizes the importance of stroke as a leading global health problem that requires urgent attention from governments, health care policy makers, international agencies, clinicians, public health specialists and individual citizens. Therefore, preventing new strokes is the core solution to the problem of growing stroke burden. Although mainstream preventative strategies should be similar in developed and developing countries (e.g. a combination of population-wide and high-risk prevention strategies), differences in the epidemiology of stroke (including differences in the prevalence and relative significance of risk factors) as well as resources available for stroke prevention should be taken into account when setting up realistic goals, priorities and means. For example, given the differences between developing and developed countries in terms of stroke burden (particularly the greater proportion of stroke in young adults and much greater burden from HS in developing countries compared to that in developed countries), a strong emphasis on early detection and management of elevated blood pressure should be a priority in developing countries. In developed countries, where the stroke burden associated with IS is noticeably greater than that in developing countries, it seems reasonable to focus more heavily on reducing behavioural risks (particularly diet, physical activity, overweight) and managing medical conditions leading to atherosclerosis. While the prevalence of behavioural and other modifiable risk factors of cardiovascular disease (CVD)29 including stroke has reached epidemic proportions worldwide,30,31 there is evidence that modifying health behaviours is feasible, improves health outcomes, reduces healthcare costs,32 can arguably reduce an individual’s risk of stroke by about 80%33,34 and reduce stroke incidence by about 50%.35 There is also evidence for more aggressive blood pressure control for stroke prevention even in people with borderline hypertension and without diabetes with a systolic blood-pressure target of less than 130 mm Hg.36,37 Another very important and prevalent risk factor for stroke the management of which has recently dramatically changed is atrial fibrillation (AF).38 As the incidence and prevalence of AF (including paroxysmal AF) is increasing,39–41 there is an urgent need to better detection and wider implementation of modern treatment for AF. However, the ever-increasing burden of stroke across the globe, large sex and racial/ethnic disparities and a trend towards more strokes in younger people in both developed and developing countries all indicate deficiencies in current stroke prevention strategies. More efficient stroke prevention strategies are urgently needed to halt and eventually reverse the stroke pandemic.3 However, before discussing new, potentially more effective stroke prevention strategies it is important to untangle causes of insufficient effectiveness of the currently used strategies.
APPROACHES TO STROKE PREVENTION
Population-wide strategies together with strategies that target high-risk individuals have been proposed as the most effective for stroke prevention.42
Population-wide strategies
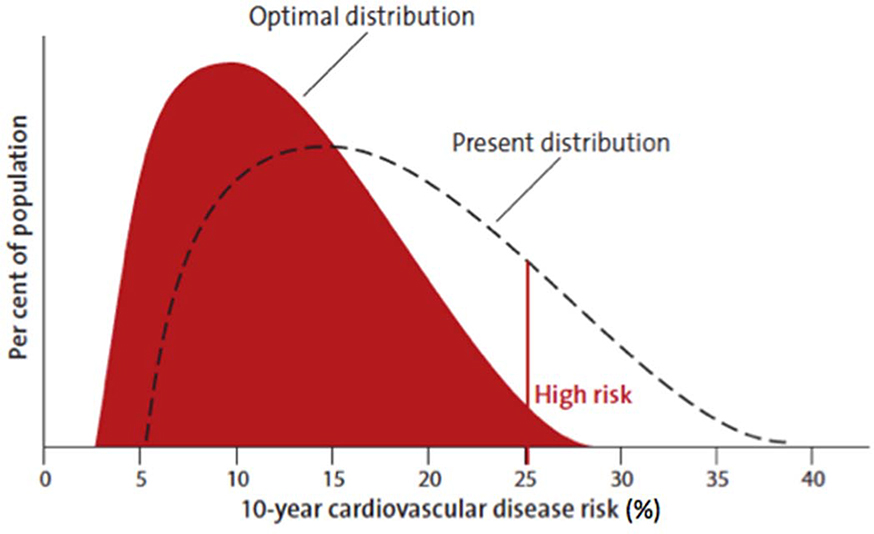
Population-wide strategies are essential because even small changes in the distribution of risk factors would lead to major reductions in stroke/CVD incidence in the population (Figure 6).43,44 The population-wide prevention strategy was suggested by Sir Geoffrey Rose in 1985 as the most effective strategy for primary CVD prevention.44 As this strategy targets several behavioural and lifestyle risk factors (including tobacco use, unhealthy diet, physical inactivity, overweight and the harmful use of alcohol) that are common for a range of major NCDs, such as heart disease, diabetes, cancer, dementia and pulmonary heart disease, this strategy will be positively impacting not only stroke risk but on all these major NCDs. Over the last 15 years, it has been included in all major CVD prevention guidelines,34,45 often in combination with high-risk approach and with several anecdotal reports of its effectiveness in selected populations.46–48 Nevertheless, there is still no country in the world where this strategy has been implemented in full at the national level. The major reasons for the lack of implementation of this strategy on a national level include costs (e.g. increasing the availability of facilities for physical activity, healthy food outlets) and the need for policy and legislative changes that are often not supported by major industries (e.g. salt reduction in processed food, reduction of exposure to smoking, alcohol, fast food).24
Figure 6.
Optimal shift in the distribution of CVD risk by combination of population-wide and high-risk prevention strategies (modified from WHO “Cardiovascular disease prevention. Translating evidence into action”.19)
An additional important benefit of population-wide prevention approaches that target the entire population rather than just those at high risk is that it may be easier and more effective to promote the maintenance of health than to reverse existing damage, therefore, sensitive timepoints at which health changes may be most impactful are likely earlier in life before subclinical risk factors have emerged.
High-risk strategies
Intensive clinical care for high-risk individuals remains the dominant paradigm in the world for prevention of stroke.49 While management strategies for primary stroke prevention in high CVD risk individuals are well established,34 they are under-utilised34,50–53 and existing methods of primary stroke prevention are not sufficiently effective.54–56 The healthcare system has been largely unsuccessful in providing relevant meaningful information to assist people in adhering to recommended lifestyle changes and medications.34,54,55,57–59 Uptake of primary prevention recommendations is particularly low in people with moderately increased risk of stroke who would benefit from lifestyle modifications.54,59,60 Inadequate CVD risk factor management54,55 and lack of effective communication between health professionals and stroke patients/family54,61–63 are implicated in underutilisation of primary stroke prevention strategies in people with moderately increased risk of stroke.61
The high-risk prevention strategy implies calculation of absolute risk of CVD over the next 5 or 10 years with the aim of identification of people at high risk of development of acute CVD (heart attack, stroke, acute peripheral artery disease), which usually defined as having a risk of ≥15% over the next 5 years or ≥30% over the next 10 year or having an established CVD (e.g. stroke, ischemic heart disease, transient ischemic attack [TIA], peripheral artery disease).64,65 Although assessment of people for absolute stroke/CVD risk is important for matching the intensity of preventing efforts with the individual’s absolute risk66 and monitoring success or failure in primary stroke/CVD prevention, there several major issues with the high-risk approach for stroke/CVD prevention. Primary stroke/CVD prevention strategy that aims primarily at high-risk individuals fails to engage into active prevention the majority of the population where most strokes and other acute CVD events occur,67,68 it also lacks personal motivation to control risk factors by people who are at low or moderate risk of CVD events. Other limitations of this strategy include costs associated with laboratory tests and doctors visit to determine the risk, and lack of population-specific algorithms for stroke and CVD prevention.24 There is evidence that even effective population-wide use of high-risk prevention strategies with aggressive pharmacological treatment in individuals with a 10-year Framingham event risk of ≥30% (6% of population) would have reduced major CVD by at most 11%,49 and if high-risk strategies are to have a major impact on CVD in the population, they need to be more widely used than previously envisaged.49 In addition, the use of high-risk prevention strategies is limited by access to medical professionals,69 and even when care is available, the adherence of patients to recommended medications and lifestyle modifications are often poor70 due to multiple reasons,71 including wrong patients’ beliefs72 (limited health literacy) and comorbidities.73
Although it is generally agreed that screening for CVD risk can be used as a guide for making clinical decisions on the intensity and effectiveness of preventative interventions,42,74 just screening of the population for CVD risk in order to provide intensive medical care to high risk individuals continues to be thought of as an important health target by many governments.75 This is particularly disappointing because there is compelling evidence from 15 large randomized controlled trials (totalling about 200,000 patients), including Cochrane systematic review and meta-analysis,76,77 that screening for high CVD risk, even in combination with some counselling,77 is not effective for reducing CVD incidence and mortality. The lack of effectiveness of just screening of the population for high CVD risk individuals and ethical concerns about promotion of screening without linkage to diagnosis and management has led some governments to explore additional ways to improve CVD prevention. For example, the use of CVD predicting algorithms in combination with incentivising clinicians for effective CVD prevention is being trialled in the USA,78–80 and the most recent evidence suggests that incentivising both clinicians and patients works.81 While shared financial incentives shown to be effective in that trial for reducing LDL-C level (US$512 per patient for physicians and US$355 for each patient enrolled in the trial) may not be feasible in less affluent societies, the findings of the importance of motivating both physicians and their patients for effective CVD prevention should not be underestimated and some other, more affordable incentives can and should be trialled in other populations.
