Abstract
Gastrointestinal stromal tumors are common mesenchymal tumors of the gastrointestinal tract. The major site of metastasis for gastrointestinal stromal tumors is the liver or peritoneum, while metastasis to the ovary is exceptionally rare. A 53-year-old woman visited the hospital for bloating and anorexia and was diagnosed with a huge gastric gastrointestinal stromal tumor and peritoneal metastasis in the pelvis on upper gastrointestinal endoscopy and abdominal enhanced computed tomography. After administration of imatinib, the tumor was significantly reduced, and we performed laparoscopic pelvic tumor resection and open proximal gastrectomy with transverse colectomy. Intraoperatively, the pelvic tumor was found to be an ovarian tumor. Microscopic examination confirmed a gastric gastrointestinal stromal tumor with ovarian metastasis. In conclusion, we experienced a rare case of gastric gastrointestinal stromal tumor with ovarian metastasis. Preoperative administration of imatinib was successful and radical resection was achieved. Although pelvic tumors are difficult to differentiate preoperatively, the possibility of ovarian metastasis from gastrointestinal stromal tumor should be considered.
Keywords: GIST, ovarian metastasis, neo adjuvant chemotherapy, imatinib
Introduction
Gastrointestinal stromal tumors (GISTs) are common mesenchymal tumors of the gastrointestinal tract.1 The most common primary site of GISTs is the stomach (56%), followed by the small intestine (32%), colon and rectum (6%), and esophagus (0.7%).2 The major site of metastasis for GISTs is the liver or peritoneum, and less frequently tumors metastasize to the lung, lymph nodes, and bone.3 Metastasis to the ovary is extremely rare. In this study, we performed proximal gastrectomy and left oophorectomy for a giant gastric GIST and pelvic tumor followed by administration of imatinib before surgery. We report a rare case of a GIST presenting an isolated ovarian metastasis that was treated by neoadjuvant chemotherapy.
Case report
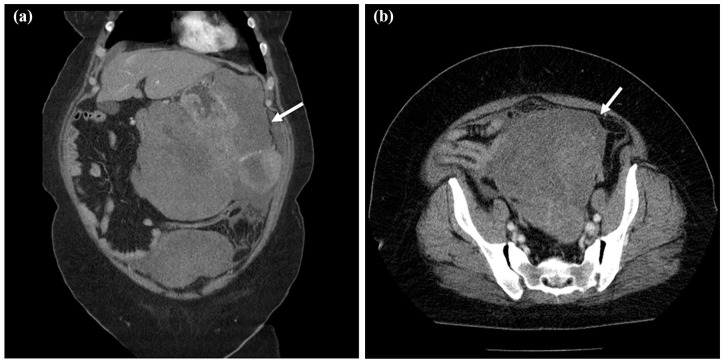
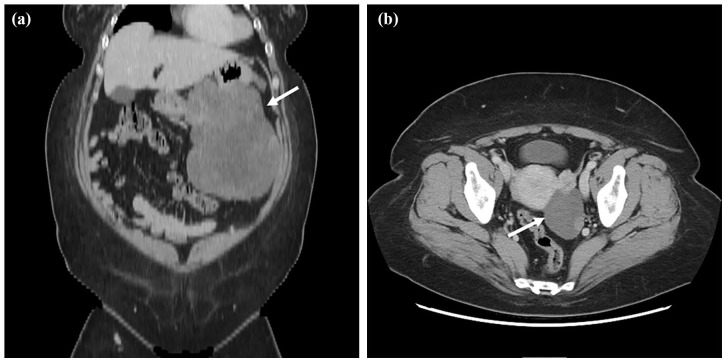
A 53-year-old woman was aware of bloating and anorexia for 2 months before visiting the hospital. She had visited another doctor previously because of persistent symptoms. Her medical history was only bronchial asthma and she had no history of abdominal surgery. The physical findings revealed bloating, softness, and no tenderness in the abdomen. Obesity was also recognized for a body mass index (BMI) of 41.7. Upper gastrointestinal endoscopy showed a huge ulcerative lesion on the posterior wall of the stomach accompanied by a small amount of bleeding (Figure 1). Biopsies of the ulcer showed a dense infiltration of short spindle cells. Expression of c-Kit was positive by immunostaining, suggesting GIST. We performed an abdominal enhanced computed tomography (CT) scan, which revealed thickening of the posterior wall of the stomach and a continuous heterogeneously enhancing mass protruding outside the stomach wall (23.4 × 21.1 cm; Figure 2(a)). The tumor largely occupied the upper to lower left abdomen, and part of tumor was notably stained around this. Furthermore, another large tumor in the pelvic cavity was also detected on the CT scan (13.8 × 13.1 cm; Figure 2(b)). The uterus was visible, but the bilateral ovaries were unclear because of the huge tumor. Blood sampling data showed Hb = 9.0 and anemia. