Abstract
Statins are recommended for first-line management of elevated cholesterol in the primary and secondary prevention of atherosclerotic cardiovascular disease. Statins may occasionally be associated with mild transaminase elevations but can also result in life-threatening liver injury. Atorvastatin is the most common cause of clinically significant liver injury in this drug class. We report a case of severe, asymptomatic liver injury in a hepatocellular pattern in a 71-year-old man occurring within 3 months of switching from simvastatin to high-intensity atorvastatin therapy. Hepatitis improved rapidly with cessation of atorvastatin and did not recur after resuming simvastatin.
Keywords: statins, atorvastatin, drug-induced liver injury, hepatocellular injury, transaminitis, simvastatin
Introduction
Statins represent the mainstay of cholesterol-lowering therapy for both primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD). The 2018 American College of Cardiology/American Heart Association Task Force report on cholesterol management recommends statins for first-line management and provides guidelines on therapy intensity based on ASCVD risk. The report also reviews common adverse effects of statins, including myopathy and abnormal liver function tests (LFTs) and notes the rarity of hepatic failure secondary to statin use.1 Additionally, the Food and Drug Administration no longer recommends routine monitoring of LFTs with statins, as prior efficacy trials have demonstrated no benefit in preventing clinically significant liver injury.2 We report a case of high-intensity atorvastatin therapy resulting in severe, asymptomatic liver injury, which was recognized only due to routine LFTs.
Case Presentation
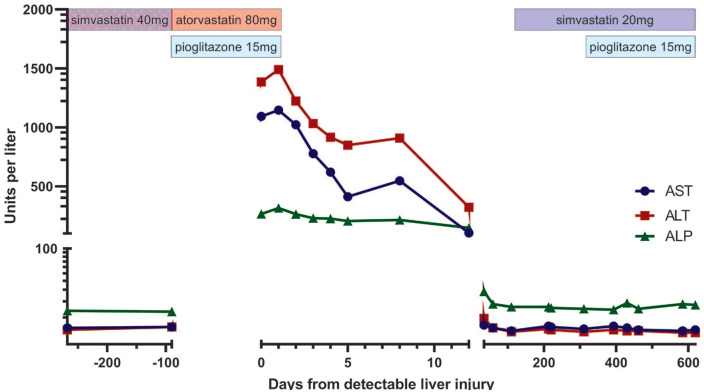
A 71-year-old African American male presented for routine follow-up with his primary care physician 3 months after initiating atorvastatin 80 mg and pioglitazone 15 mg daily. His lipid panel revealed a total cholesterol of 152 mg/dL, triglycerides of 83 mg/dL, high-density lipoprotein cholesterol of 59 mg/dL, and a calculated low-density lipoprotein cholesterol (LDL-c) of 76 mg/dL. LFTs were notable for an aspartate aminotransferase (AST) of 1093 U/L (upper limit of normal [ULN] 41 U/L), an alanine transaminase (ALT) of 1385 U/L (ULN 58 U/L), and an alkaline phosphatase (ALP) of 265 U/L (ULN 129 U/L; R Factor 11.6; Figure 1). Total bilirubin was elevated to 1.5 mg/dL (ULN 1.2 mg/dL). The patient denied myalgias, pruritus, or abdominal pain. He was admitted for further management.
Figure 1.
Liver function tests and relevant medications before, during, and after atorvastatin-induced liver injury. AST, ALT, and ALP were markedly elevated 3 months after initiation of atorvastatin and pioglitazone and normalized after discontinuing both. LFTs remained at baseline after resuming simvastatin and pioglitazone. Potential nonadherence to simvastatin represented by crosshatch pattern. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate transaminase; LFT, liver function test.
