ABSTRACT
The effect of exercise habits on the increased incidence of lifestyle-related diseases among residents of the evacuation area in Fukushima Prefecture after the Great East Japan Earthquake is not well characterized. This study examined the influence of exercise habits on the frequency of new onset of lifestyle-related diseases in the aftermath of the earthquake using data from the Fukushima Health Management Survey (FHMS). Of the 32 289 individuals (14 004 men and 18 285 women) aged 40–90 years who underwent one or more health examinations in both 2011–12 and 2014–15, those who knew whether they had any lifestyle diseases and who responded to a questionnaire about their exercise and physical activity habits were included (dyslipidemia, 8017; hypertension, 7173; and diabetes mellitus, 13140 individuals). The association between the frequency of new onset of lifestyle-related diseases in 2014–15 and the presence or absence of persistent exercise and physical activity habits (active lifestyle) was examined using the FHMS data. The frequency of new onset of dyslipidemia was significantly lower in the active lifestyle group than in the sedentary lifestyle group (P = 0.008). On univariate and multivariate logistic regression analyses, the presence of active lifestyle, obesity and the experience of evacuation showed a significant association with new onset of dyslipidemia, independent of age, sex or follow-up period. Thus maintaining physical activity and exercise habits may help prevent the new onset of dyslipidemia among residents of the evacuation area in the Fukushima Prefecture after the earthquake.
Keywords: exercise, dyslipidemia, disaster, evacuation, lifestyle
INTRODUCTION
Studies have shown an increased prevalence of obesity and lifestyle-related diseases among residents of the Fukushima Prefecture evacuation area who were forced to evacuate in the aftermath of the Great East Japan Earthquake (11 March 2011) [1–4]. This phenomenon was potentially attributable to increased stress, dwelling in temporary housing and disruption of eating habits [5–9].
There is a paucity of specific interventions to prevent lifestyle-related diseases occurring in the aftermath of natural disasters. Therefore, prevention of lifestyle-related and cardiovascular diseases following disasters is a key research imperative. The beneficial effects of increased physical activity against obesity, lifestyle diseases, cardiovascular diseases and various cancer types are well documented. Lee et al. suggested that the life expectancy of the world’s population would be extended by 0.68 years if the entire population were to be physically active [10]. Moreover, according to the World Health Organization (WHO), physical inactivity was the fourth leading risk factor for death globally in 2010 and the third leading risk factor for death due to non-communicable diseases in Japan in 2007 [11, 12].
Therefore, interventions to increase physical activity can help prevent lifestyle and cardiovascular diseases and extend healthy life expectancy among residents in the evacuation area. However, the influence of exercise habits on the frequency of new onset of lifestyle diseases following a disaster is not clear. Thus, in this study, we sought to clarify this influence using the Fukushima Health Management Survey (FHMS) data.
MATERIALS AND METHODS
STUDY GROUP
The study population comprised Japanese men and women living in communities near the Fukushima Daiichi Nuclear Power Plant in the Fukushima Prefecture, including 13 municipalities (Tamura City, Minami-soma City, Kawamata-machi, Hirono-machi, Naraha-machi, Tomioka-machi, Kawauchi-mura, Okuma-machi, Futaba-machi, Namie-machi, Katsurao-mura, Iitate-mura and Date City).
Following the disaster, the government designated a 20-km radius around the Fukushima Daiichi Nuclear Power Plant as a ‘restricted area’, with orders for compulsory evacuation. The government subsequently designated a 20–30 km area around the plant as an ‘evacuation-prepared area in case of emergency’. In addition, the area near the 30-km area where there was high-level radiation exposure (>20 mSv year–1) was termed a ‘deliberate evacuation area’.
Therefore, all residents of Hirono-machi, Naraha-machi, Tomioka-machi, Kawauchi-mura, Okuma-machi, Futaba-machi, Namie-machi, Katsurao-mura and Iitate-mura, and some residents of Tamura City, Minami-Soma City, Kawamata-machi and Date City were forced to evacuate their homes (evacuation zone). The other areas of Tamura City, Minami-Soma City, Kawamata-machi and Date City were defined as a non-evacuation zone in the present study.
Data from a specific health checkup (health checkups and guidance focusing on visceral fat obesity in individuals aged ≥40 years), a late-stage elderly patient examination and results of the Comprehensive Health Check in the FHMS were included in the analysis. The FHMS has been conducted since 19 January 2012, to evaluate the impact of radiation exposure and to determine the health status of Fukushima residents given the diffusion of radioactive substances; the objective of the survey is to inform interventions for prevention and early detection of illnesses, and to improve the future health of the residents [13].
A total of 53 193 individuals aged 40–90 years [accounting for 32.3% of the Census population aged 40–90 years in these communities in 2010 (164 714)] [3] who underwent a medical examination at least once in the period 2011–2012, and 32 289 people who underwent medical examinations in 2014 and 2015 (14 004 men and 18 285 women; mean follow-up period, 3.4 years; tracking rate, 60.7%) were included. For individuals who underwent a health checkup on two or more occasions between 2011 and 2012, the measurements taken in the fiscal year closest to the disaster were considered as baseline measurements; for individuals who underwent a health checkup on two or more occasions from 2014 to 2015, the examination results from consultation in the year furthest from the disaster were used. Furthermore, individuals who did not respond to the questionnaire about exercise and physical activity habits were excluded (n = 17 357); 14 932 individuals (6538 men and 8394 women; mean follow-up period: 3.4 years) were included. In addition, to examine the association between exercise/physical activity habits and the frequency of new onset of each lifestyle disease, individuals with a history of diabetes (n = 1792), hypertension (n = 7759) or dyslipidemia (n = 6915) from 2011 to 2012, or those with undeterminable status were excluded from the respective analyses. Ultimately, a total of 13 140 individuals (5478 men and 7662 women; mean follow-up period, 3.4 years) for diabetes, 7173 individuals (2822 men and 4351 women; mean follow-up period, 3.4 years) for hypertension and 8017 individuals (3429 men and 4588 women; mean follow-up period, 3.4 years) for dyslipidemia were targeted (Fig. 1).
