Abstract
The tear film, which includes mucins that adhere to foreign particles, rapidly clears allergens and pathogens from the ocular surface, protecting the underlying tissues. However, the tear film’s ability to efficiently remove foreign particles during blinking can also pose challenges for topical drug delivery, as traditional eye drops (solutions and suspensions) are cleared from the ocular surface before the drug can penetrate into the conjunctival and corneal epithelium. In the past 15 years, there has been an increase in the development of nanoparticles with specialized coatings that have reduced affinity to mucins and are small enough in size to pass through the mucus barrier. These mucus-penetrating particles (MPPs) have been shown to efficiently penetrate the mucus barrier and reach the ocular surface tissues. Dry eye disease (DED) is a common inflammatory ocular surface disorder that often presents with periodic flares (exacerbations). However, currently approved immunomodulatory treatments for DED are intended for long-term use. Thus, there is a need for effective short-term treatments that can address intermittent flares of DED. Loteprednol etabonate, an ocular corticosteroid, was engineered to break down rapidly after administration to the ocular surface tissues and thereby reduce risks associated with other topical steroids. KPI-121 is an ophthalmic suspension that uses the MPP technology to deliver loteprednol etabonate more efficiently to the ocular tissues, achieving in animal models a 3.6-fold greater penetration of loteprednol etabonate to the cornea than traditional loteprednol etabonate ophthalmic suspensions. In clinical trials, short-term treatment with KPI-121 0.25% significantly reduced signs and symptoms of DED compared with its vehicle (placebo). Recently approved KPI-121 0.25%, with its novel drug delivery design and ease of use, has the potential to effectively treat periodic flares of DED experienced by many patients.
Keywords: dry eye disease, KPI-121, loteprednol etabonate, mucus-penetrating particles, nanoparticles, ocular mucus, ocular surface
Introduction
Many patients with dry eye disease (DED) experience periodic flares (exacerbations) of symptoms, often in response to seasonal and environmental triggers. Until recently, there were no approved medications specifically designed for the short-term treatment of signs and symptoms of DED. Topical treatment of diseases of the ocular surface, including DED, is hampered by the difficulty of delivering drugs through the ocular surface into the anterior segment tissues. The tear film, and in particular the mucus barrier, efficiently clears topical agents from the surface of the eye before they can reach the underlying corneal and conjunctival tissues.1–3 A new technology based on mucus-penetrating particles (MPPs)1 has the potential to penetrate the mucus barrier and deliver therapeutic agents more efficiently to the ocular surface tissue. KPI-121 0.25%, an ophthalmic suspension of the ocular corticosteroid loteprednol etabonate using the MPP technology, has been approved for the short-term treatment of signs and symptoms of DED. In this article, we discuss the development of the MPP technology and the potential role of KPI-121 0.25% for the treatment of flares of DED.
The mucus barrier and challenges for topical drug delivery
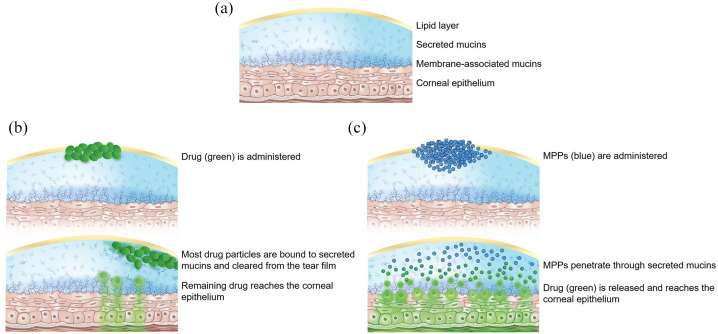
The tear film, which covers the tissues of the ocular surface, is composed of an outer lipid layer and an inner aqueous layer.3 This aqueous layer is comprised mainly of water and mucins, which are high-molecular-weight glycoproteins.2 Throughout the body, the function of mucins, or the mucus barrier, is to protect cellular surfaces and maintain water balance,2 and this is also the function of mucins on the ocular surface. In the tear film, the mucus barrier is comprised of membrane-associated mucins, which form a dense layer near the corneal epithelium, and secreted mucins, which form an outer layer and are less densely arrayed (Figure 1(a)).1 Secreted mucins, the first line of defense in the mucus barrier, move within the tear film and bind to foreign particles, including allergens and pathogens.1 The secreted mucins, with associated particles, and tear film are moved out to the nasolacrimal duct during blinking to rapidly clear the ocular surface.3
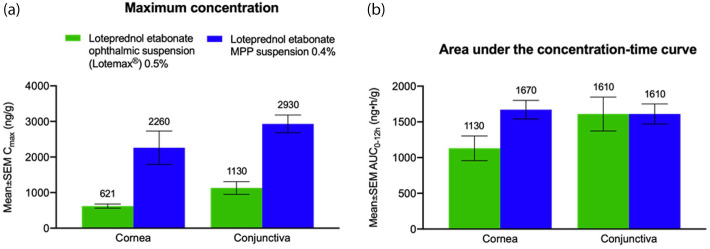
Figure 2.