A combination of population-wide and high-risk strategies
Primary prevention of stroke and other major NCDs is regarded by the UN as the most cost-effective strategy to reduce burden from these diseases.82 For example, reducing salt intake by 15% and implementing four key WHO elements of reduction in tobacco use (taxation, enforcement of smoke-free workplaces, packaging/labelling requirements, awareness campaigns) could avert 13.8 deaths over 10 years at a cost of less that US$0.4 per person per year in low-income and lower middle-income countries, and only US$0.50–1.00 per person per year in upper middle-income countries.83
It is essential for high-risk stroke prevention strategies aimed at specific high-risk populations (e.g. people with stroke or TIA, atrial fibrillation, sickle cell disease, etc.) to be accompanied by population-wide prevention strategies aimed at behavioural/lifestyle and environmental risks in which responsibilities are shared between the health sector, non-government organizations and government bodies. While certain governmental bodies have the power to inform or influence lifestyle, socio-economic and environmental factors (including tobacco control, healthy nutrition, bike lanes, availability of fruits and vegetables at corner stores, air pollution) as well as to provide adequate health services to ensure universal implementation of preventative strategies, the health system has the responsibility to identify risk factors and their proper management on an individual level.84,85 While these integrative, cross-sectoral approaches combined with behavioural modification (including stroke preparedness and lifestyle modifications) and other strategies have been recently identified as the key strategies for stroke and CVD prevention,26,59,86 the difficult question is – what could make stroke prevention work? How best to combine population-wide and high-risk prevention strategies? Here are our four major suggested strategies to improve primary stroke prevention (Box 2).
Box 2. Key priorities for effective primary stroke prevention.
Lifestyle factors
Shift the emphasis in primary stroke prevention to a more comprehensive approach that includes primary stroke prevention strategies in people with all levels of risk of CVD events
Focus on behavioural and lifestyle risk factors (including tobacco use, unhealthy diet, physical inactivity and harmful use of alcohol)
Partnering Across Sectors
Shared involvement and responsibilities of health sector, non-government organizations and government bodies for the development and implementation of population-wide and high-risk prevention strategies to control stroke/NCDs
Implement an integrated approach that incorporates community-clinical linkages to coordinate clinical strategies for high-risk individuals with community-based strategies that promote healthy behaviours and reduce health disparities
Incentivise health professionals and patients for effective stroke/NCD prevention
Electronic Health Information Technology
Implement widely accessible, affordable and validated mobile technologies for primary prevention into health systems for use by health professionals and lay people.
Reframe risk categories. In communicating absolute stroke/CVD risk, categorising people into low, moderate and high risk should be abandoned. People with 10-year absolute CVD risk less than 30% should be motivated and taught by their health professional to control their modifiable risk factors and reduce their risk of stroke/CVD to as low as possible level.
Early Life Interventions
Implement early-in-life culturally appropriate education about healthy lifestyle into standard education curricula with reinforcement across the lifespan
IMPROVING PRIMARY STROKE PREVENTION
Shifting the emphasis
The emphasis in primary stroke prevention should be shifted from high stroke/CVD absolute risk approach to a more comprehensive approach that includes primary stroke prevention strategies in people with all levels of risk of CVD events. In addition to more aggressive controlling elevated blood pressure and other metabolic/physiological risk factors (including AF), primary stroke prevention needs to focus on behavioural and lifestyle risk factors (including tobacco use, unhealthy diet, physical inactivity and the harmful use of alcohol) allowing an integrative approach to primary prevention of other major NCDs, such as heart disease, diabetes, cancer, dementia and pulmonary heart disease. This cluster of diseases and risk factors are prioritized by the World Health Organization (WHO) in its Global Action Plan on NCDs87 and included in the 2011 UN NCD Declaration, and the UN Post-2015 Sustainable Development Goals.12 In stroke/CVD occurrence predicting algorithms used by health professionals for stroke/CVD prevention educational purposes, categorising people into low, moderate and high absolute risk for the purpose of communicating the risk to the patients should be abandoned as it discourages people with low and moderate risk of CVD to be motivated to reduce their risk and engaged into primary prevention programmes.24 To illustrate increased risk for people with low absolute CVD risk, it was recommended to use a relative risk, relative risk charts, lifetime risk,24,88,89 and various risk visualization techniques.89–91 People with 10-year absolute CVD risk less than 30% should be empowered by their health professional (e.g. by showing them not only their absolute but also relative risk and the effect that each risk factor exposure reduction has on their risk) to control their modifiable risk factors, improve CVD health and reduce their risk of stroke/CVD to as low as possible level. In addition to healthy lifestyle modification92 and better adherence to the recommended medications,93,94 multidrug regimens of proven effective and cheap drugs (e.g. polypill)95 could lead to cost-effective prevention of stroke in all developing regions, potentially halving the risk of death from CVD and increasing life expectancy.96
An integrated approach
Implementing an integrated approach would incorporate community-clinical linkages that coordinate clinical strategies for high-risk individuals with community-based strategies to promote healthy behaviours and reduce health disparities.97,98 Coordinated interventions allow for community-wide prevention and management approaches that link efforts improving health across the care continuum and bridging across settings and strategies. Community-clinical linkages help ensure that people with or at high risk of chronic diseases have access to community resources and support to prevent, delay or manage chronic conditions once they occur.99 These supports include interventions such as clinician referral, community delivery and payment coverage for effective programs that increase the likelihood that people with heart disease, diabetes or pre-diabetes will be able to “follow the doctor’s orders” and take charge of their health.99 Examples include clinical strategies to promote individual smoking cessation along with smoke-free laws that reduce secondary exposure, or the use of community health workers and community pharmacists that can extend the work of the clinical provider into the community and broader population.100 Given the limited resources available for stroke and CVD prevention even in developed countries, it would be logical to place emphasis on effective population-wide interventions to control or reduce exposure to leading risk factors in the region. For example, population-wide efforts to reduce salt intake and smoking through multiple economic (e.g. taxation) and educational policies and programs have been suggested as cost-effective primary stroke and CVD strategies in developing countries.101–105 With the UN 2011 Declaration of NCDs,12 all UN-member governments have the mandate, power and responsibility to undertake actions to reduce the burden from NCDs, including stroke. The practical question is where to take resources to enable these actions? We believe that revenue from smoking and salt taxations, as well as taxations from sugar and alcohol, reduced consumption of which on a population level also shown to be beneficial for CVD and overall health,106,107 can and should be used to fund primary prevention strategies and primary prevention research of stroke and other major NCDs. Such uses of the tax revenue would also be important to unsure public acceptability of these taxes.108 There is compelling evidence that smoking,109–111 salt,83,108 sugar112–114 and alcohol115–119 taxation may be valuable strategies to improve health, especially when combined with other preventative interventions in the population, while generating a considerable revenue for governments.113,120–122 Given the already immense and fast increasing burden of stroke and other major NCDs across all countries in the world that threatens sustainability of the whole society, not acting now on primary prevention of these diseases is not acceptable. Governments can develop and implement an emergency action plan addressing the primary prevention of NCDs. It is also important to work on the implementation of what has proved working for primary stroke and NCDs prevention over the last decades in many developed countries in a broader way.
