Abstract
Patient-reported outcomes (PROs) for patients with myelofibrosis (MF) have been well characterized, but little is known about quality of life (QoL) following allogeneic stem cell transplantation (allo-SCT). Medical data and PRO measures were collected before transplant and at day 30, day 100, and 1 year after allo-SCT. PRO measures include Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF), Brief Fatigue Inventory, Global Assessment of Change, and Functional Assessment of Cancer Therapy–Bone Marrow Transplant. Forty-four patients who had baseline QoL and at least 1 post-transplant assessment were included. The median age of the patients was 62.5 years (range, 35 to 74 years). At baseline, the mean MPN Total Symptom Score was 28.0, and at day 30, day 100, and 1 year, it was 25.4, 32.3, and 24.3, respectively. However, in myeloproliferative neoplasm-specific symptoms, such as itching, night sweats, bone pain, and fever, a statistically significant improvement was observed for at least 1 time point following transplant. At day 30, 10 (26.3%) patients reported a little/moderately/very much better overall QoL since their transplant, and 26 (68.45%) had a little/moderately/very much worse QoL. At day 100, 10 (30.3%) reported better QoL and 19 (57.6%) reported worsening since transplant. By 1 year, 16 (61.5%) reported feeling better. Our study shows that there is very little change in symptom burden at 1 year following transplant in general, but MF-specific symptoms showed improvement. By 1 year, 61% felt that their QoL was better than it was before transplant.
Keywords: Myelofibrosis, Bone marrow transplant, Quality of life
INTRODUCTION
Myelofibrosis (MF) is a bone marrow disorder characterized by fibrosis of the bone marrow, splenomegaly, constitutional symptoms, leukocytosis, and anemia. Allogeneic stem cell transplant is a treatment option for patients who have primary MF, post-polycythemia vera MF, and post-essential thrombocytosis MF [1–10]. This treatment carries a significant risk profile but can result in long-term disease-free survival. Patients who are young and have a good performance status are considered for transplant, although increasing data suggest that older patients may benefit as well [11]. Timing of transplant remains a difficult decision but frequently is based on risk of disease. A well-accepted prognostic system is the Dynamic International Prognostic Scoring System [12], a prognostic score derived from hemoglobin, total WBC count, percentage of blasts, age, and constitutional symptoms. Transplant is felt to provide the most benefit when patients have intermediate-2 or high-risk disease, but younger patients who have intermediate-1 risk disease and high-risk features based on karyotype or genetic mutations such as ASXL1 may also experience a benefit [11,13]. Survival at 5 years has been reported between 38% and 75% [14,15], treatment-related mortality ranges from 25% to 40% [13], and disease relapse occurs in 15% to 20% of patients [13].
The symptom burden of MF has been well established [16–19]. Symptoms such as fatigue, abdominal pain, weight loss, pruritis, anorexia, bone pain, fever, and night sweats are very common in myeloproliferative neoplasms (MPNs) [16] and appear to be worse in patients with MF [16,19]. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) was established as a means of capturing MF-specific symptoms [20]. The MPN-SAF has been validated internationally [22]. However, subsequently, this form has been modified to include the 10 most pertinent symptoms and is called MPN Total Symptom Score (MPN-TSS) or MPN-10 [17,23]. This score has gained acceptance as a measure of symptom burden in patients with MF and is also included as part of the International Working Group (IWG) response criteria [24].
Quality of life (QoL) after transplant has been investigated in many settings. There has been very clear evidence that QoL declines in the first 30 to 100 days after transplant and improves by 1 to 2 years [25–29]. However, to our knowledge, this has never been investigated in patients with MF. Although symptom burden is central to the disease, patients generally proceed to transplant based on evidence of disease progression, and little is known regarding the impact of transplant on symptom burden.
METHODS
This study was done under an institutional review board approved protocol. Patients were enrolled within 1 to 2 months of the planned transplant. Clinical information was collected, including disease characteristics, planned conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, and stem cell source. Baseline patient-reported outcome (PRO) data were collected, including the Brief Fatigue Inventory (BFI), Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT), and MPN-SAF. At day 30, day 100, and 1 year, medical information including presence/absence of GVHD was collected, as well as disease status. The same PRO data were also measured at those time points. Assessments were done in the clinic room or in the hospital if the patient was an inpatient at that time. The day 30 and day 100 assessments were done within 1 week of the time point, and the 1-year assessment was done within a month. All the surveys were done on paper.