Fig. 1.
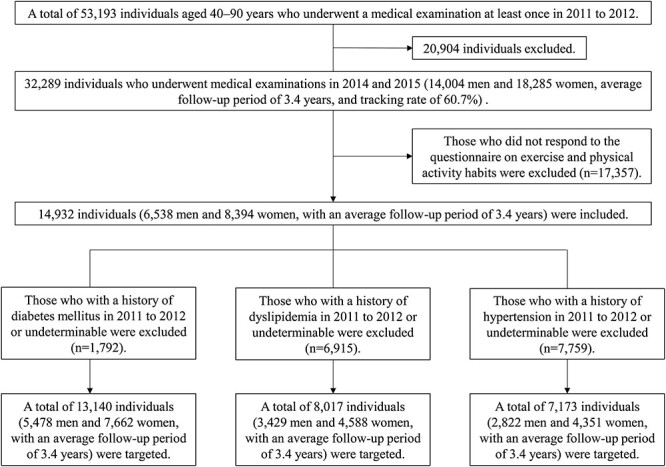
Schematic illustration of the study design and the subject selection criteria.
Data pertaining to age, residential area at the time of the disaster, use of antihypertensive agents, use of hypoglycemic agents, treatment for dyslipidemia, exercise habits, active physical activity, smoking habits, alcohol consumption, snacking habits, lack of breakfast and sleeping habits were obtained from the FHMS. In addition, height (cm), weight (kg), body mass index (BMI; kg m–2), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), glycosylated hemoglobin (HbA1c; %), fasting blood glucose (g dl–1), high-density lipoprotein (HDL) cholesterol (mg dl–1), low-density lipoprotein (LDL) cholesterol (mg dl–1) and triglycerides (mg dl–1) were examined. Fasting blood indices were defined as those obtained after fasting for at least 8 h. The HbA1c value based on the Japan Diabetes Society (JDS) was converted to the value of the National Glycohemoglobin Standardization Program (NGSP) standard using the following formula: [14].
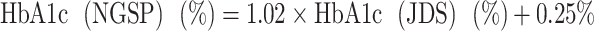 |
DEFINITION OF LIFESTYLE-RELATED DISEASES
Individuals with systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or those using medication to reduce blood pressure were defined as having ‘hypertension’.
Diabetes mellitus was defined according to the criteria in the JDS guidelines: fasting blood glucose level ≥126 mg dl–1, HbA1c level (NGSP) ≥6.5% or use of medication or insulin to reduce blood sugar.
Dyslipidemia was defined as a HDL cholesterol level of <40 mg dl–1, an LDL cholesterol level of ≥140 mg/dl, a triglyceride level of ≥150 mg dl–1 or the use of medication to reduce cholesterol or triglycerides.
To define ‘obesity’, the following formula was used to calculate BMI:
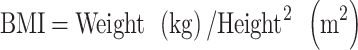 |
The WHO defines obesity as a BMI of ≥30 kg m–2 [15]. However, only ~2–3% of Japanese adults have a BMI of >30 kg m–2, which is very low compared with that in the Western population [16]. Thus, in this study, we defined obesity as a BMI of ≥25 kg m–2 as recommended by the Japan Society for the Study of Obesity [17].
Individuals who were ‘obese’ in both time periods were defined as ‘with obesity’ and individuals whose BMI was <25 kg m–2 in either of the two time periods were defined as ‘without obesity’.
DEFINITION OF LIFESTYLES AND LIFESTYLE HABITS
To assess the exercise habits, the study population was asked a question about active physical activity (‘In your daily life, do you walk or do any equivalent amount of physical activity for more than one hour a day?’) and a question about exercise habits (‘Have you been doing exercises that cause mild sweating for more than 30 minutes, twice per week, for over a year?’). Individuals who answered ‘Yes’ in both time periods of 2011–12 and 2014–15 were categorized as the ‘active lifestyle group’ and those who answered ‘No’ for both or either of the two time periods were categorized as the ‘sedentary lifestyle group’.
Smoking is associated with type 2 diabetes, hypertension and dyslipidemia [18–20]. Moreover, smokers are at a high risk of cardiovascular diseases even after cessation of smoking [21]. Regarding smoking habits, respondents were asked the question: ‘Do you habitually smoke, at present?’; individuals who answered ‘No’ for both time periods were defined as ‘without smoking habit’ and those who answered ‘Yes’ for either of the two time periods or who had a history of smoking were defined as ‘with smoking habit’.
A ‘go’ is traditionally used as a unit of alcoholic beverages in Japan. One ‘go’ corresponds to ~22 g of ethanol. One go is equivalent to a bottle of Japanese saké (180 ml), a medium-sized bottle of beer (~500 ml), a glass of double whisky (60 ml) or two glasses of wine (240 ml). In previous studies, consumption of an average of 2 go or more in a day (~44 g of alcohol or more) was defined as ‘heavy drinking’. [3] Likewise, in this study, respondents were asked two questions: ‘How often do you drink (saké, shochu, beer, wine, whisky, etc.)?’ and ‘How much alcohol do you drink per day?’ Individuals who answered ‘every day’ to the first question and ‘between 2 and less than 3 go’ or ‘3 go or more’ to the second question in both time periods were defined as ‘with a habit of heavy drinking’. All other responses were defined as ‘without a habit of heavy drinking’.
Regarding the habit of eating quickly, respondents were asked the question: ‘Is your eating speed quicker than that of others?’ Individuals who answered ‘quick’ were defined as ‘with a habit of eating quickly’, and individuals who answered ‘average’ or ‘slow’ in either of the two time periods were defined as ‘without a habit of eating quickly’.
Regarding the habit of eating snacks after meals, respondents were asked the question: ‘Do you eat snacks (late-night food other than breakfast, lunch and supper) after supper more than three times a week?’ Individuals who answered ‘Yes’ for both time periods were defined as ‘with a habit of eating a snack after meals’, and individuals who answered ‘No’ for either of the two time periods were defined as ‘without a habit of eating a snack after meals’.