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels were within normal limits; however, her carbohydrate antigen 125 (CA125) levels were highly elevated to 818 U/mL (normal = <35 U/mL). She was diagnosed with a giant GIST originating from the stomach with peritoneal metastasis. In the current case, the tumor was large and difficult to remove. Furthermore, the risk of recurrence was high, even if resection was possible. We decided to administer imatinib as preoperative chemotherapy and then perform surgery. She started imatinib at 400 mg/day for 4 months and did not present any side effects. Four months after initiation of imatinib, abdominal enhanced CT revealed that the left upper abdominal mass and the pelvic mass had markedly regressed (17.7 × 11.6 cm and 6.5 × 4.6 cm; Figure 3(a) and (b)), indicating that the imatinib treatment was effective. Bilateral ovaries were clarified, and the tumor and left ovary were in contact. CA125 had also decreased significantly to 1.3 U/mL. Next, we planned to perform laparoscopic pelvic tumor resection and proximal gastrectomy.
Figure 1.
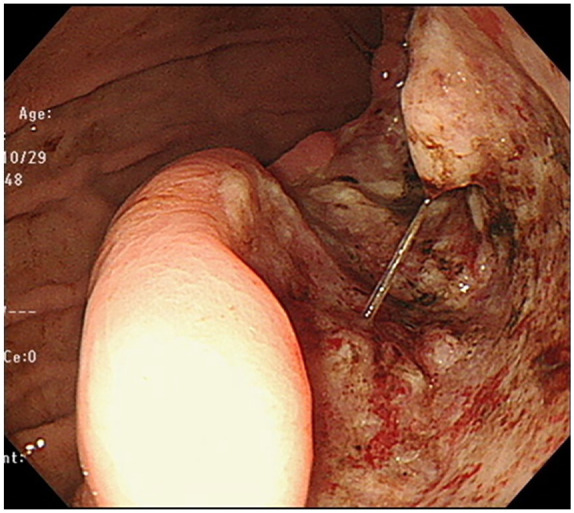
Upper gastrointestinal endoscopy revealed an ulcerative lesion on the posterior wall of the stomach.
Figure 2.
CT scan images before treatment. (a) Thickening of the posterior wall of the stomach and a continuous heterogeneously enhanced mass protruding outside the stomach wall (white arrow, 23.4 × 21.1 cm). The tumor largely occupied the upper to lower left abdomen. (b) A large tumor in the pelvic cavity was also detected on the CT scan (white arrow, 13.8 × 13.1 cm).
Figure 3.
CT scan images after imatinib administration. (a) Left upper abdominal tumor size was approximately 17.7 × 11.6 cm (white arrow). (b) Pelvic tumor size was approximately 6.5 × 4.6 cm (white arrow).
Explorative laparoscopy showed that the tumor, which was thought to be peritoneal metastasis in the pelvis, was found to be in the left ovarian tumor intraoperatively, and no peritoneal metastasis was found in the peritoneal cavity. The left ovary presented a swelling that was white, irregular, and round-shaped with adherence to the rectum but with no invasion (Figure 4). The right ovary had a normal appearance. The left ovary was resected by laparoscopic surgery without leaving tumor tissue and then gastrectomy was performed by open surgery. As the primary GIST had invaded the transverse mesocolon, we performed proximal gastrectomy with transverse colectomy, and radical resection was achieved. The surgery time was 6 h and 37 min, and blood loss was 640 mL.