Using information technology
The impact of stroke and other non-communicable disorders (NCD) that share the same risk factors with stroke, such as ischemic heart disease (IHD), dementia (cognitive decline) and type II diabetes mellitus, is huge and increasing.123–125 Collectively, these NCD account for 14.7 million of deaths (28% of deaths from all causes)125 and 290.2 million disability-adjusted life-years lost worldwide.124 The burden of NCD is likely to burgeon given the aging of the world’s population and the epidemiological transition currently observed in low- to middle-income countries.126 In addition, there is low awareness in the population about these NCD and their risk factors, particularly in developing countries. These factors, coupled with underutilization of strategies for primary prevention of NCD on an individual level and the lack of accurate data on the frequency and significance of risk factors in different countries and populations have been implicated in the ever-increasing worldwide burden of the NCD.
Recent advances in mobile (smartphone) technologies and their worldwide use (about 1.7 billion users) offer unique opportunities to utilise these technologies for improving health (including health and stroke awareness) and research capabilities. Empowering people for self-management of their risk factors through mobile health apps is another potential application of these technologies, which may be particularly appealing for less affluent populations and population with limited access to health services (e.g. rural populations). A number of stroke and other NCD Apps are currently available in App Store and Google Play and promising results are emerging from clinical trials of several smartphone-based technologies and Apps for managing particular medical conditions and risk factors (e.g., smoking cessation, depression, weight and asthma management).127–131
Primary stroke prevention should capitalize on information technology to advance prevention approaches and techniques. Electronic health information can be useful to support patients, providers and population health, such as through improved public health surveillance activities.132 Importantly, accumulating evidence demonstrates the feasibility and effectiveness of use of mobile technologies for improving healthy lifestyle133–139 and some medical conditions predisposing to stroke.140–145 Supported by WHO,146 these technologies are evolving fast147 and have shown unprecedented uptake amongst lay consumers and professionals.148 Use of widely accessible, affordable and validated technological advances,147 such as the Stroke Riskometer™ app,24,149 to recognize a person’s own risk and risk factors, to educate about warning signs of stroke that can communicate risk and the importance of addressing risk factors that speak to a various levels of health literacy and in multiple languages, to motivate the person to control their risk factors, to provide targeted advice on how to lower the risk, and to measure effectiveness of primary prevention of NCDs, is another important approach for stroke prevention that should be incorporated into the health system and used by both health professionals and lay people. Not downplaying their significance for developed countries, such mobile technologies are particularly useful for developing countries where availability/affordability of and access to health professionals are limited. Such self-care programs are also seen by the WHO150 as a vital form of prevention in those at high risk and in improving outcomes in people with NCDs.
Culturally appropriate health education
As there are significant disparities in the risk151–155 and burden of stroke among various race/ethnic groups151–155 and many life style habits are set early in life, culturally appropriate education about healthy lifestyle should be incorporated into standard education curricula and started early in life with reinforcement across the lifespan. There is evidence suggesting effectiveness of school-based stroke education of middle school children,156 and it may be possible that trained children and community leaders can be effective healthy lifestyle educators for other children. In addition to health being a physical manifestation, stroke knowledge is also a socio-cultural construct that can limit the utility of universal primary prevention strategies for at-risk groups.8–10 Therefore, it is essential to understand the views, values, norms and beliefs of peoples of varies race/ethnicity who are at risk of stroke, what is more likely to work for them in order to learn and understand stroke and its associated risk factors, and how they will use this information to modify what they do in their daily life. Evidence suggests that even if an intervention is effective, adherence to its recommendations remains poor unless it is meaningful, acceptable and takes into account the factors that make up the patients’ health care environment.157 In addition, it has been shown that people’s beliefs are important predictors of the extent to which they will engage in behaviours to reduce risk of secondary stroke.71 Therefore it is crucial that stroke prevention strategies take these factors into account to have optimal impact. This information may also be used to inform culturally appropriate preventative strategies of other major NCD that share common risk factors with stroke, such as CVD, dementia and diabetes.
Linguistic and cultural (subcultural) differences between people of different race/ethnicity in relation to social, family values and sex and gender roles should be taken into consideration when developing culturally competent health education programs.158 For example, healthy diet recommendations that are successful for stroke and CVD prevention in people of Caucasian ethnicity may not be successful for Pacific or Asian people because their food habits and traditions are very different. There are also racial/ethnic differences in the relative significance of various determinants of stroke occurrence152 that should also be taken into account when developing culturally specific health education programs. Generic guidelines for making a health education programme more culturally appropriate have been suggested.158 Development of consensus statements and national stroke prevention guidelines by recognised experts from the region to address local (cultural) issues on the basis of the best available evidence along with the effective strategies to improve stroke awareness (including campaigns to remove stigma associated with stroke) should also be encouraged.101 Mother’s health and lifestyle, normal birth weight, adequate nutrition from the first days of life and thereafter are all-important determinants of health, including risk of stroke and other major NCDs, later in the life. Healthy lifestyle habits, adequate physical activity and maintaining a healthy weight should be formed at a young age,159 reinforced throughout the lifespan (for healthy diet habits, probably during the first year of life),160 and can be successfully incorporated in preschool, school, day-care and other interventions in early life settings.161
Recent studies in other countries have shown that knowledge of stroke risk factors and warning signs is deficient in older adults (65+),162,163 minority ethnic groups,163–166 people with low levels of education163 and rural dwellers.167 Consistent, culturally sensitive and motivational stroke education campaigns throughout the year (not only at national or World Stroke Awareness day) should be given a priority. As such campaigns are unlikely to be funded by the industry, but there are opportunities for the public sector to promote stroke awareness educational campaigns (along side of other educational campaigns). There is compelling evidence that increasing population/country-specific knowledge about stroke warning signs and risk factors can reduce stroke burden in the population.168–173
FUTURE RESEARCH DIRECTIONS IN PRIMARY STROKE PREVENTION
More good quality research is urgently needed on risk factors and prevention of stroke (major stroke pathological types and ethological subtypes) in various countries and populations, including research to untangle the causes of the increasing burden of stroke in developing countries and causes of changes in trends and projections of stroke burden in men and women of different age and race/ethnicity, especially research to investigate interventions for the ageing population, and strategies for implementation that can remain cost-effective for larger and larger population. Although mortality from stroke has declined substantially in developed countries, the use of proven strategies for stroke and CVD prevention, even in wealthy countries, is far from optimum. Stroke and CVD rates can be further reduced in future not only with more widespread implementation of proven strategies, but a greatly improved understanding of regional and ethnic variations of the causes of stroke because understanding of the risk factors for stroke and stroke types (including their population-attributable risk) in different race/ethnic populations is crucial to determining priorities and strategies for targeted stroke prevention in these populations.29 While population-wide strategies for primary prevention of NCDs have been proven feasible and effective,46–48 more research is warranted on the best, culturally appropriate and cost-effective ways of implementing these strategies on a population level, including their combination with high-risk strategies, self-management and knowledge transition research. More definitive research, including digital health research and randomized controlled trials in various populations sufficiently powered by sex, race/ethnicity and various age groups, are required for behavioural and educational interventions to specifically prevent stroke. However, primary prevention research is expensive, commonly not supported by industry, often regarded as ‘non-sexy’ research by research funding agencies, and there is obvious lack of research funding even in affluent countries, especially for stroke research.174 Therefore, there is an obvious question where to get funding for primary prevention research and how it can be best managed? Given the very applied nature of such research, research initiatives could improve the evidence base for primary prevention strategies.