MPN-SAF
The MPN-SAF was established as a means of capturing MF-specific symptoms [20]. In its initial inception, it was administered with the BFI [21]. The MPN-SAF has been validated internationally [22]. However, subsequently this form has been modified to include the 10 most pertinent symptoms and is called the MPN-TSS or MPN-10 [17,23]. This score includes 9 pertinent symptoms from the MPN-SAF (concentration, early satiety, inactivity, night sweats, itching, bone pain, abdominal discomfort, weight loss, and fever), as well as queries on the worst fatigue from the BFI [21,23]. Each symptom is rated from 0 to 10, with 0 being no symptoms and 10 being a significant symptom burden; therefore, the higher the score, the worse the symptoms.
FACT-BMT
The FACT-BMT is a 50-item validated self-report questionnaire specifically designed to test QoL in bone marrow transplant (BMT) patients [29,30]. The FACT-BMT is composed of 5 subscales, including physical well-being (PWB), social/family well-being, emotional well-being (EWB), functional well-being (FWB), and additional concerns specific to patients undergoing BMT.
Global Assessment of Change
Perception of change was evaluated with 3 Global Impression of Change items. Global Impression of Change Scale (also called the Subjective Significance Scale) has been used as an anchor for determining minimally clinically significant differences in numerous oncology clinical trials within the North Central Cancer Treatment Group (N00C3, N01C3, N01CB, N03CA) [14,15].
Patient demographics and clinical characteristics were summarized by descriptive statistics. For symptom assessments and QoL measures, mean changes (±95% confidence interval) from baseline were compared by t tests and summarized over time graphically. We also evaluated patients in the highest quartile of the MPN-TSS score to see whether they experienced improvement or worsening of QoL. Correlation between MPN-TSS and FACT-BMT domains was compared by use of Pearson correlation coefficients at baseline. Baseline TSS score was compared with a general cross-sectional population of intermediate/high-risk patients with MF for comparison by t test. SAS version 9.4 (SAS Institute, Cary, NC) was used for analysis.
RESULTS
This study enrolled 50 patients; 4 patients opted to not complete baseline QoL and symptom surveys (for unknown reasons) and 2 patients did not proceed to transplant (Table 1). The median age of the remaining 44 patients was 62.5 years (range, 35 to 74 years). Most of the patients were white (n = 43, 98%) and male (n = 28, 65%). Twenty-five (58%) patients had primary MF. Eighteen patients had either polycythemia vera MF or essential thrombocytosis MF. Dynamic International Prognostic Scoring System intermediate-2 risk disease was seen in 20 patients, and 16 patients had high-risk disease. Twenty-five patients (57%) had received prior treatment with ruxolitinib. Eleven patients (27%) had a matched related donor, and 24 (59%) had a matched unrelated donor. Most patients received reduced-intensity conditioning regimens (n = 32, 84%), and all patients had calcineurin inhibitor for GVHD prophylaxis. Methotrexate was given to 12 patients, and the other patients had mycophenolate mofetil (MMF). Thirty-two patients received anti-thymocyte globulin (ATG). Thirty-eight patients completed the assessment at day 30, 34 patients at day 100, and 27 patients at 1 year. Eight patients died during the first year.
Table 1.