Pharmacokinetic parameters of loteprednol etabonate in New Zealand white rabbit cornea and conjunctiva.12 The mean ± SEM loteprednol etabonate (a) Cmax and (b) AUC0–12h for rabbits receiving a single ocular dose of loteprednol etabonate ophthalmic suspension 0.5% or loteprednol etabonate MPP suspension 0.4% is depicted. AUC0–12h, area under the concentration–time curve from time 0 to 12 hours; Cmax, maximum concentration; MPP, mucus-penetrating particle; SEM, standard error of the mean.
Figure 1.
Illustration of (a) the ocular surface and tear film before drug delivery, (b) clearing of traditional drug particles from the tear film, and (c) penetration of drug using the mucus-penetrating particle (MPP) technology.
This efficient system of clearing foreign particles from the eye can also pose a challenge for topical drug delivery. Traditional eye drops (solutions and suspensions) are cleared from the ocular surface within 1 minute, so that ⩽5% of the drug reaches anterior eye tissues (Figure 1(b)).1,3,4 In an effort to slow the clearance of drugs from the ocular surface and enhance delivery to the epithelia, mucoadhesive eye drop formulations have been developed, including polymer-based solutions, viscous gels, and mucoadhesive micro- and nanoparticles.1 Formulations that encourage drug particles to adhere to mucins may increase the residence time of drug at the mucosal layer. However, because such formulations are likely to be largely trapped by secreted mucins and cleared from the ocular surface within minutes, these formulations do not solve the problem of delivering drugs through the mucus barrier to the cornea.1,3
Another possibility for drug delivery on the eye surface is the development of formulations with very small particle size, on the order of microparticles, which might pass through the tear film without being trapped by mucin pores. However, mucins form a mesh that normally has pores of ~500 nm, so drugs in the size range of microparticles are not small enough to travel easily through the mucus barrier.1 Furthermore, even nanoparticles (smaller than 500 nm) typically have multiple sites for polyvalent interactions with mucins, so these submicron particles also become trapped by the mucus barrier before reaching the corneal epithelium.
MPPs—a new technology
A new approach to deliver drugs to mucosal surfaces, including the ocular surface, has been developed. Hanes and colleagues hypothesized that enhanced mucus penetration would facilitate prolonged retention and more uniform distribution of drug carriers at mucosal surfaces, leading to improved pharmacokinetics and therapeutic efficacy.5–8 As the deeper mucus layers (membrane-associated mucins) are cleared much more slowly than the outer layers, drug carriers that can pass through much of the mucus barrier and reach the inner layers may remain resident in the mucosal surface for a longer time and ultimately enhance drug penetration into the underlying tissue.1,5,8
To evade the entrapment of drug particles by mucins, Hanes and colleagues coated polymeric nanoparticles with a high density of a low-molecular-weight polymer that reduces particle affinity to mucins (Figure 1(c)).5 This MPP technology has been investigated to improve drug delivery to various mucosal surfaces throughout the body.1,9–11 In animal studies, the MPP technology has been shown to increase drug exposure to the ocular surface of rabbits and mini-pigs.11,12
A new advance in MPP technology was the development of drug-core MPPs, which do not require encapsulating drugs in a polymeric matrix.1 Drug-core MPPs are comprised mostly of pure drug, but they still have the MPP attributes of nanometer-scale particle size and a coating that prevents adherence to mucins. Drug-core MPPs can also be made stable for storage at room temperature as ready-to-use aqueous suspensions, and they can be formulated with excipients previously approved for ophthalmic use.1 With the development of MPP technology, it may be possible to create topical ophthalmic suspensions that can more efficiently and effectively treat diseases of the ocular surface, of which one of the most common is DED.
