Abstract
The European Commission mandated EFSA to complement its original scientific opinion on soybean MON 87769 × MON 89788 (EFSA‐GMO‐NL‐2010‐85) considering additional information on the human nutritional assessment of refined bleached deodorised oil produced from the two‐event stack soybean (RBD GM‐oil). The assessment was mainly based on a replacement scenario with a list of target foods where RBD GM‐oil is intended to be added. Intake estimations for several fatty acids present in the RBD GM‐oil, in particular γ‐linolenic acid (GLA), stearidonic acid (SDA) and linoleic acid (LA) were based on the consumption of the corresponding foods that are likely to be displaced. The assessment of LA considered the established adequate intake of 4% of total energy intake (E%) and that LA deficiency has not been observed with intakes > 1 E%. The assessment of GLA and SDA was conducted using maximum doses without adverse effects from intervention human studies as reference (4.2 grams/day for SDA and 2.8 grams/day for GLA) since no tolerable upper intake levels are set for these fatty acids. The decrease observed in the levels of LA in RBD GM‐oil as compared to oil from conventional soybean does not represent a nutritional concern as intakes were in all cases above 1 E%. For GLA, all intake estimations were below the reference dose indicating no safety concern. SDA intake estimations do not pose any safety concerns based on the overly conservative nature of the estimates, the absence of toxicological hazards and the rapid metabolism of SDA in humans. The GMO Panel concluded that the consumption of soybean MON 87769 × MON 89788 and their derived products, in particular its RBD oil, does not represent a nutritional concern in humans. A post‐market monitoring plan is recommended to confirm the predicted consumption and the application of conditions of uses considered during the pre‐market risk assessment.
Keywords: GMO, MON 87769 × MON 89788, soybean (Glycine max), altered fatty acid profile, stearidonic acid, γ‐linolenic acid
Summary
On 17 September 2015, the GMO Panel adopted a scientific opinion on application EFSA‐GMO‐NL‐2010‐85 for the placing on the market of soybean MON 87769 × MON 89788 for food and feed uses, import and processing under Regulation (EC) No 1829/2003. The two‐event stack soybean expresses two desaturases (Δ6 and Δ15) that results in an alteration of the fatty acid profile in comparison with its comparator, in particular the appearance of new fatty acids [stearidonic acid (SDA, C18:4 ω‐3) and γ‐linolenic acid (GLA, C18:3 ω‐6)] and a reduction in linoleic acid (LA, C18:2, ω‐6). Back to 2015, the GMO Panel could not complete the assessment on the impact of refined bleached deodorised oil from MON 87769 × MON 89788 soybean (RBD GM‐oil) on human health and nutrition due to the lack of an appropriate nutritional assessment provided by the applicant.
On 16 May 2019, the European Commission mandated EFSA to assess additional information received from the applicant on the human nutritional assessment of RBD oil from genetically modified soybean MON 87769 × MON 89788. Although different replacement intake scenarios were provided to assess the potential nutritional concerns linked to the consumption of the RBD GM‐oil, the assessment was mainly based on a replacement scenario with a list of target foods where the RBD GM‐oil is intended to be added. Intake estimations for several fatty acids present in the RBD GM‐oil, in particular GLA, SDA and LA were based on the consumption of the corresponding foods that are likely to be displaced.
Together with the consumption data from adult (19–64 years old) and toddler (1–4.5 years old) populations from the 2008–2012 UK National and Diet and Nutrition survey initially considered, the applicant was also asked to make use of the summary statistics of the EFSA Comprehensive European Food Consumption Database to estimate the dietary intake of SDA and GLA across European countries. The assessment of LA took into account the established adequate intake of 4% of total energy intake (E%) and that LA deficiency has not been observed with intakes > 1 E%. The assessment of GLA and SDA was conducted using maximum doses without adverse effects from intervention human studies as reference (4.2 grams/day for SDA and 2.8 grams/day for GLA) since no tolerable upper intake levels are set for these fatty acids. Taking into account the rapid conversion of SDA to EPA in humans, the safety dose of 5 g/day for the combined intake of EPA and DHA was also considered.
Although in few dietary surveys SDA intake estimates could be higher than the maximum dose without adverse effects, the overly conservative nature of the estimations together with the absence of toxicological hazards and the rapid metabolism of SDA in humans indicate that these SDA intake estimations do not pose any safety concerns. For GLA, the first intermediate in the metabolism of LA, all intake estimations were below the maximum dose without adverse effects indicating no safety concern. The decrease observed in the levels of LA in RBD GM‐oil as compared to oil from conventional soybean does not represent a nutritional concern as intakes are in all cases above 1 E%. In addition, the decrease of LA is compensated, at least partially, by the presence of GLA in the RBD GM‐oil. Taking into account all this information, the GMO Panel concluded that the consumption of soybean MON 87769 × MON 89788 and their derived products, in particular its RBD oil, does not represent a nutritional concern in humans.
The current nutritional assessment would have to be revisited if RBD GM‐oil were to be extensively used in food products not considered in this assessment, e.g. as dietary supplements or food for infants and young population. A post‐market monitoring plan is recommended to confirm the predicted consumption and the application of conditions of uses considered during the pre‐market risk assessment.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
On 17 September 2015 the GMO Panel adopted a scientific opinion on application EFSA‐GMO‐NL‐2010‐85 for the placing on the market of soybean MON 87769 × MON 89788 for food and feed uses, import and processing under Regulation (EC) No 1829/20031 (EFSA GMO Panel, 2015). In this scientific opinion, the GMO Panel could not complete the assessment on the impact of refined bleached deodorised oil (RBD) from MON 87769 × MON 89788 soybean on human health and nutrition due to the lack of an appropriate nutritional assessment provided by the applicant.
On 16 May 2019, the European Commission (EC) mandated EFSA to assess additional information received from the applicant on the human nutritional assessment of RBD oil from genetically modified soybean MON 87769 × MON 89788. EFSA was asked to complement its original scientific opinion on soybean MON 87769 × MON 89788 taking into consideration this additional information. EFSA acknowledged receipt of the mandate on 22 May 2019. To finalise the assessment, the GMO Panel asked for further information on 30 July 2019 (provided on 27 January 2020), on 31 March 2020 (provided on 30 June 2020), and on 15 July 2020 (provided on 29 January 2021). EFSA requested the EC to extend the deadline for the finalisation of the mandate on 19 February 2021; the EC accepted EFSA's request on 5 March 2021.
According to the mandate received from EC, this statement complements the EFSA scientific opinion on soybean MON 87769 × MON 89788 (EFSA GMO Panel, 2015), which is the report requested under Articles 6(6) and 18(6) of Regulation (EC) No 1829/20031 and is part of the EFSA Overall Opinion in accordance with Articles 6(5) and 18(5) of that Regulation.