The patient denied known hypotensive episodes or recent use of alcohol, herbal supplements, antibiotics, or acetaminophen. Gamma glutamyl-transferase was elevated to 551 U/L (ULN 50 U/L). Serum acetaminophen and ethanol were undetectable. Hepatitis A virus IgM/IgG, hepatitis B virus core IgM/IgG, hepatitis C virus (HCV), and human immunodeficiency virus-1 (HIV-1) antibodies were negative, as were hepatitis B surface antigen (HBsAg) and HIV-1 and HCV RNA. Anti-neutrophil cytoplasmic, anti-mitochondrial, and liver-kidney microsome antibodies were not detected. Serum IgG, copper, and ceruloplasmin levels were normal. Remaining workup was unrevealing except for a borderline-positive smooth muscle antibody titer of 1:20 (normal <1:20) and a ferritin of 1469 ng/mL (ULN 336 ng/mL). Serum albumin (3.4 g/dL) and prothrombin time international normalized ratio (1.01) were within normal limits. Liver ultrasound revealed “mildly increased echogenicity, coarsened echotexture, and attenuation of sound beam” suggestive of “hepatic steatosis and/or diffuse hepatocellular disease.” The right lobe measured 14.1 cm. A prior abdominal computerized tomography scan with contrast at age 60 years revealed only a punctate calcification, likely a granuloma, within the right lobe.
On admission, his temperature was 36.8 °C, heart rate was 67 beats per minute, blood pressure was 123/63 mm Hg, respiratory rate was 16 breaths per minute, and oxygen saturation was 97% on room air. Physical examination revealed no scleral icterus, a soft and nontender abdomen without palpable masses or organomegaly, and normal mental status. Atorvastatin and pioglitazone were discontinued on admission with rapid improvement in LFTs (see Figure 1). Liver biopsy was therefore not performed. The patient remained asymptomatic and was discharged on hospital day 4 with normalization of LFTs within 1 month. Simvastatin 20 mg daily and pioglitazone 15 mg daily were started 3 and 10 months after discharge, respectively. Contrast-enhanced computed tomography (CT) scan of the abdomen at 3 months again showed a likely granuloma but no steatosis. Serum LFTs have since remained within normal limits.
Before starting atorvastatin, the patient had been taking simvastatin 40 mg daily for 11 years for an elevated LDL-c of 137 mg/dL and a 10-year ASCVD risk of 18.9%. Other pertinent medical history included type 2 diabetes mellitus, hypertension, and stage 2 chronic kidney disease. He had no history of ASCVD. His baseline serum LFTs were within normal limits. No change in LFTs or other side effects were noted while on simvastatin. His LDL-c reached a nadir of 74 mg/dL at age 70 years.
Three months prior to this presentation, the patient’s LDL-c was elevated to 93 mg/dL, at which point simvastatin was stopped and atorvastatin 80 mg daily was started. A goal LDL-c below 70 mg/dL was targeted for secondary prevention out of concern for prior ischemic stroke resulting in bilateral superior altitudinal hemianopsia noted on visual field testing 6 months prior. Pioglitazone 15 mg daily was started simultaneously with atorvastatin for an elevated hemoglobin A1c of 8.1%. He was continued on his other medications, including gabapentin 300 mg 3 times daily for diabetic neuropathy, glipizide 20 mg twice daily, lisinopril 5 mg daily, metformin 1000 mg twice daily, omeprazole 20 mg twice daily, paroxetine 20 mg daily, and trazodone 100 mg as needed. Subsequent review of medication history revealed that he had not refilled his simvastatin for almost 6 weeks before starting on atorvastatin.
Discussion
Statin therapy may result in transient, asymptomatic elevations of AST and ALT, typically below 3 times the ULN, in 3% of patients within 3 months of initiation. Atorvastatin causes elevations in transaminases greater than 3-fold the ULN in approximately 0.5% of all cases, with an absolute risk of 1.2% with high-intensity therapy.3,4 Atorvastatin is the most common cause of clinically significant liver injury among statins with a reported incidence of 1/17 000 users.5-7
The mechanism of statin-associated liver injury remains unclear, although multiple reports of associated positive autoimmune hepatitis titers have been cited to suggest a potential immune-mediated component.2 LFTs typically reflect a mixed or cholestatic pattern of injury.5-7 Presenting symptoms include jaundice, pruritus, fever, and abdominal pain, although patients may uncommonly be asymptomatic.6,8 Indeed, whether asymptomatic transaminase elevations equate to liver injury remains controversial. The Drug-Induced Liver Injury Network (DILIN) includes asymptomatic cases (13%) secondary to statins in its database, and an expert group has defined DILI using specific laboratory parameters.8,9 Others have argued that isolated transaminitis may not reflect clinical injury or may be attributable to other causes, such as underlying metabolic disorders in patients requiring statins.10,11 Of note, our patient’s total bilirubin was 1.25 times the ULN and thus did not satisfy Hy’s law, which specifies that total bilirubin elevation twice the ULN in the setting of aminotransferase elevations greater than 3 times the ULN is associated with a mortality rate of 11%. However, he did meet R criteria (R factor 11.6), which was associated with a 9% risk of mortality or liver transplantation in a national DILI registry analysis.12
This case highlights the challenging question of monitoring for liver injury in patients receiving statin therapy. This patient, who was completely asymptomatic, was appropriately evaluated and managed for hepatitis only because of routine LFTs ordered as an outpatient. Ultrasound findings relative to prior and follow-up CT were suggestive of diffuse hepatocellular disease, which, in the setting of markedly elevated aminotransferases, was consistent with an acute hepatitis. Direct comparison between studies is unfortunately limited by the drawbacks of contrast-enhanced CT, which was performed in his case for surveillance of an adrenal incidentaloma.13 Ultrasound imaging may therefore have identified underlying nonalcoholic fatty liver disease. Statins have not been shown to cause increased rates of liver injury in patients with abnormal baseline LFTs or chronic liver disease and are considered safe for use with stable liver disease.1,2,14 However, the patient’s elevated ALP and total bilirubin suggest active inflammation sufficient to affect the biliary system. Regardless of whether the patient had underlying liver disease, this case therefore is most consistent with acute liver injury related to atorvastatin use that would have remained undetected without routine testing or until the onset of symptoms.