DED—an inflammatory disorder
DED is a disease of the ocular surface characterized by instability of the tear film that is accompanied by ocular surface inflammation and damage (TFOS DEWS II [Tear Film & Ocular Surface Society Dry Eye Workshop II] Definition and Classification Report).13 Patients with DED experience symptoms that include discomfort (eg, sensations of pain, grittiness, or stinging) and visual disturbance.13 DED is a common disorder, diagnosed in approximately 7% of the adult US population (~16.4 million people).14 Prevalence of DED increases with age, and DED is more common among women than men.14–16
Historically, cases of DED were classified as either aqueous-deficient or evaporative.13 Aqueous- (or tear-) deficient DED could be attributed to an insufficiency in tear production by the lacrimal glands or to other causes such as Sjögren’s syndrome. Evaporative DED is related to deficiencies in the meibomian (oil-producing) glands, low blink rate, contact lens wear, or other factors.13 In accordance with this understanding of DED, aqueous-deficient DED has been treated with approaches that aim to replace tears (eg, artificial and biological tear substitutes), conserve tears (eg, punctal occlusion), or stimulate tear production (eg, secretagogues). Treatments for evaporative DED have addressed lid abnormalities and meibomian gland dysfunction.17
As the understanding of DED has evolved, it has been recognized that deficiencies in tear quantity (aqueous-deficient disease) and tear quality (evaporative disease) often coexist in the same patient. Furthermore, regardless of the cause, instability of the tear film sets off a cascade of inflammatory events that becomes self-perpetuating and leads to many of the symptoms experienced by patients with DED.13,17–19 DED is now recognized as a chronic inflammatory disease in which an unstable and hyperosmolar tear film sets off a sequence of inflammatory events.20 Signaling pathways in the ocular surface epithelium and immune cells trigger production of inflammatory molecules, including proinflammatory cytokines and matrix metalloproteinases (MMPs; eg, MMP-9). Numerous extrinsic and intrinsic factors, including a desiccating environment, drying medications, exposure, aging, and autoimmune conditions, contribute to the vicious inflammatory cycle in DED.20
Unmet need for treatment of periodic flares of DED
The inflammatory nature of DED is now well established. However, it is less often recognized that DED can present as a chronic disease with periodic flares (exacerbations). In the context of early disease, DEWS II describes an initial presentation of DED that may involve intermittent symptoms or “emerging episodic dry eye.”13 However, evidence can also be found in a variety of settings for the existence of periodic flares of DED in the context of ongoing disease.
Amparo and colleagues administered DED symptom scales to patients with DED every 2 weeks for 3 months.21 A substantial minority of patients (26–32%) reported discomfort with environmental conditions such as windy conditions, low humidity, and air conditioning only “some of the time,” suggesting short-term flares in these patients when they were exposed to specific environmental triggers.21 Similarly, Iyer and colleagues found positive correlations between use of air conditioning or watching television and blurring of vision, indicating these activities were triggers for a flare of this DED symptom.22 Additional studies have found worsening of DED symptoms after prolonged reading23 and following exposure to increased ground-level ozone concentrations,24 each of which can be seen as a trigger for flares of DED symptoms.
Additional evidence for the existence of DED flares comes from studies using a controlled adverse environment (CAE), a chamber in which participants are confronted with low humidity, increased airflow, and continuous visual tasks.25 In separate studies, patients with DED exposed to a CAE experienced a worsening of symptoms and signs of DED.26,27 In three studies, an increase in inflammatory biomarkers was observed after exposure to the CAE,26–28 demonstrating a potential immune-mediated pathway for exacerbation of DED after exposure to this harsh environment.
As shown in these studies, DED flares may occur episodically in response to specific triggers. Rolando and colleagues proposed a classification of DED patients based on frequency of symptoms, with disease presentation characterized as sporadic, intermittent, persistent, or permanent (chronic).19 Sporadic DED is defined as “occasional dry eye feeling in specific situations (not every time),” and intermittent disease is characterized as “dry eye feeling all/most of the time in specific situations.”19 These definitions correspond to the observation that for some patients, symptoms of DED are experienced as exacerbations of an underlying process. As currently available immunomodulatory agents for treatment of DED are intended for chronic use, there is an unmet need for treatment of periodic flares of DED.