The World Health Federation’s recommendation for cardiovascular disease prevention (including stroke) includes conducting “large population studies in different regions of the world as the types and patterns of diet, activity, alcohol consumption, and tobacco use vary by region; regional studies are essential to document their effect in each region”.86 This recommendation recognizes the importance of region/country specific data on stroke risk and causative factors. The NIH NINDS Stroke Progress Review Group 2012 recommended three top priorities for future stroke epidemiology; (1) improving the understanding of race and ethnicity in stroke disparities; (2) evaluation of the usefulness of health information technology as a tool for epidemiology research; and (3) translating knowledge from epidemiological studies into improved health. Moreover, given race and ethnicity are confounded by socioeconomic and cultural factors, cultural contexts are essential to capture differences in contributing factors to stroke risk, such a language barriers, and access to healthcare. A study comparing CVD events in low vs high income countries has shown that while the prevalence of risk factors may be low in low-income countries, better control and more frequent use of proven therapies has mitigated the burden of stroke in high income countries, implying that contributing influences other than risk factors, such as access to and affordability of services, medications and treatments, as well as educational levels also affect outcomes.175
Given limited resources for carrying out national and international epidemiological studies (especially in developing countries), exploring new strategies for conducting such studies using mobile technologies seems appropriate. Recent advances in smartphone technologies including high processing power, storage, constant internet connection, personalised notification methods, growing worldwide uptake, and proximity to the users, offer unique opportunities to utilise these technologies for improving health and enhancing research capabilities.24 There are numerous stroke and other NCDs smartphone applications (apps) currently available in the Apple Store and Google Play. There are also several smartphone-based technologies and apps that were, or are, being used in clinical trials for managing particular medical conditions and risk factors (e.g., smoking cessation, depression, weight and asthma management),127–131 with promising results. In recognition of the importance of digital technologies for health, the UN Economic and Social Council, the International Telecommunication Union (ITU) and the WHO have recently (2013) launched a new mHealth initiative for improving NCD prevention, treatment and policy enforcement.176 A good example of such affordable, ongoing large-scale smartphone-based worldwide cross-section and prospective cohort study on stroke risk factors is a study called RIBURST (Reducing the International Burden of Stroke Using Mobile Technology Study).24
New discoveries in stroke risk factors can be expected when genetic, environmental, behavioural and medical/physiological determinants are considered in combination and their interplay, with the involvement of a multidisciplinary team of experts (including clinicians, epidemiologists, geneticists, biostatisticians, mathematicians, computer scientists). The 2nd of the top three priorities in The Stroke Progress Review Group 2012 report for future directions of stroke research includes “Focus future biomarker development on markers of small vessel disease severity and location, integrating these into multimodal studies of existing imaging markers, biochemical and genetic risks, and epidemiologic factors (including variations related to racial/ethnic/demographic group), aimed ultimately at unraveling the precise connections between vascular pathology, neurodegenerative pathology, and neurological impairment”.177
Further research is also required in advancing precision medicine approach for stroke prevention recently introduced in the US178 (including personalized prediction models of stroke), designing and clinical testing of pragmatic, potentially widely applicable (affordable) primary stroke prevention interventions in people of different sex, age and race/ethnicity, including proper assessment of promising indigenous medicines (including supplements) claiming CVD preventative properties. There is also a need for qualitative studies to explore how we can effectively design primary stroke prevention strategies to address potential barriers to implementation of medication and lifestyle changes known to reduce risk of primary stroke for those at risk.
CONCLUSIONS
As stroke is being identified as one of the prioritized NCD in the WHO and UN actions on NCDs and new recent developments in stroke prevention, primary stroke prevention has entered a new era in which UN, WHO, government bodies, medical systems and non-government organizations must work together. Our hope is that the promotion and endorsement of new, proven effective approaches for the prevention of stroke and CVD, in combination with previously endorsed, high-risk and population-wide prevention strategies, will change current practice worldwide and, as a consequence, save millions of lives. When cost-effective health-care individual interventions are complemented with population-wide prevention strategies focusing on population-wide cardiovascular health rather than disease risk, a significant impact can be made on the global NCD epidemic.150,178
KEY MESSAGES.
Although stroke incidence and mortality rates in the world declined from 1990 to 2013, in terms of absolute number of people affected by stroke, remained disabled or die from stroke the burden of stroke continues increasing at past pace throughout the world
Ever-increasing burden of stroke across the globe suggests that currently used stroke and cardiovascular disease primary prevention strategies are not sufficiently effective
For primary stroke prevention strategies to be more effective they need to be based on both population-wide and high risk prevention strategies, with the emphasis shifted from high stroke/CVD absolute risk approach to a more comprehensive approach that includes primary stroke prevention strategies in people with all levels of risk of CVD events
To be cost-effective, primary stroke prevention strategies must be integrated with primary prevention strategies of other major non-communicable diseases that share common risk factors with stroke, such as CVD, vascular dementia, and diabetes mellitus
Given the already immense and fast increasing burden of stroke and other major NCDs across all countries in the world that threatens sustainability of the whole society, not acting now on primary prevention of these diseases is not acceptable
Acknowledgements
V.L.F. was partly funded by the Health Research Council of New Zealand, Brain Research New Zealand Centre of Research Excellence and “Ageing Well” Programme of the National Science Challenge, Ministry of Business, Innovation and Employment of New Zealand. GAR has grant funding from the US National Institute on Ageing and Medtronic Philanthropy. We would like to thank Barbara Bowman, Centers for Disease Control and Prevention, for her valuable comments on early version of the manuscript.
Footnotes
Conflict of interest
Valery L. Feigin declares that Stroke Riskometer™ app is copyrighted by the Auckland University of Technology and funds resulting from the sale of the professional version of the Stroke Riskometer App will be used for further research and education for stroke prevention. All other authors declare that they have no conflict of interest.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; Centers for Disease Control and Prevention, or the U.S. Department of Health and Human Services.