Patient Demographics and Clinical Characteristics (N = 44)
| Characteristic | Value |
|---|---|
| Age, median (range), yr | 62.5 (35.0–74.0) |
| Female | 15 (34.9) |
| Male | 28 (65.1) |
| Unknown | 1 |
| White | 43 (97.7) |
| Black/African American | 1 (2.3) |
| Single | 2 (4.7) |
| Married/partner | 37 (86.0) |
| Separated | 1 (2.3) |
| Divorced | 1 (2.3) |
| Widowed | 2 (4.7) |
| Unknown | 1 |
| No | 29 (72.5) |
| Yes | 11 (27.5) |
| Unknown | 4 |
| PMF | 25 (58.1) |
| Post ET-MF | 9 (20.9) |
| Post PV-MF | 9 (20.9) |
| Unknown | 1 |
| Years since MF diagnosis, median (range) | 2.0 (0.0–21.0) |
| Yes | 31 (72.1) |
| No | 12 (27.9) |
| Unknown | 1 |
| Yes | 3 (7.0) |
| No | 40 (93.0) |
| Unknown | 1 |
| Yes | 9 (21.4) |
| No | 33 (78.6) |
| Unknown | 2 |
| 0 (low risk) | 2 (4.5) |
| 2 (intermediate 1) | 6 (13.6) |
| 3 (intermediate 2) | 10 (22.7) |
| 4 (intermediate 2) | 10 (22.7) |
| 5 (high) | 15 (34.1) |
| 6 (high) | 1 (2.3) |
| Yes | 19 (44.2) |
| No | 24 (55.8) |
| Unknown | 1 |
| Yes | 4 (9.8) |
| No | 37 (90.2) |
| Unknown | 3 |
| 10.0 (0.0–27.0) | |
| HLA-identical related | 11 (26.8) |
| HLA-mismatched related | 1 (2.4) |
| HLA-identical unrelated | 24 (58.5) |
| HLA-mismatched unrelated | 5 (12.2) |
| Unknown | 3 |
| RIC Bu/Flu | 16 (40.0) |
| RIC Flu/thiotepa/busulfan | 2 (5.0) |
| FLASMA-Bu | 8 (20.0) |
| RIC treosulfan/Flu | 3 (7.5) |
| MAC Bu/Flu | 4 (10.0) |
| RIC FBM | 7 (17.5) |
| Unknown | 4 |
| High dose | 6 (15.8) |
| Reduced/low intensity | 32 (84.2) |
| Missing | 6 |
| CNI/MMF | 28 (70.0) |
| CNI/MTX | 12 (30.0) |
| Missing | 4 |
| Yes | 32 (80.0) |
| No | 8 (20.0) |
| Unknown | 4 |
| Yes | 18 (40.0) |
| No | 26 (59.1) |
| Yes | 16 (36.4) |
| No | 28 (63.6) |
PMF indicates primary myelofibrosis; ET-MF, essential thrombocytosis myelofibrosis; PV-MF, polycythemia vera myelofibrosis; DIPSS, Dynamic International Prognostic Scoring System; RIC, reduced-intensity conditioning; Bu, Busulfan; Flu, Fludarabine; FLASMA, fludarabine, intermediate dose Ara-C, amsacrine, total body irradiation/busulfan, cyclophosphamide; MAC, myeloablative conditioning; FBM, Fludarabine, carmustine, melphalan; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil; MTX, methotrexate; ATG, anti-thymocyte globulin; HSCT, hematopoietic stem cell transplantation; CMV, cytomegalovirus.
MPN-TSS and FACT-BMT