Development of KPI-121, an MPP formulation of loteprednol etabonate
KPI-121 is an ophthalmic nanosuspension that delivers a corticosteroid, loteprednol etabonate, to the anterior eye tissues using MPP technology. Loteprednol etabonate was retro-metabolically engineered 30 years ago to reduce the common risks of topical ocular steroids, including intraocular pressure (IOP) elevation and cataract formation.29,30 To create the loteprednol etabonate molecule, the ketone at the carbon-20 position of the corticosteroid prednisolone was replaced by a cleavable 17β-chloromethyl ester.29 After exerting its therapeutic effect, loteprednol etabonate is rapidly de-esterified to an inactive carboxylic acid, reducing the risk of unwanted side effects.30 Loteprednol etabonate has additional desirable qualities for an ocular drug, including a high degree of lipophilicity, strong corticosteroid-glucocorticoid receptor binding, and a high therapeutic index.
In preclinical studies, Schopf and colleagues at Kala Pharmaceuticals investigated topical ocular delivery of loteprednol etabonate nanoparticles formulated as MPPs.11,12 In a study of rabbits receiving a single ocular administration of loteprednol etabonate ophthalmic suspension 0.5% versus loteprednol etabonate MPP suspension 0.4%, peak concentrations of loteprednol etabonate in the cornea, recorded 5 minutes after administration, were 3.6-fold higher with the MPP formulation than with the traditional ophthalmic suspension (Figure 2).12 This was true even though the concentration of drug was lower in the MPP formulation.12 Total drug available in the cornea (measured by area under the concentration-time curve [AUC]) was 1.5-fold higher, and peak concentrations of loteprednol etabonate in the conjunctiva were 2.6-fold higher with the MPP suspension.12
Schopf and colleagues also demonstrated that the AUC of loteprednol etabonate in the cornea was four-fold higher after administration of loteprednol etabonate nanoparticles engineered with MPP technology compared with conventional nanoparticles of loteprednol etabonate.11 Taken together, these studies demonstrated the potential of MPP technology to penetrate the ocular mucus barrier and deliver loteprednol etabonate to the corneal and conjunctival epithelium more efficiently than conventional ophthalmic suspensions and nanosuspensions lacking the mucus-penetrating attributes. The MPP formulation of loteprednol etabonate ophthalmic suspension developed for clinical use, KPI-121, thus has favorable properties for treatment of ocular surface disease.
KPI-121 clinical development
Development of the technology underlying the commercially available MPP formulation of loteprednol etabonate ophthalmic suspension, KPI-121, has been described in detail by Popov.1 KPI-121 was approved in 2018 by the U.S. Food and Drug Administration (FDA) in a 1% concentration (INVELTYS®, Kala Pharmaceuticals, Inc., Watertown, MA) for treatment of postoperative inflammation and pain following ocular surgery.31 Two phase-3, randomized, double-masked, vehicle-controlled, parallel-group trials (ClinicalTrials.gov identifiers, NCT02163824 and NCT02793817) compared 14 days of twice-daily administration of KPI-121 1% or vehicle for treatment of patients with postsurgical inflammation and pain following cataract surgery.32 Each study achieved the primary efficacy endpoints of complete resolution of ocular inflammation by slit-lamp biomicroscopy and complete resolution of subject-rated ocular pain.32
KPI-121 0.25% was approved by the FDA in 2020 for short-term treatment (up to 2 weeks) of the signs and symptoms of DED (EYSUVIS®, Kala Pharmaceuticals, Inc.).33 The 0.25% concentration was selected for treatment of DED because it had an improved pharmacokinetic profile in the reduced dose strength compared with that in conventional loteprednol etabonate suspension 0.5% without the MPP drug delivery technology. KPI-121 0.25% was investigated for treatment of DED in one phase-2 trial (NCT02188160) and three phase-3 trials: STRIDE 1 (Safety and Efficacy of KPI-121 in Subjects with DED; NCT02813265), STRIDE 2 (NCT02819284), and STRIDE 3 (NCT03616899). Each of the four trials was a multicenter, double-masked, randomized, vehicle-controlled, parallel-group study in patients with DED. Across the four trials, total enrollment included more than 2,800 patients, making this the largest clinical development program for DED. Each phase-3 study assessed KPI-121 0.25% versus vehicle control (placebo) administered four times daily for 14 days. In STRIDE 1 and STRIDE 2, the primary outcome measures were the changes from baseline to day 15 in bulbar conjunctival hyperemia (a sign of DED) and patient-reported ocular discomfort (a symptom of DED) at week 2. In STRIDE 3, the primary outcome measure was the change from baseline to day 15 in ocular discomfort at week 2; conjunctival hyperemia was a secondary endpoint. Conjunctival hyperemia was chosen as an endpoint in these trials based on its role in DED and use in past clinical studies.34,35