Regarding skipping breakfast, respondents were asked the question: ‘Do you skip breakfast more than three times a week?’ Individuals who answered ‘Yes’ for both time periods were defined as ‘skipping breakfast’, and those who answered ‘No’ for either of the two time periods were defined as ‘not skipping breakfast’.
Regarding sleep habits, respondents were asked the question: ‘Do you sleep well and enough?’ Individuals who answered ‘No’ for both time periods were defined as ‘lacking sleep’, and those who answered ‘Yes’ for either of the two time periods were defined as ‘not lacking sleep’.
RELATIONSHIP BETWEEN THE ONSET OF LIFESTYLE-RELATED DISEASES AND LIFE HABITS
Individuals who already had hypertension, diabetes mellitus or dyslipidemia as of 2011–12 were excluded, and the relationship as of 2014–15 between the onset of various lifestyle-related diseases and an active lifestyle was studied. Moreover, differences in the plasma lipid profile between the two lifestyle groups (active and sedentary) were examined. Finally, univariate and multivariate logistic regression analyses were performed to identify whether an active lifestyle was independently linked to lifestyle-related diseases.
STATISTICAL METHODS
Statistical software SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for analyses. Outcomes are presented as ± SD or as frequency (percentage). First, the association between the frequency of new onset of lifestyle-related diseases and the presence or absence of an active lifestyle was evaluated using the χ2 test. The objective variables were the presence or absence of dyslipidemia, hypertension and diabetes, and the explanatory variable was the presence or absence of an active lifestyle.
Next, the difference of lifestyle habits between individuals with and without dyslipidemia was assessed by χ2 test. The objective variable was the presence or absence of dyslipidemia, and the explanatory variables were age in 2011–12, follow-up period, sex, presence or absence of smoking habits, drinking habits, diabetes mellitus, hypertension, eating snacks, eating quickly, skipping breakfast, obesity, sleep habit and experience of evacuation.
Moreover, the plasma lipid profile of individuals with an active lifestyle and sedentary lifestyle were compared using the unpaired t-test at two time points to investigate the effect of active lifestyle on the plasma lipid profile. The objective variable was the presence or absence of active lifestyle and the explanatory variables were triglycerides, LDL cholesterol and HDL cholesterol.
Finally, regression models were used to obtain age- and sex-adjusted and multiple adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the association between each factor and dyslipidemia.
The objective variable was the presence or absence of dyslipidemia, and the explanatory variables were age, follow-up period, sex, presence or absence of active lifestyle, skipping breakfast, obesity and experience of evacuation.
Two-sided P values <0.05 were considered indicative of statistical significance.
ETHICAL CONSIDERATIONS
Informed consent was obtained from the community representatives to conduct the epidemiological study based on the Council for International Organizations of Medical Science guidelines [22]. The Ethics Committee of the Fukushima Medical University approved this study (#1319).
RESULTS
ASSOCIATION BETWEEN THE FREQUENCY OF NEW ONSET OF LIFESTYLE-RELATED DISEASES AND ACTIVE LIFESTYLE
The association between the frequency of new onset of lifestyle-related diseases and active lifestyle is shown in Table 1. A total of 865 (6.6%) individuals out of the 13 140 without diabetes mellitus, 1320 (18.4%) out of the 7173 without hypertension and 1868 (23.3%) out of the 8017 without dyslipidemia in 2011–12 developed new lifestyle-related diseases in 2014–15. The frequency of new onset of dyslipidemia in the active lifestyle group was significantly lower than that in the sedentary lifestyle group (P = 0.008). The frequency of new onset of diabetes mellitus or hypertension showed no significant differences between the two lifestyle groups (P = 0.740 and P = 0.222, respectively).
Table 1.
Relationship between active lifestyle and lifestyle diseases.
| All subjects | No onset of a disease | Onset of a disease | P value | ||
|---|---|---|---|---|---|
| Active lifestyle | Diabetes mellitus (n = 13 140) | ||||
| No | 11 801 (89.8) | 11 027 (89.8) | 774 (89.5) | 0.740 | |
| Yes | 1339 (10.2) | 1248 (10.2) | 91 (10.5) | ||
| Hypertension (n = 7173) | |||||
| No | 6508 (90.7) | 5322 (90.9) | 1186 (89.9) | 0.222 | |
| Yes | 665 (9.3) | 531 (9.1) | 134 (10.2) | ||
| Dyslipidemia (n = 8017) | |||||
| No | 7135 (89.0) | 5441 (88.5) | 1694 (90.7) | 0.008 | |
| Yes | 882 (11.0) | 708 (11.5) | 174 (9.3) | ||
Data are expressed as frequency (percentage). Between-group differences were assessed using the χ2 test. Two-sided P values <0.05 were considered indicative of statistical significance. BMI, body mass index.
Next, we examined the influence of active lifestyle on the plasma lipid profile (Table 2). In 2011–2012, triglycerides, LDL cholesterol in the active lifestyle group were not significantly different from those in the sedentary lifestyle group (P = 0.299, P = 0.587, respectively). HDL cholesterol levels in the active lifestyle group were significantly higher than that in the sedentary lifestyle group (P = 0.036). In contrast, triglyceride and LDL cholesterol levels in the active lifestyle group were significantly lower than those in the sedentary lifestyle group in 2014–15 (P = 0.028 and P = 0.003, respectively). HDL cholesterol in the active lifestyle group tended to be high (P = 0.069). These data indicated that an active lifestyle suppressed the deterioration of the plasma lipid profile and helped prevent the new onset of dyslipidemia. Therefore, we conducted further analyses to evaluate the association between the frequency of new onset of dyslipidemia and other lifestyle factors.
Table 2.