Citations
- 1.Feigin VL et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383, 245–254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA et al. Demographic and epidemiologic drivers of global cardiovascular mortality. New Engl. J. Med 372, 1333–1341, doi: 10.1056/NEJMoa1406656 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology 45, 161–176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJL & Lopez AD Measuring the global burden of disease. New Engl. J. Med 369, 448–457, doi: 10.1056/NEJMra1201534 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Mensah GA, Norrving B & Feigin VL The Global Burden of Stroke. Neuroepidemiology 45, 143–145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth GA et al. Methods for Estimating the Global Burden of Cerebrovascular Diseases. Neuroepidemiology 45, 146–151 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Truelsen T et al. Causes of Death Data in the Global Burden of Disease Estimates for Ischemic and Hemorrhagic Stroke. Neuroepidemiology 45, 152–160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnamurthi RV et al. Stroke Prevalence, Mortality and Disability-Adjusted Life Years in Children and Youth Aged 0–19 Years: Data from the Global and Regional Burden of Stroke 2013. Neuroepidemiology 45, 177–189 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthi RV et al. Stroke Prevalence, Mortality and Disability-Adjusted Life Years in Adults Aged 20–64 Years in 1990–2013: Data from the Global Burden of Disease 2013 Study. Neuroepidemiology 45, 190–202 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Barker-Collo S et al. Sex Differences in Stroke Incidence, Prevalence, Mortality and Disability-Adjusted Life Years: Results from the Global Burden of Disease Study 2013. Neuroepidemiology 45, 203–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigin VL, Mensah GA, Norrving B, Murray CJL & Roth GA Atlas of the Global Burden of Stroke (1990–2013): The GBD 2013 Study. Neuroepidemiology 45, 230–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norrving B et al. Stroke Prevention Worldwide - What Could Make It Work. Neuroepidemiology 45, 215–220 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth GA et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 132, 1667–1678, doi: 10.1161/CIRCULATIONAHA.114.008720 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Maher D, Waswa L, Baisley K, Karabarinde A & Unwin N Epidemiology of hypertension in low-income countries: A cross-sectional population-based survey in rural Uganda. Journal of Hypertension 29, 1061–1068, doi: 10.1097/HJH.0b013e3283466e90 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim MM & Damasceno A Hypertension in developing countries. The Lancet 380, 611–619, doi: 10.1016/S0140-6736(12)60861-7 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Kumara WAN, Perera T, Dissanayake M, Ranasinghe P & Constantine GR Prevalence and risk factors for resistant hypertension among hypertensive patients from a developing country. BMC Research Notes 6, doi: 10.1186/1756-0500-6-373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehndiratta MM Stroke in Asia: geographical variations and temporal trends. Journal of neurology, neurosurgery, and psychiatry 85, 1308–1312, doi: 10.1136/jnnp-2013-306992 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL & Parag V Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. The Lancet Neurology 8, 355–369 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Bernick C et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology 57, 1222–1229 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Shafe ACE & Cowie MR UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 1, doi: 10.1136/bmjopen-2011-000269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soljak M et al. Does higher quality primary health care reduce stroke admissions? a national cross-sectional study. British Journal of General Practice 61, e801–e807, doi: 10.3399/bjgp11X613142 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissela BM et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 79, 1781–1787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George MG, Tong X, Kuklina EV & Labarthe DR Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Annals of Neurology 70, 713–721, doi: 10.1002/ana.22539 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Feigin VL et al. New Strategy to Reduce the Global Burden of Stroke. Stroke 46, 1740–1747, doi: 10.1161/STROKEAHA.115.008222 (2015). [DOI] [PubMed] [Google Scholar]
- 25.United Nations General Assembly. Resolution adopted by the General Assembly: 66/2: Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases. Adopted September 19, 2011. Published January 24, 2012 edn, (2012).
- 26.Boden-Albala B & Quarles LW Education strategies for stroke prevention. Stroke 44, S48–S51, doi: 10.1161/STROKEAHA.111.000396 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Lyle CA, Bernard TJ & Goldenberg NA Childhood arterial ischemic stroke: A review of etiologies, antithrombotic treatments, prognostic factors, and priorities for future research. Seminars in Thrombosis and Hemostasis 37, 786–793, doi: 10.1055/s-0031-1297169 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray CJL et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. The Lancet 386, 2145–2191, doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell MJ et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. The Lancet 376, 112–123 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Go AS et al. Heart Disease and Stroke Statistics - 2014 Update: A report from the American Heart Association. Circulation 129, e28–e292, doi: 10.1161/01.cir.0000441139.02102.80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim SS et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380, 2224–2260, doi: 10.1016/S0140-6736(12)61766-8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spring B et al. Better population health through behavior change in adults: A call to action. Circulation 128, 2169–2176, doi: 10.1161/01.cir.0000435173.25936.e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose G Strategy of prevention: lessons from cardiovascular disease. British Medical Journal Clinical Research Ed 282, 1847–1851 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein LB et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association.[Erratum appears in Stroke. 2011 Feb;42(2):e26]. Stroke 42, 517–584 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Tikk K et al. Primary preventive potential for stroke by avoidance of major lifestyle risk factors: The European prospective investigation into cancer and nutrition-heidelberg cohort. Stroke 45, 2041–2046, doi: 10.1161/STROKEAHA.114.005025 (2014). [DOI] [PubMed] [Google Scholar]
- 36.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New Engl. J. Med 373, 2103–2116, doi:doi: 10.1056/NEJMoa1511939 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ettehad D et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. The Lancet, doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 38.Ruff CT et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. The Lancet 383, 955–962, doi: 10.1016/S0140-6736(13)62343-0 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Martinez C et al. Increasing incidence of non-valvular atrial fibrillation in the UK from 2001 to 2013. Heart 101, 1748–1754, doi: 10.1136/heartjnl-2015-307808 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Miyasaka Y et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114, 119–125, doi: 10.1161/CIRCULATIONAHA.105.595140 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Stefansdottir H, Aspelund T, Gudnason V & Arnar DO Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace 13, 1110–1117, doi: 10.1093/europace/eur132 (2011). [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Prevention of Cardiovascular Disease: Guidelines for assessment and management of cardiovascular risk. (WHO, 2007). [Google Scholar]
- 43.Preventing chronic diseases: a vital investment. World Health Organization, http://www.who.int/chp/chronic_disease_report/en/ (2005). [Google Scholar]
- 44.Rose G Sick individuals and sick populations. International Journal of Epidemiology 14, 32–38 (1985). [DOI] [PubMed] [Google Scholar]
- 45.Pearson TA et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. [Review] [37 refs]. Circulation 106, 388–391 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Puska P Successful prevention of non-communicable diseases: 25 Year experiences with North Karelia project in Finland. Public Health Medicine 4, 5–7 (2002). [Google Scholar]
- 47.Miura K Epidemiology and prevention of hypertension in Japanese: How could Japan get longevity? EPMA Journal 2, 59–64, doi: 10.1007/s13167-011-0069-y (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Record NB et al. Community-wide cardiovascular disease prevention programs and health outcomes in a rural county, 1970–2010. JAMA - Journal of the American Medical Association 313, 147–155, doi: 10.1001/jama.2014.16969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emberson J, Whincup P, Morris R, Walker M & Ebrahim S Evaluating the impact of population and high-risk strategies for the primary prevention of cardiovascular disease. European Heart Journal 25, 484–491, doi: 10.1016/j.ehj.2003.11.012 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Mehta S et al. Under-utilisation of preventive medication in patients with cardiovascular disease is greatest in younger age groups (PREDICT-CVD 15). J Prim Health Care 3, 93–101 (2011). [PubMed] [Google Scholar]
- 51.Goldstein LB et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group.[Erratum appears in Circulation. 2006 Nov 28;114(22):e617]. Circulation 113, e873–923 (2006). [DOI] [PubMed] [Google Scholar]
- 52.New Zealand Guidelines G Life after stroke. New Zealand guideline for management of stroke. (Stroke Foundation New Zealand Inc., 2003). [Google Scholar]