In STRIDE 1, STRIDE 2, and STRIDE 3, treatment with KPI-121 0.25% resulted in significantly greater reduction in conjunctival hyperemia compared with vehicle (p < 0.0001 for between-group comparison in each trial).36 Ocular discomfort was reduced more with KPI-121 0.25% than with vehicle in STRIDE 1 (p < 0.0001), and STRIDE 3 (p = 0.0002). Significant improvement in total corneal fluorescein staining was also observed with KPI-121 0.25% versus vehicle in STRIDE 2 (p = 0.0134) and STRIDE 3 (p = 0.0042). The most frequently reported adverse event was instillation site pain.36
Potential role of KPI-121 0.25% in treatment of DED
A large proportion of patients with DED generally present with mild signs and symptoms that are adequately controlled with conservative approaches, including environmental modifications, lubricant eye drops, and lid hygiene.17 These patients often do not require chronic therapy with immunomodulatory agents such as cyclosporine A or lifitegrast, which are more appropriate for treatment of patients with chronic symptoms and more advanced disease.
However, as described, many patients with generally mild DED have periodic flares on a seasonal or episodic basis, often in response to environmental triggers. These patients may benefit from additional short-term therapy when their DED symptoms flare up. In patients with episodic disease, appropriate therapy at the time of a flare could potentially break the vicious circle of inflammation early in its process and help prevent further damage to the ocular surface.19 Flares of DED can be viewed as similar to exacerbations that occur in the context of other chronic inflammatory and autoimmune disorders, including asthma, systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease. As one example, patients with allergic conjunctivitis typically experience relief of symptoms with daily use of a combined antihistamine/mast cell stabilizer. However, patients often have breakthrough symptoms caused by allergens that acutely increase inflammation, and these flares are typically treated with a short course of topical corticosteroids. Similarly, flares of otherwise mild DED may benefit from short-term steroid therapy.
KPI-121 0.25% has a favorable pharmacological profile for the short-term treatment of signs and symptoms of DED, especially during flares. KPI-121 delivers the ocular corticosteroid loteprednol etabonate, which breaks down quickly, potentially decreasing harmful side effects such as elevated IOP and cataract formation.30 In animal studies, MPP technology allowed KPI-121 to achieve high penetration of loteprednol etabonate into the target cornea and conjunctival tissue.12 In the STRIDE trials, KPI-121 0.25% successfully resolved both signs and symptoms of DED after 2 weeks of treatment.36
When a patient presents in clinical practice with an episode of ocular surface discomfort and a flare of DED is diagnosed, a short course of KPI-121 0.25% may relieve the patient’s symptoms and potentially calm the vicious cycle of inflammation. Patients should be counseled to discontinue the use of contact lenses while being treated with steroids. Currently, it is likely that the population of patients with milder DED that becomes symptomatic two or three times per year are undertreated, either because they decline long-term therapy or because clinicians fail to identify these patients. With a proven treatment for short-term treatment of signs and symptoms of DED that has a favorable risk–benefit profile, eye care practitioners are now able to appropriately treat mild DED that manifests as periodic flares. Many ophthalmologists will be comfortable prescribing a short course of ocular corticosteroids, which may have a low risk of adverse effects, a few times per year.