At baseline, the mean MPN-TSS was 28.0, and at day 30, day 100, and 1 year, it was 25.4, 32.3, and 24.35, respectively (Table 2). Although there was a slight decline between baseline and 1 year, the change in MPN-TSS was not significant (Figure 1). Over the course of the first year, generally symptoms became worse at day 30 and returned close to a baseline level by 1 year. When looking at specific symptoms, there were a few notable findings. First, symptoms such as early satiety, abdominal pain, weight loss, and inactivity worsened significantly at day 30 but returned to baseline by day 100 and 1 year. MPN-specific symptoms, such as itching, night sweats, bone pain, and fever, improved significantly in at least 1 time point following transplant (Figure 2). Significant changes were appreciated in patients in the highest quartile based on baseline TSS score (12 patients with scores at baseline greater than or equal to 37) at day 100 (Figure 1B).
Table 2.
MPN-SAF Scores by Visit, Mean (SD)
| Characteristic | Baseline (n = 43) | Day 30 (n = 38) | Day 100 (n = 34) | Year 1 (n = 27) |
|---|---|---|---|---|
| BFI | 3.6 (1.9) | 5.1 (2.1) | 3.9 (2.2) | 3.8 (2.7) |
| Worst fatigue (BFI) | 5.8 (2.5) | 6.6 (2.3) | 5.4 (2.2) | 4.9 (2.6) |
| Early satiety | 3.1 (2.8) | 5.1 (3.0) | 3.5 (2.5) | 3.0 (3.1) |
| Abdominal pain | 1.4 (2.0) | 2.7 (2.9) | 1.6 (2.5) | 1.7 (2.4) |
| Abdominal discomfort | 1.5 (1.8) | 3.0 (3.1) | 1.8 (2.4) | 2.0 (2.5) |
| Inactivity | 4.0 (2.9) | 5.4 (2.7) | 3.6 (2.6) | 3.8 (2.9) |
| Headache | 2.3 (2.9) | 1.3 (2.4) | 1.3 (2.1) | 1.8 (2.8) |
| Concentration | 3.5 (3.1) | 4.2 (3.2) | 3.3 (2.5) | 2.7 (2.7) |
| Dizziness | 2.0 (2.4) | 2.8 (2.8) | 2.5 (2.9) | 2.4 (2.5) |
| Numbness | 1.8 (2.4) | 1.7 (2.5) | 1.7 (2.2) | 1.8 (2.2) |
| Insomnia | 4.4 (3.0) | 5.5 (2.9) | 3.1 (2.4) | 3.9 (3.3) |
| Sad mood | 2.0 (2.6) | 2.7 (2.7) | 1.8 (2.2) | 3.0 (3.2) |
| Sexuality | 4.1 (3.5) | 4.9 (3.8) | 4.1 (4.0) | 4.7 (3.7) |
| Cough | 1.2 (1.8) | 2.2 (2.5) | 0.7 (1.4) | 2.0 (2.7) |
| Night sweats | 3.1 (3.3) | 0.8 (1.9) | 0.4 (1.1) | 1.5 (2.9) |
| Itching | 1.7 (2.6) | 1.9 (2.7) | 0.6 (0.9) | 2.0 (2.6) |
| Bone pain | 2.3 (2.8) | 1.2 (1.9) | 1.7 (2.2) | 1.8 (2.5) |
| Fever | 0.8 (1.8) | 0.8 (1.9) | 0.1 (0.3) | 0.2 (0.6) |
| Weight loss | 2.0 (2.8) | 3.3 (3.6) | 5.0 (3.4) | 2.1 (3.3) |
| Overall QoL | 4.2 (2.7) | 4.0 (2.4) | 5.1 (2.7) | 4.8 (3.2) |
| MPN-SAF TSS | 28.0 (15.5) | 25.4 (10.6) | 32.3 (14.9) | 24.3 (20.3) |
Figure 1.
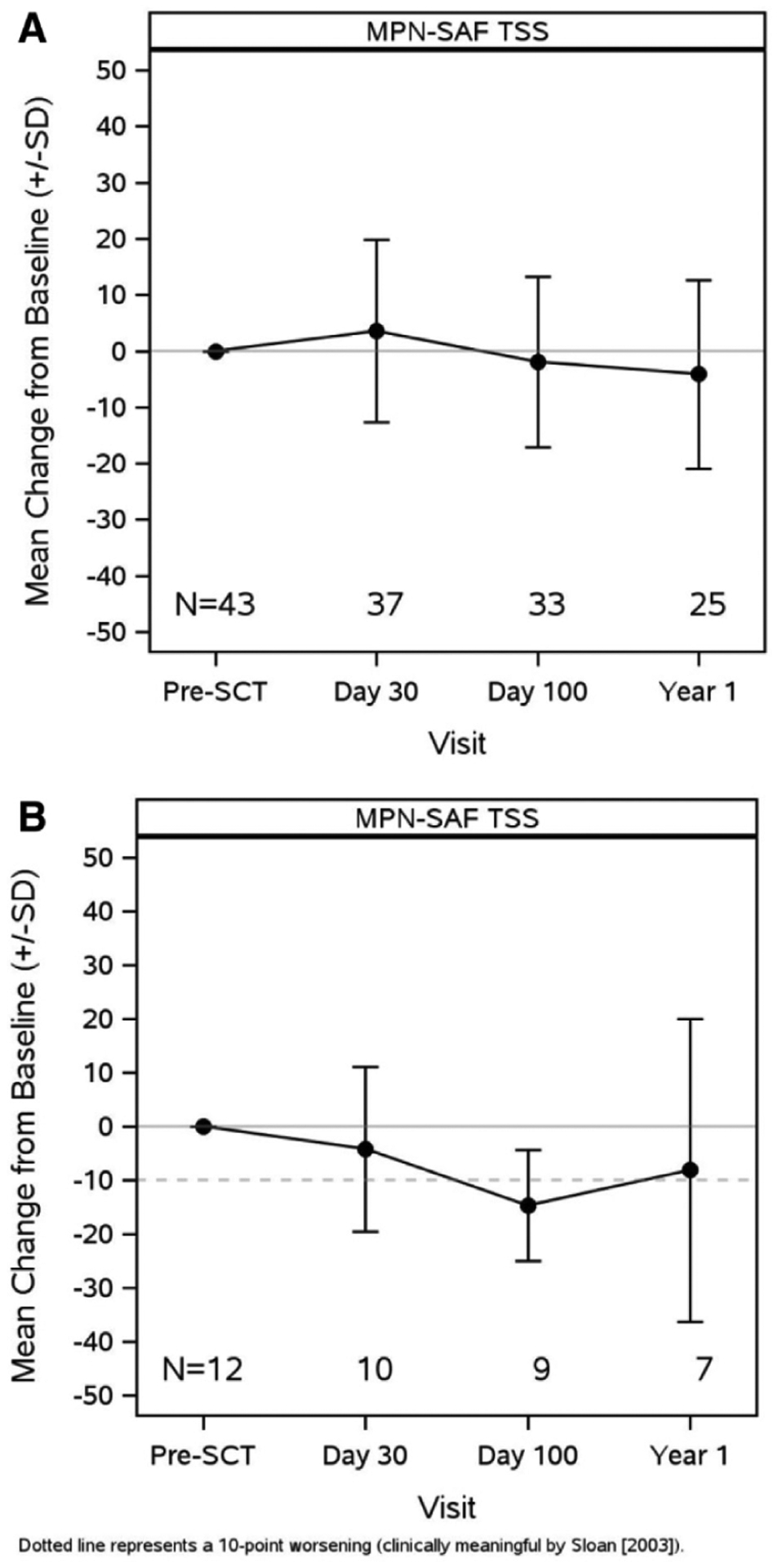
MPN-SAF TSS changes from baseline (A) in all patients and (B) in those with highest TSS.