Comparison of plasma lipid profile and exercise habits.
| 2011–12 | 2014–15 | |||||
|---|---|---|---|---|---|---|
| Factors | Sedentary lifestyle (n = 7135) | Active lifestyle (n = 882) | P value | Sedentary lifestyle (n = 7135) | Active lifestyle (n = 882) | P value |
| Triglyceride (mg dl–1) | 85 ± 29 | 83 ± 29 | 0.299 | 93 ± 45 | 90 ± 42 | 0.028 |
| LDL cholesterol (mg dl–1) | 109 ± 20 | 109 ± 20 | 0.587 | 115 ± 26 | 112 ± 24 | 0.003 |
| HDL cholesterol (mg dl–1) | 64 ± 14 | 65 ± 15 | 0.036 | 64 ± 15 | 65 ± 16 | 0.069 |
Data are expressed as mean ± SD. Between-group differences were assessed using the unpaired t-test. Two-sided P values <0.05 were considered indicative of statistical significance. LDL, low-density lipoprotein; HDL, high-density lipoprotein.
RELATIONSHIP BETWEEN THE FREQUENCY OF NEW ONSET OF DYSLIPIDEMIA AND OTHER LIFESTYLE FACTORS
To clarify the lifestyle habits among individuals with dyslipidemia, the study participants were classified into two groups according to the presence or absence of dyslipidemia. The results are shown in Table 3.
Table 3.
Relationship between dyslipidemia and lifestyle.
| Factors | All subjects (n = 8017) | No dyslipidemia (n = 6149) | Dyslipidemia (n = 1868) | P value | |
|---|---|---|---|---|---|
| Age (years) | 62 ± 8 | 63 ± 8 | 62 ± 8 | <0.001 | |
| Follow-up period (person-years) | 3.4 ± 0.6 | 3.4 ± 0.6 | 3.4 ± 0.6 | 0.155 | |
| Sex | Male | 3429 (42.8) | 2636 (42.9) | 793 (42.5) | 0.750 |
| Female | 4588 (57.2) | 3513 (57.1) | 1075 (57.5) | ||
| Smoking habits | No | 5027 (62.8) | 3874 (63.1) | 1153 (61.9) | 0.341 |
| Yes | 2979 (37.2) | 2268 (36.9) | 711 (38.1) | ||
| Drinking habits | No | 6204 (95.5) | 4792 (95.5) | 1413 (95.7) | 0.699 |
| Yes | 289 (4.5) | 226 (4.5) | 63 (4.3) | ||
| Diabetes mellitus | No | 6739 (84.1) | 5158 (83.9) | 1581 (84.6) | 0.437 |
| Yes | 1278 (15.9) | 991 (16.1) | 287 (15.4) | ||
| Hypertension | No | 3201 (39.9) | 2469 (40.2) | 732(39.2) | 0.462 |
| Yes | 4814 (60.1) | 3679(59.8) | 1135 (60.8) | ||
| Eating snacks (≥3 days/week) | No | 7720 (96.5) | 5919 (96.5) | 1801 (96.5) | 0.972 |
| Yes | 284 (3.6) | 218 (3.6) | 66 (3.5) | ||
| Eating quickly | No | 6671 (83.3) | 5124 (83.5) | 1547 (83.0) | 0.609 |
| Yes | 1334 (16.7) | 1016 (16.5) | 318 (17.1) | ||
| Skipping breakfast (≥3 days/week) | No | 7798 (97.6) | 5997 (97.8) | 1801 (96.8) | 0.019 |
| Yes | 195 (2.4) | 136 (2.2) | 59 (3.2) | ||
| Obesity (BMI ≥25) | No | 6179 (77.1) | 4839 (78.7) | 1340 (71.7) | <0.001 |
| Yes | 1838 (22.9) | 1310 (21.3) | 528 (28.3) | ||
| Sleep habit | Not lacking sleep | 6594 (82.4) | 5086 (82.8) | 1513 (81.0) | 0.084 |
| Lacking sleep | 1411 (17.6) | 1057 (17.2) | 354 (19.0) | ||
| Experience of evacuation | No | 5229 (65.2) | 4072 (66.2) | 1157 (61.9) | <0.001 |
| Yes | 2788 (34.8) | 2077 (33.8) | 711 (38.1) |
Data are expressed as mean ± SD or as frequency (percentage). Between-group differences were assessed using the unpaired t-test or χ2 test. Two-sided P values <0.05 were considered indicative of statistical significance. BMI, body mass index.
Age in 2011–12 in the dyslipidemia group was significantly lower than that in the non-dyslipidemia group (P < 0.001). The follow-up period and sex showed no significant association with the frequency of new onset of dyslipidemia (P = 0.155 and P = 0.750, respectively). The frequency of new onset of dyslipidemia in individuals who skipped breakfast was significantly greater than in those who did not skip breakfast (P = 0.019). The frequency of new onset of dyslipidemia in individuals from the evacuation area was significantly higher than that in individuals from other regions (P < 0.001). Presence or absence of smoking habits (P = 0.341), drinking habits (P = 0.699), diabetes mellitus (P = 0.437), hypertension (P = 0.462), eating snacks (P = 0.972), eating quickly (P = 0.609), and sleep habit (P = 0.084) showed no significant association with the frequency of new onset of dyslipidemia.
ASSOCIATION BETWEEN ACTIVE LIFESTYLE AND THE FREQUENCY OF NEW ONSET OF DYSLIPIDEMIA
To investigate the association between active lifestyle and the frequency of new onset of dyslipidemia, we performed multivariate logistic regression analyses after adjusting for age and sex (Table 4). Based on the results of univariate analysis, sex, age, presence or absence of obesity, evacuation and active lifestyle were included in the multivariate analysis. In addition, given the potential influence of the difference in individual follow-up periods on the frequency of new onset of dyslipidemia, the follow-up period was also included as a variable. Based on results of the age- and sex-adjusted logistic regression analysis, age, obesity, evacuation and active lifestyle showed a significant association with the frequency of new onset of dyslipidemia. However, sex and follow-up period showed no significant association with the frequency of new onset of dyslipidemia. However, those factors were added to the multivariate logistic regression analysis to eliminate the effect of differences in sex, age and follow-up period. Multivariate logistic regression analysis was performed using sex, age, follow-up period, obesity, evacuation and active lifestyle as adjustment factors; active lifestyle (OR 0.82; 95% CI 0.68–0.97; P = 0.024), obesity (OR 1.46; 95% CI 1.29–1.64; P < 0.001) and evacuation (OR 1.14; 95% CI 1.02–1.28; P = 0.019) were significantly related to the frequency of new onset of dyslipidemia, independent of age, sex or follow-up period.