A common challenge in the diagnosis of DILI is establishing causality, and atorvastatin similarly cannot be identified unequivocally as the cause of this patient’s presentation. The workup as described above was largely unrevealing. Hepatitis E virus, cytomegalovirus, and Epstein-Barr virus were not tested for and could have caused an asymptomatic hepatitis, although these would be less common viral etiologies. An elevated ferritin in the setting of acute liver injury is nonspecific, and the patient had no other sequelae suggestive of hemochromatosis except long-standing type 2 diabetes mellitus. The patient may have had chronic nonalcoholic steatosis progressing to transient steatohepatitis, which was incidentally detected on laboratory testing and seen on ultrasound, coincidentally only while on atorvastatin. However, the temporal relationship of the initiation and cessation of atorvastatin therapy with his liver injury and subsequent rapid improvement, respectively, strongly suggest a causal role for atorvastatin in this case.
Pioglitazone was started and discontinued simultaneously with atorvastatin, raising the possibility of pioglitazone-associated liver injury. Isolated case reports have described pioglitazone-associated liver injury ranging in presentation from asymptomatic transaminitis, as in our patient, to fulminant hepatic failure.15,16 However, LFTs remained within normal limits even after re-challenging the patient with pioglitazone therapy with concurrent simvastatin use. Pioglitazone is less commonly associated with transaminase elevations (0.26%) greater than 3 times the ULN compared to atorvastatin. Pioglitazone is also not known to interact with CYP3A4, which metabolizes atorvastatin, reducing the likelihood that this combination resulted in hepatocellular injury as opposed to atorvastatin alone.2
Atorvastatin-associated liver injury may present with positive autoantibody titers and may uncommonly require immunosuppression.17,18 Our patient did have a detectable SMA of 1:20, although titers below 1:80 are of unclear significance, and immunosuppression was not required.19
Importantly, medication review revealed that the patient had not filled his simvastatin prescription for at least 6 weeks prior to starting atorvastatin, raising the possibility of nonadherence. Exploring this discrepancy prior to resorting to high-dose atorvastatin may have prevented this presentation with a comparable lipid-lowering effect, as simvastatin 40 mg daily had previously lowered his LDL-c to 74 mg/dL. Since resuming simvastatin at 20 mg daily, the patient’s LDL-c has ranged from 76.0 to 104.8 mg/dL. Re-challenging with atorvastatin for improved LDL-c may be dangerous and led to multiorgan failure and death in a patient whose first trial of atorvastatin resulted only in transient jaundice.20 Continued lifestyle counseling and, if necessary, a cautious increase in simvastatin dose with a plan for LFT monitoring would be more appropriate in this patient. Alternatively, rosuvastatin has been shown to achieve comparable reduction in LDL-c to atorvastatin with greater reduction in atheroma volume and decreased incidence of aminotransferase elevations (2.1% vs 0.7%).21
In conclusion, high-intensity atorvastatin therapy is the most common cause of both incidental transaminase elevations and DILI among the various statins. This patient’s presentation was consistent with asymptomatic hepatocellular injury related to high-dose atorvastatin, with comprehensive workup revealing no alternative cause of his acute liver injury. Although current guidelines recommend against routine surveillance of asymptomatic statin users for liver injury, this patient may have experienced a delayed presentation with more advanced hepatic disease had his LFTs not been evaluated on follow-up.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Informed consent for patient information to be published in this article was not obtained because all discernible personally identifying information regarding the patient in this case report was removed to the best of our judgment.