KPI-121 0.25% may also be helpful for patients receiving chronic immunomodulatory treatment for DED, especially those with underlying autoimmune or inflammatory conditions. When a patient starts topical immunomodulatory therapy, KPI-121 0.25% has the potential to be used as induction therapy to quell ocular surface inflammation and DED symptoms until the new therapeutic agent takes effect. Similarly, in patients using chronic immunomodulatory therapy for DED who nonetheless experience periodic episodes of breakthrough symptoms, KPI-121 0.25% could be effective in pulsed doses to treat episodic DED signs and symptoms. With its capacity to deliver drugs efficiently to the corneal epithelium, KPI-121 0.25% might also be used in place of traditional topical ophthalmic corticosteroids to optimize the ocular surface before cataract, or refractive surgery as a stable ocular surface is of utmost importance when obtaining biometric measurements for intraocular lens power calculations to ensure patients obtain superior postoperative visual outcomes. Similarly, KPI-121 0.25% has the potential to treat flares of DED after surgery. Ultimately, additional uses of KPI-121 0.25% may be explored in future studies.
Conclusion
MPP technology offers the possibility to deliver therapeutics efficiently to the ocular surface tissues. KPI-121 0.25%, recently approved for short-term treatment of signs and symptoms of DED, uses MPP to deliver a custom-engineered ocular corticosteroid, loteprednol etabonate, to the corneal and conjunctival epithelium. In clinical trials, KPI-121 0.25% reduced signs and symptoms of DED compared with vehicle when administered for 2 weeks. KPI-121 0.25% has the potential to effectively treat periodic flares of DED with a low risk of side effects.
Acknowledgments
Editorial support for this article was provided by Lisa Baker, PhD, and Esther Tazartes, MS, of the Global Outcomes Group.
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.K.G. is a consultant to Alcon, Allergan, HanAll Biopharma, Johnson & Johnson Vision, Kala Pharmaceuticals, Inc., New World Medical, Inc., Novartis, Ocular Therapeutix, Oyster Point Pharma, ReGenTree, LLC, Sight Sciences, Sun Pharmaceutical Industries Ltd., TearLab, TissueTech, Inc., and Zeiss. N.V. reports no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Editorial support for this paper was funded by Kala Pharmaceuticals, Inc.
ORCID iD: Preeya K. Gupta  https://orcid.org/0000-0003-0895-7870
https://orcid.org/0000-0003-0895-7870
Contributor Information
Preeya K. Gupta, Department of Ophthalmology, Duke University Eye Center, 4709 Creekstone Drive, Suite 100, Durham, NC 27703, USA.
Nandini Venkateswaran, Massachusetts Eye and Ear Infirmary, Department of Ophthalmology, Harvard Medical School, Boston, MA, USA.
References
- 1. Popov A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J Ocul Pharmacol Ther 2020; 36: 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. Int J Pharm 2013; 453: 56–64. [DOI] [PubMed] [Google Scholar]
- 3. Taherali F, Varum F, Basit AW. A slippery slope: on the origin, role and physiology of mucus. Adv Drug Deliv Rev 2018; 124: 16–33. [DOI] [PubMed] [Google Scholar]
- 4. Ruponen M, Urtti A. Undefined role of mucus as a barrier in ocular drug delivery. Eur J Pharm Biopharm 2015; 96: 442–446. [DOI] [PubMed] [Google Scholar]
- 5. Yang M, Lai SK, Wang YY, et al. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew Chem Int Ed Engl 2011; 50: 2597–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang BC, Dawson M, Lai SK, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci USA 2009; 106: 19268–19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang YY, Lai SK, Suk JS, et al. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed Engl 2008; 47: 9726–9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev 2009; 61: 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popov A, Enlow E, Bourassa J, et al. Mucus-penetrating nanoparticles made with “mucoadhesive” poly(vinyl alcohol). Nanomedicine 2016; 12: 1863–1871. [DOI] [PubMed] [Google Scholar]
- 10. Popov A, Schopf L, Bourassa J, et al. Enhanced pulmonary delivery of fluticasone propionate in rodents by mucus-penetrating nanoparticles. Int J Pharm 2016; 502: 188–197. [DOI] [PubMed] [Google Scholar]