Figure 2.
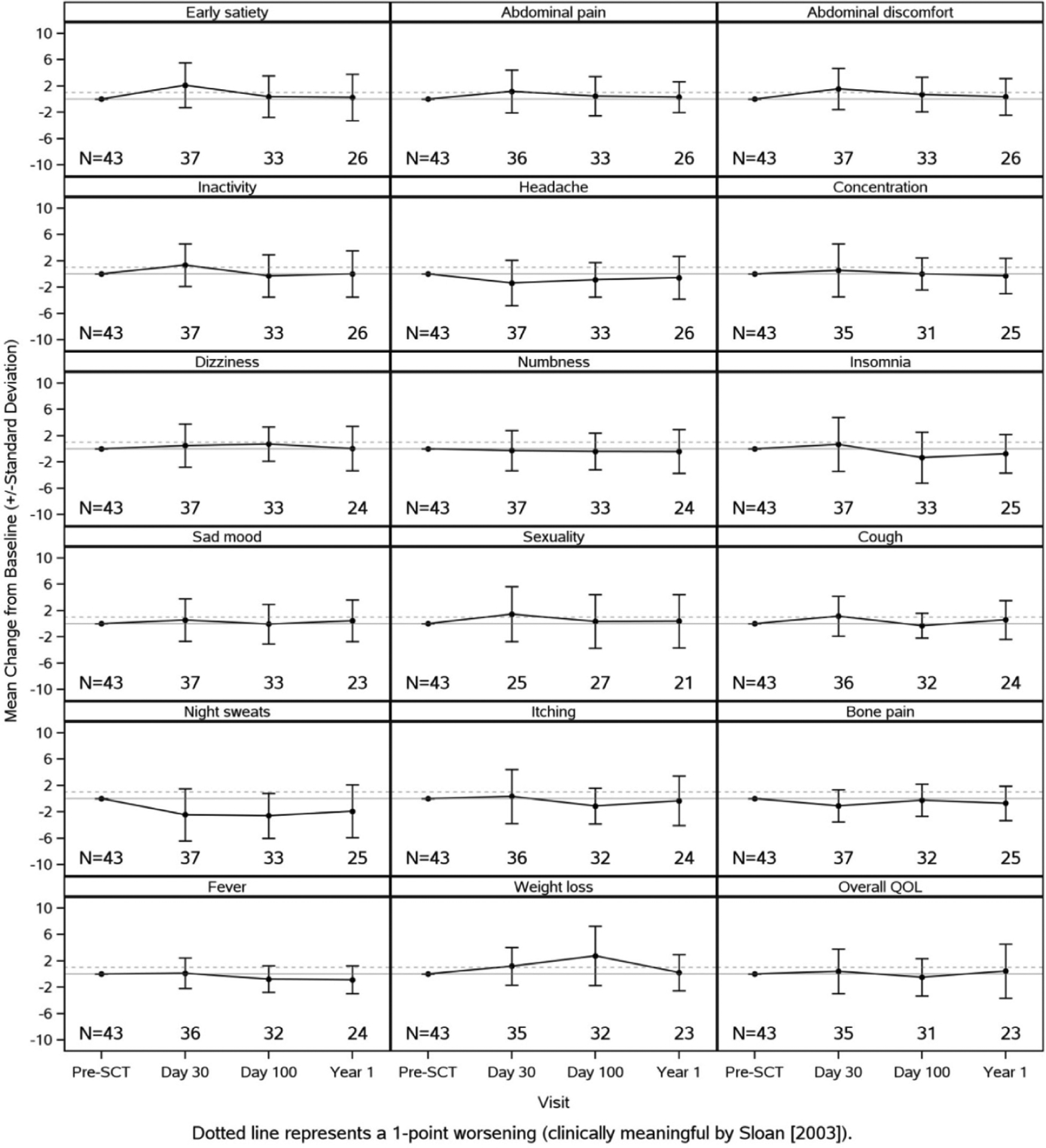
MPN-SAF changes from baseline for symptoms.