Table 4.
Results of univariate and multivariate logistic regression analyses showing factors associated with the frequency of new onset of dyslipidemia.
| Factors | Classification | No dyslipidemia (n = 6149) | Dyslipidemia (n = 1868) | Age- and sex- adjusted OR (95% CI) | P value | Multiple adjusted OR (95% CI) a (n = 8017) | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Continuous | 63 ± 8 | 62 ± 8 | 0.99 | (0.98–0.99) | <0.001 | 0.99 | (0.98–0.99) | <0.001 |
| Follow-up period (person-years) | Continuous | 3.4 ± 0.6 | 3.4 ± 0.6 | 1.06 | (0.98–1.16) | 0.151 | 1.04 | (0.95–1.14) | 0.377 |
| Sex | Men | 2636 (42.9) | 793 (42.5) | Ref. | Ref. | ||||
| Women | 3513 (57.1) | 1075 (57.5) | 1.00 | (0.90–1.11) | 0.926 | 0.99 | (0.89–1.11) | 0.907 | |
| Active lifestyle | No | 5441 (88.5) | 1694 (90.7) | Ref. | Ref. | ||||
| Yes | 708 (11.5) | 174 (9.3) | 0.82 | (0.69–0.98) | 0.031 | 0.82 | (0.68–0.97) | 0.024 | |
| Skipping breakfast (≥3 days/week) | No | 5997 (97.8) | 1801 (96.8) | Ref. | |||||
| Yes | 136 (2.2) | 59 (3.2) | 1.34 | (0.98–1.83) | 0.071 | ||||
| Obesity (BMI ≥25) | No | 4839 (78.7) | 1340 (71.7) | Ref. | Ref. | ||||
| Yes | 1310 (21.3) | 528 (28.3) | 1.47 | (1.31–1.66) | <0.001 | 1.46 | (1.29–1.64) | <0.001 | |
| Experience of evacuation | No | 4072 (66.2) | 1157 (61.9) | Ref. | Ref. | ||||
| Yes | 2077 (33.8) | 711 (38.1) | 1.19 | (1.07–1.32) | 0.002 | 1.14 | (1.02–1.28) | 0.019 | |
aAdjusted for age, follow-up period, sex, active lifestyle, obesity and experience of evacuation. 95% CI, 95% confidence interval; OR, odds ratio; Ref, reference; BMI, body mass index. Logistic regression model. Two-sided P values <0.05 were considered indicative of statistical significance.
In addition, we conducted two separate multivariate logistic regression analyses based on the presence or absence of evacuation (Table 5). The results showed a significant association of active lifestyle with the frequency of new onset of dyslipidemia in non-evacuees (n = 5229, OR 0.78; 95% CI 0.62–0.98; P = 0.030), independent of age, sex or follow-up period. The ORs for active lifestyle was comparable between non-evacuees and evacuees, but not significantly different among evacuees (n = 2778, OR 0.88; 95% CI 0.66–1.17; P = 0.371). However, the results suggested that an active lifestyle could be effective in reducing the new onset of dyslipidemia in evacuees.
Table 5.
Results of multivariate logistic regression analysis showing factors associated with the frequency of new onset of dyslipidemia disaggregated by the presence or absence of experience of evacuation.
| Non-evacuee (n = 5229) | Evacuee (n = 2788) | ||||||
|---|---|---|---|---|---|---|---|
| Factors | Classification | Multivariate OR (95% CI)a | P value | Multivariate OR (95% CI)a | P value | ||
| Age (years) | Continuous | 0.99 | (0.98–1.00) | 0.013 | 0.98 | (0.97–0.99) | 0.001 |
| Follow-up period (person-years) | Continuous | 1.04 | (0.94–1.16) | 0.419 | 1.04 | (0.88–1.22) | 0.669 |
| Sex | Men | Ref. | Ref. | ||||
| Women | 1.05 | (0.92–1.20) | 0.501 | 0.91 | (0.76–1.08) | 0.279 | |
| Active lifestyle | No | Ref. | Ref. | ||||
| Yes | 0.78 | (0.62–0.98) | 0.030 | 0.88 | (0.66–1.17) | 0.371 | |
| Obesity (BMI ≥ 25) | No | Ref. | Ref. | ||||
| Yes | 1.38 | (1.19–1.62) | <0.001 | 1.56 | (1.30–1.88) | <0.001 | |
aAdjusted for age, follow-up period, sex, active lifestyle and obesity. 95% CI, 95% confidence interval; OR, odds ratio; Ref, reference; BMI, body mass index. Logistic regression model. Two-sided P values <0.05 were considered indicative of statistical significance.
DISCUSSION
In this study, 1868 (23.3%) out of 8017 individuals without dyslipidemia in 2011–12 developed dyslipidemia in 2014–15. Out of the 14,932 individuals studied in this study, the number of individuals with dyslipidemia decreased from 6410 (42.9%) in 2011–2012 to 6065 (40.6%) in 2014–2015. The results of this study suggested that the prevelance of dyslipidemia was slightly lower than immediately after the Great East Japan Earthquake. Previously, Takahashi et al. compared the results of the FHMS between 2011–12 and 2013–14, and found a significant increase in the prevalence of dyslipidemia over this period (from 49.8% to 53.3%) [23]. These results suggested that more continuous observation is needed to discuss the increase or decrease of lipid abnormalities after the Great East Japan Earthquake.