Figure 4.
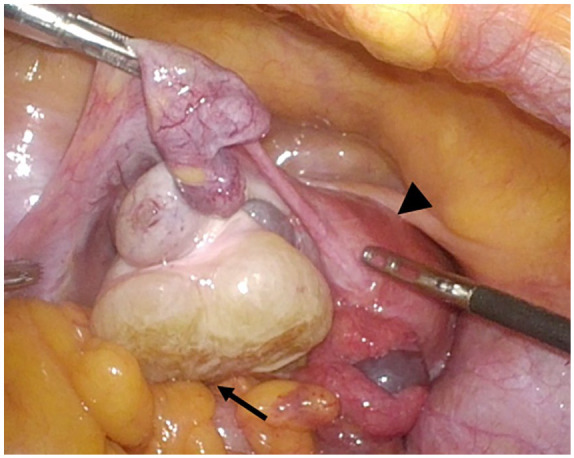
Laparoscopic image showing the pelvic tumor originating from the left ovary. The left ovarian tumor presented a swelling that had white regions and was of an irregular and round shape with adherence to the rectum but exhibited no invasion (black arrow). Black arrowhead indicates uterus.
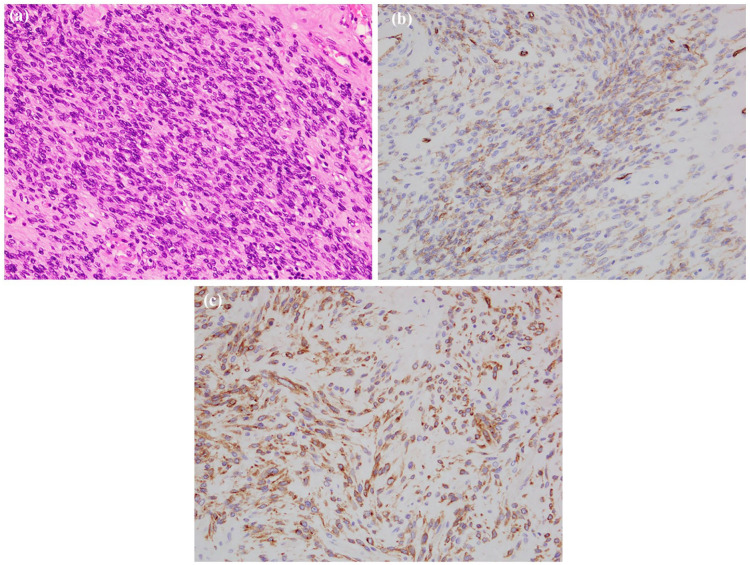
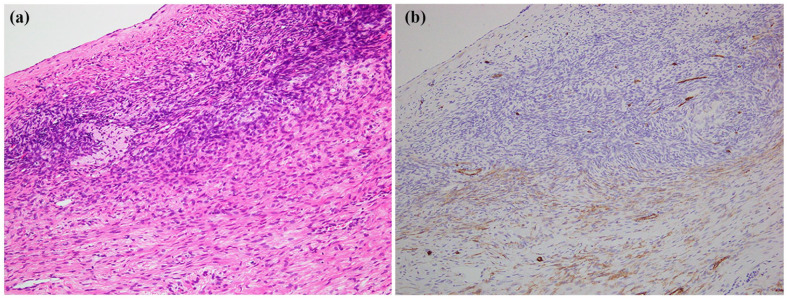
Histopathological analysis revealed the gastric tumor was 255 mm in size and was located in the subserosa of the stomach and projected to the omentum bursa. The tumor formed an ulceration at the posterior wall of the upper region of the stomach. Histologically, half of the tumor consisted of hyaline cells with visible spindle cells densely proliferating in the marginal region of the tumor (Figure 5(a)). A focal area with an accumulation of foamy cells and deposition of the hemosiderin was observed. In immunohistochemistry, the tumor cells were focally positive for CD34 (Figure 5(b)), c-Kit (Figure 5(c)), and DOG1, and negative for SMA and S-100. The ovarian tumor was 77 mm in size and yellowish translucent with a focal white and brown area on the cut surface. Histologically, most of the tumor was hyalinized with focal spindle cells coarsely proliferating. Spindle cells had proliferated in the fascicle adjacent to the ovarian stroma in the marginal region (Figure 6(a)). There was also focal necrosis with calcification and hemosiderin-laden macrophages. In immunohistochemistry for CD34, the tumor spindle cells were positive, but the ovarian stroma was negative (Figure 6(b)). Immunostaining showed that c-Kit was focally positive in ovarian tumor.