At baseline, the mean FACT-BMT was 101.1, and at day 30, day 100, and 1 year, it was 91.7, 96.7, and 99.4, respectively (Table 3). As expected, there was a significant decline in the FACT-BMT total score at day 30 but a return to close to baseline by 1 year.
Table 3.
FACT-BMT Scores by Visit, Mean (SD)
| Characteristic | Baseline (n = 44) | Day 30 (n = 38) | Day 100 (n = 33) | Year 1 (n = 26) |
|---|---|---|---|---|
| Physical well-being | 19.7 (6.0) | 15.2 (7.4) | 18.4 (6.8) | 20.8 (6.2) |
| Social/family well-being | 22.7 (3.9) | 23.2 (3.7) | 22.2 (4.6) | 20.4 (5.6) |
| Emotional well-being | 17.3 (4.3) | 18.8 (3.8) | 18.2 (4.5) | 17.2 (5.6) |
| Functional well-being | 14.2 (6.0) | 10.8 (5.6) | 12.8 (5.6) | 15.1 (6.7) |
| FACT-G | 73.9 (14.1) | 68.0 (13.5) | 71.7 (17.1) | 73.6 (20.7) |
| BMT additional concerns | 27.2 (4.4) | 23.5 (5.0) | 25.0 (5.9) | 25.8 (7.7) |
| FACT-BMT | 101.1 (18.1) | 91.7 (17.1) | 96.7 (22.0) | 99.4 (27.9) |
| Trial outcome index | 61.1 (14.0) | 49.8 (15.0) | 56.2 (16.6) | 61.7 (19.5) |
Correlation between MPN-TSS and FACT-BMT domains was low for EWB (r = −0.10) and social well-being (r = −0.31) but higher for FWB (r = −0.44), BMT subscale (r = −0.61), and PWB (r = −0.76). Overall FACT-BMT score had a high inverse correlation with TSS (r = −0.71).
Global Assessment of Change
The Global Assessment of Change was performed at different intervals. At day 30, 10 (26.3%) patients reported a little/moderately/very much better overall QoL since their transplant, and 26 (68.45%) had a little/moderately/very much worse QoL. At day 100, 10 (30.3%) reported better QoL and 19 (57.6%) reported worsening since transplant. By 1 year, 16 (61.5%) reported feeling better and 6 (23.1%) reported worsening.
Ten of 38 (27.0%) patients had GVHD at day 30, 29% at day 100, and 37% at 1 year (Table 4). We evaluated the impact of GVHD on QoL as an exploratory analysis. Patients without GVHD at day 30 had significant changes in early satiety (mean change, 1.6), night sweats (mean change, −2.6), and bone pain (mean change, −1.2). Patients with GVHD had significant changes in early satiety (mean change, 3.7), abdominal pain (mean change, 2.1), abdominal discomfort (mean change, 2.6), night sweats (mean change, −2.4), and weight loss (mean change, 2.0). For FACT-BMT, patients without GVHD had decreased PWB (mean change, −3.1) and BMT subscale (mean change, −2.6) score at 30 days, indicating worse QoL. For GVHD patients, significant decreases were also seen in PWB (mean change, −8.5), FWB (mean change, −6.1), EWB (mean change, 3.5), and BMT subscale (mean change, −6.1), and these differences were somewhat larger in size but not statistically significant (data not shown).
Table 4.