In the Japanese National Health and Nutrition Survey (2017) conducted by the Ministry of Health, Labor and Welfare, the proportion of adult men and women with an active lifestyle (exercises that cause mild sweating for >30 min, twice per week, for over a year) was 37.8% and 27.3%, respectively [24]. Although the conditions were different, 174 out of 1868 (9.3%) individuals with dyslipidemia in this study had healthy exercise habits. This percentage was low compared with the average for the entire country. Murakami et al. conducted an analysis of National Nutrition Survey (2010) data; despite the relatively small sample size, they found that the number of steps taken by residents of temporary housing in the H district of the Iwate Prefecture was less than the average number of steps taken from 2006 to 2010 by residents of the Iwate Prefecture [25, 26]. In contrast, Nagai et al. found that the proportion of individuals who exercised more than twice a week (including for <1 year) in the FHMS was almost equivalent to the national average found in the National Health Nutrition Survey of 2011 [27]. The discrepancies in the reported exercise habits in previous studies are likely to be attributable to the use of different definitions for exercise habits and the different methods used to evaluate the amount of physical activity. Thus, future studies should evaluate the influence of the earthquake on exercise habits using homogeneous definitions and evaluation methods.
Physical inactivity decreases lipid oxidation and hormone-sensitive lipase activity, and reduces systemic fatty oxidation. Those who were evacuated had a significantly greater incidence of low HDL cholesterolemia than those who were not evacuated, and the prevalence of low HDL cholesterolemia increased from 6.0% to 7.2% in the individuals from the evacuation area [28]. High LDL cholesterol and low HDL cholesterol levels may lead to coronary heart disease [29–31]. Therefore, these results suggest that physical inactivity increases the risk of cardiovascular disease by causing deterioration in the plasma lipid profile in individuals from the evacuation area. We further found that maintaining an active lifestyle from baseline suppressed the deterioration in the plasma lipid profile. An active lifestyle was also significantly associated with a reduction of new onset of dyslipidemia, independent of sex, age or follow-up period. Exercise has been shown to improve systemic lipid metabolism and decrease the gene expression levels related to fatty acid transport and lipid metabolism in skeletal muscle [32].
Increased regular physical activity has been shown to reduce the risk of obesity, high blood pressure, diabetes mellitus [33–34], cardiovascular disease [35] and various types of cancers [36]. Several studies have demonstrated improvement in the plasma lipid profile with aerobic exercise, resistance training or a combination of the two [37–41]. These findings indicate that adoption of an active lifestyle can help improve the plasma lipid profile and prevent the new onset of dyslipidemia; this can be expected to reduce the incidence of cardiovascular disease. In the future, to reduce dyslipidemia incidence in individuals from the evacuation area, more aggressive interventions designed to inspire an active lifestyle are recommended.
In addition to exercise habits, those who perform active physical activity had a reduced frequency of new onset of dyslipidemia. In recent studies, ‘sedentary behavior’ (defined as ‘any waking behavior characterized by an energy expenditure of ≤1.5 METs while in a sitting or reclining posture’ [42]) was found to have a harmful effect on the health status [43–46]. Implementation of mid- and high-intensity physical activity, which has been gaining attention in epidemiological studies on exercise, represent ~5% of the daily awake time, whereas low-intensity physical activity represents ~35–40% and sedentary behaviors represent 55–60% of the daily awake time [47, 48]. Therefore, replacing sedentary behaviors with even low-intensity physical activity for a long duration can accrue considerable health benefits. Studies have also shown that prolonging the standing time instead of sitting or lying down time prevents weight gain by increasing energy consumption and may even promote weight reduction in the long term [49]. Therefore, to prevent dyslipidemia in individuals in the evacuation area, we should aim to establish healthy exercise habits as well as encourage a reduction in sedentary behavior.
A previous study documented an increase in HbA1c level after the earthquake; the findings indicated a significant association between evacuation and incidence of diabetes mellitus [2]. Recently, Ohira et al. postulated that the evacuation of the Fukushima Prefecture after the Great East Japan Earthquake was related to an increased risk of hypertension among men [50]. Ohira et al. also found an increase in the proportion of obese individuals in the Fukushima Prefecture evacuation areas after the Great East Japan Earthquake [3]. Hashimoto et al. reported that evacuees had a higher BMI, greater waist circumference and higher triglyceride and fasting plasma glucose levels than non-evacuees after the Great East Japan Earthquake; in addition, evacuation was associated with increased incidence of metabolic syndrome [4]. Likewise, our study also found a significant association between living in an evacuation area and dyslipidemia; thus, experiencing an evacuation may be a risk factor for dyslipidemia. We recommend continued follow-up of individuals who were actually evacuated in order to monitor the incidence of lifestyle-related diseases.
Metabolic syndrome is known to increase the risk of cardiovascular disease [51]. However, lifestyle interventions for patients with metabolic syndrome can reduce obesity and therefore also reduce the risk of cardiovascular disease in the long term [52]. Our study suggests the need to provide comprehensive treatment for metabolic syndrome to maintain and promote the health of evacuees who are considered to be at high risk of developing cardiovascular diseases.
The present study has some limitations worth noting. In this study, data pertaining to exercise or physical activity habits were missing for 17 357 (53.8%) of 32 289 individuals who underwent medical examination in both 2011–12 and 2014–15. Consequently, there were many deficiencies in the answers to inquiries about active physical exercise and exercise habits at baseline; this also limited the number of individuals included in the analysis. It is possible that our data do not accurately reflect the activity levels of people from the entire evacuation area. Owing to the selection or information bias, our results may not be entirely representative of the residents of the Fukushima evacuation zone. For a more accurate evaluation of the amount of physical activity in the evacuation area, future studies should also include detailed investigations using equipment such as pedometers which semi-quantitatively measure the number of steps taken and the physical activity performed.
As another possible limitation of this study, the lifestyle survey was performed by using questionnaires, which may not accurately reflect the actual amount of physical activity and exercise. Therefore, it was not possible to determine an appropriate amount of physical activity and exercise to prevent dyslipidemia.
In addition, in this study, an active lifestyle showed no significant association with diabetes or hypertension. This may be attributable to the higher proportion of individuals with an active lifestyle among those with newly developed diabetes or hypertension. It is also plausible that individuals who tend to have higher blood sugar and blood pressure start exercising before the onset, but do not control the onset. However, this study was a cross-sectional analysis that did not permit any causal inferences. Future studies should positively consider the items used for the judgment only for those who were within the normal range at the baseline.