- 53.Goldstein LB et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Circulation 113, e873–923 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Cox JL, Carr B, Vallis TM, Szpilfogel C & O’Neill BJ A Novel Approach to Cardiovascular Health by Optimizing Risk Management (ANCHOR): A Primary Prevention Initiative Examining the Impact of Health Risk Factor Assessment and Management on Cardiac Wellness. Canadian Journal of Cardiology 27, 809–817 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Zannad F et al. Prevention of cardiovascular disease guided by total risk estimations - Challenges and opportunities for practical implementation: Highlights of a CardioVascular Clinical Trialists (CVCT) Workshop of the ESC Working Group on CardioVascular Pharmacology and Drug Therapy. European Journal of Preventive Cardiology 19, 1454–1464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebrahim S et al. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database of Systematic Reviews, CD001561 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Mc Namara KP et al. Engaging community pharmacists in the primary prevention of cardiovascular disease: Protocol for the pharmacist assessment of adherence, risk and treatment in cardiovascular disease (PAART CVD) pilot study. BMC Health Services Research 10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selak V et al. IMProving Adherence using Combination Therapy (IMPACT): design and protocol of a randomised controlled trial in primary care. Contemporary Clinical Trials 32, 909–915 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Gorelick PB Primary prevention of stroke: impact of healthy lifestyle. Circulation 118, 904–906 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Jackson R et al. Alcohol and ischaemic heart disease: probably no free lunch. Lancet 366, 1911–1912 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Powers BJ et al. The effectiveness of personalized coronary heart disease and stroke risk communication. American Heart Journal 161, 673–680 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Sheridan SL et al. The effect of giving global coronary risk information to adults: a systematic review. Archives of Internal Medicine 170, 230–239 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Guarneri M, Mercado N & Suhar C Integrative approaches for cardiovascular disease. Nutrition in Clinical Practice 24, 701–708 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Alliance NVDP Guidelines for the management of absolute cardiovascular disease risk. (National Health and Medical Research Council of Australia, Canberra, 2012). [Google Scholar]
- 65.Heuschmann PUMDMPH, Grieve APPD, Toschke AMMDMPHM, Rudd AGF & Wolfe CDAMDF Ethnic Group Disparities in 10-Year Trends in Stroke Incidence and Vascular Risk Factors: The South London Stroke Register (SLSR). Stroke 39, 2204–2210 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Goff DC Jr et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Journal of the American College of Cardiology 63, 2935–2959, doi: 10.1016/j.jacc.2013.11.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brindle P et al. Predictive accuracy of the Framingham coronary risk score in British men: Prospective cohort study. British Medical Journal 327, 1267–1270 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dalton ARH, Soljak M, Samarasundera E, Millett C & Majeed A Prevalence of cardiovascular disease risk amongst the population eligible for the NHS Health Check Programme. European Journal of Preventive Cardiology 20, 142–150, doi: 10.1177/1741826711428797 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Jansen J et al. General practitioners’ use of absolute risk versus individual risk factors in cardiovascular disease prevention: An experimental study. BMJ Open 4, doi: 10.1136/bmjopen-2014-004812 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health O World Health Organization Fact Sheet. (World Health Organization, 2003). [Google Scholar]
- 71.Zullig LL et al. Patient-reported medication adherence barriers among patients with cardiovascular risk factors. Journal of Managed Care Pharmacy 21, 479–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKenzie SJ, McLaughlin D, Clark J & Doi SAR The Burden of Non-Adherence to Cardiovascular Medications Among the Aging Population in Australia: A Meta-Analysis. Drugs and Aging 32, 217–225, doi: 10.1007/s40266-015-0245-1 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Wong MCS et al. The association between multimorbidity and poor adherence with cardiovascular medications. International Journal of Cardiology 177, 477–482, doi: 10.1016/j.ijcard.2014.09.103 (2014). [DOI] [PubMed] [Google Scholar]
- 74.McLaren L, McIntyre L & Kirkpatrick S Rose’s population strategy of prevention need not increase social inequalities in health. International Journal of Epidemiology 39, 372–377, doi: 10.1093/ije/dyp315 (2010). [DOI] [PubMed] [Google Scholar]
- 75.The 2015/16 health targets. (New Zealand Ministry of Health; http://www.health.govt.nz/new-zealand-health-system/health-targets, 2015). [Google Scholar]
- 76.Krogsbøll LT, Jørgensen KJ, Grønhøj Larsen C & Gøtzsche PC General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. BMJ (Online) 345, doi: 10.1136/bmj.e7191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jørgensen T et al. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ (Online) 348, doi: 10.1136/bmj.g3617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sacco RL, Frieden TR, Blakeman DE, Jauch EC & Mohl S What the million hearts initiative means for stroke: A presidential advisory from the American heart association/American stroke association. Stroke 43, 924–928, doi: 10.1161/STR.0b013e318248f00e (2012). [DOI] [PubMed] [Google Scholar]
- 79.Frieden TR & Berwick DM The “million hearts” initiative - Preventing heart attacks and strokes. New Engl. J. Med 365, e27.21–e27.24, doi: 10.1056/NEJMp1110421 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Sanghavi DM & Conway PH Paying for prevention: A novel test of medicare value-based payment for cardiovascular risk reduction. JAMA - Journal of the American Medical Association 314, 123–124, doi: 10.1001/jama.2015.6681 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Asch DA et al. Effect of financial incentives to physicians, patients, or both on lipid levels: A randomized clinical trial. JAMA - Journal of the American Medical Association 314, 1926–1935, doi: 10.1001/jama.2015.14850 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Probst-Hensch N, Tanner M, Kessler C, Burri C & Kunzli N Prevention--a cost-effective way to fight the non-communicable disease epidemic: an academic perspective of the United Nations High-level NCD Meeting. Swiss Medical Weekly 141, w13266 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Asaria P, Chisholm D, Mathers C, Ezzati M & Beaglehole R Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet 370, 2044–2053 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Khatib R et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. The Lancet 10.1016/S0140-6736(15)00469-9, doi: 10.1016/S0140-6736(15)00469-9 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Notara V, Panagiotakos DB & Pitsavos CE Secondary prevention of acute coronary syndrome. Socio-economic and lifestyle determinants: a literature review. Central European journal of public health 22, 175–182 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Yusuf S, Wood D, Ralston J & Reddy KS The World Heart Federation’s vision for worldwide cardiovascular disease prevention. The Lancet 386, 399–402, doi: 10.1016/S0140-6736(15)60265-3 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Global action plan for the prevention and control of noncommunicable diseases 2013–2020.http://www.who.int/nmh/events/ncd_action_plan/en/, 2015).
- 88.Cooney MT, Dudina A, D’Agostino R & Graham IM Cardiovascular risk-estimation systems in primary prevention: Do they differ? do they make a difference? can we see the future? Circulation 122, 300–310, doi: 10.1161/CIRCULATIONAHA.109.852756 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Mancia G et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Journal of hypertension 31, 1281–1357, doi: 10.1097/01.hjh.0000431740.32696.cc (2013). [DOI] [PubMed] [Google Scholar]
- 90.Hill S et al. Absolute risk representation in cardiovascular disease prevention: Comprehension and preferences of health care consumers and general practitioners involved in a focus group study. BMC Public Health 10, doi: 10.1186/1471-2458-10-108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fagerlin A, Zikmund-Fisher BJ & Ubel PA Helping patients decide: Ten steps to better risk communication. Journal of the National Cancer Institute 103, 1436–1443, doi: 10.1093/jnci/djr318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maniar S in PanVascular Medicine, Second Edition 1695–1718 (2015). [Google Scholar]
- 93.Wald DS & Raiman L Medication adherence in cardiovascular disease: How to address one of the challenges of preventive medicine. Primary Care Cardiovascular Journal 6, 60–62, doi: 10.3132/pccj.2013.012 (2013). [DOI] [Google Scholar]
- 94.Bowry ADK, Shrank WH, Lee JL, Stedman M & Choudhry NK A systematic review of adherence to cardiovascular medications in resource-limited settings. Journal of General Internal Medicine 26, 1479–1491, doi: 10.1007/s11606-011-1825-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webster R & Rodgers A Polypill: Progress and Challenges to Global Use—Update on the Trials and Policy Implementation. Current Cardiology Reports 17, doi: 10.1007/s11886-015-0673-x (2015). [DOI] [PubMed] [Google Scholar]
- 96.Gaziano TA, Opie LH & Weinstein MC Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet 368, 679–686, doi: 10.1016/S0140-6736(06)69252-0 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Community‐Clinical Linkages to Improve Hypertension Identification, Management, and Control. (Association of State and Territorial Health Officials 2015, 2015). [Google Scholar]
- 98.Foltz JL, Belay B & Blackburn GL Improving the weight of the nation by engaging the medical setting in obesity prevention and control. Journal of Law, Medicine and Ethics 41, 19–26, doi: 10.1111/jlme.12105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chronic Disease Prevention and Health Promotion Domains. http://www.cdc.gov/chronicdisease/about/foa/docs/four-domains-nov2012.pdf (2012).