GVHD Outcomes at Day 30, Day 100, and Year 1
| Characteristic | n (%) |
|---|---|
| GVHD | (n = 38) |
| No | 27 (73.0) |
| Yes | 10 (27.0) |
| Missing | 1 |
| Grade I | 4 (40.0) |
| Grade II | 1 (10.0) |
| Grade III | 3 (30.0) |
| Grade IV | 2 (20.0) |
| GVHD | (n = 33) |
| No | 22 (71.0) |
| Yes | 9 (29.0) |
| Missing | 2 |
| Grade I (mild) | 2 (25.0) |
| Grade II (moderate) | 4 (50.0) |
| Grade III (severe) | 1 (12.5) |
| Grade IV (life-threatening) | 1 (12.5) |
| Missing | 1 |
| Acute | 6 (85.7) |
| Chronic | 1 (14.3) |
| Missing | 2 |
| GVHD | (n = 26) |
| No | 15 (62.5) |
| Yes | 9 (37.5) |
| Missing | 2 |
| Grade I (mild) | 3 (33.3) |
| Grade II (moderate) | 1 (16.7) |
| Grade III (severe) | 3 (50.0) |
| Missing | 2 |
| Acute | 2 (25.0) |
| Chronic | 6 (75.0) |
| Missing | 1 |
DISCUSSION
Our study evaluated the QoL in patients with MF who have undergone allogeneic stem cell transplantation. There was a statistically significant improvement in MF-specific symptoms such as itching, night sweats, bone pain, and fever observed during at least 1 point following transplant. FACT-BMT showed marked worsening in symptoms at day 30 but then improvement to baseline at 1 year, which is similar to what has been described in other transplant studies [27,28]. MPN-TSS showed a slight decline at 1 year but did not reach statistical significance. Despite this, by 1 year, 61% felt that their QoL was better than it was before transplant.
One of the biggest barriers to transplantation in patients with MF is concern over QoL [31]. In a recent survey done on patients with MF, there was a reluctance to proceed with transplant due to concerns over QoL. The concept that patients will experience a significant decline in QoL following transplant is not validated based on our findings. Although at day 30, both FACT-BMT and MPN-TSS suggest worsening of symptoms, these improve over the course of the year and are similar to where they were before admission for transplantation.
Potential transplant factors that affect QoL include conditioning intensity as well as presence or absence of GVHD. In this study, there were very few myeloablative conditioning (MAC) transplants, making it difficult to do an accurate comparison. In a study by Gupta et al. [25] evaluating patients who received a transplant for acute myelogenous leukemia/myelodysplastic syndrome with either reduced-intensity conditioning or MAC conditioning, no significant differences were appreciated over the first year after transplant in QoL. In our study, patients with GVHD had changes in symptoms and QoL after transplant similar to those without GVHD, although numbers in the group were small and many limit generalizability.
There are several challenges with using MPN-SAF in the peritransplant setting. First, patients generally proceed with transplant due to progressive disease as characterized by change in peripheral blood counts or other markers of risk, such as karyotype abnormalities or molecular mutations. At the present time, we are uncertain how PROs affect timing of hematopoietic cell transplantation or how they interact with the more objective measures of disease. Second, interpretation of the MPN-SAF in the post-transplant setting is challenging. Many of the symptoms that are problematic in patients with MF are also present in patients following transplant. Therefore, although there was worsening at day 30, this likely does not reflect symptoms caused by MF but more due to transplantation. The decline in the immediate post-transplant period has been reported in several studies. Notably, even these symptoms returned to baseline by 1 year. The symptoms that improved significantly after transplant appear to be MF-specific symptoms such as pruritis, night sweats, bone pain, and fever. Interestingly, those with the highest symptom burden experienced significant improvements over time. Although these changes do not meet the standard IWG criteria for clinical benefit, clinical benefit in the first year following transplant is generally defined by OS and relapse-free survival.
Our study is limited by small numbers, which make it difficult to identify statistically significant trends. In addition, our population was mostly white, with a higher percentage of males, which may affect the generalizability of our results. Likewise, a variety of conditioning regimens was used before transplant, and therefore it may be difficult to tell whether changes in QoL or symptoms were due to transplant or conditioning. However, the MPN-TSS was similar to that of a cohort of patients whose data were collected over the years in studies to validate the MPN-TSS, suggesting that from a symptom burden standpoint, they are similar to patients with MF of a similar age. Missing surveys at the post-transplant time points were common, and it is unknown why the surveys were not completed but could be due to reasons related to the patients’ clinical status. Also, we followed patients for only 1 year. Several studies show that the QoL continues to increase for several years after transplant [27,32,33], and even at 2 years, only 71% of patients felt as if they recovered from their transplant [33]. Therefore, longer follow-up may be needed.