- 11. Schopf LR, Popov AM, Enlow EM, et al. Topical ocular drug delivery to the back of the eye by mucus-penetrating particles. Transl Vis Sci Technol 2015; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schopf L, Enlow E, Popov A, et al. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther 2014; 3: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017; 15: 276–283. [DOI] [PubMed] [Google Scholar]
- 14. Farrand KF, Fridman M, Stillman IO, et al. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol 2017; 182: 90–98. [DOI] [PubMed] [Google Scholar]
- 15. Schaumberg DA, Dana R, Buring JE, et al. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol 2009; 127: 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schaumberg DA, Sullivan DA, Buring JE, et al. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 2003; 136: 318–326. [DOI] [PubMed] [Google Scholar]
- 17. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf 2017; 15: 575–628. [DOI] [PubMed] [Google Scholar]
- 18. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017; 15: 438–510. [DOI] [PubMed] [Google Scholar]
- 19. Rolando M, Zierhut M, Barabino S. Should we reconsider the classification of patients with dry eye disease? Ocul Immunol Inflamm. Epub ahead of print 12 November 2019. DOI: 10.1080/09273948.2019.1682618. [DOI] [PubMed] [Google Scholar]
- 20. Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology 2017; 124: S4–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amparo F, Dana R. Web-based longitudinal remote assessment of dry eye symptoms. Ocul Surf 2018; 16: 249–253. [DOI] [PubMed] [Google Scholar]
- 22. Iyer JV, Lee SY, Tong L. The dry eye disease activity log study. ScientificWorldJournal 2012; 2012: 589875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karakus S, Agrawal D, Hindman HB, et al. Effects of prolonged reading on dry eye. Ophthalmology 2018; 125: 1500–1505. [DOI] [PubMed] [Google Scholar]
- 24. Kim Y, Paik HJ, Kim MK, et al. Short-term effects of ground-level ozone in patients with dry eye disease: a prospective clinical study. Cornea 2019; 38: 1483–1488. [DOI] [PubMed] [Google Scholar]
- 25. Ousler GW, 3rd, Rimmer D, Smith LM, et al. Use of the controlled adverse environment (CAE) in clinical research: a review. Ophthalmol Ther 2017; 6: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. López-Miguel A, Tesón M, Martín-Montañez V, et al. Dry eye exacerbation in patients exposed to desiccating stress under controlled environmental conditions. Am J Ophthalmol 2014; 157: 788–798. [DOI] [PubMed] [Google Scholar]
- 27. Teson M, Gonzalez-Garcia MJ, Lopez-Miguel A, et al. Influence of a controlled environment simulating an in-flight airplane cabin on dry eye disease. Invest Ophthalmol Vis Sci 2013; 54: 2093–2099. [DOI] [PubMed] [Google Scholar]
- 28. Fernandez I, Lopez-Miguel A, Enriquez-de-Salamanca A, et al. Response profiles to a controlled adverse desiccating environment based on clinical and tear molecule changes. Ocul Surf 2019; 17: 502–515. [DOI] [PubMed] [Google Scholar]
- 29. Alberth M, Wu WM, Winwood D, et al. Lipophilicity, solubility and permeability of loteprednol etabonate: a novel, soft anti-inflammatory corticosteroid. J Biopharma Sci 1991; 2: 115–125. [Google Scholar]
- 30. Bodor N, Loftsson T, Wu WM. Metabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonate. Pharm Res 1992; 9: 1275–1278. [DOI] [PubMed] [Google Scholar]
- 31. Kala Pharmaceuticals Inc. INVELTYS [package insert], 2020. https://inveltys.com/pdf/inveltys-prescribing-information.pdf
- 32. Kim T, Sall K, Holland EJ, et al. Safety and efficacy of twice daily administration of KPI-121 1% for ocular inflammation and pain following cataract surgery. Clin Ophthalmol 2019; 13: 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kala Pharmaceuticals Inc. EYSUVIS [package insert], 2020. https://www.eysuvis.com/pdf/prescribing-information.pdf
- 34. Benitez-Del-Castillo JM, Moreno-Montañés J, Jiménez-Alfaro I, et al. Safety and efficacy clinical trials for SYL1001, a novel short interfering RNA for the treatment of dry eye disease. Invest Ophthalmol Vis Sci 2016; 57: 6447–6454. [DOI] [PubMed] [Google Scholar]
- 35. McMonnies CW. Conjunctival tear layer temperature, evaporation, hyperosmolarity, inflammation, hyperemia, tissue damage, and symptoms: a review of an amplifying cascade. Curr Eye Res 2017; 42: 1574–1584. [DOI] [PubMed] [Google Scholar]
- 36. Holland E, Nichols K, Foulks G, et al. Efficacy and safety of KPI-121 0.25% for short term relief in dry eye (STRIDE). In: ASCRS•ASOA symposium & congress, Virtual Meeting, 16–17 May 2020. [Google Scholar]