- 100.Guide to Community Preventive Services. Cardiovascular disease prevention and control: interventions engaging community health workers. http://www.thecommunityguide.org/cvd/CHW.html (2015).
- 101.Feigin VL Stroke in developing countries: can the epidemic be stopped and outcomes improved? The Lancet Neurology 6, 94–97 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Chandra V et al. in Neurological Disorders (eds Jamison DT et al.) Ch. Disease Control Priorities in Developing Countries, 627–643 (Oxford University Press and World bank, 2006). [Google Scholar]
- 103.Zhao X et al. Using a low-sodium, high-potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: A patient-blinded randomized controlled trial. PLoS ONE 9, doi: 10.1371/journal.pone.0110131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo B, Wan L, Liang L & Li T The effects of educational campaigns and smoking bans in public places on smokers’ intention to quit smoking: Findings from 17 cities in China. BioMed Research International 2015, doi: 10.1155/2015/853418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Selmer RM et al. Cost and health consequences of reducing the population intake of salt. Journal of Epidemiology and Community Health 54, 697–702, doi: 10.1136/jech.54.9.697 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson RK et al. Dietary sugars intake and cardiovascular health a scientific statement from the american heart association. Circulation 120, 1011–1020, doi: 10.1161/CIRCULATIONAHA.109.192627 (2009). [DOI] [PubMed] [Google Scholar]
- 107.Martineau F, Tyner E, Lorenc T, Petticrew M & Lock K Population-level interventions to reduce alcohol-related harm: An overview of systematic reviews. Preventive Medicine 57, 278–296, doi: 10.1016/j.ypmed.2013.06.019 (2013). [DOI] [PubMed] [Google Scholar]
- 108.Wilson N Salt tax could reduce population’s salt intake [6]. British Medical Journal 329, 918 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hopkins DP et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. American Journal of Preventive Medicine 20, 16–66 (2001). [DOI] [PubMed] [Google Scholar]
- 110.Goodchild M, Perucic AM & Nargis N Modelling the impact of raising tobacco taxes on public health and finance. Bulletin of the World Health Organization 94, 250–257, doi: 10.2471/BLT.15.164707 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.WHO report on the global tobacco epidemic, 2015: raising taxes on tobacco. (World Health Organization, Geneva, 2015). [Google Scholar]
- 112.Ruff RR & Zhen C Estimating the effects of a calorie-based sugar-sweetened beverage tax on weight and obesity in New York City adults using dynamic loss models. Annals of Epidemiology 25, 350–357, doi: 10.1016/j.annepidem.2014.12.008 (2015). [DOI] [PubMed] [Google Scholar]
- 113.Briggs ADM et al. Overall and income specific effect on prevalence of overweight and obesity of 20% sugar sweetened drink tax in UK: Econometric and comparative risk assessment modelling study. BMJ (Online) 347, doi: 10.1136/bmj.f6189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reducing consumption of sugar-sweetened beverages to reduce the risk of unhealthy weight gain in adults http://www.who.int/elena/bbc/ssbs_adult_weight/en/ (WHO, 2014). [Google Scholar]
- 115.Meier PS et al. Estimated Effects of Different Alcohol Taxation and Price Policies on Health Inequalities: A Mathematical Modelling Study. PLoS Medicine 13, doi: 10.1371/journal.pmed.1001963 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delcher C, Maldonado-Molina MM & Wagenaar AC Effects of alcohol taxes on alcohol-related disease mortality in New York State from 1969 to 2006. Addictive Behaviors 37, 783–789, doi: 10.1016/j.addbeh.2012.02.019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wagenaar AC, Tobler AL & Komro KA Effects of alcohol tax and price policies on morbidity and mortality: A systematic review. American Journal of Public Health 100, 2270–2278, doi: 10.2105/AJPH.2009.186007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaloupka FJ, Grossman M & Saffer H The effects of price on alcohol consumption and alcohol-related problems. Alcohol Research and Health 26, 22–34 (2002). [PMC free article] [PubMed] [Google Scholar]
- 119.Elder RW et al. The Effectiveness of Tax Policy Interventions for Reducing Excessive Alcohol Consumption and Related Harms. American Journal of Preventive Medicine 38, 217–229, doi: 10.1016/j.amepre.2009.11.005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doran CM, Byrnes JM, Higashi H & Truong K Revenue implications to the Vietnamese government of using taxes to curb cigarette smoking. Addictive Behaviors 35, 1089–1093, doi: 10.1016/j.addbeh.2010.07.010 (2010). [DOI] [PubMed] [Google Scholar]
- 121.Andreyeva T, Chaloupka FJ & Brownell KD Estimating the potential of taxes on sugar-sweetened beverages to reduce consumption and generate revenue. Preventive Medicine 52, 413–416, doi: 10.1016/j.ypmed.2011.03.013 (2011). [DOI] [PubMed] [Google Scholar]
- 122.Doran CM, Byrnes JM, Cobiac LJ, Vandenberg B & Vos T Estimated impacts of alternative Australian alcohol taxation structures on consumption, public health and government revenues. Medical Journal of Australia 199, 619–622, doi: 10.5694/mja13.10605 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Feigin V, A. B, Arroll B & R. K Stroke prevention in New Zealand: can we do better? International Journal of Stroke accepted for publication (2013). [DOI] [PubMed] [Google Scholar]
- 124.Murray CJL et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380, 2197–2223, doi: 10.1016/S0140-6736(12)61689-4 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Lozano R et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 380, 2095–2128, doi: 10.1016/S0140-6736(12)61728-0 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cappuccio F Epidemiologic transition, migration and cardiovascular disease. International Journal of Epidemiology 33, 387–388 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Licskai C, Sands TW & Ferrone M Development and pilot testing of a mobile health solution for asthma self-management: asthma action plan smartphone application pilot study. Canadian Respiratory Journal 20, 301–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brouillette RM et al. Feasibility, reliability, and validity of a smartphone based application for the assessment of cognitive function in the elderly. PLoS ONE [Electronic Resource] 8, e65925 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buller DB, Borland R, Bettinghaus EP, Shane JH & Zimmerman DE Randomized Trial of a Smartphone Mobile Application Compared to Text Messaging to Support Smoking Cessation. Telemedicine Journal and e-Health December 18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ly KH, Carlbring P & Andersson G Behavioral activation-based guided self-help treatment administered through a smartphone application: study protocol for a randomized controlled trial. Trials [Electronic Resource] 13, 62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carter MC, Burley VJ, Nykjaer C & Cade JE Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. Journal of Medical Internet Research 15, e32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.CDC Office of Public Health Scientific Services. Surveillance Strategy: A strategy for improving the Centers for Disease Control and Prevention’s activities in public health surveillance. http://www.cdc.gov/ophss/docs/cdc-surveillance-strategy-final.pdf (2014).