2. Data and methodologies
2.1. Data
In the preparation of this statement, the GMO Panel took into account the human nutritional assessment provided by the applicant, the additional information requested during the risk assessment, relevant peer‐reviewed scientific publications and the EFSA scientific opinions on applications EFSA‐GMO‐UK‐2009‐76 (EFSA GMO Panel, 2014) and EFSA‐GMO‐NL‐2010‐85 (EFSA GMO Panel, 2015).
2.2. Methodologies
The GMO Panel carried out a scientific risk assessment of the additional information taking into account the appropriate principles described in its guidelines and EFSA statements for the risk assessment of GM plants and derived food and feed (EFSA GMO Panel, 2011; EFSA, 2019).
3. Assessment
3.1. Introduction
The two‐event stack soybean MON 87769 × MON 89788 is produced by conventional crossing of the soybean lines MON 87769 and MON 89788.
The single event MON 89788 expresses the CP4 protein 5‐enolpyruvylshikimate‐3‐phosphate synthase (EPSPS) that confers glyphosate tolerance to the plant (EFSA GMO Panel, 2008).
The single event MON 87769 expresses the Δ15 desaturase protein from Neurospora crassa (NcΔ15D) and the Δ6 desaturase protein from Primula juliae (PjΔ6D) (EFSA GMO Panel, 2014). The Δ6 desaturase promotes the conversion of α‐linolenic acid [C18:3, ω‐3] (ALA) to octadecatetraenoic acid [C18:4 (ω‐3)], also known as stearidonic acid (SDA); at the same time, the Δ6 desaturase also catalyses the conversion of linoleic acid [C18:2, ω‐6] (LA) to γ‐linolenic acid [C18:3 (ω‐6)] (GLA). The Δ15 desaturase participates in the conversion of LA to ALA and allows a higher accumulation of SDA in the seeds; hence also lowers the substrate pool for GLA production by lowering LA levels (Figure 1).
Figure 1.
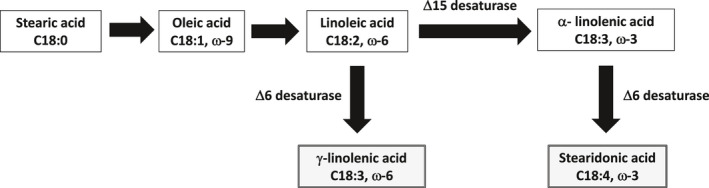
Fatty acid biosynthesis in plants with the two desaturases (Δ6 and Δ15) introduced in soybean MON 87769 × MON 89788
GLA is produced in humans as the first intermediate in the metabolism of LA to arachidonic acid (C20:4, ω‐6); this first step, catalysed by Δ6 desaturase, is believed to be the rate‐limiting step in the metabolic pathway. Once GLA is formed or ingested, it is rapidly metabolised to dihomo‐γ‐linolenic acid which undergoes oxidative metabolism by cyclooxygenases and lipoxygenases to produce eicosanoids (Kapoor and Huang, 2006).
SDA is an intermediate in the formation of the long chain omega‐3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid [C20:5, ω‐3] (EPA) and docosahexaenoic acid [C22:6, ω‐3] (DHA). However, in humans, the bioconversion of ALA to SDA is considered to be the rate limiting reaction in the pathway (Baker et al., 2016). EPA biosynthesis from SDA is considered much more efficient than that from ALA as the first catalytic step is not needed (Whelan et al., 2012; Walker et al., 2013), with a conversion efficiency between 3:1 and 6:1 (SDA:EPA) (Lemke et al., 2010). This means that an intake of around 750 mg SDA/day could theoretically have the same effect as an intake of 125–250 mg EPA/day.
There is not much information on the baseline dietary intake of SDA and GLA; these fatty acids are typically not included in nutrient composition tables as they are not major components of the human diet. The major dietary source of SDA is seafood although it is a very minor fatty acid in fish as compared to EPA and DHA (Whelan, 2009). Both GLA and SDA can be found in particular seed oils (e.g. borage, blackcurrant) at relatively high amounts (~ 10–20%) (EFSA NDA Panel 2015; Sergeant et al., 2016).
The human nutritional assessment of the single event MON 87769 consisted of different scenarios (EFSA GMO Panel, 2014). Initially, a targeted scenario was used to estimate the total intake of MON 87769 soybean oil and specifically of SDA from a defined list of 36 foods where the applicant targets to add MON 87769 soybean oil. Then, the intake of different fatty acids (palmitic acid, oleic acid, LA, SDA, GLA) was also assessed in another scenario after replacing the consumption of conventional vegetable oil by the amount of MON 87769 soybean oil estimated in the targeted scenario. In both scenarios, consumption data from the 2000–2001 UK National and Diet and Nutrition survey (adults, 19–64 years old) were used. A separate third scenario assessed the changes in fatty acid intake (with focus on SDA and LA) after replacing conventional soybean with soybean MON 87769 in the production of soybean food, using consumption data retrieved from the EFSA Comprehensive European Food Consumption Database (EFSA consumption database) from France and Denmark. Adverse health effects related to the presence of total trans‐fatty acids in MON 87769 soybean oil were not expected due to their low amounts (well below 2% total fat) that are similar to those quantified in the conventional soybean oil. The GMO Panel concluded that there were no safety concerns in the adult population following the consumption of MON 87769 soybean oil. The GMO Panel also mentioned that the quantitative dietary estimates used during the assessment should be revisited if the oil produced by soybean MON 87769 was to be extensively used in food products not considered during the assessment, e.g. as dietary supplements (EFSA GMO Panel, 2014).
In 2015, the GMO Panel delivered the scientific opinion on the two‐event stack soybean MON 87769 × MON 89788 (EFSA GMO Panel, 2015). No safety concerns were identified during the molecular characterisation, the comparative analysis (agronomic, phenotypic and compositional characteristics) and the toxicological and allergenicity assessments. There were no concerns regarding the use of feeding stuffs derived from defatted toasted MON 87769 × MON 89788 soybean meal, and no safety concerns with regard to the environment from the import and processing of soybean MON 87769 × MON 89788 were identified. Regarding the nutritional relevance of soybean MON 87769 × MON 89788, the applicant referred to the nutritional assessment provided for the single event based on the similarity of the fatty acid profile of the seeds and the refined bleached deodorised (RBD) oil in both the single and the double stack. The applicant was asked to provide a dietary exposure assessment based on the compositional analysis of the RBD oil produced from the two‐event stack soybean (RBD GM‐oil), taking into account different intake scenarios, covering low and high consumer groups. However, the applicant did not provide these data. Therefore, the GMO Panel could not complete the food safety assessment of soybean MON 87769 × MON 89788 because of the lack of an appropriate human nutritional assessment (EFSA GMO Panel, 2015).