ORCID iD: Amit Saha  https://orcid.org/0000-0001-6304-2361
https://orcid.org/0000-0001-6304-2361
References
- 1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Halegoua-De Marzio VJND. Hepatotoxicity of cardiovascular and antidiabetic drugs. In: Kaplowitz LDD Neil, ed. Drug-Induced Liver Disease. 3rd ed. Academic Press; 2013:519-540. [Google Scholar]
- 3. LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-1435. doi: 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 4. Newman CB, Palmer G, Silbershatz H, Szarek M. Safety of atorvastatin derived from analysis of 44 completed trials in 9416 patients. Am J Cardiol. 2003;92:670-676. doi: 10.1016/s0002-9149(03)00820-8 [DOI] [PubMed] [Google Scholar]
- 5. Bjornsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56:374-380. doi: 10.1016/j.jhep.2011.07.023 [DOI] [PubMed] [Google Scholar]
- 6. Bjornsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int. 2017;37:173-178. doi: 10.1111/liv.13308 [DOI] [PubMed] [Google Scholar]
- 7. Perdices EV, Medina-Caliz I, Hernando S, et al. Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig. 2014;106:246-254. [PubMed] [Google Scholar]
- 8. Russo MW, Hoofnagle JH, Gu J, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60:679-686. doi: 10.1002/hep.27157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806-815. doi: 10.1038/clpt.2011.58 [DOI] [PubMed] [Google Scholar]
- 10. Cohen DE, Anania FA, Chalasani N; National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C-81C. doi: 10.1016/j.amjcard.2005.12.014 [DOI] [PubMed] [Google Scholar]
- 11. Sniderman AD. Is there value in liver function test and creatine phosphokinase monitoring with statin use? Am J Cardiol. 2004;94:30F-34F. doi: 10.1016/j.amjcard.2004.07.052 [DOI] [PubMed] [Google Scholar]
- 12. Robles-Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147:109-118.e5. doi: 10.1053/j.gastro.2014.03.050 [DOI] [PubMed] [Google Scholar]
- 13. Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26:1637-1653. doi: 10.1148/rg.266065004 [DOI] [PubMed] [Google Scholar]
- 14. Bays H, Cohen DE, Chalasani N, Harrison SA; The National Lipid Association’s Statin Safety Task Force. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 suppl):S47-S57. doi: 10.1016/j.jacl.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 15. Chase MP, Yarze JC. Pioglitazone-associated fulminant hepatic failure. Am J Gastroenterol. 2002;97:502-503. doi: 10.1111/j.1572-0241.2002.05516.x [DOI] [PubMed] [Google Scholar]
- 16. Maeda K. Hepatocellular injury in a patient receiving pioglitazone. Ann Intern Med. 2001;135:306. doi: 10.7326/0003-4819-135-4-200108210-00029 [DOI] [PubMed] [Google Scholar]
- 17. Alla V, Abraham J, Siddiqui J, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. 2006;40:757-761. doi: 10.1097/00004836-200609000-00018 [DOI] [PubMed] [Google Scholar]
- 18. Pelli N, Setti M, Ceppa P, Toncini C, Indiveri F. Autoimmune hepatitis revealed by atorvastatin. Eur J Gastroenterol Hepatol. 2003;15:921-924. doi: 10.1097/00042737-200308000-00014 [DOI] [PubMed] [Google Scholar]
- 19. Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ; Practice Parameters Committee of the American College of Gastroenterology. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950-967. doi: 10.1038/ajg.2014.131 [DOI] [PubMed] [Google Scholar]
- 20. Sreenarasimhaiah J, Shiels P, Lisker-Melman M. Multiorgan failure induced by atorvastatin. Am J Med. 2002;113:348-349. doi: 10.1016/s0002-9343(02)01178-6 [DOI] [PubMed] [Google Scholar]
- 21. Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078-2087. doi: 10.1056/NEJMoa1110874 [DOI] [PubMed] [Google Scholar]