- 133.Spring B et al. Multiple behavior changes in diet and activity: A randomized controlled trial using mobile technology. Arch. Intern. Med 172, 789–796, doi: 10.1001/archinternmed.2012.1044 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Recio-Rodríguez JI et al. Effectiveness of a smartphone application for improving healthy lifestyles, a randomized clinical trial (EVIDENT II): Study protocol. BMC Public Health 14, doi: 10.1186/1471-2458-14-254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bond DS et al. B-MOBILE - A smartphone-based intervention to reduce sedentary time in overweight/obese individuals: A within-subjects experimental trial. PLoS ONE 9, doi: 10.1371/journal.pone.0100821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Glynn LG et al. Effectiveness of a smartphone application to promote physical activity in primary care: The SMART MOVE randomised controlled trial. British Journal of General Practice 64, e384–e391, doi: 10.3399/bjgp14X680461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin CK et al. Efficacy of SmartLossSM, a smartphone-based weight loss intervention: Results from a randomized controlled trial. Obesity 23, 935–942, doi: 10.1002/oby.21063 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stephens JD, Yager AM & Allen J Smartphone Technology and Text Messaging for Weight Loss in Young Adults: A Randomized Controlled Trial. Journal of Cardiovascular Nursing, doi: 10.1097/JCN.0000000000000307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eyles H et al. Using mobile technology to support lower-salt food choices for people with cardiovascular disease: Protocol for the SaltSwitch randomized controlled trial. BMC Public Health 14, doi: 10.1186/1471-2458-14-950 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piette JD et al. Hypertension management using mobile technology and home blood pressure monitoring: Results of a randomized trial in two low/middle-income countries. Telemedicine. e-Health 18, 613–620, doi: 10.1089/tmj.2011.0271 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Block G et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: A randomized controlled trial among persons with prediabetes. Journal of Medical Internet Research 17, doi: 10.2196/jmir.4897 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodgers A et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tobacco Control 14, 255–261, doi: 10.1136/tc.2005.011577 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Free C et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): A single-blind, randomised trial. The Lancet 378, 49–55, doi: 10.1016/S0140-6736(11)60701-0 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chow CK et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: A randomized clinical trial. JAMA - Journal of the American Medical Association 314, 1255–1263, doi: 10.1001/jama.2015.10945 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Proudfoot J et al. Impact of a mobile phone and web program on symptom and functional outcomes for people with mild-to-moderate depression, anxiety and stress: A randomised controlled trial. BMC Psychiatry 13, doi: 10.1186/1471-244X-13-312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.World Health Organization. in Global Observatory for eHealth series - Volume 3 1–99 (WHO, Geneva, 2011). [Google Scholar]
- 147.Burke LE et al. Current Science on Consumer Use of Mobile Health for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation 132, 1157–1213, doi: 10.1161/CIR.0000000000000232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nasser FB & Trevena L There’s an App for That: A Guide for Healthcare Practitioners and Researchers on Smartphone Technology. Online Journal of Public Health Informatics 7, e218, doi: 10.5210/ojphi.v7i2.5522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morgan J The power of the App: can mobile-technology save lives? The Lancet Neurology DOI 10.1016/S1474-4422(15)00297-5, doi:10.1016/S1474–4422(15)00297–5 (2015). [DOI] [PubMed] [Google Scholar]
- 150.WHO. Global status report on noncommunicable diseases 2010. Description of the global burden of NCDs, their risk factors and determinants. (World Health Organization, 2011). [Google Scholar]
- 151.Fustinoni O & Biller J Ethnicity and stroke : beware of the fallacies.[see comment]. Stroke. 31, 1013–1015 (2000). [DOI] [PubMed] [Google Scholar]
- 152.Sacco RL et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 147, 259–268 (1998). [DOI] [PubMed] [Google Scholar]
- 153.Stewart JA, Dundas R, Howard RS, Rudd AG & Wolfe CD Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ 318, 967–971 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Rossum CT, van de MH, Breteler MM, Grobbee DE & Mackenbach JP Socioeconomic differences in stroke among Dutch elderly women: the Rotterdam Study. Stroke 30, 357–362 (1999). [DOI] [PubMed] [Google Scholar]
- 155.Feigin VL et al. 30-Year trends in stroke rates and outcome in Auckland, New Zealand (1981–2012): A multi-ethnic population-based series of studies. PLoS ONE 10, doi: 10.1371/journal.pone.0134609 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mullen Conley K et al. Kids Identifying and Defeating Stroke (KIDS): development and implementation of a multiethnic health education intervention to increase stroke awareness among middle school students and their parents. Health promotion practice 11, 95–103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Adherence to long-term therapies: evidence for action. (World Health Organization, 2003). [Google Scholar]
- 158.Davis PC Jr & Rankin LL Guidelines for making existing health education programs more culturally appropriate. American Journal of Health Education 37, 250–252 (2006). [Google Scholar]
- 159.Peñalvo JL et al. The Program SI! intervention for enhancing a healthy lifestyle in preschoolers: First results from a cluster randomized trial. BMC Public Health 13, doi: 10.1186/1471-2458-13-1208 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wen X, Kong KL, Eiden RD, Sharma NN & Xie C Sociodemographic differences and infant dietary patterns. Pediatrics 134, e1387–e1398, doi: 10.1542/peds.2014-1045 (2014). [DOI] [PubMed] [Google Scholar]
- 161.Foltz JL et al. Population-level intervention strategies and examples for obesity prevention in children. Annual Review of Nutrition 32, 391–415, doi: 10.1146/annurev-nutr-071811-150646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hickey A et al. Stroke awareness in the general population: knowledge of stroke risk factors and warning signs in older adults. BMC Geriatrics 9, 35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jones SP, Jenkinson AJ, Leathley MJ & Watkins CL Stroke knowledge and awareness: an integrative review of the evidence. Age & Ageing 39, 11–22 (2010). [DOI] [PubMed] [Google Scholar]
- 164.Ellis C, Wolff J & Wyse A Stroke awareness among low literacy Latinos living in the South Carolina low country. Journal of Immigrant & Minority Health 11, 499–504 (2009). [DOI] [PubMed] [Google Scholar]
- 165.Ryan T Stroke awareness among British ethnic minorities. British Journal of Community Nursing 15, 381–384 (2010). [DOI] [PubMed] [Google Scholar]
- 166.Ellis C & Egede LE Racial/ethnic differences in stroke awareness among veterans.[Summary for patients in Ethn Dis. 2008 Spring;18(2):237; PMID: 18507282]. Ethnicity & Disease 18, 198–203 (2008). [PubMed] [Google Scholar]
- 167.Alkadry MG & Tower LE The effect of rurality and gender on stroke awareness of adults in West Virginia. Journal of Health & Human Services Administration 33, 63–93 (2010). [PubMed] [Google Scholar]
- 168.Hodgson C Community outreach for stroke education. Stroke 39, 2189–2190 (2008). [DOI] [PubMed] [Google Scholar]
- 169.Sullivan KA & Katajamaki A Stroke education: promising effects on the health beliefs of those at risk. Topics in Stroke Rehabilitation 16, 377–387 (2009). [DOI] [PubMed] [Google Scholar]
- 170.Tadros A et al. Emergency medical services-based community stroke education: pilot results from a novel approach. Stroke 40, 2134–2142 (2009). [DOI] [PubMed] [Google Scholar]
- 171.Kim YS et al. Stroke awareness decreases prehospital delay after acute ischemic stroke in Korea. BMC Neurology 11, 2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bray JE, Mosley I, Bailey M, Barger B & Bladin C Stroke public awareness campaigns have increased ambulance dispatches for stroke in Melbourne, Australia. Stroke 42, 2154–2157 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Boden-Albala BMPHD & Quarles LWMPH Education Strategies for Stroke Prevention. Stroke 44(6_suppl_1) Supplement, S48–S51 (2013). [DOI] [PubMed] [Google Scholar]
- 174.Rothwell PM Lack of research funding for stroke. International Journal of Stroke 2, 73, doi: 10.1111/j.1747-4949.2007.00125.x (2007). [DOI] [PubMed] [Google Scholar]
- 175.Yusuf S et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. New Engl. J. Med 371, 818–827, doi: 10.1056/NEJMoa1311890 (2014). [DOI] [PubMed] [Google Scholar]
- 176.BE HE@LTHY, BE MOBILE, <http://www.itu.int/en/ITU-D/ICT-Applications/eHEALTH/Pages/Be_Healthy.aspx> (2014).
- 177.Eyres S, Carey A, Gilworth G, Neumann V & Tennant A Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clinical Rehabilitation 19, 878–887, doi:DOI: 10.1191/0269215505cr905oa (2005). [DOI] [PubMed] [Google Scholar]
- 178.Collins FS & Varmus H A New Initiative on Precision Medicine. New Engl. J. Med 372, 793–795, doi:doi: 10.1056/NEJMp1500523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]