On 16 May 2019, the European Commission mandated EFSA to assess additional information received from the applicant on human nutritional assessment of RBD oil from genetically modified soybean MON 87769 × MON 89788. EFSA was asked to complement its original scientific opinion on soybean MON 87769 × MON 89788 taking into consideration this additional information.
3.2. Compositional analysis and effect of processing
The composition data used in this nutritional assessment were already provided as part of the application dossier of soybean MON 87769 × MON 89788 and as additional information during the risk assessment (EFSA GMO Panel, 2015). Composition data were available for both seeds from soybean MON 87769 × MON 89788 and its derived RBD oil (Table 1); this oil is the main processed product from soybean for human consumption as well as the most relevant from a nutritional point of view in this application. The compositional changes are consistent with the intended trait and mainly refer to the presence of two new polyunsaturated fatty acids [SDA (C18:4, ω‐3) and GLA (C18:3, ω‐6)], and to the decrease in around 50% in the content of LA (C18:2, ω‐6). As observed for MON 87769, the modified fatty acid composition of soybean MON 87769 × MON 89788 seeds is reflected in the composition of the RBD GM‐oil.
Table 1.
Fatty acid composition of seeds from the non‐GM comparator, and of seeds and RBD oil produced from soybean MON 87769 × MON 89788
| % total fatty acids | |||
|---|---|---|---|
| Soybean MON 87769 × MON 89788a | Non‐GM comparator | ||
| Seedsb | RBD GM‐oilc | Seedsb | |
| Palmitic acid (C16:0) | 12.32 | 12.36 | 11.80 |
| Stearic acid (C18:0) | 4.22 | 4.27 | 4.12 |
| Oleic acid (C18:1) | 18.10 | 18.10 | 20.37 |
| Linoleic acid (LA, C18:2) | 25.42 | 25.28 | 54.25 |
| γ‐linolenic acid (GLA, C18:3) | 6.49 | 6.45 | –e |
| α‐linolenic acid (ALA, C18:3) | 10.70 | 10.56 | 8.68 |
| Stearidonic acid (SDA, C18:4) | 21.62 | 21.38 | –e |
| Arachidic acid (C20:0) | 0.34 | 0.35 | 0.31 |
| Eicosenoic acid (C20:1) | 0.18 | 0.23 | 0.16 |
| Behenic acid (C22:0) | 0.28 | 0.29 | 0.30 |
| Trans‐ALAd | 0.20 | 0.29 | –e |
| Trans‐SDAd | 0.14 | 0.24 | –e |
Soybean MON 87769 × MON 89788 treated with the intended herbicide (glyphosate).
Technical dossier application for authorisation of soybean MON 87769 × MON 89788.
Additional information 03/06/2015 (risk assessment for authorisation of soybean MON 87769 × MON 89788).
Similar levels of trans‐fatty acids levels were found in RBD oil from seeds of soybean MON 87769 and in RBD oil from conventional soybean seeds (EFSA GMO Panel, 2014).
Below limit of quantification (LOQ = 0.02%).
Information on the oxidative stability of the RBD GM‐oil was already provided and considered adequate by the GMO Panel in the context of the risk assessment of the single event MON 87769 (EFSA GMO Panel, 2014). Since the composition of the RBD GM‐oil from MON 87769 × MON 89788 and MON 87769 soybean is comparable, this conclusion remains valid.
As commented above, no adverse health effects related to the presence of total trans‐fatty acids in MON 87769 soybean oil are expected (EFSA GMO Panel, 2014). As specified in Commission Regulation 2019/6492, a maximum of 2 grams/100 grams fat of trans‐fatty acids other than trans‐fatty acids naturally occurring in fat of animal origin is allowed in food intended for the final consumer and food intended for supply to retail. Since the composition of the RBD GM‐oil from MON 87769 × MON 89788 and MON 87769 soybean is comparable, this conclusion remains valid.
No additional information on other food processed commodities was provided as part of the European Commission mandate. The available composition data on food processed commodities other than RBD GM‐oil refer back to the risk assessment of the single event MON 87769 (EFSA GMO Panel, 2014), and consist of data for protein isolates and crude lecithin. While the fat content of protein isolates is rather low (< 3%), soybean lecithin mainly consists of phospholipids (~ 65–75%) together with triglycerides and smaller amounts of other substances (Scholfield, 1981). Soybean lecithin is produced during the refinement of soybean oil and it is extensively used as food additive. During the current assessment and following a request for additional information from the GMO Panel, the applicant confirmed that the composition of the lecithin derived from MON 87769 × MON 89788 soybean is expected to be similar to that of MON 87769 soybean.3 Considerations on the possible impact of the use of lecithin as food additive on the total intake of fatty acids are further discussed in Section 3.3.2.
3.3. Human nutritional assessment
3.3.1. Toxicology
At present, no tolerable upper intake levels (UL) are set for ω‐3/ω‐6 PUFAs since no consistent evidence exists that the intake of any of these fatty acids has detrimental effects on health for any population group (EFSA NDA Panel, 2010a). The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) considers that supplemental intakes of EPA and DHA combined at doses up to 5 g/day (without specifying EPA:DHA ratios) and supplemental intakes of EPA alone up to 1.8 g/day do not raise safety concerns for the adult population. Supplemental intakes of DHA alone up to about 1 g/day are also considered as not raising safety concerns for the general population (EFSA NDA Panel, 2012).
During the current nutritional assessment of SDA (ω‐3 PUFA) and GLA (ω‐6 PUFA), the human studies assessed by the GMO Panel during the risk assessment of the single event MON 89788 were used as reference since no other relevant studies on these two fatty acids were identified (EFSA GMO Panel, 2014). Based on intervention human studies, two doses were confirmed as adequate for the nutritional assessment: 4.2 grams/day for SDA (Lemke et al., 2010) and 2.8 grams/day for GLA (Zurier et al., 1996; Kenny et al., 2000). These are maximum doses used in human studies without reports of adverse effects.
3.3.2. Dietary intake assessment
The additional information initially provided by the applicant consisted of a nutritional assessment of soybean MON 87769 × MON 89788 that included a dietary intake assessment of diverse fatty acids using consumption data from adult (19–64 years old) and toddler (1–4.5 years old) populations from the 2008–2012 UK National and Diet and Nutrition survey.4,5
Although the small changes in fatty acids such as palmitic acid, stearic acid and oleic acid were also assessed, the assessment focused on the changes observed in LA (C18:2, ω‐3), GLA (C18:3, ω‐6) and SDA (C18:4, ω‐3). To assess the potential nutritional concerns linked to the consumption of the RBD GM‐oil, two different scenarios were provided: a replacement of all consumed vegetable oils by the RBD GM‐oil (scenario A) and a replacement of vegetable oil by the RBD GM‐oil in targeted food categories (scenario B). As compared to the nutritional assessment provided in the risk assessment of the single event MON 87769, more recent consumption data were used, with consumption data from a young population (toddlers). Dietary intake estimates covered both low (5th percentile) and high (95th percentile) consumers.