Diet also plays an important role in the prevention and treatment of dyslipidemia. However, as a possible limitation of our study, we did not find a significant association between dietary habits and dyslipidemia. In contrast, a previous study has suggested that psychological distress and the living environment after the Great East Japan Earthquake led to a disruption of eating habits [8, 9]. Therefore, we suspect that disruption of eating habits due to the stress induced by a changed living environment may have contributed to the new onset of dyslipidemia in the evacuation area. In future, we recommend not only investigating the dietary habits but also assessing the actual dietary intake, and to correct dietary imbalance by providing dietary instructions to individuals.
In addition, smoking habits did not show a significant association with the frequency of new onset of dyslipidemia. However, some studies have reported that smoking has a direct effect on lipid metabolism [53, 54]. In a study based on NIPPON DATA 80, smoking ≥21 cigarettes per day was found to be a risk factor for stroke in men and women [21]. In the study by Lubin et al., a 20 cigarettes per day habit was associated with a 2.1-fold higher relative risk of cardiovascular disease, based on the Atherosclerosis Risk in Communities study results [55]. Thus, smoking habits may cause dyslipidemia and cardiovascular disease. Future studies should also examine the association between smoking habits, lifestyle-related diseases and the incidence of cardiovascular disease among victims of the Great East Japan Earthquake through a longer follow-up.
In summary, in this study, active lifestyle, obesity, and evacuation were related to the new onset of dyslipidemia among recidents of the evacuation area in the Fukushima Prefecture after the Great East Japan Earthquake, independent of sex, age or follow-up period. Our study highlights the need for targeted interventions to inculcate healthy exercise habits in order to prevent the new onset of dyslipidemia in the Fukushima evacuation area.
ACKNOWLEDGEMENTS
We thank the staff of the Fukushima Health Management Survey for their cooperation. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Fukushima Prefecture government. The authors would like to thank Enago (www.enago.jp) for the English language review.
FUNDING
This survey was supported by the National Health Fund for Children and Adults Affected by the Nuclear Incident for design and conduct of the study.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest with respect to this research study and paper.
SUPPLEMENT FUNDING
This supplement has been funded by the Program of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University.
AUTHOR CONTRIBUTIONS
F.H. and T.O. contributed to the design of the present study. T.O., A.S., M.H., M.S., A.T., J.K., S.Y., S.H., Y.K., G.K., H.O. and K.K. were responsible for data collection and overseeing the study procedures. The analysis was conducted by F.H. The manuscript was written by F.H. T.O., K.O., H.N., A.S., M.H., M.S., A.T., J.K., S.Y., S.H., Y.K., G.K., H.O. and K.K. made significant contributions to the critical interpretation of the results in terms of important practical content. All authors read and approved the final version of the manuscript.
REFERENCES
- 1. Tsubokura M, Takita M, Matsumura T et al. Changes in metabolic profiles after the great East Japan earthquake: a retrospective observational study. BMC Public Health 2013;13:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Satoh H, Ohira T, Hosoya M et al. Evacuation after the Fukushima Daiichi nuclear power plant accident is a cause of diabetes: results from the Fukushima Health Management Survey. J Diabetes Res 2015;2015:627390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohira T, Hosoya M, Yasumura S et al. Effect of evacuation on body weight after the great East Japan earthquake. Am J Prev Med 2016;50:553–60. [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto S, Nagai M, Fukuma S et al. Influence of post-disaster evacuation on incidence of metabolic syndrome. J Atheroscler Thromb 2017;24:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakai A, Ohira T, Hosoya M et al. Life as an evacuee after the Fukushima Daiichi nuclear power plant accident is a cause of polycythemia: the Fukushima Health Management Survey. BMC Public Health 2014;14:1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzuki H, Ohira T, Takeishi Y et al. Increased prevalence of atrial fibrillation after the great East Japan earthquake: results from the Fukushima Health Management Survey. Int J Cardiol 2015;198:102–5. [DOI] [PubMed] [Google Scholar]
- 7. Kunii Y, Suzuki Y, Shiga T et al. Severe psychological distress of evacuees in evacuation zone caused by the Fukushima Daiichi nuclear power plant accident: the Fukushima Health Management Survey. PLoS One 2016;11:e0158821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uemura M, Ohira T, Yasumura S et al. Association between psychological distress and dietary intake among evacuees after the great East Japan earthquake in a cross-sectional study: the Fukushima Health Management Survey. BMJ Open 2016;6:e011534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Ohira T, Abe M et al. Evacuation after the great East Japan earthquake was associated with poor dietary intake: the Fukushima Health Management Survey. J Epidemiol 2017;27:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee IM, Shiroma EJ, Lobelo F et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO:Global Health Risks . 2009. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (12 April 2019, date last accessed).
- 12. Ikeda N, Inoue M, Iso H et al. Adult mortality attributable to preventable risk factors for non-communicable diseases and injuries in Japan: a comparative risk assessment. PLoS Med 2012;9:e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasumura S, Hosoya M, Yamashita S et al. Study protocol for the Fukushima Health Management Survey. J Epidemiol 2012;22:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kashiwagi A, Kasuga M, Araki E et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012;3:39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Obesity . Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity, Geneva. 3. Global Prevalence and Secular Trends in Obesity. Geneva: World Health Organization, 1997. [PubMed] [Google Scholar]
- 16. Yoshiike N, Matsumura Y, Zaman MM et al. Descriptive epidemiology of body mass index in Japanese adults in a representative sample from the National Nutrition Survey 1990–1994. Int J Obes Relat Metab Disord 1998;22:684–68. [DOI] [PubMed] [Google Scholar]
- 17. Examination Committee of Criteria for ‘Obesity Disease’ in Japan . Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002;66:987–92. [DOI] [PubMed] [Google Scholar]
- 18. Willi C, Bodenmann P, Ghali WA et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–64. [DOI] [PubMed] [Google Scholar]
- 19. Groppelli A, Omboni S, Parati G et al. Blood pressure and heart rate response to repeated smoking before and after beta-blockade and selective alpha 1 inhibition. J Hypertens Suppl 1990;8:S35–40. [PubMed] [Google Scholar]
- 20. Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ 1989;298:784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ueshima H, Choudhury SR, Okayama A et al. Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke 2004;35:1836–41. [DOI] [PubMed] [Google Scholar]
- 22. International guidelines for ethical review of epidemiological studies. Law Med Healthcare 1991;19:247–58. [PubMed] [Google Scholar]
- 23. Takahashi A, Ohira T, Uemura M et al. Changes in hepatobiliary enzyme abnormality after the great East Japan earthquake: the Fukushima Health Management Survey. Sci Rep 2017;7:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ministry of Health, Labour and Welfare . National Health and Nutrition Survey. http://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html (7 February 2020, date last accessed).