Figure 5.
Pathology of the gastric gastrointestinal stromal tumor (GIST). (a) Histology of the viable region. Spindle cells with oval nuclei arranges in fascicles. Original magnification, ×200. (b) Immunohistochemistry – tumor cells positive for CD34 and (c) immunohistochemistry – tumor cells positive for c-Kit.
Figure 6.
Pathology of the ovarian tumor. (a) Histology of the marginal region. Spindle cells proliferating in the fascicle adjacent to the ovarian stroma. Original magnification, ×100. (b) Immunohistochemistry for CD34. Same as (a). Spindle tumor cells were positive for CD34, but the ovarian stroma was negative.
Based on the above, we diagnosed ovarian metastasis of gastric GIST. The patient was discharged from the hospital with a normal postoperative course and is currently receiving imatinib as adjuvant chemotherapy. There has been no recurrence for 10 months after the operation.
Discussion
GIST originates from the intestinal cells of Cajal within the muscle layer, which are the pacemaker cells of gastrointestinal movement.4 GIST expresses the CD117 antigen (c-Kit) and gains functional mutations responsible for activating the growth of these tumors.5 The liver, omentum, or peritoneum are the most frequent sites of metastasis, and metastasis to the ovary is exceedingly rare. In the current case, the ovarian tumor showed spindle-shaped cells similar to gastric GIST, and immunohistochemistry was positive for CD34 and c-Kit; therefore, we diagnosed the ovarian tumor as metastasis of GIST. To our knowledge, ovarian metastasis from GIST has been previously described in only six cases in two series.6,7 In previous reports, patients have been middle-aged to elderly (44−81 years old; median = 54 years old). Four cases were bilateral ovarian metastasis and two were unilateral cases. Five cases were from small bowel GISTs and one was from a stomach GIST, the same as our patient. This is in accordance with the recognized site-dependent behavior of GISTs, with those of small bowel origin more likely to recur or metastasize than those of gastric origin.8,9
Ovarian metastasis from malignant tumors located elsewhere is known as a Krukenberg tumor. Krukenberg tumor is an unusual metastatic tumor of the ovary first described by Friedrich Krukenberg, and Schlagenhaufer stated that the most common primary site is the gastrointestinal tract.10,11 These tumors are relatively uncommon, are bilateral in 80% of cases, and account for only 1%−2% of all ovarian tumors.12–15 The stomach was previously reported to be the site of most primary tumors responsible for this tumor,16 while recent literature indicates an increased frequency of Krukenberg tumors of colorectal origin.17–19 Three possible pathways of metastasis to the ovary have been considered: via peritoneal spread, via lymphatic pathways, and via vascular pathways.20,21 In the current case, peritoneal spread was negative because the ovarian capsule was preserved and the external surface of the ovaries lacked tumor seedings, while other lesions including peritoneal metastasis were absent. Presence of a Krukenberg tumor in gastric cancer is generally considered to be caused by lymphatic metastasis.21 However, the frequency of lymph node metastasis is low in GIST and was not observed in this case. In our case, the transvascular pathway was most suspected because hematological metastasis such as liver metastases is common for GISTs. However, there are many unclear points, and further studies are required.