Dietary intake – Scenario A
The scenario A assumed that all vegetable oils (not only soybean oil) consumed in the UK directly or indirectly (frying oil, baked goods, sauces, mayonnaise, margarine, salad dressings, etc.) will be replaced by the RBD GM‐oil. This is an extremely unrealistic scenario also taking into account the composition of the RBD GM‐oil, with a relatively high content of PUFAs, that makes it not suitable for frying as already stated in the scientific opinion of the single event (EFSA GMO Panel, 2014). This scenario retrieves food supply data from FAOSTAT databases6 for the type of vegetable oils consumed in the UK, and the contribution of these oils to the total fat intake as a parameter to assess the relevance of vegetable oil in the total intake of fatty acids. The fatty acid profile of the different vegetable oils used for the baseline scenario was taken from McCance and Widdowson's Composition of Foods Integrated Dataset (CoFIDS).7 Three replacement levels of the vegetable oils with the RBD GM‐oil were assessed (100%, 50% and 25%). The outcome of this scenario shows that with a 100% replacement level, the estimated intake of GLA and SDA might be well above the maximum dose without adverse effects for these two fatty acids in humans (4.2 grams/day for SDA and 2.8 grams/day for GLA). This outcome should be cautiously interpreted considering 1) the extremely unrealistic nature of this scenario that undoubtedly overestimates the intakes of GLA and SDA and 2) the doses for GLA and SDA are not health‐based guidance values but maximum doses without adverse effects.
The reduction in approximately 50% in the content of LA in the RBD GM‐oil as compared to the oil from conventional soybean seeds was also assessed within this scenario. LA intake represents ~ 99% of the total intake of n‐6 PUFAs in the human diet (Sioen et al., 2017); it cannot be synthesised by the body and it is considered an essential fatty acid required to maintain metabolic integrity. EFSA has proposed an adequate intake (AI) for LA of 4% of the total energy intake (E%), although LA deficiency has not been observed with intakes > 1 E% (EFSA NDA Panel, 2010a,b). Apart from vegetable oils, cereals and cereal products and meat, in particular chicken, are also relevant contributors to the intake of LA (Wood et al., 2008). The estimated intakes of LA do not represent a nutritional concern as intakes were in all cases above 1 E%. When concluding, the GMO Panel also took into account the presence of GLA in the RBD GM oil (~ 6% total fatty acids), the first intermediate in the metabolism of LA.
Dietary intake – Scenario B
The scenario B based the dietary intake assessment in the selection of a list of target foods (see Appendix A) where the applicant intends to add the RBD GM‐oil at levels that will provide around 375 mg SDA per serving. Assuming a conversion efficiency of SDA into EPA between 3:1 and 6:1 (Lemke et al., 2010), the consumption of four servings/day would provide 1,500 mg SDA, equivalent to 250–500 mg EPA/day. For EPA and DHA, EFSA has set an AI level of 250 mg EPA + DHA/day for adults, based on considerations of cardiovascular health (EFSA NDA Panel, 2010a,b).
Different sources of data were used in this scenario together with the 2008–2012 UK National and Diet and Nutrition survey: the Food Standards Agency risk recipes database (RRAD) to identify the type of fat used (vegetable/animal), FAOSTAT food supply data for UK for the type of vegetable oils consumed in the UK,6 and CoFIDS7 for the fatty acid profile of the different vegetable oils. The applicant targeted eight possible oils to be replaced: corn oil, cottonseed oil, olive oil, peanut oil, rapeseed oil, sesame oil, soybean oil and sunflower oil; based on FAOSTAT data, these oils represent 93% of the total vegetable oil consumed in the UK.8 In each target food, the vegetable oil was partially or entirely replaced by up to 1.75 grams of RBD GM‐oil to provide up to 375 mg of SDA; the intake of seven fatty acids (palmitic acid, stearic acid, oleic acid, ALA, LA, GLA, SDA) was compared before and after the replacement taking into account the contribution of vegetable oils to the total fat intake. The relatively minor changes in the levels of palmitic acid, stearic acid, oleic acid and ALA did not provoke relevant variations in the intake of these fatty acids after the replacement; for LA, all estimated intakes were well above 1 E%. The highest intakes of SDA and GLA were estimated in the adult population, with 4 grams/day for SDA and 1.2 grams/day, respectively (95th percentile), in both cases below the maximum doses without adverse effects used as reference.
During the risk assessment of scenario B, the GMO Panel asked to complement this scenario with dietary intakes from other European countries where the consumption pattern of the selected target commodities might differ from that of the UK population. The applicant was asked to make use of the summary statistics of the EFSA consumption database9 to estimate the intake of SDA and GLA across diverse European countries. A default body weight of 70 kg (EFSA Scientific Committee, 2012) was used to express the reference doses for SDA and GLA in body weight basis (40 and 60 mg/kg body weight per day for GLA and SDA, respectively). The consumption data to estimate the dietary intake of SDA and GLA were also retrieved in body weight basis from the EFSA consumption database. The target food categories as selected by the applicant are described in Appendix A; those food not listed in the appendix were not considered in the intake estimations and, therefore, in the nutritional assessment of soybean MON 87769 × MON 89788. Among those not considered as target food are food for infants and children (e.g. infant and follow‐on formulae) and food supplements.10 A thorough mapping was done by the applicant to match the selected target food to similar food categories reported in the EFSA consumption database. The GMO Panel considers the mapping as overly conservative since the target foods include a vast number of food commodities;10 as an example, together with all types of breakfast cereals and biscuits, all types of cheese (except cottage‐type cheese) were included since some cheese foods were considered as target food.3
Dietary intakes of SDA and GLA in mg/kg body weight (bw) per day were provided for high consumers (95th percentile) in the adult population (adults, elderly, very elderly, lactating women and pregnant women). For SDA, most of the 95th percentile intake estimations (~ 90%) were below or within 10% the maximum dose without adverse effects. For GLA, all dietary intake estimates (95th percentile) were well below the maximum dose without adverse effects. For the young population (infants, toddlers, other children and adolescents), the applicant indicated the absence of specific maximum references doses for SDA and GLA in this population group. The applicant provided a direct comparison between the maximum reference dose without adverse effects in adults and the SDA and GLA intake (both in g/day), supporting the absence of safety concerns with diverse studies in children with high doses of EPA and DHA without any reported adverse effects (Sorgi et al., 2007).