- 25. Murakami H, Yoshimura E, Ishikawa-Takata K et al. Validity and reproducibility of a physical activity questionnaire used for health surveying among victims of the great East Japan earthquake. Nihon Koshu Eisei Zasshi 2013;60:222–30 (in Japanese). [PubMed] [Google Scholar]
- 26. Murakami H, Yoshimura E, Ishikawa-Takata K et al. The longitudinal change in physical activity among great East Japan earthquake victims living in temporary housing. Nihon Koshu Eisei Zasshi 2014;61:86–92 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 27. Nagai M, Ohira T, Yasumura S et al. Association between evacuation condition and habitual physical activity in great East Japan earthquake evacuees: the Fukushima Health Management Survey. Nihon Koshu Eisei Zasshi 2016;63:3–10 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 28. Satoh H, Ohira T, Nagai M et al. Hypo-high-density lipoprotein cholesterolemia is caused by evacuation after the Fukushima Daiichi nuclear power plant accident: results from the Fukushima Health Management Survey. Intern Med 2016;55:1967–76. [DOI] [PubMed] [Google Scholar]
- 29. Chen G, Levy D. Contributions of the Framingham Heart Study to the epidemiology of coronary heart disease. JAMA Cardiol 2016;1:825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gordon T, Castelli WP, Hjortland MC et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707–14. [DOI] [PubMed] [Google Scholar]
- 31. Wilson PW. High-density lipoprotein, low-density lipoprotein and coronary artery disease. Am J Cardiol 1990;66:7A–10A. [DOI] [PubMed] [Google Scholar]
- 32. Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity (Silver Spring) 2009;17:S27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nemoto K, Gen-no H, Masuki S et al. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc 2007;82:803–11. [DOI] [PubMed] [Google Scholar]
- 34. Smith AD, Crippa A, Woodcock J et al. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose–response meta-analysis of prospective cohort studies. Diabetologia 2016;59:2527–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noda H, Iso H, Toyoshima H et al. Walking and sports participation and mortality from coronary heart disease and stroke. J Am Coll Cardiol 2005;46:1761–70. [DOI] [PubMed] [Google Scholar]
- 36. Moore SC, Lee IM, Weiderpass E et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016;176:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferguson MA, Alderson NL, Trost SG et al. Effects of four different single exercise sessions on lipids, lipoproteins, and lipoprotein lipase. J Appl Physiol (1985) 1998;85:1169–74. [DOI] [PubMed] [Google Scholar]
- 38. Banz WJ, Maher MA, Thompson WG et al. Effects of resistance versus aerobic training on coronary artery disease risk factors. Exp Biol Med (Maywood) 2003;228:434–40. [DOI] [PubMed] [Google Scholar]
- 39. Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med 2014;44:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lira FS, Yamashita AS, Uchida MC et al. Low and moderate, rather than high intensity strength exercise induces benefit regarding plasma lipid profile. Diabetol Metab Syndr 2010;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fett CA, Fett WC, Marchini JS. Circuit weight training vs jogging in metabolic risk factors of overweight/obese women. Arq Bras Cardiol 2009;93:519–25. [DOI] [PubMed] [Google Scholar]
- 42. Sedentary Behaviour Research Network . Letter to the editor: standardized use of the terms ‘sedentary’ and ‘sedentary behaviours’. Appl Physiol Nutr Metab 2012;37:540–2. [DOI] [PubMed] [Google Scholar]
- 43. Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA 2011;305:2448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katzmarzyk PT, Church TS, Craig CL et al. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 2009;41:998–1005. [DOI] [PubMed] [Google Scholar]
- 45. Thorp AA, Owen N, Neuhaus M et al. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011. Am J Prev Med 2011;41:207–15. [DOI] [PubMed] [Google Scholar]
- 46. Wijndaele K, Brage S, Besson H et al. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC Norfolk study. Int J Epidemiol 2011;40:150–9. [DOI] [PubMed] [Google Scholar]
- 47. Owen N. Sedentary behavior: understanding and influencing adults’ prolonged sitting time. Prev Med 2012;55:535–9. [DOI] [PubMed] [Google Scholar]
- 48. Santos-Lozano A, Marín PJ, Torres-Luque G et al. Technical variability of the GT3X accelerometer. Med Eng Phys 2012;34:787–90. [DOI] [PubMed] [Google Scholar]
- 49. Saeidifard F, Medina-Inojosa JR, Supervia M et al. Differences of energy expenditure while sitting versus standing: a systematic review and meta-analysis. Eur J Prev Cardiol 2018;25:522–38. [DOI] [PubMed] [Google Scholar]
- 50. Ohira T, Hosoya M, Yasumura S et al. Evacuation and risk of hypertension after the great East Japan earthquake: the Fukushima Health Management Survey. Hypertension 2016;68:558–64. [DOI] [PubMed] [Google Scholar]
- 51. Mottillo S, Filion KB, Genest J et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–32. [DOI] [PubMed] [Google Scholar]
- 52. Ilanne-Parikka P, Eriksson JG, Lindström J et al. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish diabetes prevention study. Diabetes Care 2008;31:805–7. [DOI] [PubMed] [Google Scholar]
- 53. Maeda K, Noguchi Y, Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med 2003;37:283–90. [DOI] [PubMed] [Google Scholar]
- 54. Slagter SN, van Vliet-Ostaptchouk JV, Vonk JM et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med 2013;11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lubin JH, Couper D, Lutsey PL et al. Risk of cardiovascular disease from cumulative cigarette use and the impact of smoking intensity. Epidemiology 2016;27:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]