In summary, we have found, in a small group of patients, that trajectory of QoL after transplant is similar to that seen in a general transplant population. Furthermore, at 1 year, the QoL measures we obtained showed a return to baseline QoL, and the MPN-TSS suggests improvement in MPN-specific symptoms. This is a small sample, and these findings will need to be validated in a larger study with longer follow-up.
ACKNOWLEDGMENTS
Financial disclosure:
The authors have nothing to disclose.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Abelsson J, Merup M, Birgegard G, et al. The outcome of allo-HSCT for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transplant. 2012;47:380–386. [DOI] [PubMed] [Google Scholar]
- 2.Alchalby H, Yunus D-R, Zabelina T, et al. Risk models predicting survival after reduced-intensity transplantation for myelofibrosis. Br J Haematol. 2012;157:75–85. [DOI] [PubMed] [Google Scholar]
- 3.Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2010;16:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeg HJ, Bredeson C, Farnia S, et al. Hematopoietic cell transplantation as curative therapy for patients with myelofibrosis: long-term success in all age groups. Biol Blood Marrow Transplant. 2015;21:1883–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta V, Malone AK, Hari PN, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2014;20:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerbauy DMB, Gooley TA, Sale GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant. 2007;13:355–365. [DOI] [PubMed] [Google Scholar]
- 7.Keyzner A, Han S, Shapiro S, et al. Outcome of allogeneic hematopoietic stem cell transplantation for patients with chronic and advanced phase myelofibrosis. Biol Blood Marrow Transplant. 2016;22:2180–2186. [DOI] [PubMed] [Google Scholar]
- 8.Kröger N, Giorgino T, Scott BL, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood. 2015;125:3347–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lussana F, Rambaldi A, Finazzi MC, et al. Allogeneic hematopoietic stem cell transplantation in patients with polycythemia vera or essential thrombocythemia transformed to myelofibrosis or acute myeloid leukemia: a report from the MPN Subcommittee of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rondelli D, Goldberg JD, Isola L, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–2133. [DOI] [PubMed] [Google Scholar]
- 12.Passamonti F, Cervantes F, Vannucchi AM, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116:2857–2858. [DOI] [PubMed] [Google Scholar]
- 13.Jain T, Mesa RA, Palmer JM. Allogeneic stem cell transplantation in myelofibrosis. Biol Blood Marrow Transplant. 2017;23:1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kröger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:5264–5270. [DOI] [PubMed] [Google Scholar]
- 15.Gupta V, Gotlib J, Radich JP, et al. Janus kinase inhibitors and allogeneic stem cell transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2014;20:1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesa RA, Scherber RM, Geyer HL. Reducing symptom burden in patients with myeloproliferative neoplasms in the era of Janus kinase inhibitors. Leuk Lymphoma. 2015;56:1989–1999. [DOI] [PubMed] [Google Scholar]
- 17.Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014;123:3803–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53:441–444. [DOI] [PubMed] [Google Scholar]
- 19.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs). Cancer. 2007;109:68–76. [DOI] [PubMed] [Google Scholar]
- 20.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85:1186–1196. [DOI] [PubMed] [Google Scholar]
- 22.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. [DOI] [PubMed] [Google Scholar]
- 23.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative Neoplasm (MPN) Symptom Assessment Form Total Symptom Score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta V, Panzarella T, Li L, et al. A prospective study comparing the outcomes and health-related quality of life in adult patients with myeloid malignancies undergoing allogeneic transplantation using myeloablative or reduced-intensity conditioning. Biol Blood Marrow Transplant. 2012;18:113–124. [DOI] [PubMed] [Google Scholar]
- 26.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bevans MF, Marden S, Leidy NK, et al. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2006;38:101. [DOI] [PubMed] [Google Scholar]
- 28.McQuellon RP, Russell GB, Rambo TD, et al. Quality of life and psychological distress of bone marrow transplant recipients: the ‘time trajectory’ to recovery over the first year. Bone Marrow Transplantation. 1998;21:477. [DOI] [PubMed] [Google Scholar]
- 29.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplantation. 1997;19:357–368. [DOI] [PubMed] [Google Scholar]
- 30.Cella D, Tulsky D, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 31.Palmer J, Scherber R, Girardo M, et al. Patient perspectives regarding allogeneic bone marrow transplant in myelofibrosis. Biol Blood Marrow Transplant. 2019;25:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: patient education, evaluation and intervention. Br J Haematol. 2010;148:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Fairclough D, Parsons SK, et al. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19:242–252. [DOI] [PubMed] [Google Scholar]