To further support the nutritional assessment, the GMO Panel estimated dietary intakes of SDA and GLA for both the young and the adult populations using individual consumption data. The target foods described in Appendix A were mapped in‐house into the different food categories in the EFSA consumption database; a list of the different foods considered in the intake assessment is shown in the Supporting information. Detailed estimated daily intakes of SDA and GLA for each population group within the different European dietary surveys are also available in the Supporting information. All dietary intake estimates for GLA (95th percentile) were well below the maximum dose without adverse effects as shown in Figure 2. For SDA, around 35% of the 95th percentile intake estimations were above the maximum dose without adverse effects, mostly in the young population (Figure 3). When concluding on the nutritional assessment of SDA, the GMO Panel took into account the known rapid conversion of SDA to EPA in the organism that leads to undetectable or negligible accumulation of SDA in the organism (James et al., 2003). Assuming the most conservative scenario (conversion ratio SDA to EPA of 3:1), the resulting concentrations of EPA in the organism derived from the SDA intake estimations would be in all cases well below the dose of 5 g/day (71 mg/kg bw per day) considered as safe by the EFSA NDA Panel (EFSA NDA Panel, 2012).
Figure 2.
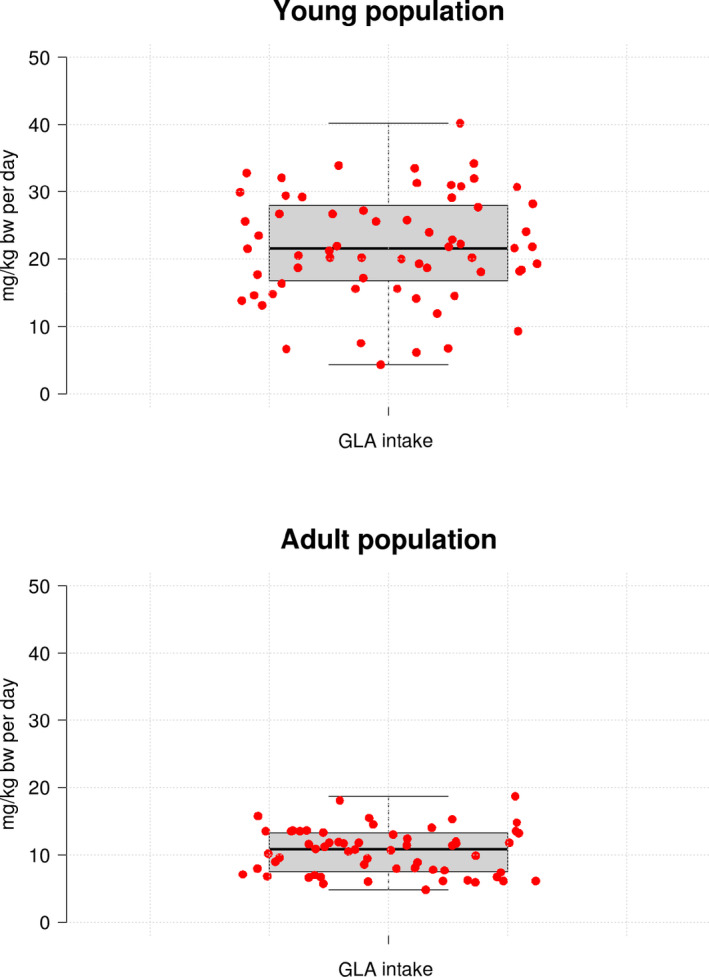
Dietary intake estimations of GLA (95th percentiles) in (a) the young population (infants, toddlers, other children and adolescents) and (b) the adult population (adults, elderly, very elderly, pregnant women and lactating women) across different European countries. Whiskers in the box‐plot represent the minimum and the maximum estimates, top and bottom of the box represent first and third quartiles, respectively, and the median is indicated with a horizontal line in the interior of the box
Figure 3.
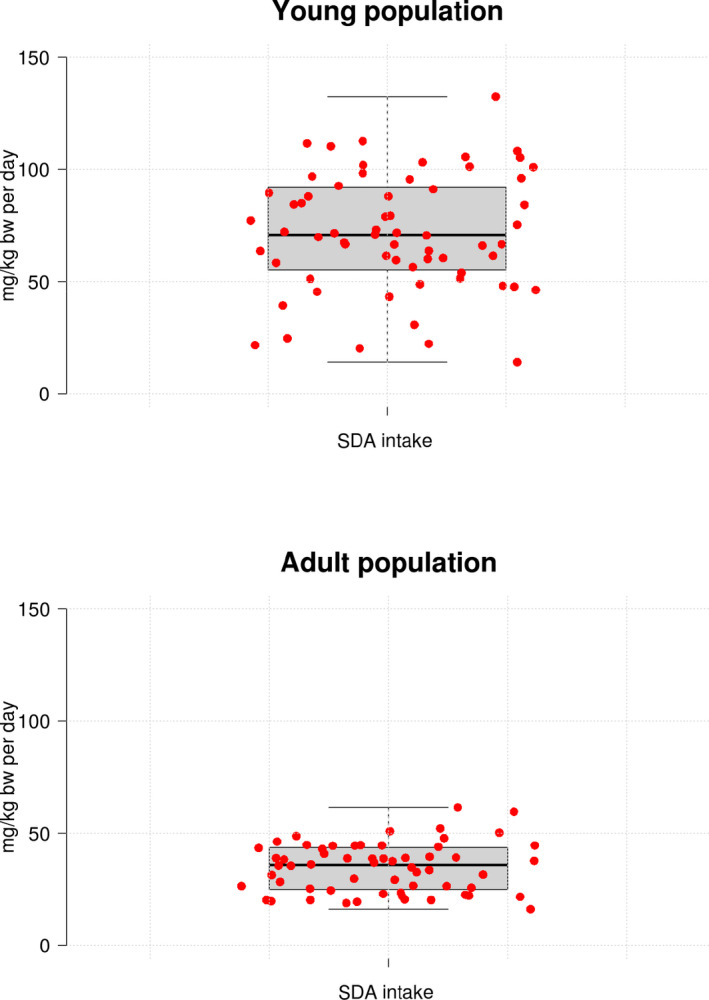
Dietary intake estimations of SDA (95th percentiles) in the young population (infants, toddlers, other children and adolescents) and in the adult population (adults, elderly, very elderly, pregnant women and lactating women) across different European countries. Whiskers in the box‐plot represent the minimum and the maximum estimates, top and bottom of the box represent first and third quartiles, respectively, and the median is indicated with a horizontal line in the interior of the box
The applicant was also asked to assess how the use of lecithin from soybean MON 87769 × MON 89788 as food additive might impact the intake of SDA and GLA. Under the overly conservative assumption that all lecithin in the market is derived from soybean MON 87769 × MON 89788, and considering 1) the lecithin consumption as estimated in the 2017 EFSA scientific opinion (EFSA ANS Panel et al., 2017), 2) the composition of soybean lecithin (Scholfield, 1981) and 3) the levels of SDA and GLA in lecithin (EFSA GMO Panel, 2014), maximum intakes (high consumers, 95th percentile) up to 0.73 g/day and 0.25 g/day for SDA and GLA, respectively, were estimated.