There are various types of ovarian tumors including benign and borderline disease, and it is often difficult to diagnose them before surgery. Furthermore, in the gynecological region, when large ovarian tumors are in contact with the uterus or bladder, it is difficult to distinguish them from uterine bladder tumors in images.22 In the current case, the pelvic tumor was large, and it was difficult to identify the ovary. Imatinib treatment significantly reduced the tumor, and the ovaries could be identified and were found to be in contact with the ovaries. The pelvic tumor was thought to be of the same tissue type because imatinib is also effective against pelvic tumors. Previously, only one case of primary ovarian GIST was reported.23 In our case, there was a large GIST in the stomach, and both the stomach and ovarian tumors shrank with imatinib administration. Considering these clinical findings, it is reasonable to assume that the ovarian lesion is a metastasis of gastric GIST. However, the possibility of ovarian primary cannot be completely ruled out, and it is a subject for further investigation.
The gold standard of treatment for localized GISTs is surgical resection. Laparoscopic surgery was previously recommended for GISTs that are less than 5 cm. Recently, the long-term survival of patients was reported to mainly depend on the nature of the tumor itself,24 and the surgical method should be determined in each case considering the site, size, and presence of other organ invasions. Preoperative imatinib administration should be considered to reduce the tumor size and to preserve the organ because the purpose of surgical treatment is to achieve R0 resection. Particularly, when lesions are large, in difficult locations (e.g. low rectum, esophagus, gastroesophageal junction, and duodenum), or have a tendency to rupture, preoperative administration of imatinib may effectively downsize the tumor.25 There have been no reports of curative resection of GISTs with ovarian metastasis treated with preoperative chemotherapy. Our result suggests preoperative chemotherapy may be useful even in the treatment of ovarian metastasis. In our case, laparoscopic examination confirmed the regressed ovarian tumor, and diagnosed no other metastasis, thus radical resection was possible. Previous reports suggest that surgery in combination with sunitinib (tyrosine kinase receptor inhibitor) may increase progression-free survival and overall survival in highly selected patients.26,27 When the response to imatinib is poor, sunitinib administration may be considered as preoperative chemotherapy.
There are several reports for the prognosis of GIST. DeMatteo et al.28 reported that the 5-year survival rate in patients who underwent complete resection was 54%, and a tumor size of >10 cm was the worst prognostic factor. Regarding ovarian metastasis, patients with a prognosis of between 1 and 6.5 years (mean = 2.8 years) from the time of ovarian tumor diagnosis indicated that the prognosis of ovarian metastasis of GIST is poor.6 As Irving et al.6 did not mention the use of imatinib in their report, the effect and prognosis of its use for metastasis to the ovary require further study. Imatinib administration for 3 years as adjuvant therapy for patients with high-risk GIST who have undergone complete tumor resection is recommended,29 and thus we plan to use imatinib for a certain period after surgery.
In conclusion, we experienced a rare case of gastric GIST with ovarian metastasis. Preoperative administration of imatinib was successful and radical resection was possible. Although pelvic tumors may be difficult to differentiate preoperatively, ovarian metastasis of GISTs should be considered.
Acknowledgments
The author thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Consent to publish: All authors consent to the publication of the manuscript in this journal.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Takahisa Yamaguchi  https://orcid.org/0000-0001-8086-739X
https://orcid.org/0000-0001-8086-739X
Availability of data and materials: The data set supporting the conclusions of this article is included within the article.