Due to the relatively low levels of fat present in the different soybean processed commodities (e.g. tofu, soybean milk, etc.) and its much lower consumption as compared to the RBD GM‐oil, its relevance on the intake of fatty acids is considered negligible and sufficiently covered by the dietary intake scenarios provided. In addition and as specified in the application dossier, soybean MON 87769 × MON 89788 will employ an identity preservation system developed for specialty soybean products to preserve market value, ensure purity and to direct this specialty soybean to its intended use and away from commodity processing.
3.4. Post‐market monitoring and labelling
3.4.1. Post‐market monitoring of GM food/feed
In accordance with Article 6(5)(e) of Regulation (EC) No 1829/20031 and considering the altered fatty acid profile and the risk assessment conducted for soybean MON 87769 × MON 89788, a proposal for a post‐market monitoring (PMM) plan needs to be provided. EFSA recommends that the PMM plan should initially focus on the collection of import data to Europe of soybean MON 87769 × MON 89788 and/or its products, in particular RBD GM‐oil. If imports are identified, consumption data should be collected (e.g. through dietary surveys) on conventional foods and foods containing RBD GM‐oil to confirm the predicted consumption data and to verify that the conditions of use are those considered during the pre‐market risk assessment.
3.4.2. Labelling11
No proposal for labelling was provided by the applicant as part of the application dossier of soybean MON 87769 × MON 89788. However, the EFSA GMO Panel confirms that the nutritional composition of soybean MON 87769 × MON 89788 is different from its conventional counterpart, in particular due to the presence of two new fatty acids [stearidonic acid (C18:4, ω‐3) and γ‐linolenic acid (C18:3, ω‐6)] and the decrease of linoleic acid (C18:2, ω‐6). In addition and based on its fatty acid profile, the intended uses of soybean MON 87769 × MON 89788 will probably differ from those of its conventional counterpart. Therefore, a proposal for specific labelling is required in accordance with Article 13(2)(a) and Article 25(2)(c) of Regulation (EC) No 1829/2003.1
3.5. Conclusions
The GMO Panel was mandated to assess additional information on human nutritional assessment of the genetically modified soybean MON 87769 × MON 89788 to complement its original scientific opinion adopted in 2015 (EFSA GMO Panel, 2015). The additional information comprises a human nutritional assessment of RBD oil from genetically modified soybean MON 87769 × MON 89788.
During the assessment, the GMO Panel considers the dietary intake estimations of different fatty acids (based on composition and consumption data) but also the most recent information available (e.g. metabolism of fatty acids, human intervention studies, etc.). At present, no UL are set for any of the two main new fatty acids identified in soybean MON 87769 × MON 8978 (SDA and GLA), only maximum doses without adverse effects derived from human studies. These doses were considered during the nutritional assessment together with the safety dose of 5 g/day for EPA and DHA, taking into account the rapid conversion of SDA to EPA in the organism. Although in few dietary surveys SDA intake estimates could be higher than the maximum dose without adverse effects, the overly conservative nature of the intake estimations together with the absence of toxicological hazards and the rapid metabolism of SDA in humans indicate that these SDA intake estimations do not pose any safety concerns. For GLA, the first intermediate in the metabolism of LA, all intake estimations were below the maximum dose without adverse effects indicating no safety concern. The decrease observed in the levels of LA in RBD GM‐oil as compared to oil from conventional soybean does not represent a nutritional concern as intakes were in all cases above 1 E%. In addition, the decrease of LA is compensated, at least partially, by the presence of GLA in the RBD GM‐oil. Taking into account all this information, the GMO Panel concluded that the consumption of soybean MON 87769 × MON 89788 and their derived products, in particular its RBD oil, does not represent a nutritional concern in humans.
The GMO Panel notes that in certain crops, as identified for soybean MON 87769 × MON 89788, the genetic modifications can result in food products (e.g. RBD GM‐oil) with a composition that significantly differs from their conventional counterpart food products. In these cases, dietary intake estimations used in the nutritional assessment should take into account the nutrient composition of the GM‐food products when selecting the foods likely to be displaced (Codex Alimentarius, 2009).
The current nutritional assessment would have to be revisited if RBD GM‐oil were to be extensively used in food products not considered in this assessment, e.g. as dietary supplements or food for infants and young population. A PMM plan is recommended to confirm the predicted consumption and the application of conditions of uses considered during the pre‐market risk assessment.
4. Documentation as provided to EFSA
Mandate from European Commission (EC) received on 16 May 2019 concerning a request to assess new additional information related to the application for authorisation of food and feed containing, consisting of and produced from genetically modified soybean MON 87796 × MON 89788 (EFSA‐GMO‐NL‐2010‐85).
Mandate accepted by EFSA on 22 May 2019.
Request for supplementary information to the applicant, 30 July 2019.
Receipt of supplementary information from the applicant, 27 January 2020.
Request for supplementary information to the applicant, 31 March 2020.
Request for supplementary information to the applicant, 30 June 2020.
Request for supplementary information to the applicant, 15 July 2020.
Receipt of supplementary information from the applicant, 29 January 2021.
Request to EC to extend the deadline of the mandate, from 22 February 2021 to 22 April 2021, 19 February 2021.
Acceptance of the deadline extension requested to EC, 9 March 2021.
Abbreviations
- ALA
alpha‐linolenic acid
- ANS Panel
EFSA Panel on Food Additives and Nutrient Sources added to Food
- bw
body weight
- DHA
docosahexaenoic acid
- E%
percentage of energy intake
- EPA
eicosapentaenoic acid
- GLA
gamma‐linolenic acid
- GM
genetically modified
- GMO Panel
EFSA Panel on Genetically Modified Organisms
- GMO
genetically modified organism
- LA
linoleic acid
- NDA Panel
EFSA Panel on Dietetic Products, Nutrition and Allergies
- OECD
Organisation for Economic Co‐operation and Development
- PMM
post‐market monitoring
- PUFA
polyunsaturated fatty acids
- RBD
refined bleached deodorised
- SDA
stearidonic acid
- UL
tolerable upper intake levels
Appendix A – List of target food categories as selected by the applicant and considered during the nutritional assessment of soybean MON 87769 × MON 89788. RBD GM‐oil produced from soybean MON 87769 × MON 89788 is to be added to the target foods to obtain the concentrations of stearidonic acid (SDA) and gamma linolenic acid (GLA) as described in the table
1.
| % of vegetable oil in food | Amount of RBD GM‐oil/serving (g) | Amount of SDA per serving (g) | Amount of GLA per serving (g) | Concentration of SDA (mg/g of food) | Concentration of GLA (mg/g of food) | |
|---|---|---|---|---|---|---|
| Baked goods and baking mixes‐bars | 10% | 1.75 | 0.375 | 0.114 | 9.38 | 2.85 |
| Baked goods and baking mixes‐biscuits, bagels, etc. | 13% | 1.75 | 0.375 | 0.114 | 6.82 | 2.07 |
| Baked goods and baking mixes‐breads | 7% | 1.75 | 0.375 | 0.114 | 7.50 | 2.28 |
| Baked goods and baking mixes‐cakes heavy weight | 13% | 1.75 | 0.375 | 0.114 | 3.00 | 0.91 |
| Baked goods and baking mixes‐cakes light weight | 19% | 1.75 | 0.375 | 0.114 | 6.70 | 2.04 |
| Baked goods and baking mixes‐cakes medium weight | 21% | 1.75 | 0.375 | 0.114 | 4.69 | 1.43 |
| Baked goods and baking mixes‐cookies | 19% | 1.75 | 0.375 | 0.114 | 12.50 | 3.80 |
| Baked goods and baking mixes‐crackers | 19% | 1.75 | 0.375 | 0.114 | 12.50 | 3.80 |
| Breakfast cereals & Grains‐breakfast cereals | 5% | 1.75 | 0.375 | 0.114 | 6.82 | 2.07 |
| Cheeses (cottage cheese excluded) | 8% | 1.75 | 0.375 | 0.114 | 12.50 | 3.80 |
| Dairy Product Analogues | 10% | 1.39 | 0.297 | 0.090 | 19.79 | 6.02 |
| Fats and oils‐dressings for salads | 53% | 1.75 | 0.375 | 0.114 | 12.50 | 3.80 |
| Fats and oils‐Margarines/Spreads | 100% | 1.75 | 0.375 | 0.114 | 26.79 | 8.14 |
| Fats and oils‐mayonnaise | 76% | 1.75 | 0.375 | 0.114 | 25.00 | 7.60 |
| Fish products‐entrees with sauce | 9% | 1.75 | 0.375 | 0.114 | 2.68 | 0.81 |
| Gelatin, Puddings and fillings‐pudding | 4% | 1.75 | 0.375 | 0.114 | 3.13 | 0.95 |
| Grain Products and Pastas‐Pasta | 4% | 1.75 | 0.375 | 0.114 | 2.68 | 0.81 |
| Gravies and sauces‐Main Entrée Sauces (e.g. spaghetti sauce) | 5.4% | 1.75 | 0.375 | 0.114 | 3.00 | 0.91 |
| Hard candies | 5% | 0.66 | 0.141 | 0.043 | 9.38 | 2.85 |
| Meat products‐entrees with sauce | 2% | 1.75 | 0.375 | 0.114 | 2.68 | 0.81 |
| Nuts and nut products‐peanut butter | 4% | 1.10 | 0.234 | 0.071 | 7.32 | 2.23 |
| Plant proteins‐soymilk | 3% | 1.75 | 0.375 | 0.114 | 1.56 | 0.48 |
| Poultry products‐entrees with sauces | 4% | 1.75 | 0.375 | 0.114 | 2.68 | 0.81 |
| Snack foods | 13% | 1.75 | 0.375 | 0.114 | 12.50 | 3.80 |
| Soft candy‐candy bars | 12% | 1.75 | 0.375 | 0.114 | 9.38 | 2.85 |
| Soups and soup mixes | 3% | 1.75 | 0.375 | 0.114 | 1.53 | 0.47 |
Supporting information
Dietary intake GLA
Dietary intake SDA
Suggested citation: EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms) , Naegeli H, Bresson JL, Dalmay T, Dewhurst IC, Epstein MM, Firbank LG, Guerche P, Hejatko J, Moreno FJ, Mullins E, Nogué F, Rostoks N, Sánchez Serrano JJ, Savoini G, Veromann E, Veronesi F, Frenzel T and Gómez Ruiz JÁ, 2021. Scientific Opinion on the statement complementing the EFSA Scientific Opinion on application (EFSA‐GMO-NL‐2010-85) for authorisation of food and feed containing, consisting of and produced from genetically modified soybean MON 87769 × MON 89788. EFSA Journal 2021;19(5):6589, 18 pp. 10.2903/j.efsa.2021.6589
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00329
Panel members: Hanspeter Naegeli, Jean‐Louis Bresson, Tamas Dalmay, Ian Crawford Dewhurst, Michelle M Epstein, Leslie George Firbank, Philippe Guerche, Jan Hejatko, Francisco Javier Moreno, Ewen Mullins, Fabien Nogué, Nils Rostoks, Jose Juan Sánchez Serrano, Giovanni Savoini, Eve Veromann, and Fabio Veronesi.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to thank the support provided by the GMO standing Working Group on Food and Feed Safety Assessment and by EFSA staff members Sonia Hernandez Valero, Hanne Pedersen and Yustina Olshevska Grigorov
Adopted: 14 April 2021
Notes
Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. OJ L 268, 18.10.2003, p. 1–23.
Commission Regulation (EU) 2019/649 of 24 April 2019 amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards trans fat, other than trans fat naturally occurring in fat of animal origin. OJ L 110, 25.4.2019, p. 17–20.
Additional information on 24 January 2020.
Additional information on 22 May 2019.
Dietary data included in the assessment were submitted to EFSA when the UK was a member of the EU.
http://www.fao.org/faostat/en/#data/FBS (accessed: February 2017, data 2011–2013). FAOSTAT Food Balance Sheets (FBS) provide information on country's food supply during a specified reference period (food availability) considering the quantity of foodstuffs produced in the country, the quantity imported and any change in stocks.
McCance and Widdowson's The Composition of Foods Integrated Dataset (CoFIDS). http://www.food.gov.uk/science/dietarysurveys/dietsurveys/. Data accessed: February 2017.
% of vegetable oil supply from FAOSTAT food supply data (2011–2013): corn oil (2.4%), cottonseed oil (0%), olive oil (5.5%), peanut oil (0.2%), rapeseed oil (47.3%), sesame oil (0.21%), soybean oil (23.9%) and sunflower oil (13.1%).
http://www.efsa.europa.eu/en/food-consumption/comprehensive-database. The EFSA consumption database contains dietary surveys from more than 20 European countries, different age classes and vulnerable population groups such as lactating and pregnant women.
Additional information on 29 January 2021.
Dossier Part IV – Labelling Proposal.