References
- 1. Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018; 24: 2806–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soreide K, Sandvik OM, Soreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol 2016; 40: 39–46. [DOI] [PubMed] [Google Scholar]
- 3. Patnaik S, Jyotsnarani Y, Rammurti S. Radiological features of metastatic gastrointestinal stromal tumors. J Clin Imaging Sci 2012; 2: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998; 152(5): 1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 5. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279: 577–580. [DOI] [PubMed] [Google Scholar]
- 6. Irving JA, Lerwill MF, Young RH. Gastrointestinal stromal tumors metastatic to the ovary a report of five cases. Am J Surg Pathol 2005; 29(7): 920–926. [DOI] [PubMed] [Google Scholar]
- 7. De Leo A, Nannini M, Dondi G, et al. Unusual bilateral ovarian metastases from ileal gastrointestinal stromal tumor (GIST): a case report. BMC Cancer 2018; 18: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol 1999; 23(1): 82–87. [DOI] [PubMed] [Google Scholar]
- 9. Ueyama T, Guo KJ, Hashimoto H, et al. A clinicopathologic and immunohistochemical study of gastrointestinal stromal tumors. Cancer 1992; 69: 947–955. [DOI] [PubMed] [Google Scholar]
- 10. Spinelli C, Liloia C, Piscioneri J, et al. An unusual evolution of Krukenberg tumour: a case report. J Clin Diagn Res 2016; 10(10): PD07–PD11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang R, Tang J, Cheng X, et al. Surgical treatment for patients with different origins of Krukenberg tumours: outcomes and prognostic factors. Eur J Surg Oncol 2009; 35: 92–97. [DOI] [PubMed] [Google Scholar]
- 12. Moghazy D, Al-Hendy O, Al-Hendy A. Krukenberg tumour presenting as back pain and a positive urine pregnancy test: a case report and literature review. J Ovarian Res 2014; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fazzari C, Fedele F, Pizzi G, et al. Krukenberg tumour of the ovary: a case report with light microscopy, immunohistochemistry and electron microscopy study. Anticancer Res 2008; 28(2B): 1417–1420. [PubMed] [Google Scholar]
- 14. Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med 2006; 130(11): 1725–1730. [DOI] [PubMed] [Google Scholar]
- 15. Sandhu S, Arafat O, Patel H, et al. Krukenberg tumor: a rare cause of ovarian torsion. J Clin Imaging Sci 2012; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yada-Hashimoto N, Yamamoto T, Kamiura S, et al. Metastatic ovarian tumors: a review of 64 cases. Gynecol Oncol 2003; 89(2): 314–317. [DOI] [PubMed] [Google Scholar]
- 17. Jeung YJ, Ok HJ, Kim WG, et al. Krukenberg tumors of gastric origin versus colorectal origin. Obstet Gynecol Sci 2015; 58: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu F, Zhao X, Mi B, et al. Clinical characteristics and prognostic analysis of Krukenberg tumor. Mol Clin Oncol 2015; 3(6): 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. En IS, Hwarng G, Hwei G, et al. Palliative surgery for Krukenberg tumors – 12-year experience and review of the literature. World J Clin Oncol 2018; 9: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Disaia P, Creasman W. Clinical gynecologic oncology. 5th ed. St. Louis, MO: Mosby, 1997, pp. 369–371. [Google Scholar]
- 21. Kakushima N, Kamoshida T, Hirai S, et al. Early gastric cancer with Krukenberg tumor and review of cases of intramucosal gastric cancers with Krukenberg tumor. J Gastroenterol 2003; 38(12): 1176–1180. [DOI] [PubMed] [Google Scholar]
- 22. Ijeri SK, Rathod PS, Kundargi R, et al. Gastrointestinal stromal tumor mimicking as ovarian tumor in gynaecologic oncology. Indian J Surg Oncol 2016; 7(1): 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaballa KM, Metwally IH, Refky B, et al. Ovarian gastrointestinal stromal tumor: does this diagnosis exist? Eur J Gynaecol Oncol 2017; 38(1): 147–149. [PubMed] [Google Scholar]
- 24. Chen QL, Pan Y, Cai JQ, et al. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: an updated systematic review and meta-analysis. World J Surg Oncol 2014; 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012; 19(4): 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bamboat ZM, DeMatteo RP. Metastasectomy for gastrointestinal stromal tumors. J Surg Oncol 2014; 109(1): 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scanchez-Hidalgo JM, Duran-Martinez M, Molero-Payan R, et al. Gastrointestinal stromal tumors: a multidisciplinary challenge. World J Gastroenterol 2018; 24(18): 1925–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231(1): 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012; 307: 1265–1272. [DOI] [PubMed] [Google Scholar]