References
- Baker EJ, Miles EA, Burdge GC, Yaqoob P and Calder PC, 2016. Metabolism and functional effects of plant‐derived ω‐3 fatty acids in humans. Progress in Lipid Research, 64, 30–56. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Commission , 2009. Foods derived from modern biotechnology. 2nd Edition. Rome, Italy: FAO/WHO. [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Opinion of the Scientific Panel on Genetically Modified Organisms on an application (Reference EFSA-GMO‐NL-2006‐36) for the placing on the market of glyphosate‐tolerant soybean MON 89788 for food and feed uses, import and processing under Regulation (EC) 1829/2003 from Monsanto. EFSA Journal 2008;6(7):758, 23 pp. 10.2903/j.efsa.2008.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Lambre C, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Oskarsson A, Parent‐Massin D, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Brimer L, Altieri A, Christodoulidou A, Lodi F and Dusemund B, 2017. Scientific opinion on the re‐evaluation of lecithins (E 322) as a food additive. EFSA Journal 2017;15(4):4742, 74 pp. 10.2903/j.efsa.2017.4742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms), 2011. Guidance for risk assessment of food and feed from genetically modified plants. EFSA Journal 2011;9(5):2150, 37 pp. 10.2903/j.efsa.2011.2150 [DOI] [Google Scholar]
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms), 2014. Scientific Opinion on application (EFSA‐GMO‐UK‐2009‐76) for the placing on the market of soybean MON 87769 genetically modified to contain stearidonic acid, for food and feed uses, import and processing under Regulation (EC) No 1829/2003 from Monsanto. EFSA Journal 2014;12(5):3644, 41 pp. 10.2903/j.efsa.2014.3644 [DOI] [Google Scholar]
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms), 2015. Scientific Opinion on an application (EFSA‐GMO‐NL‐2010‐85) for the placing on the market of MON 87769 × MON 89788 soybean, genetically modified to contain stearidonic acid and be tolerant to glyphosate for food and feed uses, import and processing under Regulation (EC) No 1829/2003 from Monsanto. EFSA Journal 2015;13(10):4256, 25 pp. 10.2903/j.efsa.2015.4256 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies), 2010a. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010;8(3):1461, 107 pp. 10.2903/j.efsa.2010.1461 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies), 2010b. Scientific Opinion on the substantiation of health claims related to eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), docosapentaenoic acid (DPA) and maintenance of normal cardiac function (ID 504, 506, 516, 527, 538, 703, 1128, 1317, 1324, 1325), maintenance of normal blood glucose concentrations (ID 566), maintenance of normal blood pressure (ID 506, 516, 703, 1317, 1324), maintenance of normal blood HDL‐cholesterol concentrations (ID 506), maintenance of normal (fasting) blood concentrations of triglycerides (ID 506, 527, 538, 1317, 1324, 1325), maintenance of normal blood LDL‐cholesterol concentrations (ID 527, 538, 1317, 1325, 4689), protection of the skin from photo‐oxidative (UV‐induced) damage (ID 530), improved absorption of EPA and DHA (ID 522, 523), contribution to the normal function of the immune system by decreasing the levels of eicosanoids, arachidonic acid‐derived mediators and pro‐inflammatory cytokines (ID 520, 2914), and “immunomodulating agent” (4690) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(10):1796, 32 pp. 10.2903/j.efsa.2010.1796 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies), 2012. Scientific Opinion related to the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA Journal 2012;10(7):2815, 48 pp. 10.2903/j.efsa.2012.2815. Available online: www.efsa.europa.eu/efsajourna [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition, and Allergies), 2015. Scientific Opinion on the safety of refined Buglossoides oil as a novel food ingredient. EFSA Journal 2015;13(2):4029, 21 pp. 10.2903/j.efsa.2015.4029 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Gomez Ruiz JA, Bresson J‐L, Frenzel T and Paoletti C, 2019. Statement on the human dietary exposure assessment to newly expressed proteins in GM foods. EFSA Journal 2019;17 (7):5802, 18 pp. 10.2903/j.efsa.2019.5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MJ, Ursin VM and Cleland LG, 2003. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n− 3 fatty acids. The American journal of clinical nutrition, 77, 1140–1145. [DOI] [PubMed] [Google Scholar]
- Kapoor R and Huang YS, 2006. Gamma linolenic acid: an antiinflammatory omega‐6 fatty acid. Current Pharmaceutical Biotechnology, 7, 531–534. [DOI] [PubMed] [Google Scholar]
- Kenny F, Pinder S, Ellis I, Gee J, Nicholson R, Bryce R and Robertson J, 2000. Gamma linolenic acid with tamoxifen as primary therapy in breast cancer. International Journal of Cancer, 85, 643–648. [DOI] [PubMed] [Google Scholar]
- Lemke SL, Vicini JL, Su H, Goldstein DA, Nemeth MA, Krul ES and Harris WS, 2010. Dietary intake of stearidonic acid‐enriched soybean oil increases the omega‐3 index: randomized, double‐blind clinical study of efficacy and safety. American Journal of Clinical Nutrition, 92, 766–775. [DOI] [PubMed] [Google Scholar]
- Scholfield CR, 1981. Composition of soybean lecithin. Journal of American Oil Chemistry Society, 58, 889–892. [Google Scholar]
- Sergeant S, Rahbar E and Chilton FH, 2016. Gamma‐linolenic acid, dihommo‐gamma linolenic, eicosanoids and inflammatory processes. European Journal of Pharmacology, 785, 77–86. 10.1016/j.ejphar.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioen I, van Lieshout L, Eilander A, Fleith M, Lohner S, Szommer A, Petisca C, Eussen S, Forsyth S, Calder PC, Campoy C and Mensink RP, 2017. Systematic review on N‐3 and N‐6 polyunsaturated fatty acid intake in European countries in light of the current recommendations ‐ focus on specific population groups. Annals of Nutrition and Metabolism, 70, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgi PJ, Hallowell EM, Hutchins HL and Sears B, 2007. Effects of an open‐label pilot study with high‐dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutrition Journal, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CG, Jebb SA and Calder PC, 2013. Stearidonic acid as a supplemental source of ω‐3 polyunsaturated fatty acids to enhance status for improved human health. Nutrition, 29, 363–369. [DOI] [PubMed] [Google Scholar]
- Whelan J, 2009. Dietary stearidonic acid is a long chain (n‐3) polyunsaturated fatty acid with potential health benefits. The Journal of Nutrition, 139, 5–10. [DOI] [PubMed]
- Whelan J, Gouffon J and Zhao Y, 2012. Effects of dietary stearidonic acid on biomarkers of lipid metabolism. The Journal of Nutrition, 142, 630S–634S. 10.3945/jn.111.149138 [DOI] [PubMed] [Google Scholar]
- Wood JD, Enser M, Richardson RI and Whittington FM, 2008. Fatty Acids in Meat and Meat Products. In: Chow CK (ed.). Fatty Acids in Foods and their Health Implications. CRC Press, Taylor & Francis Group, Boca Raton, FL. 87–107. [Google Scholar]
- Zurier R, Rossetti R, Jacobson E, De Marco D, Liu N, Temming J, White B and Laposata M, 1996. Gamma‐linolenic acid treatment of rheumatoid arthritis—a randomized, placebo‐controlled trial. Arthritis and Rheumatism, 39, 1808–1817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dietary intake GLA
Dietary intake SDA


